Published online May 19, 2023. doi: 10.5498/wjp.v13.i5.144
Peer-review started: December 16, 2022
First decision: January 12, 2023
Revised: February 11, 2023
Accepted: April 17, 2023
Article in press: April 17, 2023
Published online: May 19, 2023
Processing time: 153 Days and 13.6 Hours
Resilience to psychological stress is defined as adaption to challenging life experiences and not the absence of adverse life events. Determinants of resilience include personality traits, genetic/epigenetic modifications of genes involved in the stress response, cognitive and behavioral flexibility, secure attachment with a caregiver, social and community support systems, nutrition and exercise, and alignment of circadian rhythm to the natural light/dark cycle. Therefore, resilience is a dynamic and flexible process that continually evolves by the intersection of different domains in human’s life; biological, social, and psychological. The objective of this minireview is to summarize the existing knowledge about the multitude factors and molecular alterations that result from resilience to stress response. Given the multiple contributing factors in building resilience, we set out a goal to identify which factors were most supportive of a causal role by the current literature. We focused on resilience-related molecular alterations resulting from mind-body homeostasis in connection with psychosocial and environmental factors. We conclude that there is no one causal factor that differentiates a resilient person from a vulnerable one. Instead, building resilience requires an intricate network of positive experiences and a healthy lifestyle that contribute to a balanced mind-body connection. Therefore, a holistic approach must be adopted in future research on stress response to address the multiple elements that promote resilience and prevent illnesses and psychopathology related to stress allostatic load.
Core Tip: There are multiple reviews in the literature that address different factors contributing to resilience, an adaptation to stress. To our knowledge, none of these reviews takes into consideration the complexity of the system that leads to allostasis or allostatic load, an indicator of physiologic “wear and tear” resulting from repeated exposure to stress and inability to cope. The purpose of this review is to shed light on the complexity of the system and discuss the molecular mechanisms that may contribute to resilience. Lastly, we conclude by emphasizing the need for a comprehensive approach to reduce stress allostatic load.
- Citation: Chbeir S, Carrión V. Resilience by design: How nature, nurture, environment, and microbiome mitigate stress and allostatic load. World J Psychiatry 2023; 13(5): 144-159
- URL: https://www.wjgnet.com/2220-3206/full/v13/i5/144.htm
- DOI: https://dx.doi.org/10.5498/wjp.v13.i5.144
According to the American Psychological Association (APA), “resilience is the process and outcome of successfully adapting to difficult or challenging life experiences, especially through mental, emotional, and behavioral flexibility and adjustment to external and internal demands”. Adaptation to adversities depends on several factors: Individual’s engagement and view of the world, the availability of social and communal resources, and the use of specific coping strategies (APA, 2022, https://www.apa.org/topics/resilience). It is worth noting that resilience and coping are inter-related but have different constructs with respect to their impact on behavioral changes[1]. While coping manages stressful events by using cognitive and behavioral strategies[2], resilience refers to the adaptive capacity to recover from traumatic or stressful situations[3].
Research in humans over the last two decades has demonstrated that resilience and use of adaptive coping skills in the face of adversity in both children and adults are the main factors that protect from developing mental and physical illnesses. While personal characteristics such as personality traits of persistence and determination, and cognitive flexibility are key elements, perceived parental care, adolescent peer relationships, adult romantic relationships, community support systems, dietary lifestyle, exercise, and circadian rhythm are all implicated in building resilience[4-11]. Resilience is an active adaptive process that helps mitigate negative social, psychological, and biological consequences of extreme stress[12]. The adaptive behavioral manifestations of resilient people are described as enhanced internal locus of control, self-efficacy, happiness, life satisfaction, the ability to derive a sense of life meaning, ability to foster social and communal interactions, and problem-solving skills[13,14].
Fostering resilience early in life may be an effective preventative measure prior to the development of trauma[15]. It is estimated that 60% of men and 50% of women would experience at least one potentially traumatic event in their lifetime, however only 8% of women and 4% in men develop post-traumatic stress disorder (PTSD) (National Center of PTSD, 2022, https://www.ptsd.va.gov/). The development of resilience is an ongoing dynamic process throughout the lifespan in both children and adults, even in those who have suffered from adverse early life experiences, to successfully adapt to or overcome traumatic stress-related illnesses[16,17]. Indeed, resilience can aid in developing positive changes in the aftermath of traumatic events, such as gaining the capacity to relate to others, personal strength, spirituality, and life appreciation[18]. Resilience is implicated in changes in brain regions involved with the social networks that promote empathy, social connectedness, and modulation of central responses to stress[19].
The objective of this review is to summarize the existing knowledge about the multitude factors and molecular alterations that result from resilience to stress response. Our aim is to create awareness for future research studies on allostatic load and stress response about the intricate network contributing to resilience. The methodology we used is Literature Review performed through PubMed, PsycINFO and Scopus databases for peer-reviewed English-language articles published prior to December 2022 using the following keywords: Chronic stress, allostatic load, trauma, biomarkers, circadian rhythm, resilience, neurobiology, genetic, epigenetic, attachment, oxytocin, gut microbiome, diet, mindfulness, exercise, and psychotherapy. We will first describe the existing knowledge about the biologic aspects of the stress response and implications of chronic stress. Then we will present the different factors that contribute to resilience and associated molecular changes.
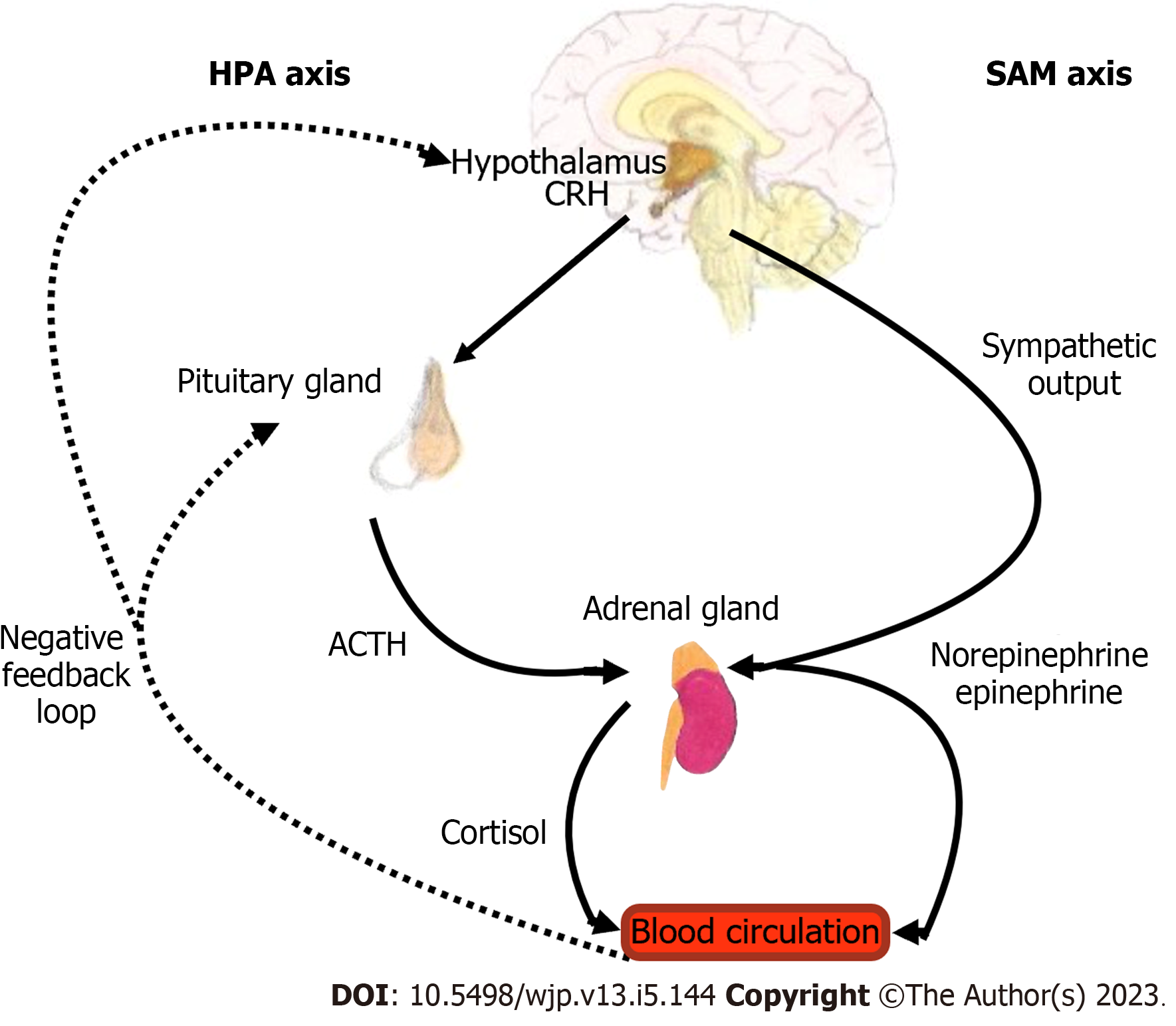
The stress response involves a neurohormonal mechanism activated by the cross talk of the hypothalamic-pituitary-adrenal (HPA)-axis and the sympathetic-adrenal-medullary (SAM)-axis that results in widespread hormonal, neurochemical, and physiological alterations. The SAM axis activates the peripheral sympathetic nervous system to release epinephrine and norepinephrine[20,21]. HPA axis activation leads to a cascade of events whereby corticotropin releasing hormone released from the hypothalamus stimulates the release of adrenocorticotropic (ACTH) from the pituitary gland, which in turn results in production of glucocorticoids (GCs) from the adrenal cortex. GCs, also known as cortisol in mammals and corticosterone in rodents, regulate cellular function by interacting with steroid receptors that modulate the neural circuitry and neuroendocrine systems involved in behavioral responses to stress[21]. These receptors, glucocorticoid receptors (GR) and mineralocorticoid receptors (MR), are expressed throughout the brain, mostly in the prefrontal cortex (PFC), hippocampus, amygdala, and other limbic and midbrain structures[17,22]. Under conditions of threat, HPA activation leads to increased release of GCs to promote acute survival by mobilizing stored energy, hence contributing to a state of increased arousal and vigilance. The stress response is then terminated by a negative feedback loop that attenuates the HPA-axis at the level of GR, causing GCs levels to return to normal (Figure 1)[23]. In contrast to GR, MR modulates basal and stress-induced HPA-axis activity to appraise stress and fear-related memories. Enhanced expression and function of MR may improve resilience to traumatic stress and reduces the risk for psychiatric disorders[21]. Studies have shown that MR dampen glucocorticoid receptor sensitivity to stress via regulation of FK506-binding protein 5 (FKBP5), a potent negative regulator of glucocorticoid signaling that plays an important role in fine-tuning the MR:GR balance in the hippocampus[24].
