Published online May 19, 2025. doi: 10.5498/wjp.v15.i5.102706
Revised: January 26, 2025
Accepted: February 14, 2025
Published online: May 19, 2025
Processing time: 185 Days and 23.8 Hours
Depression, non-suicidal self-injury (NSSI), and suicide attempts (SA) often co-occur during adolescence and are associated with long-term adverse health outcomes. Unfortunately, neural mechanisms underlying self-injury and SA are poorly understood in depressed adolescents but likely relate to the structural abnormalities in brain regions.
To investigate structural network communication within large-scale brain networks in adolescents with depression.
We constructed five distinct network communication models to evaluate structural network efficiency at the whole-brain level in adolescents with depression. Diffusion magnetic resonance imaging data were acquired from 32 healthy controls and 85 depressed adolescents, including 17 depressed adolescents without SA or NSSI (major depressive disorder group), 27 depressed adolescents with NSSI but no SA (NSSI group), and 41 depressed adolescents with SA and NSSI (NSSI + SA group).
Significant differences in structural network communication were observed across the four groups, involving spatially widespread brain regions, particularly encompassing cortico-cortical connections (e.g., dorsal posterior cingulate gyrus and the right ventral posterior cingulate gyrus; connections based on precentral gyrus) and cortico-subcortical circuits (e.g., the nucleus accumbens-frontal circuit). In addition, we examined whether compromised communication efficiency was linked to clinical symptoms in the depressed adolescents. We observed significant correlations between network communication efficiencies and clinical scale scores derived from depressed adolescents with NSSI and SA.
This study provides evidence of structural network communication differences in depressed adolescents with NSSI and SA, highlighting impaired neuroanatomical communication efficiency as a potential contributor to their symptoms. These findings offer new insights into the pathophysiological mechanisms underlying the comorbidity of NSSI and SA in adolescent depression.
Core Tip: This study investigates structural network communication differences in depressed adolescents, highlighting impaired brain network efficiency in those with non-suicidal self-injury and suicide attempts. Using diffusion magnetic resonance imaging, the research identifies disrupted cortico-cortical and cortico-subcortical circuits, particularly in regions such as the posterior cingulate gyrus and nucleus accumbens. The findings suggest that compromised neural communication is linked to clinical symptoms, offering new insights into the neurobiological mechanisms behind the comorbidity of non-suicidal self-injury and suicide attempts in depression.
- Citation: Wang S, Qin JL, Yang LL, Ji YY, Huang HX, Gao XS, Zhou ZM, Guo ZR, Wu Y, Tian L, Ni HJ, Zhou ZH. Structural network communication differences in drug-naive depressed adolescents with non-suicidal self-injury and suicide attempts. World J Psychiatry 2025; 15(5): 102706
- URL: https://www.wjgnet.com/2220-3206/full/v15/i5/102706.htm
- DOI: https://dx.doi.org/10.5498/wjp.v15.i5.102706
Depression, non-suicidal self-injury (NSSI), and suicide represent significant public health challenges in adolescents[1-3], often co-occurring during this developmental stage and contributing to long-term adverse health outcomes[4,5]. Depression is widely recognized as one of the primary risk factors for suicidal thoughts and behaviors[6]. Frequent NSSI incidents are associated with an elevated risk of suicide attempts (SA)[7], a critical predictor of completed suicide[8]. Suicidal behavior often originates from NSSI. However, the etiologies of suicidal behavior and NSSI are multifaceted and only partially overlap, suggesting the involvement of both shared and distinct vulnerability factors in the development of SA and NSSI[9].
Depression is conceptualized as a disconnection syndrome[10]. Consistent with this perspective, diffusion magnetic resonance imaging (dMRI) studies have identified widespread abnormalities in white matter structures in depression, encompassing both local micro-structure integrity[11] and system-level connectome disruptions[12]. Structural connectivity constrains and facilitates neural information transfer, which in turn gives rise to synchronization between neural elements[13]. Hence, decreases in structural connectivity might influence anatomically disconnected pairs of regions’ communication, which could have an effect on the brain’s overall signal traffic[14]. There is evidence to support the idea that examining models of indirect connectivity can offer a deeper understanding of psychosis symptomatology than what can be determined solely by direct anatomical connections[15]. Thus, examining not only direct connections but also indirect communication pathways can offer a more thorough understanding of disruptions in connectivity in depression. The neural mechanisms underlying self-injury and SA in depressed adolescents remain poorly understood but are likely associated with structural abnormalities in specific brain regions. To date, limited evidence from a handful of dMRI studies has suggested an association between white matter abnormalities and NSSI or SA in depression[16,17]. For example, by using tract-based spatial statistics with a region of interest analysis, Hu et al[16] found that reduced cingulum integrity in the left dorsal cingulum was negatively associated with the severity of NSSI in depressed adolescents with NSSI.
The architecture of structural brain networks influences patterns of interaction and signaling between neurons and brain areas, and the ensuing communication dynamics are critical for brain function[18]. To capture information flow between nodes that are directly linked and those that are not, a variety of network communication models have been proposed to investigate structural network communication in large-scale brain networks[18]. These models cover a wide range of neural signaling conceptualizations. For instance, shortest paths[19] and navigation[20] deterministically route information using centralized and decentralized strategies, respectively. In contrast, diffusion[21] and search information model communication[22] from the stochastic perspective of random walk processes. Finally, communicability[23] implements a broadcasting model of signaling, in which signals are simultaneously propagated along multiple network fronts. Network communication offers a theoretical framework that can contribute to understanding behavior and brain dynamics in complex mental disorders[24]. Nevertheless, no previous study has examined information transfer between both directly connected and unconnected nodes using communication models mentioned above in depressed adolescents. Therefore, investigating how large-scale neural signaling differs in depressed adolescents using different communication models, and whether these possible differences are related to symptom profiles in depressed adolescents might help to improve our understanding of the pathophysiology of comorbidity between NSSI and SA in depression.
By establishing network communication models including shortest path efficiency, navigation efficiency, diffusion efficiency, search information and communicability, we intended to explore structural network communication in large-scale brain networks in depressed adolescents. We hypothesized that depressed adolescents with NSSI and SA would show different patterns of impaired whole-brain communication. Drawing on previous studies[16,17], we further hypothesized that symptom profiles (i.e., clinical scale scores) would correlate with abnormal communication metrics in depressed adolescents.