When the HPA axis is activated, dehydroepiandrosterone (DHEA) is also released by the adrenal glands along with GCs. DHEA is an important mediator of the HPA axis since it facilitates the N-methyl-D-aspartate receptor function, antagonizes g-Aminobutyric Acid A receptors, and facilitates metabolism of cortisol to the inactive metabolite cortisone. DHEA-Sulfate (DHEA-S), a more potent form than DHEA, plays a neuroprotective role by inhibiting GC effects at the level of the GR as well as supporting neurogenesis[25-28]. Cortisol/DHEA-S ratio represents a balance between the catabolic effects of cortisol and the anabolic, regenerative function of DHEA-S[27,29]. In fact, a higher DHEA-S to cortisol ratio predicted stress resilience in male military personnel, lowering symptoms of PTSD and showing greater improvement over time[30]. On the other hand, a lower resting DHEA/cortisol ratio has been associated with childhood trauma[26].
Multiple studies have demonstrated the close connection between the circadian rhythm and stress systems to maintain allostasis. The homeostasis of the HPA axis, which produces the primary mediators in adaptation to stress, is closely regulated by the circadian rhythm output[31]. The nearly 24-h periodic peripheral rhythms are controlled by the master circadian pacemaker located in the hypothalamic suprachiasmatic nucleus (SCN). The SCN generates a daily rhythm of transcription and translation feedback loop that align with the 24-h external light-dark environment[32]. The central clock in the SCN orchestrates peripheral clocks at the cellular level to synchronize physiological and behavioral rhythms and regulates the activity of various humoral and neuronal allostatic mediators, among which GCs that show a robust time-of-day dependence[33-35]. These robust circadian dynamics of the allostatic mediators enable the host to flexibly respond and adapt to various physiological stressors[36,37]. In this current modern society and lifestyle that humans live in, where light at night is widespread due to adoption of electrical light, we developed a deranged temporal adaptation that our physiological systems have not evolved to cope with. The chronic disruption of circadian rhythms predisposes to physiologic and behavioral changes that can lead to maladaptive allostatic mechanisms and compromising resilience[32,36].
Animal studies have shown that disruption of the circadian rhythm by misalignment to the natural light/dark cycle in mice results in neurobehavioral changes resulting in decreased complexity of neurons in the prefrontal cortex, the brain center involved in executive and emotional control, and reduction in cognitive flexibility[38]. Even short-term circadian misalignment has been shown to disrupt memory consolidation in response to fear-conditioning that could compromise resilience[39]. The bidirectional communication between the central clock and HPA axis is also evident by circadian disruption in response to early life stress that contributes to metabolic derangement occurring later in life[40]. Hence, re-alignment of circadian rhythms by following the natural light/dark cycle can enhance allostatic adaptive resilience and can be especially beneficial for individuals with psychiatric disorders who struggle with sleep disturbances[41-43]. Interestingly, timing of Trauma Exposure therapy to specific trauma-associated cues can have a different outcome in the process of healing. One study found that exposure to trauma cues is more efficacious when administered during the morning time compared to the evening[44,45].
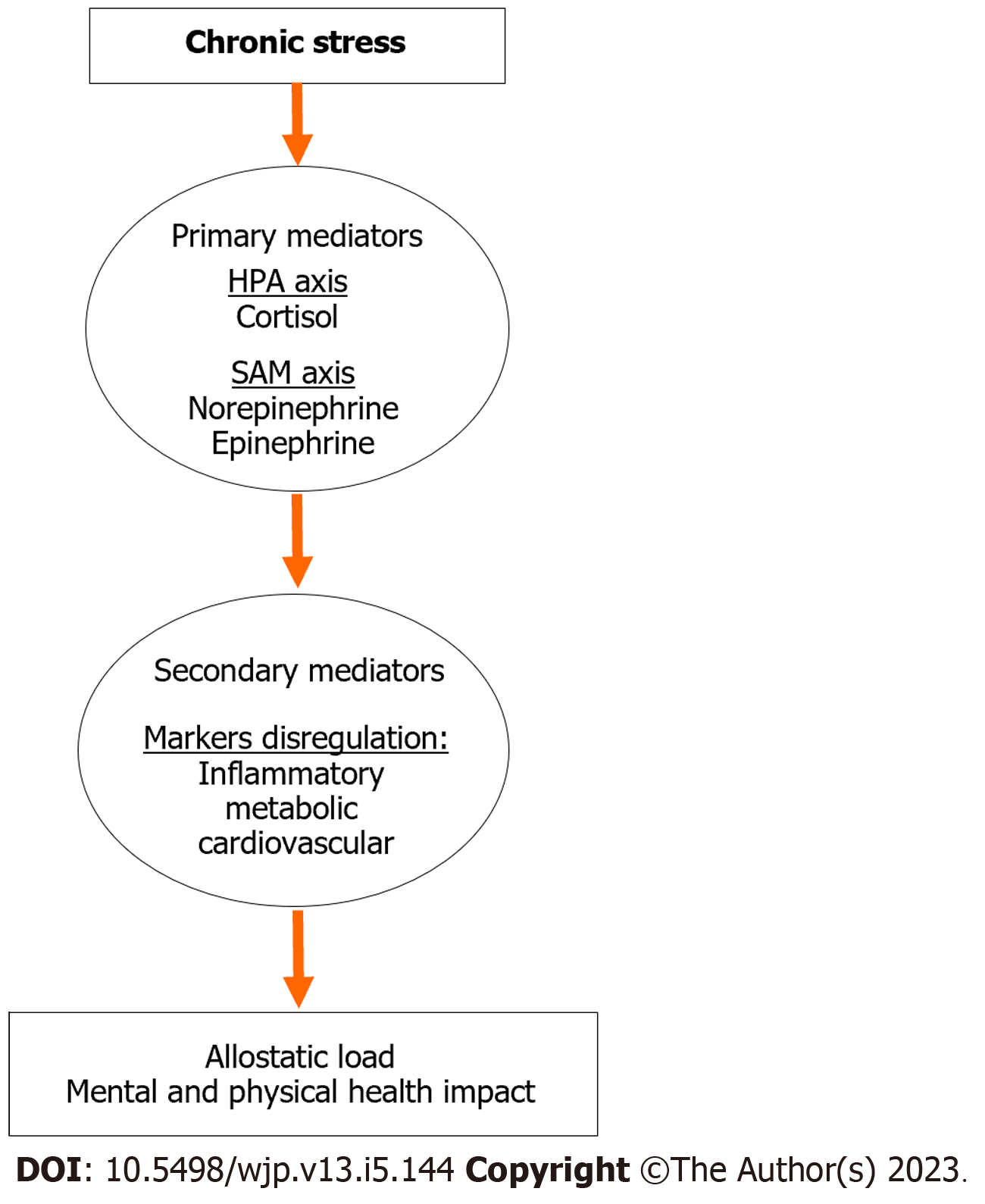
As presented above, exposure to stress triggers several biological mechanisms in the body that release stress hormones as primary mediators in order to adapt to short term or acute stress. Maintaining stability through change is a phenomenon known as allostasis[46,47]. Allostasis is considered a beneficial adaptive mechanism that promotes host survival through the appropriate activation of energetic resources[48]. However, repeated exposure to stress, also known as chronic stress, can result in prolonged activation of the HPA and SAM axes, which may lead to activation of secondary inflammatory and metabolic mediators, and ultimately lead to deleterious effect on metabolic, immune, and cardiovascular functions as well as brain and behavior (Figure 2)[49-52]. The cumulative effects of chronic stress reduce the ability of the host to flexibly cope with subsequent stressful situations, which lead the system to shift from allostasis to allostatic load or physiological “wear and tear”[53-56].