We recruited 105 adolescents with depression from The Affiliated Mental Health Center of Jiangnan University, China. Depressed adolescents met the criteria for major depressive disorder in the fifth edition of Diagnostic and Statistical Manual of Mental Disorders[25] and were classified into three groups: 20 depressed adolescents without SA or NSSI (major depressive disorder group), 35 depressed adolescents with NSSI but no SA (NSSI group), and 50 depressed adolescents with SA and NSSI (NSSI + SA group). At the time of their magnetic resonance imaging (MRI) scans, all depressed adolescents were drug-naive. Two independent Ottawa Self-Injury Inventory sub-scales were used to assess the frequency and functions of NSSI behaviors[26]. The evaluation of SA was assessed with the Beck Scale for Suicide Ideation (BSS)[27]. Additionally, depressed adolescents’ current levels of depression and anxiety were assessed using the 17-item Hamilton Depression Scale (HAMD-17) and the 14-item Hamilton Anxiety Scale (HAMA-14)[28,29]. Healthy controls (HC) were recruited from the local community via advertisements and were free of a history or current diagnosis of any psychiatric disorder. All participants were assessed to be right-handed using the Edinburgh Handedness Inventory[30]. A brief structured clinical interview tool, the Mini International Neuropsychiatric Interview[31], was used to screen for several psychiatric disorders. Exclusion criteria included intracranial pathology, brain injury, neurological illness, alcohol or substance abuse, contraindications to MRI, and excessive head motion in the subsequent data analysis. Finally, 85 depressed adolescents and 32 HC were included in the imaging analysis (Table 1). All participants were given detailed information regarding the purpose and procedures of the study. Formal written consents were obtained from participants and their parents or legal guardians before participation. The study was approved by the Medical Ethics Committee of The Affiliated Mental Health Center of Jiangnan University and performed in accordance with the Declaration of Helsinki.
| Variables | NSSI + SA | NSSI | MDD | HC | P value |
| Gender (male/female) | 6/35 | 4/23 | 7/10 | 12/20 | 0.03a |
| Age (years) | 15.59 ± 2.16 | 15.70 ± 2.64 | 16.41 ± 2.35 | 20.18 ± 2.67 | < 0.001b |
| Education (years) | 8.66 ± 1.77 | 9.44 ± 2.72 | 10.05 ± 1.91 | 14.34 ± 2.42 | < 0.001b |
| HAMD-17 | 22.83 ± 4.75 | 18.37 ± 5.04 | 16.71 ± 5.11 | - | < 0.001b |
| HAMA-14 | 21.51 ± 7.57 | 20.44 ± 8.20 | 17.82 ± 8.40 | 0.002b |
MRI scans were acquired with a Magnetom Skyra 3.0T MRI (Siemens Medical System, Erlangen, Germany) at the Department of Medical Imaging, Huadong Sanatorium, Wuxi, China. All participants obtained dMRI data and high-resolution three-dimensional T1-weighted images. Foam pads were used to reduce head motion and noise from the scanner. T1-weighted images were acquired using a 3D-MPRAGE sequence with the following parameters: Repetition time/echo time = 2530/2.98 milliseconds, 192 sagittal slices, thickness = 1 mm, flip angle = 7°, matrix = 256 × 256, voxel size = 1 mm × 1 mm × 1 mm, and field of view (FOV) 256 mm. Whole brain, high angular resolution diffusion imaging was acquired using a spin-echo echo-planar imaging pulse sequence with the following parameters: Echo time = 78 milliseconds, repetition time = 11600 milliseconds, voxel size = 2 mm × 2 mm × 2 mm, 75 axial slices, FOV 224 mm, b-value = 1000 second/mm2 in 64 non-collinear gradient directions. A single non-diffusion-weighted b0 image was also obtained.
We visually inspected the T1-weighted (T1W) and dMRI images of all subjects to detect any signal dropouts or artifacts. Next, we pre-processed the images via the well-established pipeline, described as follows: For both T1W and dMRI data, the procedure began with axial alignment, centering, Gibbs ringing removal based on Local Subvoxel-Shifts[32], and intensity inhomogeneity correction via N4ITK[33]. For dMRI data, we also included the following steps: (1) Marchenko-Pastur principal-component analysis denoising[34] to improve the signal-to-noise ratio without reducing spatial resolution; (2) FSL’s eddy correct tool was used for eddy current correction[35]; (3) Brain mask generation using a con
We conducted whole-brain tractography using a probability fiber tracking algorithm, which fits a mixture of fiber orientation distribution function to the diffusion signal. The fiber orientation distribution function was estimated using the multi-shell multi-tissue constrained spherical deconvolution model[36]. Whole-brain streamlines were generated using the iFOD2 algorithm[37] with the following parameters: 50 million seeds, random placement in gray and white matter volume, Runge-Kutta 4th order integration, 15° and 30° angle, respectively, constrained by anatomical tissues and cropping at gray-white matter interface. The resulting tractograms were filtered with the spherical-deconvolution informed filtering of the tractograms algorithm[38] to reduce the false positive connections to 5 million streamlines[39].