While it is beyond the scope of this minireview to discuss all the structural, biological and genetic modifications in response to chronic or developmental stress, we will briefly discuss few structural changes and gene variants involved in the stress response, which may result in either negative or positive associations to resilience. Animal studies have shown structural changes in different brain regions whereby chronic stress increases dendritic spine number or branching in the amygdala and nucleus accumbens, reduces dendritic arborization and glutamatergic dendritic spine density of pyramidal neurons in PFC and hippocampus, and decreases hippocampus neurogenesis[57].
The impact of the environment and specifically early life stress profoundly alters key genes involved in stress response via epigenetic modification. Many of these genes can be modified through epigenetic alterations and variation in microRNAs (miRNA), short non-coding RNAs detected in body fluids, in response to environmental influences[58,59]. Little is known about the role of miRNA in psychiatric disorders and resilience. One animal study showed that the systemic knockdown of miRNA-144-3p reduced the depression-like phenotype in stress-susceptible mice[60]. Another study demonstrated that knockdown of miRNA-144 and miRNA-33 in the hippocampus increased the proportion of resilient female animals[61]. Studies also showed that low maternal care is associated with epigenetic modification by increased methylation of the gene encoding GR (NR3C1), leading to decreased GR expression in the hippocampus[62,63]. Another important player in regulating the stress response is the FKBP5 gene. This gene modulates intracellular glucocorticoid signaling and homeostatic regulation of the stress response[64]. Studies have shown that epigenetic modifications induced by early life stress or single nucleotide polymorphisms (SNPs) in human FKBP5 gene result in differential induction of the FKBP5 protein upon glucocorticoid stimulation, thereby adding to the variability of stress perception and response, and increasing the risk of developing stress-related psychiatric disorders[65,66].
While early-life stress can induce permanent changes in behavioral and physiological responses to stress through epigenetic modification, DNA methylation is potentially reversible through intervention. Using blood samples from human, methylation levels of specific regions within FKBP5 and brain derived neurotrophic factor (BDNF) were changed in response to psychotherapy and were associated with recovery and improvement[67,68]. For example, Exposure-based Cognitive Behavioral Therapy results in a decrease in FKBP5 methylation and leads to a positive treatment response[69]. Furthermore, maternal stroking and tactile stimulation can normalize DNA methylation in the leukocytes of infants who had been exposed to high levels of pre- and postnatal maternal depression[70]. Mindfulness practice is well known to improve cognitive and social functioning, and reduce symptoms of depression and anxiety[71,72]. The mechanisms whereby mindfulness regulates the stress response is through activation of brain regions involved in interoception, self-awareness, emotion regulation, and threat detection[73-75]. Mindfulness practice has been shown to alter different immune and endocrine pathways, as well as epigenetic methylation of the FKBP5 gene in adults with PTSD[76-78].
These findings are in line with animal studies demonstrating that intervention by enrichment can reverse the epigenetic, plasticity, and behavioral deficits in rats exposed to early life stress[79]. Indeed, our group has recently published a new treatment approach, Cue Centered Therapy (CCT) for complex developmental trauma in children and adolescents. CCT emphasizes resiliency and positive adaptation factors by using the life timeline as a core component for both positive and negative events in youth’s life. CCT has shown effectiveness in improving functioning and reducing child and parent post-traumatic stress. Treatment outcome research of CCT have demonstrated PTSD symptom improvement as measured by cortical activation patterns using functional near-infrared spectroscopy, a non-invasive neuroimaging technique[80,81].
Another important molecular determinant of resilience is oxytocin (OXT), which is closely tied to attachment. Humans are considered social mammals that develop various forms of social attachments and bonds from infancy throughout life[82,83]. The influence of secure attachment to a caregiver modulates physiology and behavior and is essential for a healthy psychological development and well-being. Attachment is a psychological/behavioral construct for infant’s self-regulation that is promoted and facilitated by caregiver's ongoing emotional availability. Infants internalize their interactions with their caregiver and build internal working models that represent their attachment figures in relation to themselves[82]. Attachment styles during early life predict moderate stability of attachment over the years but can be susceptible to change by significant relationship experiences[84]. The different attachment styles are believed to have a profound shaping of an individual’s emotional and psychosocial functioning as well as resilience or predisposition to psychopathology[85-87].
It is established that sudden separation of children from their parents, interpreted as abandonment, threatens the attachment bond and results in profound sense of shame and complex emotions especially in the absence of adequate support[88,89]. In addition to the impact of separation on secure attachment, transgenerational maternal experiences with unresolved attachment to their own caregivers can influence the quality of bonding of those mothers to their children. Children of mothers who exhibit higher distress and disruptive behavior exhibited a significantly higher cortisol compared to ones with no disruptive behavior[90]. These differences in cortisol levels and behavior may be related to gene polymorphism of the OXT and OXT receptor (OXT-R) in children from mothers with negative maternal experiences. These genes moderate the stress response of children, and polymorphisms can be associated with the disorganized behavior independent of maternal experiences[91].
The OXT system activated through social interaction is thought to have an important implication in building resilience by playing an important role in the regulation of affective and social behaviors as well as modulating stress response[92-94]. OXT is a neuropeptide produced by hypothalamic paraventricular, supraoptic, and accessory nuclei, and is released to the posterior pituitary gland and ultimately to the peripheral blood circulation[95,96]. In addition to OXT’s role in parturition and lactation[97], the neuropeptide plays a key role in promotion of postnatal sensitive maternal caregiving to optimize infant’s social and emotional development[98-100]. Higher levels of OXT have been linked with increased attention to social cues[101] while lower levels have been seen in maltreated children[102]. A positive association between OXT level and interpersonal bonding and affiliation, as well as stress modulation in interpersonal situations is well established. In animal models, OXT facilitates mating-induced pair bonds via mesolimbic dopamine system, however, variation in striatal OXT-R density predicts resilience and susceptibility to neonatal social neglect[103,104]. Growing evidence from animal and human studies showed that OXT signaling early in life by parental nurturing can buffer against physical and emotional stressors and help establish the neural networks needed during adult life to form social bonds[105-110].
Maternal care during early life, which overlaps with behaviors involving OXT in mother and infant through embedded hidden regulators, maintains the stress hyporesponsive period and direct HPA maturation during heightened plasticity[62]. It has been established that early life stress in human and non-human primates is associated with changes in cerebrospinal fluid OXT level and social behavior, as well as the ability of parental presence to attenuate stress response to a novel environment[111-113]. Animal studies have shown that OXT controls the secretion of ACTH under stress condition and enhance the long-term HPA axis response to stress in adult rats, which may act as a feedback regulator to enhance recovery from stress-related symptoms[114-116].
OXT seems to play an important role in fear modulation where it strengthens fear memory to predictable or cued fear while attenuating fear memory to unpredictable, diffuse threats (contextual fear and non-cued fear)[117-122]. Thus, OXT fosters adaptive defensive behaviors and accurate fear discrimination of relevant and imminent threats, yet reducing sustained fear responses to distant threats[123]. Animal studies have shown that administration of OXT by intracerebroventricular injection or intranasally facilitated cued fear extinction and reduced chronic stress-induced deficits in fear extinction in male rats, respectively. In contrast, administration of OXT-R antagonist impaired fear extinction in male rats[124,125]. These studies emphasized the role of OXT in reducing sustained fear responses and anxiety-like behaviors while strengthening fear responses to relevant and predictable threats. Nuclei of the central amygdala are the main output that connect to the brainstem to eventually mediate the fear response, including freezing behavior. OXT-R transmission in the amygdala switches from passive freezing to active escape behaviors in confrontation with an imminent, but escapable threat[126-128]. OXT also mediates affiliate and prosocial behaviors. OXT-R signaling facilitates social transmission of fear between familiar conspecifics, which might serve as warning system of impeding threat[129,130].
SNPs of the OXT-R gene, rs53576, has been shown to be associated with individual differences in social and emotional abilities, predisposition to psychopathology, and environmental adversity in the prediction of anxiety and depression[131-138]. Human brain-imaging studies in repeated childhood trauma and emotional neglect have demonstrated structural and functional variations in the amygdala, hypothalamus and cingulate gyrus in response to OXT-R gene polymorphism leading to variations in social and behavioral outcomes[139]. Furthermore, epigenetic alterations to specific OXT-R gene polymorphisms can attenuate resting parasympathetic tone and increasing central amygdala grey volume, thereby altering traumatic stress reactivity[140].
There is an extensive connection between the mind and body in which the wellbeing of one influences the other. A major element of this mind-body connection is the brain-gut axis. Indeed, there is an association between early life adversities and changes in the brain-gut axis that may occur via pathways related to glutamatergic excitotoxicity and oxidative stress, predisposing to negative mood and stress[141].