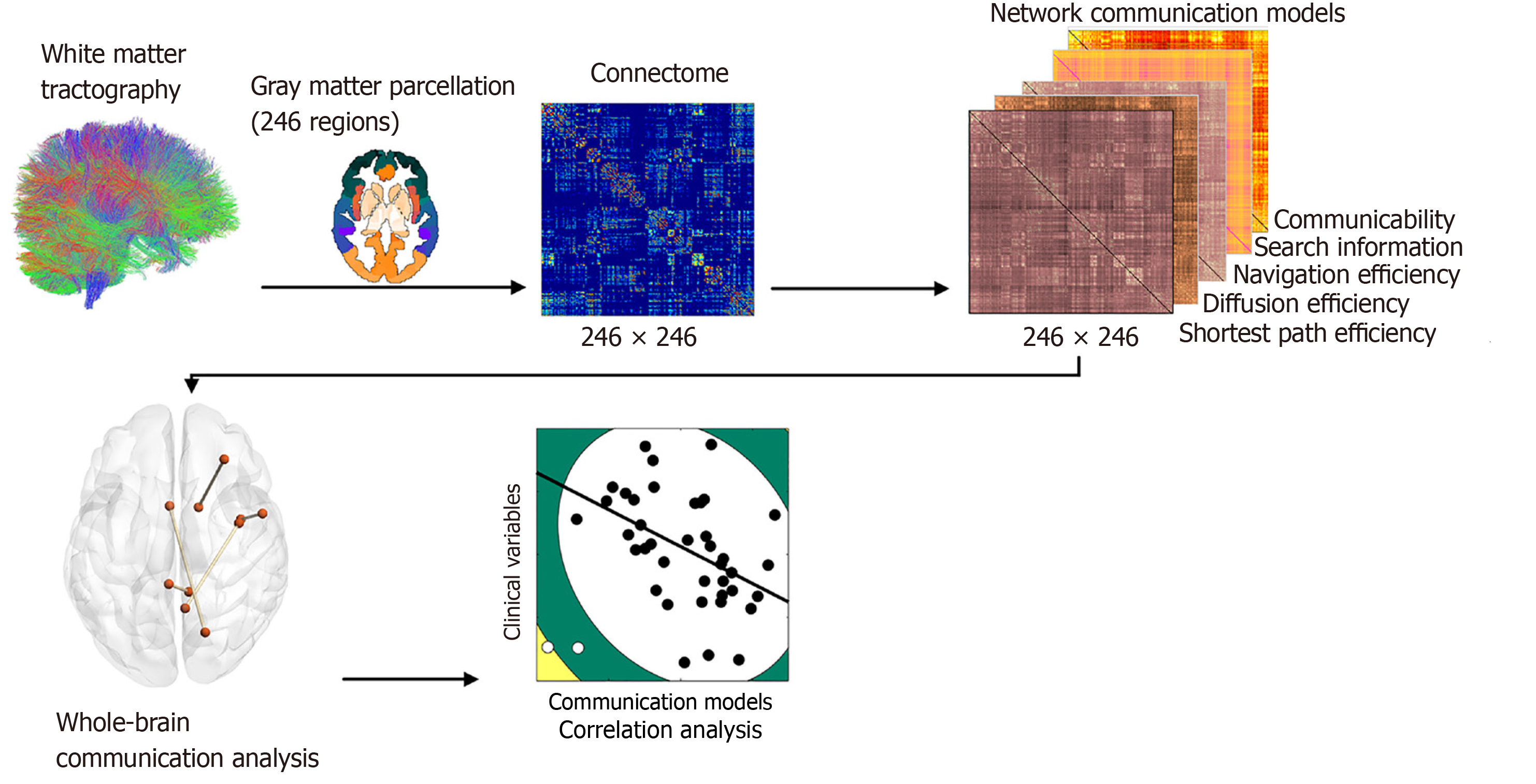
Figure 1 depicts the flowchart of this work. Specifically, network nodes were based on the 246 cortical and subcortical regions comprising the brainnetome atlas[40]. Using the inverse of the transform information, this atlas in the Montreal Neurologic Institute space was registered into each subject’s native space. Edges were defined as interregional fibers between each pair of nodes and met the conditions: (1) At least two double-ended fibers passed through pairwise nodes; and (2) The length of the passing fibers was greater than 10 mm. Each element of the weighted connectivity matrix W was populated with streamline counts between the corresponding pair of regions.
We computed five popular network communication models, namely, shortest-path efficiency, navigation efficiency, diffusion efficiency, search information, and communicability. Their formal mathematical definitions and meanings have been described in detail elsewhere[13,41]. In brief, the connectivity measures used in this study capture different aspects of brain network function. Shortest paths represent the most efficient routes for information transfer, highlighting rapid and energy-efficient communication. Navigation simulates decentralized routing based on local information, reflecting adaptive signal transmission in dynamic networks. Diffusion models random signal propagation, is relevant for ex
To detect statistical significance of group differences in demographic variables between the patients and HC group, the χ2 analysis and one-way analysis of variance were used as appropriate by SPSS 25.0 software (SPSS, Chicago, IL, United States). The Mann-Whitney U test was used to compare the difference of scale scores between NSSI + SA and SA for each of the measures. Using the F-test in conjunction with a false discovery rate (FDR) method within the network-based statistic framework, we conducted a comparative analysis across four groups for five distinct network communication models[45]. Notably, our analysis employed a significance threshold of P < 0.05 and incorporated 5000 permutations to ensure a comprehensive whole-brain comparison. The FDR can be more sensitive to focal effects involving single, isolated connections. The FDR enables rejection of the null hypothesis at the level of individual connections, while controlling the FDR; that is, the proportion of false positive connections among all positive connections. After the above comparison, we used the Wilcoxon signed-rank test to conduct post-hoc for the five communication models. It should be noted that age, gender and education year were treated as covariables, which were regressed out of all the communication models. The observed connections with significant between-group differences across the groups were subsequently subjected to a correlation analysis with the clinical variables of each patient cohort. This approach aimed to elucidate potential associations between network communication models and clinical manifestations. Regressing the aforementioned covariates from both the connections and clinical variables separately, we subsequently conducted correlation analysis using the Spearman method.
Demographic and clinical characteristics of the adolescents are presented in Table 1. There were significant differences in the gender, age, education level, HAMD-17 and HAMA-14 scores among the groups (P < 0.05). The results showed that there was no significant difference in the Ottawa self-injury inventory-frequency between the NSSI + SA and NSSI groups (P > 0.05) (Table 2). Regarding Ottawa self-injury inventory-functions, except for anti-suicide, interpersonal influence and sensation seeking, the remaining indicators were significantly different between the NSSI + SA and NSSI groups (P < 0.05). BSS scores in the NSSI + SA group are summarized in Table 3.
| Variables | NSSI + SA | NSSI | Z value (P value) |
| OSI-frequency | |||
| In the past one month | 2 (1-3) | 2 (1-2) | -0.32 (0.74)a |
| In the past six months | 3 (1-3) | 2 (1-3) | -0.41 (0.68)a |
| In the past one year | 2 (1-3) | 2 (1-3) | -5.11 (0.61)a |
| One year ago | 2 (0-3) | 1 (0-3) | -0.52 (0.61)a |
| OSI-functions | |||
| Affect regulation | 19 (14-23) | 16 (9-18) | -2.37 (0.018)a |
| Anti-dissociation | 9 (7-11) | 7 (4-8) | -3.23 (0.001)a |
| Anti-suicide | 0 (0-5) | 1 (1-6) | -3.36 (0.71)a |
| Interpersonal boundaries | 1 (0-3) | 0 (0-2) | -2.33 (0.02)a |
| Interpersonal influence | 6 (2-12) | 5 (3-9) | -0.45 (0.66)a |
| Self-punishment | 3 (2-4) | 2 (0-3) | -2.37 (0.018)a |
| Sensation seeking | 2 (0-4) | 0 (0-1) | -1.58 (0.114)a |
| Addictive features | 2 (0-3) | 1 (0-2) | -2.35 (0.019)a |
| Variables | Median |
| BSS-screen (in the past one week) | 12.0 (9.5-13.5) |
| BSS-screen (previous most depressed) | 15.0 (13.5-15.0) |
| Total BSS score (in the past one week) | 51.5 (40.9-66.7) |
| Total BSS score (previous most depressed) | 75.8 (68.2-84.9) |
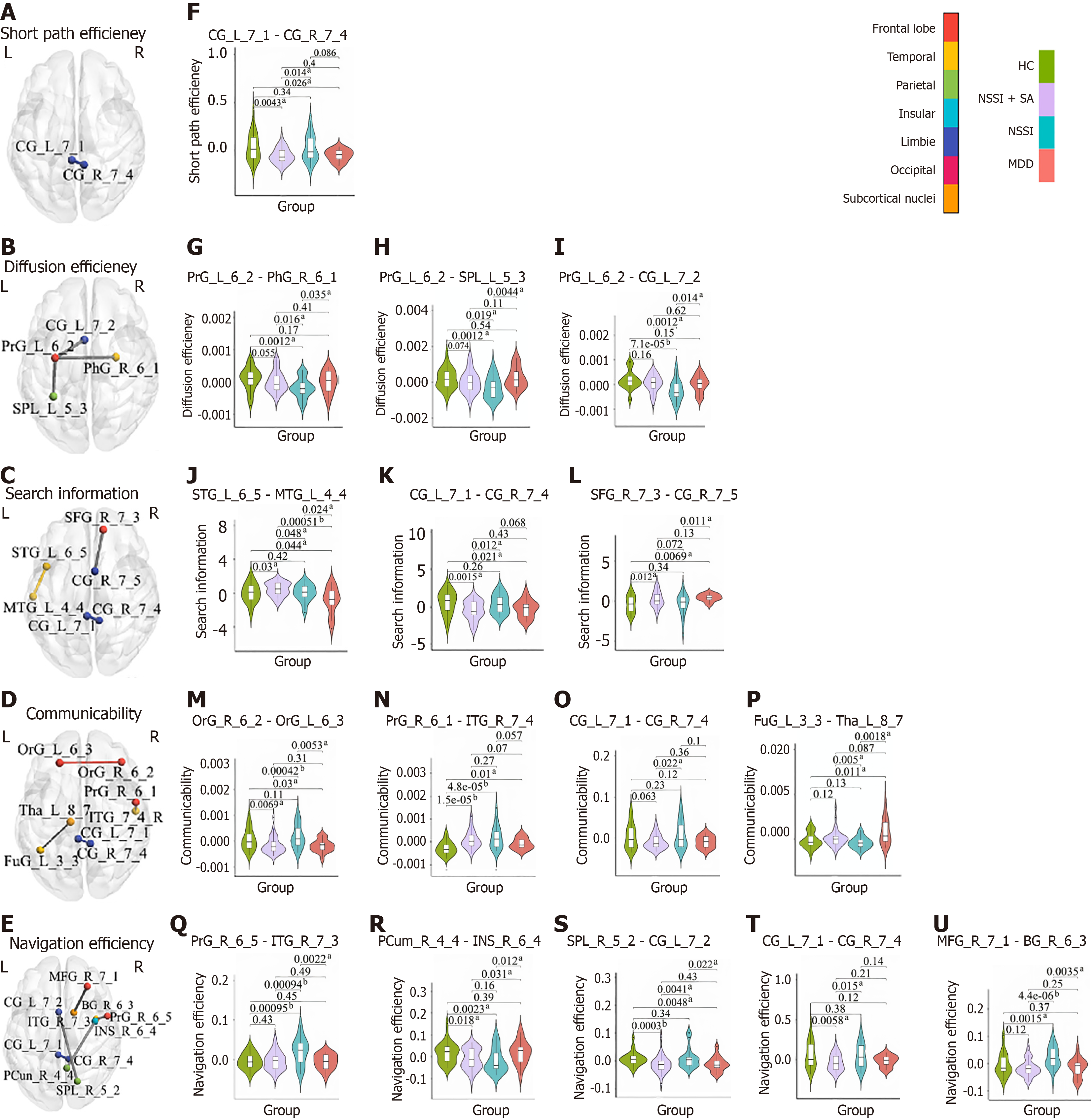
Table 4 presents the significantly different connections identified by the F-test statistical analysis. In the analysis of communicability, four distinct connections exhibited significant variance across the HC, NSSI + SA, NSSI, and major depressive disorder groups, particularly involving the frontal regions such as the orbital and precentral gyrus (PrG), the cingulum, and temporal regions including the inferior temporal gyrus (ITG) and fusiform gyrus, as well as the thalamus. Regarding diffusion efficiency, three connections demonstrated significant differences primarily associated with the left PrG. In the domain of navigation efficiency, five edges were identified with significant disparities, encompassing the cingulum, frontal regions (middle frontal gyrus, and PrG), the right nucleus accumbens (NAc) (BG_R_6_3), and the right insula (INS_R_6_4). Furthermore, in the measure of search information, three connections showed significant differences, which included the cingulum, temporal regions (middle temporal gyrus and superior temporal gyrus), and the superior frontal gyrus. Lastly, only one connection in shortest path efficiency revealed a significant difference, located within the cingulum. Figure 2A-E illustrate the spatial distribution of these results, presented from axial perspectives of the brain. Additionally, Figure 2F-U, presents the post-hoc results of these connections. Among them, a consistent pattern emerges across the three significant group-difference edges of diffusion efficiency, with the NSSI group exhibiting a notably lower value compared to the HC and the other groups. Conversely, the specific edges (i.e., PrG_R_6_5-ITG_R_7_3, and middle frontal gyrus_R_7_1-BG_R_6_3) of navigation efficiency reveal a significant increase in values for the NSSI when contrasted with the HC and the remaining groups.