Maternal diet and resilience: The quality of dietary interventions during a critical period of neural development predicts the function of the brain-gut axis and plays a key role in building resilience to stress. Earlier studies showed that maternal nutrition during pregnancy plays a fundamental role in intrauterine developmental programming and predicts child’s resilience to stress and vulnerability to psychiatric disorders, such as anxiety and depression. Maternal malnutrition during fetal development has a detrimental long-term impact on the physical, cognitive, and emotional development[142,143]. A deficit in maternal dietary protein during pregnancy alters the brain neurochemistry and behavior by reducing 5-hydroxytryptamine 1A receptor and the responsiveness to stress during adult life[144]. It has been shown that branched-chain amino acids such as leucine, isoleucine or valine are essential nutrients that promote resilience via activation of hippocampal BDNF signaling[145]. An unbalanced diet during pregnancy is linked to heightened HPA axis responses to stress and higher cortisol levels as well as epigenetic changes in genes controlling glucocorticoid action in adult offspring[146-148].
Microbiome and gut-brain axis: The gut microbiota plays a major role in shaping how the body responds to stress. Animal studies have shown that Germ-free mice with absent microbiota have significant variations in their stress response caused by abnormal development of the HPA axis that was reversed by inoculation of Bifidobacteria infantis[149]. Exposure to early life adversity is correlated with changes in microbial diversity of the gut where taxonomic abundances predicted PFC activity[150].
The microbiome-derived short-chain fatty acids (SCFA) are the most studied metabolites because they ameliorate the gut-brain axis and stress-induced cortisol release in humans and rodents[151]. The composition of diet is essential because it can impact gut-brain pathways involved in stress response. A healthy diet rich in fibers, phytochemicals, or live bacteria can increase microbial diversity and enhances production of SCFA and other bioactive compounds[152,153]. Western diet, lacking in dietary fibers, is associated with suboptimal gut microbiota composition and a low-grade systemic inflammation that can predispose to mental illness, gastrointestinal and metabolic disorders, and obesity[154,155]. In addition to diet, exercise has been shown to increase SCFA availability, thereby influencing microbiome composition[156]. Gut microbes also play a major role in synthesizing key neuroactive molecules such as serotonin, gamma-aminobutyric acid, and catecholamines like norepinephrine and dopamine. For example, Lactobacillus can stimulate the conversion of dietary tryptophan into 5-hydroxytryptamine by enterochromaffin cells, which then interacts with the autonomic nervous system and conveys the signal to other brain structures, such as the hypothalamus, nucleus accumbens, and ventral tegmental area[157-159].
Microbiome-targeted therapies known as “psychobiotics” that include administration of live organisms, fecal microbial transplants, and dietary interventions to reshape the microbiome composition and function have beneficial effects on brain and behavior[152,160,161]. Administration of the probiotic organisms Bifidobacterium and Lactobacillus is known to confer resilience in social defeat model and has been tested in clinical depression[161,162]. Additionally, probiotics supplementation results in higher DHEA-to-corticosterone fecal metabolite ratios and reduces microglia immunoreactivity in the basolateral amygdala in rodent models, thereby mitigating the pervasive effects of early life stress on anxiety and depressive behavior as well as HPA axis activity[163].
Modulating the microbiota–gut–brain-axis through diet is also a promising approach to prevent and treat mental health disorders. Consumption of a Mediterranean diet resulted in a significant improvement in depressive symptoms after 12 weeks of dietary intervention compared to control group among patients with major depressive disorder[164,165]. Studies have also shown that adherence to Mediterranean diet, consumption of fruits and vegetable-based dietary pattern and dietary polyphenols were positively associated with psychological resilience[166]. Polyphenols are stress-modifying phytochemicals composed of hydroxylated phenyl moieties and are abundant in fruits, vegetables, tea, caffeine, curcumin, herbs, citrus peel, and grape seeds. They have anti-inflammatory actions, which may be involved in fighting psychosocial stress. Therefore, polyphenols are considered adaptogens (stress response modifiers that have beneficial effects to protect from chronic diseases) because of their ability to adapt to and survive external stressors[167-171]. Polyphenols are also considered ‘prebiotics’ because of their ability to enhance the growth of specific beneficial bacteria that produce bioactive phenolic acids, which promotes cognitive resilience to depression and anxiety[172-175].
Exercise and resilience: Exercise is well known for its benefit in enhancing resilience and longevity. Physical activity through exercise activates endocrine, paracrine, and autocrine pathways through the release of exerkines (signaling particles that have a potential role in improving health in response to exercise), cytokines, nucleic acids, lipids, and metabolites from multiple organs[176]. Exercise is well known to improve brain function, most notably the effect on learning and memory. Indeed, aerobic exercise increases hippocampal volume and improves memory, increases plasma levels of BDNF, and delays the onset of neuro-degenerative conditions in older adults human studies[177-179]. These findings are in line with animal studies on rodents where chronic exercise resulted in upregulation of BDNF in the hippocampus, leading to hippocampal neurogenesis, neural plasticity, and improved cognition and mood[180]. A possible mechanism of the upregulation of BDNF in hippocampus is through the release of irisin from myokines, which plays an important role in hippocampal neurogenesis, increased neurotrophin levels, and enhanced mood and cognition[181]. Another benefit of exercise is the release of adiponectin from adipocyte that seems to have neuroprotective effects by crossing the blood–brain barrier and resulting in increased neurogenesis and reduced depression-like behaviors[182]. Kynurenic acid, another molecule of interest produced in muscles in response to chronic aerobic exercise, has been shown to protect the brain from stress-induced depression[183]. Vigorous exercise is also associated with lower emotional distress, depression, and anxiety[184,185].
Studies have linked the adaptative changes in opioid systems to regular exercise, which reduces noradrenergic-induced stress responses. Regular exercise activates the endogenous opioid in the peripheral and central nervous system and is implicated in mood improvement[186,187]. The stress-reducing effect of exercise in response to both physical and psychological challenge is reversed by administration of naloxone, an opioid antagonist[188,189].
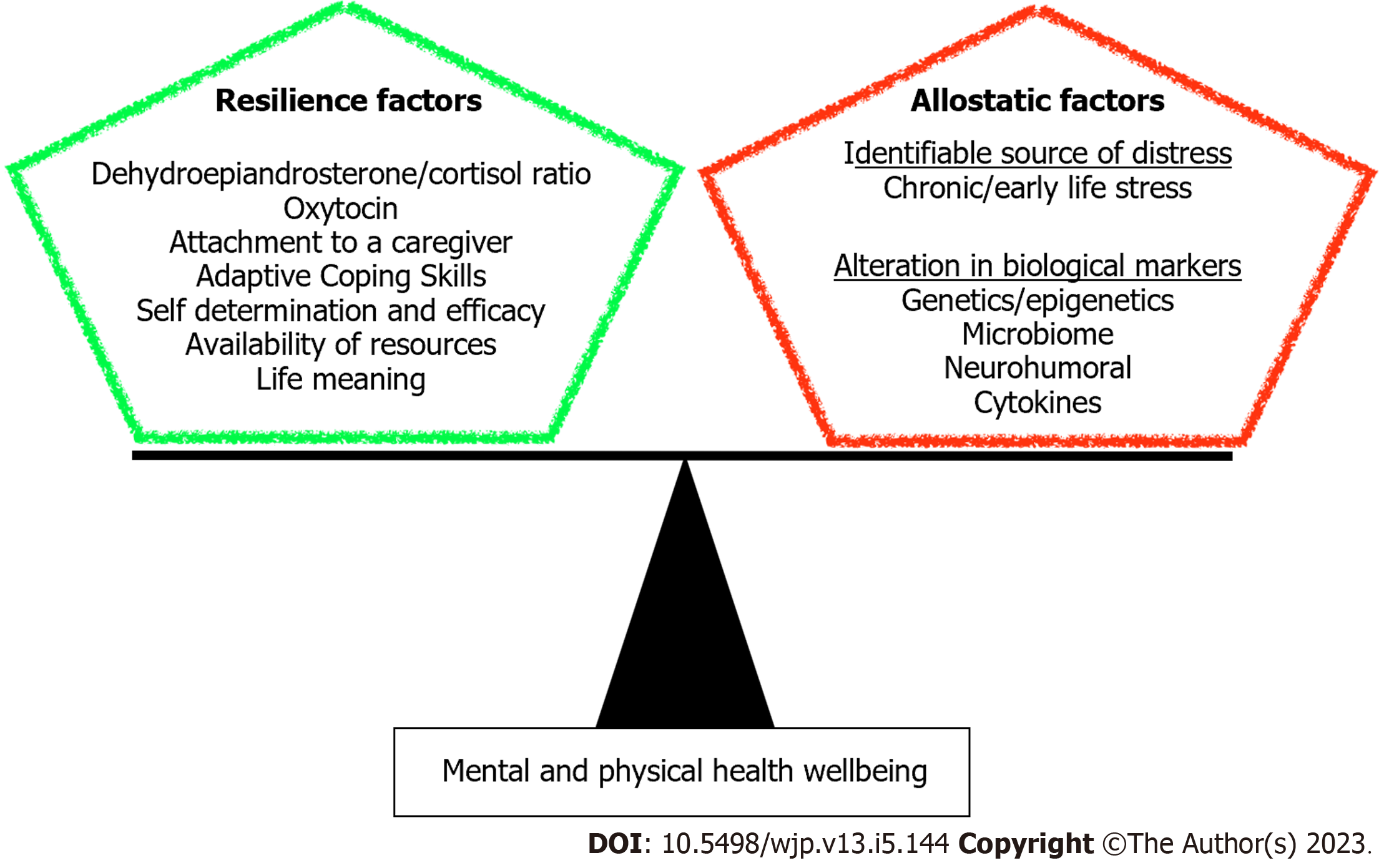
We have addressed in this minireview the biological basis of resilience as an outcome of structural and molecular alterations resulting from various determinants. Understanding the molecular aspect of resilience can provide insight for therapeutic discoveries and interventions to promote resilience in face of adverse life events. Here we showed that building resilience requires an intricate network of positive experiences and healthy lifestyle that contribute to a balanced mind-body connection (Figure 3). Indeed, positive childhood experiences such as interpersonal social and emotional support can mitigate the impact of adverse childhood experiences, thereby reducing the risk for adult onset depression and poor mental health[190].
Clinical and preclinical investigations showed that resilience is implicated in molecular alterations and changes in brain regions involved with the social networks that promote empathy, social connectedness, and modulation of central responses to stress. DNA methylation in response to early life stress is potentially reversible through intervention. Resilience promoted by psychotherapy and mindfulness practices reverses the epigenetic modification of FKBP5 and BDNF genes caused by stress response and alters cortical activation to improve PTSD symptoms[76-78,80,81]. Investigations also showed that DHEA-S, OXT and enhanced expression of MR can be used as important predictors of resilience[21,24,30]. OXT, a neuropeptide tied to attachment, plays a key role in promotion of postnatal sensitive maternal caregiving to optimize infant’s social and emotional development and establish the neural networks needed during adult life to form social bonds[105-110]. OXT is also a feedback regulator of the HPA axis that enhance recovery from stress-related symptoms[114].
Recent studies also showed the association between early life adversities and circadian rhythm disruption as well as changes in brain-gut axis that may occur via pathways related to glutamatergic excitotoxicity and oxidative stress, predisposing to metabolic derangement, negative mood and stress[37,141]. Re-alignment of circadian rhythms to the natural light/dark cycle is an important health behavior that enhances resilience and adaptation to various stressors[41-43]. Additionally, a healthy brain-gut axis plays an important role in building resilience where gut microbial diversity, healthy diet and exercise can ameliorate the gut-brain axis and stress-induced cortisol release in humans and rodents. Administration of probiotics confer resilience in social defeat model and results in higher DHEA-to-corticosterone fecal metabolite[161,162], thereby mitigating the pervasive effects of early life stress on anxiety and depressive behavior as well as HPA axis activity[163].
Despite the advances in studying resilience and the multitude of contributing factors, there are still gaps in literature about genetic determinants that differentiate resilient individuals from vulnerable ones. Here we reviewed that epigenetic modification of FKBP5 and BDNF genes in response to stress can be reversed by interventions to reduce stress[67,68]. Our group is currently conducting a large-scale study to determine what other genes are implicated in resilience in response to CCT psychotherapy. The advances in miRNA research can also be a powerful tool that can serve as a therapeutic target to improve resilience.
This minireview emphasized the dynamic process of resilience and showed that it continually evolves by the intersection of different domains in human’s life; nature, nurture, environment, and microbiome. Despite the large number of research studies on resilience to stress, none have established one causal factor that differentiates a resilient person from a vulnerable one. This minireview demonstrated that a holistic approach, both clinically and for research purposes, must be adopted to address the multiple elements that promote resilience and prevent physical illnesses and psychopathology. Our future direction is to further understand the role of epigenetic gene silencing in chronic stress in order to identify potential resilience genes that can be reactivated by psychotherapy and the other resilience-promoting interventions mentioned above.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Psychiatry
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Liu XQ, China; Muneoka K, Japan S-Editor: Chen YL L-Editor: A P-Editor: Cai YX
| 1. | Bonanno GA, Diminich ED. Annual Research Review: Positive adjustment to adversity--trajectories of minimal-impact resilience and emergent resilience. J Child Psychol Psychiatry. 2013;54:378-401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 403] [Cited by in RCA: 350] [Article Influence: 29.2] [Reference Citation Analysis (0)] |
| 2. | Folkman S, Moskowitz JT. Coping: pitfalls and promise. Annu Rev Psychol. 2004;55:745-774. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1490] [Cited by in RCA: 1232] [Article Influence: 58.7] [Reference Citation Analysis (0)] |
| 3. | Steinhardt M, Dolbier C. Evaluation of a resilience intervention to enhance coping strategies and protective factors and decrease symptomatology. J Am Coll Health. 2008;56:445-453. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 412] [Cited by in RCA: 369] [Article Influence: 21.7] [Reference Citation Analysis (0)] |
| 4. | Caspi A, McClay J, Moffitt TE, Mill J, Martin J, Craig IW, Taylor A, Poulton R. Role of genotype in the cycle of violence in maltreated children. Science. 2002;297:851-854. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3078] [Cited by in RCA: 2111] [Article Influence: 91.8] [Reference Citation Analysis (0)] |
| 5. | Collishaw S, Pickles A, Messer J, Rutter M, Shearer C, Maughan B. Resilience to adult psychopathology following childhood maltreatment: evidence from a community sample. Child Abuse Negl. 2007;31:211-229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 438] [Cited by in RCA: 386] [Article Influence: 21.4] [Reference Citation Analysis (0)] |
| 6. | Masten AS. Ordinary magic. Resilience processes in development. Am Psychol. 2001;56:227-238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 232] [Article Influence: 9.7] [Reference Citation Analysis (6)] |
| 7. | Quinton D, Rutter M, Liddle C. Institutional rearing, parenting difficulties and marital support. Psychol Med. 1984;14:107-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 125] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 8. | Werner EE. The children of Kauai: resiliency and recovery in adolescence and adulthood. J Adolesc Health. 1992;13:262-268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 103] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 9. | Charney DS, Dejesus G, Manji HK. Cellular plasticity and resilience and the pathophysiology of severe mood disorders. Dialogues Clin Neurosci. 2004;6:217-225. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 10. | Buschdorf JP, Meaney MJ. Epigenetics/Programming in the HPA Axis. Compr Physiol. 2015;6:87-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 44] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 11. | Albakr L, Alqahtani FY, Aleanizy FS, Alomrani A, Badran M, Alhindas H, Al-Mohanna F. Improved delivery of miR-1296 Loaded cationic nanoliposomes for effective suppression of triple negative breast cancer. Saudi Pharm J. 2021;29:446-455. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 12. | Citak C, Erten E. Impact of Childhood Trauma and Attachment on Resilience in Remitted Patients with Bipolar Disorder. J Affect Disord. 2021;280:219-227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 13. | Campbell-Sills L, Cohan SL, Stein MB. Relationship of resilience to personality, coping, and psychiatric symptoms in young adults. Behav Res Ther. 2006;44:585-599. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 652] [Cited by in RCA: 572] [Article Influence: 28.6] [Reference Citation Analysis (0)] |
| 14. | Connor KM, Davidson JR, Lee LC. Spirituality, resilience, and anger in survivors of violent trauma: a community survey. J Trauma Stress. 2003;16:487-494. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 194] [Cited by in RCA: 144] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 15. | Nugent NR, Sumner JA, Amstadter AB. Resilience after trauma: from surviving to thriving. Eur J Psychotraumatol. 2014;5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 26] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 16. | Yehuda R, Flory JD. Differentiating biological correlates of risk, PTSD, and resilience following trauma exposure. J Trauma Stress. 2007;20:435-447. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 70] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 17. | Russo SJ, Murrough JW, Han MH, Charney DS, Nestler EJ. Neurobiology of resilience. Nat Neurosci. 2012;15:1475-1484. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 804] [Cited by in RCA: 743] [Article Influence: 57.2] [Reference Citation Analysis (0)] |
| 18. | Tedeschi RG, Calhoun LG. The Posttraumatic Growth Inventory: measuring the positive legacy of trauma. J Trauma Stress. 1996;9:455-471. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 240] [Cited by in RCA: 968] [Article Influence: 33.4] [Reference Citation Analysis (0)] |
| 19. | Sharma SR, Gonda X, Dome P, Tarazi FI. What's Love Got to do with it: Role of oxytocin in trauma, attachment and resilience. Pharmacol Ther. 2020;214:107602. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 30] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 20. | Romero LM, Platts SH, Schoech SJ, Wada H, Crespi E, Martin LB, Buck CL. Understanding stress in the healthy animal - potential paths for progress. Stress. 2015;18:491-497. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 69] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 21. | Godoy LD, Rossignoli MT, Delfino-Pereira P, Garcia-Cairasco N, de Lima Umeoka EH. A Comprehensive Overview on Stress Neurobiology: Basic Concepts and Clinical Implications. Front Behav Neurosci. 2018;12:127. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 432] [Cited by in RCA: 449] [Article Influence: 64.1] [Reference Citation Analysis (0)] |
| 22. | ter Heegde F, De Rijk RH, Vinkers CH. The brain mineralocorticoid receptor and stress resilience. Psychoneuroendocrinology. 2015;52:92-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 135] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 23. | Smith SM, Vale WW. The role of the hypothalamic-pituitary-adrenal axis in neuroendocrine responses to stress. Dialogues Clin Neurosci. 2006;8:383-395. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 855] [Cited by in RCA: 1092] [Article Influence: 60.7] [Reference Citation Analysis (0)] |