| Network communication models | Node name | Anatomical descriptions1 | Node name | Anatomical descriptions | F value (P value) | Eta-squared (η²) |
| Communicability | OrG_R_6_2 | Orbital area | OrG_L_6_3 | Lateral orbital area | 8.620 (3.35e-05) | 0.185 |
| PrG_R_6_1 | Head and face region of PrG | ITG_R_7_4 | Intermediate lateral area of ITG | 6.903 (2.61e-04) | 0.154 | |
| CG_L_7_1 | Dorsal area of CG | CG_R_7_4 | Ventral area of CG | 6.871 (2.71e-04) | 0.153 | |
| FuG_L_3_3 | Lateroventral area of FuG | Tha_L_8_7 | Caudal temporal Tha | 7.581 (1.15e-04) | 0.166 | |
| Diffusion efficiency | PrG_L_6_2 | Caudal dorsolateral area of PrG | PhG_R_6_1 | Rostral area of PhG | 7.017 (2.27e-04) | 0.156 |
| PrG_L_6_2 | Caudal dorsolateral area of PrG | SPL_L_5_3 | Lateral area of SPL | 6.298 (5.45e-04) | 0.142 | |
| PrG_L_6_2 | Caudal dorsolateral area of PrG | CG_L_7_2 | Rostroventral area of CG | 8.450 (4.10e-05) | 0.182 | |
| Navigation efficiency | PrG_R_6_5 | Tongue and larynx region of PrG | ITG_R_7_3 | Rostral area of ITG | 7.899 (7.88e-05) | 0.172 |
| PCun_R_4_4 | PCun | INS_R_6_4 | Ventral dysgranular and granular insula | 7.436 (1.37e-04) | 0.164 | |
| SPL_R_5_2 | Caudal area of SPL | CG_L_7_2 | Rostroventral area of CG | 7.440 (1.36e-04) | 0.164 | |
| CG_L_7_1 | Dorsal area of CG | CG_R_7_4 | Ventral area of CG | 7.362 (1.50e-04) | 0.162 | |
| MFG_R_7_1 | Dorsal area of MFG | BG_R_6_3 | Nucleus accumbens | 6.486 (4.33e-04) | 0.146 | |
| Search information | STG_L_6_5 | Lateral area of STG | MTG_L_4_4 | Anterior superior temporal sulcus | 6.995 (2.33e-04) | 0.155 |
| CG_L_7_1 | Dorsal area of CG | CG_R_7_4 | Ventral area of CG | 6.419 (4.70e-04) | 0.145 | |
| SFG_R_7_3 | Lateral area of SFG | CG_R_7_5 | Caudodorsal area of CG | 6.270 (5.64e-04) | 0.142 | |
| Shortest path efficiency | CG_L_7_1 | Dorsal area of CG | CG_R_7_4 | Ventral area of CG | 8.579 (3.52e-05) | 0.184 |
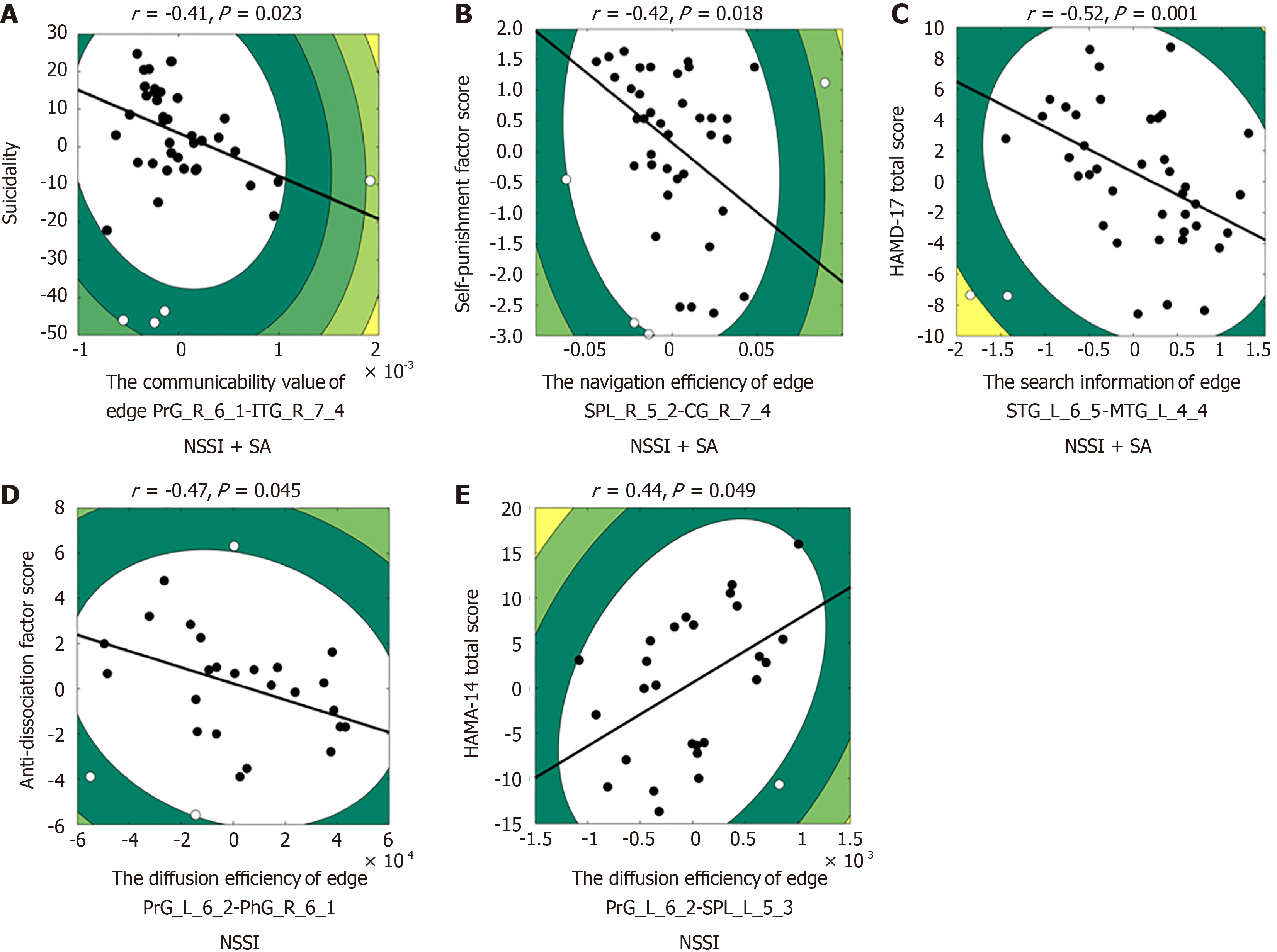
We examined the relationship between the mean communication efficiency values of significant connections that we found to be altered in depressed adolescents and symptom profiles within corresponding groups. With regard to the NSSI + SA group, Spearman correlation analyses revealed significant negative relationships between communicability scores (edge PrG_R_6_1-ITG_R_7_4) and suicidality derived from BSS-screen (previous most depressed) (r = -0.41, P = 0.023; Figure 3A), between navigation efficiency [edge superior parietal lobule (SPL)_R_5_2-cingulate gyrus (CG)_R_7_4] and Self-Punishment scores (r = -0.42, P = 0.018; Figure 3B), between the search information (edge STG_L_6_5-middle temporal gyrus_L_4_4) and HAMD-17 total scores (r = -0.52, P = 0.001; Figure 3C). With regard to the NSSI group, Spearman correlation analyses revealed a significant negative relationship between diffusion efficiency (edge PrG_L_6_2-parahippocampal gyrus_R_6_1) and anti-dissociation scores (r = -0.47, P = 0.045; Figure 3D), and a significant positive relationship between diffusion efficiency (edge PrG_L_6_2-SPL_L_5_3) and HAMA-14 total scores (r = 0.44, P = 0.049; Figure 3E).