| 24. | Hartmann J, Bajaj T, Klengel C, Chatzinakos C, Ebert T, Dedic N, McCullough KM, Lardenoije R, Joëls M, Meijer OC, McCann KE, Dudek SM, Sarabdjitsingh RA, Daskalakis NP, Klengel T, Gassen NC, Schmidt MV, Ressler KJ. Mineralocorticoid receptors dampen glucocorticoid receptor sensitivity to stress via regulation of FKBP5. Cell Rep. 2021;35:109185. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 60] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 25. | Hu Y, Cardounel A, Gursoy E, Anderson P, Kalimi M. Anti-stress effects of dehydroepiandrosterone: protection of rats against repeated immobilization stress-induced weight loss, glucocorticoid receptor production, and lipid peroxidation. Biochem Pharmacol. 2000;59:753-762. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 85] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 26. | Yehuda R, Brand SR, Golier JA, Yang RK. Clinical correlates of DHEA associated with post-traumatic stress disorder. Acta Psychiatr Scand. 2006;114:187-193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 100] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 27. | Maninger N, Wolkowitz OM, Reus VI, Epel ES, Mellon SH. Neurobiological and neuropsychiatric effects of dehydroepiandrosterone (DHEA) and DHEA sulfate (DHEAS). Front Neuroendocrinol. 2009;30:65-91. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 594] [Cited by in RCA: 552] [Article Influence: 34.5] [Reference Citation Analysis (0)] |
| 28. | Mouthaan J, Sijbrandij M, Luitse JS, Goslings JC, Gersons BP, Olff M. The role of acute cortisol and DHEAS in predicting acute and chronic PTSD symptoms. Psychoneuroendocrinology. 2014;45:179-186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 71] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 29. | Mocking RJ, Pellikaan CM, Lok A, Assies J, Ruhé HG, Koeter MW, Visser I, Bockting CL, Olff M, Schene AH. DHEAS and cortisol/DHEAS-ratio in recurrent depression: State, or trait predicting 10-year recurrence? Psychoneuroendocrinology. 2015;59:91-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 43] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 30. | Pitman RK, Rasmusson AM, Koenen KC, Shin LM, Orr SP, Gilbertson MW, Milad MR, Liberzon I. Biological studies of post-traumatic stress disorder. Nat Rev Neurosci. 2012;13:769-787. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1056] [Cited by in RCA: 1045] [Article Influence: 80.4] [Reference Citation Analysis (0)] |
| 31. | Spencer RL, Chun LE, Hartsock MJ, Woodruff ER. Glucocorticoid hormones are both a major circadian signal and major stress signal: How this shared signal contributes to a dynamic relationship between the circadian and stress systems. Front Neuroendocrinol. 2018;49:52-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 79] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 32. | Nelson RJ, Chbeir S. Dark matters: effects of light at night on metabolism. Proc Nutr Soc. 2018;77:223-229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 50] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 33. | Buijs RM, van Eden CG, Goncharuk VD, Kalsbeek A. The biological clock tunes the organs of the body: timing by hormones and the autonomic nervous system. J Endocrinol. 2003;177:17-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 270] [Cited by in RCA: 256] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 34. | Urbanski HF. Role of circadian neuroendocrine rhythms in the control of behavior and physiology. Neuroendocrinology. 2011;93:211-222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 36] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 35. | Rao R, Androulakis IP. The physiological significance of the circadian dynamics of the HPA axis: Interplay between circadian rhythms, allostasis and stress resilience. Horm Behav. 2019;110:77-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 48] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 36. | Karatsoreos IN, McEwen BS. Timing is everything: a collection on how clocks affect resilience in biological systems. F1000Res. 2014;3:273. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 37. | Koch CE, Leinweber B, Drengberg BC, Blaum C, Oster H. Interaction between circadian rhythms and stress. Neurobiol Stress. 2017;6:57-67. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 118] [Cited by in RCA: 164] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 38. | Karatsoreos IN, Bhagat S, Bloss EB, Morrison JH, McEwen BS. Disruption of circadian clocks has ramifications for metabolism, brain, and behavior. Proc Natl Acad Sci U S A. 2011;108:1657-1662. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 368] [Cited by in RCA: 411] [Article Influence: 29.4] [Reference Citation Analysis (0)] |
| 39. | Loh DH, Navarro J, Hagopian A, Wang LM, Deboer T, Colwell CS. Rapid changes in the light/dark cycle disrupt memory of conditioned fear in mice. PLoS One. 2010;5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 74] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 40. | Maniam J, Antoniadis C, Morris MJ. Early-Life Stress, HPA Axis Adaptation, and Mechanisms Contributing to Later Health Outcomes. Front Endocrinol (Lausanne). 2014;5:73. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 172] [Cited by in RCA: 205] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 41. | Rao RT, Pierre KK, Schlesinger N, Androulakis IP. The Potential of Circadian Realignment in Rheumatoid Arthritis. Crit Rev Biomed Eng. 2016;44:177-191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 42. | Asarnow LD, McGlinchey E, Harvey AG. The effects of bedtime and sleep duration on academic and emotional outcomes in a nationally representative sample of adolescents. J Adolesc Health. 2014;54:350-356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 113] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 43. | Hickie IB, Naismith SL, Robillard R, Scott EM, Hermens DF. Manipulating the sleep-wake cycle and circadian rhythms to improve clinical management of major depression. BMC Med. 2013;11:79. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 95] [Cited by in RCA: 94] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 44. | Pace-Schott EF, Spencer RM, Vijayakumar S, Ahmed NA, Verga PW, Orr SP, Pitman RK, Milad MR. Extinction of conditioned fear is better learned and recalled in the morning than in the evening. J Psychiatr Res. 2013;47:1776-1784. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 66] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 45. | Meuret AE, Rosenfield D, Bhaskara L, Auchus R, Liberzon I, Ritz T, Abelson JL. Timing matters: Endogenous cortisol mediates benefits from early-day psychotherapy. Psychoneuroendocrinology. 2016;74:197-202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 27] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 46. | McEwen BS. Interacting mediators of allostasis and allostatic load: towards an understanding of resilience in aging. Metabolism. 2003;52:10-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 227] [Cited by in RCA: 216] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 47. | Sterling P, Eyer J. Biological basis of stress-related mortality. Soc Sci Med E. 1981;15:3-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 97] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 48. | McEwen BS, Gianaros PJ. Central role of the brain in stress and adaptation: links to socioeconomic status, health, and disease. Ann N Y Acad Sci. 2010;1186:190-222. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1191] [Cited by in RCA: 1034] [Article Influence: 68.9] [Reference Citation Analysis (0)] |
| 49. | McEwen BS, Brinton RE, Sapolsky RM. Glucocorticoid receptors and behavior: implications for the stress response. Adv Exp Med Biol. 1988;245:35-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 38] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 50. | de Kloet ER, Joëls M, Holsboer F. Stress and the brain: from adaptation to disease. Nat Rev Neurosci. 2005;6:463-475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3042] [Cited by in RCA: 3201] [Article Influence: 160.1] [Reference Citation Analysis (0)] |
| 51. | Korte SM, Koolhaas JM, Wingfield JC, McEwen BS. The Darwinian concept of stress: benefits of allostasis and costs of allostatic load and the trade-offs in health and disease. Neurosci Biobehav Rev. 2005;29:3-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 714] [Cited by in RCA: 680] [Article Influence: 32.4] [Reference Citation Analysis (0)] |
| 52. | Lupien SJ, Schramek TE. The differential effects of stress on memory consolidation and retrieval: a potential involvement of reconsolidation? et al. 120:735-738. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 53. | McEwen BS, Stellar E. Stress and the individual. Mechanisms leading to disease. Arch Intern Med. 1993;153:2093-2101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 268] [Cited by in RCA: 271] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 54. | Ganzel BL, Morris PA, Wethington E. Allostasis and the human brain: Integrating models of stress from the social and life sciences. Psychol Rev. 2010;117:134-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 249] [Cited by in RCA: 205] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 55. | Juster RP, McEwen BS, Lupien SJ. Allostatic load biomarkers of chronic stress and impact on health and cognition. Neurosci Biobehav Rev. 2010;35:2-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1391] [Cited by in RCA: 1573] [Article Influence: 104.9] [Reference Citation Analysis (0)] |