To the best of our knowledge, this study is the first to investigate structural network communication in large-scale brain networks in depressed adolescents. Using dMRI-based probabilistic tractography, we constructed five distinct network communication models that incorporate both direct and indirect connections to evaluate the structural network efficiency across the whole brain level in depressed adolescents with SA and NSSI. We identified structural network com
In conclusion, the present study provided evidence that structural network communication differences in depressed adolescents with NSSI and SA, and the compromised communication efficiency in neuroanatomical networks were associated with the symptoms of depressed adolescents. In particular, our data highlight aberrant structural network communication efficiencies within PCC subregions, PrG-based connections and the NAc-frontal circuit, which offers new insight into the pathophysiological mechanisms underlying the comorbidity between NSSI and SA in depression.
Sincere appreciation is extended to all adolescents for their valuable participation.
| 1. | Ho TC, Gifuni AJ, Gotlib IH. Psychobiological risk factors for suicidal thoughts and behaviors in adolescence: a consideration of the role of puberty. Mol Psychiatry. 2022;27:606-623. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 47] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 2. | Jin MK, Wang XY, Wang RX, Cheng SY, Yang SY, Zhang SL, Lv SB. A systematic review and meta-analysis of factors related to non-suicidal self-injury among Chinese adolescents. Psychiatry Res. 2023;326:115329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 16] [Reference Citation Analysis (0)] |
| 3. | Mufson L, Rynn MA. Primary Care: Meeting the Mental Health Care Needs of Adolescents With Depression. J Am Acad Child Adolesc Psychiatry. 2019;58:389-391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 4. | Grossberg A, Rice T. Depression and Suicidal Behavior in Adolescents. Med Clin North Am. 2023;107:169-182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 52] [Article Influence: 26.0] [Reference Citation Analysis (0)] |
| 5. | Thapar A, Eyre O, Patel V, Brent D. Depression in young people. Lancet. 2022;400:617-631. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 372] [Article Influence: 124.0] [Reference Citation Analysis (0)] |
| 6. | Ribeiro JD, Huang X, Fox KR, Franklin JC. Depression and hopelessness as risk factors for suicide ideation, attempts and death: meta-analysis of longitudinal studies. Br J Psychiatry. 2018;212:279-286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 285] [Cited by in RCA: 529] [Article Influence: 75.6] [Reference Citation Analysis (0)] |
| 7. | Poudel A, Lamichhane A, Magar KR, Khanal GP. Non suicidal self injury and suicidal behavior among adolescents: co-occurrence and associated risk factors. BMC Psychiatry. 2022;22:96. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 45] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 8. | Klonsky ED, May AM, Saffer BY. Suicide, Suicide Attempts, and Suicidal Ideation. Annu Rev Clin Psychol. 2016;12:307-330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 592] [Cited by in RCA: 909] [Article Influence: 101.0] [Reference Citation Analysis (0)] |
| 9. | Beauchaine TP, Hinshaw SP, Bridge JA. Nonsuicidal Self-Injury and Suicidal Behaviors in Girls: The Case for Targeted Prevention in Preadolescence. Clin Psychol Sci. 2019;7:643-667. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 43] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 10. | Liao Y, Huang X, Wu Q, Yang C, Kuang W, Du M, Lui S, Yue Q, Chan RC, Kemp GJ, Gong Q. Is depression a disconnection syndrome? Meta-analysis of diffusion tensor imaging studies in patients with MDD. J Psychiatry Neurosci. 2013;38:49-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 297] [Cited by in RCA: 302] [Article Influence: 25.2] [Reference Citation Analysis (0)] |
| 11. | Chen G, Guo Y, Zhu H, Kuang W, Bi F, Ai H, Gu Z, Huang X, Lui S, Gong Q. Intrinsic disruption of white matter microarchitecture in first-episode, drug-naive major depressive disorder: A voxel-based meta-analysis of diffusion tensor imaging. Prog Neuropsychopharmacol Biol Psychiatry. 2017;76:179-187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 65] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 12. | Gong Q, He Y. Depression, neuroimaging and connectomics: a selective overview. Biol Psychiatry. 2015;77:223-235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 296] [Cited by in RCA: 362] [Article Influence: 36.2] [Reference Citation Analysis (0)] |
| 13. | Seguin C, Razi A, Zalesky A. Inferring neural signalling directionality from undirected structural connectomes. Nat Commun. 2019;10:4289. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 72] [Cited by in RCA: 68] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 14. | Korgaonkar MS, Fornito A, Williams LM, Grieve SM. Abnormal structural networks characterize major depressive disorder: a connectome analysis. Biol Psychiatry. 2014;76:567-574. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 251] [Cited by in RCA: 273] [Article Influence: 24.8] [Reference Citation Analysis (0)] |
| 15. | Parkes L, Moore TM, Calkins ME, Cieslak M, Roalf DR, Wolf DH, Gur RC, Gur RE, Satterthwaite TD, Bassett DS. Network Controllability in Transmodal Cortex Predicts Positive Psychosis Spectrum Symptoms. Biol Psychiatry. 2021;90:409-418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 37] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 16. | Hu C, Jiang W, Wu Y, Wang M, Lin J, Chen S, Shang Y, Xie J, Kong Y, Yuan Y. Microstructural abnormalities of white matter in the cingulum bundle of adolescents with major depression and non-suicidal self-injury. Psychol Med. 2024;54:1113-1121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 17. | Wei S, Womer FY, Edmiston EK, Zhang R, Jiang X, Wu F, Kong L, Zhou Y, Tang Y, Wang F. Structural alterations associated with suicide attempts in major depressive disorder and bipolar disorder: A diffusion tensor imaging study. Prog Neuropsychopharmacol Biol Psychiatry. 2020;98:109827. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 27] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 18. | Avena-Koenigsberger A, Misic B, Sporns O. Communication dynamics in complex brain networks. Nat Rev Neurosci. 2017;19:17-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 387] [Cited by in RCA: 452] [Article Influence: 56.5] [Reference Citation Analysis (0)] |
| 19. | Kaiser M, Hilgetag CC. Nonoptimal component placement, but short processing paths, due to long-distance projections in neural systems. PLoS Comput Biol. 2006;2:e95. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 508] [Cited by in RCA: 426] [Article Influence: 22.4] [Reference Citation Analysis (0)] |
| 20. | Seguin C, van den Heuvel MP, Zalesky A. Navigation of brain networks. Proc Natl Acad Sci U S A. 2018;115:6297-6302. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 83] [Cited by in RCA: 104] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 21. | Goñi J, Avena-Koenigsberger A, Velez de Mendizabal N, van den Heuvel MP, Betzel RF, Sporns O. Exploring the morphospace of communication efficiency in complex networks. PLoS One. 2013;8:e58070. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 102] [Cited by in RCA: 100] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 22. | Goñi J, van den Heuvel MP, Avena-Koenigsberger A, Velez de Mendizabal N, Betzel RF, Griffa A, Hagmann P, Corominas-Murtra B, Thiran JP, Sporns O. Resting-brain functional connectivity predicted by analytic measures of network communication. Proc Natl Acad Sci U S A. 2014;111:833-838. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 404] [Cited by in RCA: 397] [Article Influence: 33.1] [Reference Citation Analysis (0)] |
| 23. | Crofts JJ, Higham DJ. A weighted communicability measure applied to complex brain networks. J R Soc Interface. 2009;6:411-414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 96] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 24. | de Lange SC, Scholtens LH; Alzheimer’s Disease Neuroimaging Initiative, van den Berg LH, Boks MP, Bozzali M, Cahn W, Dannlowski U, Durston S, Geuze E, van Haren NEM, Hillegers MHJ, Koch K, Jurado MÁ, Mancini M, Marqués-Iturria I, Meinert S, Ophoff RA, Reess TJ, Repple J, Kahn RS, van den Heuvel MP. Shared vulnerability for connectome alterations across psychiatric and neurological brain disorders. Nat Hum Behav. 2019;3:988-998. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 72] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 25. | American Psychological Association. Diagnostic and statistical manual of mental disorders: DSM-5™. 5th ed. United States: American Psychiatric Publishing Inc, 2013. |
| 26. | Nixon MK, Levesque C, Preyde M, Vanderkooy J, Cloutier PF. The Ottawa Self-Injury Inventory: Evaluation of an assessment measure of nonsuicidal self-injury in an inpatient sample of adolescents. Child Adolesc Psychiatry Ment Health. 2015;9:26. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 70] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 27. | Beck AT, Robert AS. BSI, Beck scale for suicide ideation: Manual. Australia: Pearson, 1991. |
| 28. | Hamilton M. Development of a rating scale for primary depressive illness. Br J Soc Clin Psychol. 1967;6:278-296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5785] [Cited by in RCA: 5845] [Article Influence: 100.8] [Reference Citation Analysis (0)] |
| 29. | HAMILTON M. The assessment of anxiety states by rating. Br J Med Psychol. 1959;32:50-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6169] [Cited by in RCA: 6789] [Article Influence: 102.9] [Reference Citation Analysis (0)] |
| 30. | Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97-113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26222] [Cited by in RCA: 27735] [Article Influence: 513.6] [Reference Citation Analysis (0)] |
| 31. | Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, Hergueta T, Baker R, Dunbar GC. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59 Suppl 20:22-33;quiz 34. [PubMed] |
| 32. | Kellner E, Dhital B, Kiselev VG, Reisert M. Gibbs-ringing artifact removal based on local subvoxel-shifts. Magn Reson Med. 2016;76:1574-1581. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 589] [Cited by in RCA: 952] [Article Influence: 95.2] [Reference Citation Analysis (0)] |
| 33. | Tustison NJ, Avants BB, Cook PA, Zheng Y, Egan A, Yushkevich PA, Gee JC. N4ITK: improved N3 bias correction. IEEE Trans Med Imaging. 2010;29:1310-1320. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4946] [Cited by in RCA: 3774] [Article Influence: 251.6] [Reference Citation Analysis (0)] |
| 34. | Veraart J, Novikov DS, Christiaens D, Ades-Aron B, Sijbers J, Fieremans E. Denoising of diffusion MRI using random matrix theory. Neuroimage. 2016;142:394-406. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 776] [Cited by in RCA: 1266] [Article Influence: 140.7] [Reference Citation Analysis (0)] |
| 35. | Jenkinson M, Beckmann CF, Behrens TE, Woolrich MW, Smith SM. FSL. Neuroimage. 2012;62:782-790. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6462] [Cited by in RCA: 8108] [Article Influence: 579.1] [Reference Citation Analysis (0)] |
| 36. | Jeurissen B, Tournier JD, Dhollander T, Connelly A, Sijbers J. Multi-tissue constrained spherical deconvolution for improved analysis of multi-shell diffusion MRI data. Neuroimage. 2014;103:411-426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 749] [Cited by in RCA: 948] [Article Influence: 86.2] [Reference Citation Analysis (0)] |
| 37. | Tournier JD, Smith R, Raffelt D, Tabbara R, Dhollander T, Pietsch M, Christiaens D, Jeurissen B, Yeh CH, Connelly A. MRtrix3: A fast, flexible and open software framework for medical image processing and visualisation. Neuroimage. 2019;202:116137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 817] [Cited by in RCA: 1676] [Article Influence: 279.3] [Reference Citation Analysis (0)] |
| 38. | Smith RE, Tournier JD, Calamante F, Connelly A. SIFT: Spherical-deconvolution informed filtering of tractograms. Neuroimage. 2013;67:298-312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 437] [Cited by in RCA: 520] [Article Influence: 40.0] [Reference Citation Analysis (0)] |
| 39. | Smith RE, Tournier JD, Calamante F, Connelly A. The effects of SIFT on the reproducibility and biological accuracy of the structural connectome. Neuroimage. 2015;104:253-265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 174] [Cited by in RCA: 187] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 40. | Fan L, Li H, Zhuo J, Zhang Y, Wang J, Chen L, Yang Z, Chu C, Xie S, Laird AR, Fox PT, Eickhoff SB, Yu C, Jiang T. The Human Brainnetome Atlas: A New Brain Atlas Based on Connectional Architecture. Cereb Cortex. 2016;26:3508-3526. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1239] [Cited by in RCA: 1918] [Article Influence: 213.1] [Reference Citation Analysis (0)] |
| 41. | Seguin C, Tian Y, Zalesky A. Network communication models improve the behavioral and functional predictive utility of the human structural connectome. Netw Neurosci. 2020;4:980-1006. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 64] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 42. | Rubinov M, Sporns O. Complex network measures of brain connectivity: uses and interpretations. Neuroimage. 2010;52:1059-1069. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6976] [Cited by in RCA: 7454] [Article Influence: 465.9] [Reference Citation Analysis (0)] |
| 43. | Latora V, Marchiori M. Efficient behavior of small-world networks. Phys Rev Lett. 2001;87:198701. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3311] [Cited by in RCA: 2376] [Article Influence: 99.0] [Reference Citation Analysis (0)] |