| 56. | Schulkin J. Social allostasis: anticipatory regulation of the internal milieu. Front Evol Neurosci. 2011;2:111. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 56] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 57. | Christoffel DJ, Golden SA, Russo SJ. Structural and synaptic plasticity in stress-related disorders. Rev Neurosci. 2011;22:535-549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 215] [Cited by in RCA: 253] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 58. | Niitsu K, Rice MJ, Houfek JF, Stoltenberg SF, Kupzyk KA, Barron CR. A Systematic Review of Genetic Influence on Psychological Resilience. Biol Res Nurs. 2019;21:61-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 59. | Ryan M, Ryznar R. The Molecular Basis of Resilience: A Narrative Review. Front Psychiatry. 2022;13:856998. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 24] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 60. | van der Zee YY, Eijssen LMT, Mews P, Ramakrishnan A, Alvarez K, Lardner CK, Cates HM, Walker DM, Torres-Berrío A, Browne CJ, Cunningham A, Cathomas F, Kronman H, Parise EM, de Nijs L, Shen L, Murrough JW, Rutten BPF, Nestler EJ, Issler O. Blood miR-144-3p: a novel diagnostic and therapeutic tool for depression. Mol Psychiatry. 2022;27:4536-4549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 37] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 61. | Krispil-Alon M, Jovasevic V, Radulovic J, Richter-Levin G. Sex-specific roles of hippocampal microRNAs in stress vulnerability and resilience. Transl Psychiatry. 2022;12:503. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 62. | Weaver IC, Cervoni N, Champagne FA, D'Alessio AC, Sharma S, Seckl JR, Dymov S, Szyf M, Meaney MJ. Epigenetic programming by maternal behavior. Nat Neurosci. 2004;7:847-854. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4264] [Cited by in RCA: 3781] [Article Influence: 180.0] [Reference Citation Analysis (0)] |
| 63. | Turecki G, Meaney MJ. Effects of the Social Environment and Stress on Glucocorticoid Receptor Gene Methylation: A Systematic Review. Biol Psychiatry. 2016;79:87-96. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 543] [Cited by in RCA: 438] [Article Influence: 48.7] [Reference Citation Analysis (0)] |
| 64. | Zannas AS, Wiechmann T, Gassen NC, Binder EB. Gene-Stress-Epigenetic Regulation of FKBP5: Clinical and Translational Implications. Neuropsychopharmacology. 2016;41:261-274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 304] [Cited by in RCA: 409] [Article Influence: 45.4] [Reference Citation Analysis (0)] |
| 65. | Klengel T, Mehta D, Anacker C, Rex-Haffner M, Pruessner JC, Pariante CM, Pace TW, Mercer KB, Mayberg HS, Bradley B, Nemeroff CB, Holsboer F, Heim CM, Ressler KJ, Rein T, Binder EB. Allele-specific FKBP5 DNA demethylation mediates gene-childhood trauma interactions. Nat Neurosci. 2013;16:33-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 970] [Cited by in RCA: 999] [Article Influence: 76.8] [Reference Citation Analysis (0)] |
| 66. | Ising M, Depping AM, Siebertz A, Lucae S, Unschuld PG, Kloiber S, Horstmann S, Uhr M, Müller-Myhsok B, Holsboer F. Polymorphisms in the FKBP5 gene region modulate recovery from psychosocial stress in healthy controls. Eur J Neurosci. 2008;28:389-398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 230] [Cited by in RCA: 241] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 67. | Perroud N, Salzmann A, Prada P, Nicastro R, Hoeppli ME, Furrer S, Ardu S, Krejci I, Karege F, Malafosse A. Response to psychotherapy in borderline personality disorder and methylation status of the BDNF gene. Transl Psychiatry. 2013;3:e207. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 223] [Cited by in RCA: 199] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 68. | Yehuda R, Daskalakis NP, Desarnaud F, Makotkine I, Lehrner AL, Koch E, Flory JD, Buxbaum JD, Meaney MJ, Bierer LM. Epigenetic Biomarkers as Predictors and Correlates of Symptom Improvement Following Psychotherapy in Combat Veterans with PTSD. Front Psychiatry. 2013;4:118. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 221] [Cited by in RCA: 231] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 69. | Roberts S, Keers R, Breen G, Coleman JRI, Jöhren P, Kepa A, Lester KJ, Margraf J, Scheider S, Teismann T, Wannemüller A, Eley TC, Wong CCY. DNA methylation of FKBP5 and response to exposure-based psychological therapy. Am J Med Genet B Neuropsychiatr Genet. 2019;180:150-158. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 44] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 70. | Murgatroyd C, Quinn JP, Sharp HM, Pickles A, Hill J. Effects of prenatal and postnatal depression, and maternal stroking, at the glucocorticoid receptor gene. Transl Psychiatry. 2015;5:e560. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 127] [Cited by in RCA: 118] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 71. | Zenner C, Herrnleben-Kurz S, Walach H. Mindfulness-based interventions in schools-a systematic review and meta-analysis. Front Psychol. 2014;5:603. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 436] [Cited by in RCA: 291] [Article Influence: 26.5] [Reference Citation Analysis (0)] |
| 72. | Biegel GM, Brown KW, Shapiro SL, Schubert CM. Mindfulness-based stress reduction for the treatment of adolescent psychiatric outpatients: A randomized clinical trial. J Consult Clin Psychol. 2009;77:855-866. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 385] [Cited by in RCA: 400] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 73. | Hölzel BK, Ott U, Hempel H, Hackl A, Wolf K, Stark R, Vaitl D. Differential engagement of anterior cingulate and adjacent medial frontal cortex in adept meditators and non-meditators. Neurosci Lett. 2007;421:16-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 336] [Cited by in RCA: 307] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 74. | Tomasino B, Fabbro F. Increases in the right dorsolateral prefrontal cortex and decreases the rostral prefrontal cortex activation after-8 wk of focused attention based mindfulness meditation. Brain Cogn. 2016;102:46-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 62] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 75. | Zeidan F, Emerson NM, Farris SR, Ray JN, Jung Y, McHaffie JG, Coghill RC. Mindfulness Meditation-Based Pain Relief Employs Different Neural Mechanisms Than Placebo and Sham Mindfulness Meditation-Induced Analgesia. J Neurosci. 2015;35:15307-15325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 220] [Article Influence: 24.4] [Reference Citation Analysis (0)] |
| 76. | Kaliman P, Alvarez-López MJ, Cosín-Tomás M, Rosenkranz MA, Lutz A, Davidson RJ. Rapid changes in histone deacetylases and inflammatory gene expression in expert meditators. Psychoneuroendocrinology. 2014;40:96-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 135] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 77. | Bishop JR, Lee AM, Mills LJ, Thuras PD, Eum S, Clancy D, Erbes CR, Polusny MA, Lamberty GJ, Lim KO. Methylation of FKBP5 and SLC6A4 in Relation to Treatment Response to Mindfulness Based Stress Reduction for Posttraumatic Stress Disorder. Front Psychiatry. 2018;9:418. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 59] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 78. | Creswell JD, Pacilio LE, Lindsay EK, Brown KW. Brief mindfulness meditation training alters psychological and neuroendocrine responses to social evaluative stress. Psychoneuroendocrinology. 2014;44:1-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 182] [Cited by in RCA: 174] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 79. | Wang A, Nie W, Li H, Hou Y, Yu Z, Fan Q, Sun R. Epigenetic upregulation of corticotrophin-releasing hormone mediates postnatal maternal separation-induced memory deficiency. PLoS One. 2014;9:e94394. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 60] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 80. | Kletter H, Matlow R, Tanovic S, Carrion V. Cue-Centered Therapy for Youth Experiencing Posttraumatic Symptoms. Curr Treat Options Psychiatry. 2021;8:125-140. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 81. | Espil FM, Balters S, Li R, McCurdy BH, Kletter H, Piccirilli A, Cohen JA, Weems CF, Reiss AL, Carrion VG. Cortical activation predicts posttraumatic improvement in youth treated with TF-CBT or CCT. J Psychiatr Res. 2022;156:25-35. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 82. | Bowlby J. Attachment and loss: retrospect and prospect. Am J Orthopsychiatry. 1982;52:664-678. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1255] [Cited by in RCA: 992] [Article Influence: 23.1] [Reference Citation Analysis (0)] |
| 83. | Harlow HF, Zimmermann RR. Affectional responses in the infant monkey; orphaned baby monkeys develop a strong and persistent attachment to inanimate surrogate mothers. Science. 1959;130:421-432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 565] [Cited by in RCA: 392] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 84. | Feeney BC, Cassidy J, Ramos-Marcuse F. The generalization of attachment representations to new social situations: predicting behavior during initial interactions with strangers. J Pers Soc Psychol. 2008;95:1481-1498. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 74] [Cited by in RCA: 49] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 85. | Cohn DA. Child-mother attachment of six-year-olds and social competence at school. Child Dev. 1990;61:152-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 20] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 86. | Masten AS, Coatsworth JD. The development of competence in favorable and unfavorable environments. Lessons from research on successful children. Am Psychol. 1998;53:205-220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 120] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 87. | Wyman PA, Cowen EL, Work WC, Hoyt-Meyers L, Magnus KB, Fagen DB. Caregiving and developmental factors differentiating young at-risk urban children showing resilient vs stress-affected outcomes: a replication and extension. Child Dev. 1999;70:645-659. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 91] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 88. | Wood LCN. Impact of punitive immigration policies, parent-child separation and child detention on the mental health and development of children. BMJ Paediatr Open. 2018;2:e000338. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 47] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 89. | Sohlberg S, Claesson K, Birgegard A. Memories of mother, complementarity and shame: predicting response to subliminal stimulation with "Mommy and I are one". Scand J Psychol. 2003;44:339-346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 90. | Köhler-Dauner F, Roder E, Krause S, Buchheim A, Gündel H, Fegert JM, Ziegenhain U, Waller C. Reduced caregiving quality measured during the strange situation procedure increases child's autonomic nervous system stress response. Child Adolesc Psychiatry Ment Health. 2019;13:41. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 91. | Buchheim A, Ziegenhain U, Kindler H, Waller C, Gündel H, Karabatsiakis A, Fegert J. Identifying Risk and Resilience Factors in the Intergenerational Cycle of Maltreatment: Results From the TRANS-GEN Study Investigating the Effects of Maternal Attachment and Social Support on Child Attachment and Cardiovascular Stress Physiology. Front Hum Neurosci. 2022;16:890262. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 92. | Neumann ID. Stimuli and consequences of dendritic release of oxytocin within the brain. Biochem Soc Trans. 2007;35:1252-1257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 125] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 93. | Neumann ID. Oxytocin: the neuropeptide of love reveals some of its secrets. Cell Metab. 2007;5:231-233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 94. | Olff M, Frijling JL, Kubzansky LD, Bradley B, Ellenbogen MA, Cardoso C, Bartz JA, Yee JR, van Zuiden M. The role of oxytocin in social bonding, stress regulation and mental health: an update on the moderating effects of context and interindividual differences. Psychoneuroendocrinology. 2013;38:1883-1894. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 412] [Cited by in RCA: 428] [Article Influence: 35.7] [Reference Citation Analysis (0)] |