| 44. | Estrada E, Hatano N. Communicability in complex networks. Phys Rev E Stat Nonlin Soft Matter Phys. 2008;77:036111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 422] [Cited by in RCA: 238] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 45. | Zalesky A, Fornito A, Bullmore ET. Network-based statistic: identifying differences in brain networks. Neuroimage. 2010;53:1197-1207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1577] [Cited by in RCA: 1956] [Article Influence: 130.4] [Reference Citation Analysis (0)] |
| 46. | Van Essen DC, Smith SM, Barch DM, Behrens TE, Yacoub E, Ugurbil K; WU-Minn HCP Consortium. The WU-Minn Human Connectome Project: an overview. Neuroimage. 2013;80:62-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4246] [Cited by in RCA: 3529] [Article Influence: 294.1] [Reference Citation Analysis (0)] |
| 47. | Leech R, Smallwood J. The posterior cingulate cortex: Insights from structure and function. Handb Clin Neurol. 2019;166:73-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 58] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 48. | Li W, Wang C, Lan X, Fu L, Zhang F, Ye Y, Liu H, Wu K, Lao G, Chen J, Li G, Zhou Y, Ning Y. Aberrant Dynamic Functional Connectivity of Posterior Cingulate Cortex Subregions in Major Depressive Disorder With Suicidal Ideation. Front Neurosci. 2022;16:937145. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 49. | Lang AN, Zhong Y, Lei W, Xiao Y, Hang Y, Xie Y, Lv Z, Zhang Y, Liu X, Liang M, Zhang C, Zhang P, Yang H, Wu Y, Wang Q, Yang K, Long J, Liu Y, Wang S, Tang Y, Lei M, Zhang D, Ouyang L, Zhang L, Wang C. Neural mechanism of non-adaptive cognitive emotion regulation in patients with non-suicidal self-injury. Compr Psychiatry. 2024;133:152487. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Reference Citation Analysis (0)] |
| 50. | Yan R, Huang Y, Shi J, Zou H, Wang X, Xia Y, Zhao S, Zhou H, Chen Y, Li X, Wu X, Yao Z, Lu Q. Alterations of regional spontaneous neuronal activity and corresponding brain circuits related to non-suicidal self-injury in young adults with major depressive disorder. J Affect Disord. 2022;305:8-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 10] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 51. | Cullen KR, Schreiner MW, Klimes-Dougan B, Eberly LE, LaRiviere LL, Lim KO, Camchong J, Mueller BA. Neural correlates of clinical improvement in response to N-acetylcysteine in adolescents with non-suicidal self-injury. Prog Neuropsychopharmacol Biol Psychiatry. 2020;99:109778. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 52. | Mohebi A, Pettibone JR, Hamid AA, Wong JT, Vinson LT, Patriarchi T, Tian L, Kennedy RT, Berke JD. Dissociable dopamine dynamics for learning and motivation. Nature. 2019;570:65-70. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 281] [Cited by in RCA: 447] [Article Influence: 74.5] [Reference Citation Analysis (0)] |
| 53. | Salgado S, Kaplitt MG. The Nucleus Accumbens: A Comprehensive Review. Stereotact Funct Neurosurg. 2015;93:75-93. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 215] [Cited by in RCA: 326] [Article Influence: 32.6] [Reference Citation Analysis (0)] |
| 54. | West EA, Moschak TM, Carelli RM. Distinct Functional Microcircuits in the Nucleus Accumbens Underlying Goal-Directed Decision-Making. In: Morris R, Bornstein A, Shenhav A, editors. Goal-Directed Decision Making. Amsterdam: Elsevier, 2018: 199-219. |
| 55. | van Duijvenvoorde ACK, Westhoff B, de Vos F, Wierenga LM, Crone EA. A three-wave longitudinal study of subcortical-cortical resting-state connectivity in adolescence: Testing age- and puberty-related changes. Hum Brain Mapp. 2019;40:3769-3783. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 49] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 56. | Rappaport BI, Kandala S, Luby JL, Barch DM. Brain Reward System Dysfunction in Adolescence: Current, Cumulative, and Developmental Periods of Depression. Am J Psychiatry. 2020;177:754-763. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 48] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 57. | Schlaepfer TE, Cohen MX, Frick C, Kosel M, Brodesser D, Axmacher N, Joe AY, Kreft M, Lenartz D, Sturm V. Deep brain stimulation to reward circuitry alleviates anhedonia in refractory major depression. Neuropsychopharmacology. 2008;33:368-377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 660] [Cited by in RCA: 632] [Article Influence: 37.2] [Reference Citation Analysis (0)] |
| 58. | Zalesky A, Fornito A, Seal ML, Cocchi L, Westin CF, Bullmore ET, Egan GF, Pantelis C. Disrupted axonal fiber connectivity in schizophrenia. Biol Psychiatry. 2011;69:80-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 351] [Cited by in RCA: 362] [Article Influence: 25.9] [Reference Citation Analysis (0)] |
| 59. | Liu X, He C, Fan D, Zang F, Zhu Y, Zhang H, Zhang Z, Zhang H, Xie C. Alterations of core structural network connectome associated with suicidal ideation in major depressive disorder patients. Transl Psychiatry. 2021;11:243. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 25] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 60. | Wang Q, He C, Wang Z, Fan D, Zhang Z, Xie C; REST-meta-MDD Consortium. Connectomics-based resting-state functional network alterations predict suicidality in major depressive disorder. Transl Psychiatry. 2023;13:365. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 61. | Schumacher A, Campisi SC, Khalfan AF, Merriman K, Williams TS, Korczak DJ. Cognitive functioning in children and adolescents with depression: A systematic review and meta-analysis. Eur Neuropsychopharmacol. 2024;79:49-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 14] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 62. | Lau JY, Waters AM. Annual Research Review: An expanded account of information-processing mechanisms in risk for child and adolescent anxiety and depression. J Child Psychol Psychiatry. 2017;58:387-407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 81] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 63. | Bresin K, Schoenleber M. Gender differences in the prevalence of nonsuicidal self-injury: A meta-analysis. Clin Psychol Rev. 2015;38:55-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 275] [Cited by in RCA: 385] [Article Influence: 38.5] [Reference Citation Analysis (0)] |
| 64. | Sher L. Gender differences in suicidal behavior. QJM. 2022;115:59-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 65. | Wilkinson PO, Qiu T, Jesmont C, Neufeld SAS, Kaur SP, Jones PB, Goodyer IM. Age and gender effects on non-suicidal self-injury, and their interplay with psychological distress. J Affect Disord. 2022;306:240-245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 54] [Article Influence: 18.0] [Reference Citation Analysis (0)] |