| 95. | Sofroniew MV. Morphology of vasopressin and oxytocin neurones and their central and vascular projections. Prog Brain Res. 1983;60:101-114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 247] [Cited by in RCA: 247] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 96. | Swanson LW, Sawchenko PE. Hypothalamic integration: organization of the paraventricular and supraoptic nuclei. Annu Rev Neurosci. 1983;6:269-324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1568] [Cited by in RCA: 1565] [Article Influence: 37.3] [Reference Citation Analysis (0)] |
| 97. | Uvnäs-Moberg K, Ekström-Bergström A, Berg M, Buckley S, Pajalic Z, Hadjigeorgiou E, Kotłowska A, Lengler L, Kielbratowska B, Leon-Larios F, Magistretti CM, Downe S, Lindström B, Dencker A. Maternal plasma levels of oxytocin during physiological childbirth - a systematic review with implications for uterine contractions and central actions of oxytocin. BMC Pregnancy Childbirth. 2019;19:285. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 151] [Cited by in RCA: 178] [Article Influence: 29.7] [Reference Citation Analysis (0)] |
| 98. | Feldman R, Bakermans-Kranenburg MJ. Oxytocin: a parenting hormone. Curr Opin Psychol. 2017;15:13-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 106] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 99. | Galbally M, Lewis AJ, Ijzendoorn Mv, Permezel M. The role of oxytocin in mother-infant relations: a systematic review of human studies. Harv Rev Psychiatry. 2011;19:1-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 212] [Cited by in RCA: 177] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 100. | Scatliffe N, Casavant S, Vittner D, Cong X. Oxytocin and early parent-infant interactions: A systematic review. Int J Nurs Sci. 2019;6:445-453. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 71] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 101. | Nishizato M, Fujisawa TX, Kosaka H, Tomoda A. Developmental changes in social attention and oxytocin levels in infants and children. Sci Rep. 2017;7:2540. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 47] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 102. | Suzuki S, Fujisawa TX, Sakakibara N, Fujioka T, Takiguchi S, Tomoda A. Development of Social Attention and Oxytocin Levels in Maltreated Children. Sci Rep. 2020;10:7407. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 103. | Bosch OJ, Young LJ. Oxytocin and Social Relationships: From Attachment to Bond Disruption. Curr Top Behav Neurosci. 2018;35:97-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 98] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 104. | Macdonald K, Macdonald TM. The peptide that binds: a systematic review of oxytocin and its prosocial effects in humans. Harv Rev Psychiatry. 2010;18:1-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 475] [Cited by in RCA: 383] [Article Influence: 25.5] [Reference Citation Analysis (0)] |
| 105. | Barrett CE, Arambula SE, Young LJ. The oxytocin system promotes resilience to the effects of neonatal isolation on adult social attachment in female prairie voles. Transl Psychiatry. 2015;5:e606. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 93] [Cited by in RCA: 95] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 106. | Rilling JK, Young LJ. The biology of mammalian parenting and its effect on offspring social development. Science. 2014;345:771-776. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 362] [Cited by in RCA: 334] [Article Influence: 30.4] [Reference Citation Analysis (0)] |
| 107. | Uvnäs-Moberg K. Oxytocin may mediate the benefits of positive social interaction and emotions. Psychoneuroendocrinology. 1998;23:819-835. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 633] [Cited by in RCA: 568] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 108. | Neumann ID. Involvement of the brain oxytocin system in stress coping: interactions with the hypothalamo-pituitary-adrenal axis. Prog Brain Res. 2002;139:147-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 261] [Cited by in RCA: 269] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 109. | Smith AS, Wang Z. Hypothalamic oxytocin mediates social buffering of the stress response. Biol Psychiatry. 2014;76:281-288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 256] [Cited by in RCA: 266] [Article Influence: 24.2] [Reference Citation Analysis (0)] |
| 110. | Ditzen B, Heinrichs M. Psychobiology of social support: the social dimension of stress buffering. Restor Neurol Neurosci. 2014;32:149-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 131] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 111. | Heim C, Newport DJ, Mletzko T, Miller AH, Nemeroff CB. The link between childhood trauma and depression: insights from HPA axis studies in humans. Psychoneuroendocrinology. 2008;33:693-710. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1122] [Cited by in RCA: 1139] [Article Influence: 67.0] [Reference Citation Analysis (0)] |
| 112. | Doom JR, Hostinar CE, VanZomeren-Dohm AA, Gunnar MR. The roles of puberty and age in explaining the diminished effectiveness of parental buffering of HPA reactivity and recovery in adolescence. Psychoneuroendocrinology. 2015;59:102-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 51] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 113. | Winslow JT, Noble PL, Lyons CK, Sterk SM, Insel TR. Rearing effects on cerebrospinal fluid oxytocin concentration and social buffering in rhesus monkeys. Neuropsychopharmacology. 2003;28:910-918. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 242] [Cited by in RCA: 233] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 114. | Gibbs DM. Stress-specific modulation of ACTH secretion by oxytocin. Neuroendocrinology. 1986;42:456-458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 44] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 115. | Nakashima T, Noguchi T, Furukawa T, Yamasaki M, Makino S, Miyata S, Kiyohara T. Brain oxytocin augments stress-induced long-lasting plasma adrenocorticotropic hormone elevation in rats. Neurosci Lett. 2002;321:161-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 116. | Engert V, Koester AM, Riepenhausen A, Singer T. Boosting recovery rather than buffering reactivity: Higher stress-induced oxytocin secretion is associated with increased cortisol reactivity and faster vagal recovery after acute psychosocial stress. Psychoneuroendocrinology. 2016;74:111-120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 71] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 117. | Missig G, Ayers LW, Schulkin J, Rosen JB. Oxytocin reduces background anxiety in a fear-potentiated startle paradigm. Neuropsychopharmacology. 2010;35:2607-2616. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 63] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 118. | Ayers LW, Missig G, Schulkin J, Rosen JB. Oxytocin reduces background anxiety in a fear-potentiated startle paradigm: peripheral vs central administration. Neuropsychopharmacology. 2011;36:2488-2497. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 67] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 119. | Ayers L, Agostini A, Schulkin J, Rosen JB. Effects of oxytocin on background anxiety in rats with high or low baseline startle. Psychopharmacology (Berl). 2016;233:2165-2172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 120. | Moaddab M, Dabrowska J. Oxytocin receptor neurotransmission in the dorsolateral bed nucleus of the stria terminalis facilitates the acquisition of cued fear in the fear-potentiated startle paradigm in rats. Neuropharmacology. 2017;121:130-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 35] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 121. | Janeček M, Dabrowska J. Oxytocin facilitates adaptive fear and attenuates anxiety responses in animal models and human studies-potential interaction with the corticotropin-releasing factor (CRF) system in the bed nucleus of the stria terminalis (BNST). Cell Tissue Res. 2019;375:143-172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 55] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 122. | Martinon D, Lis P, Roman AN, Tornesi P, Applebey SV, Buechner G, Olivera V, Dabrowska J. Oxytocin receptors in the dorsolateral bed nucleus of the stria terminalis (BNST) bias fear learning toward temporally predictable cued fear. Transl Psychiatry. 2019;9:140. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 33] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 123. | Olivera-Pasilio V, Dabrowska J. Oxytocin Promotes Accurate Fear Discrimination and Adaptive Defensive Behaviors. Front Neurosci. 2020;14:583878. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 35] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 124. | Toth I, Neumann ID, Slattery DA. Central administration of oxytocin receptor ligands affects cued fear extinction in rats and mice in a timepoint-dependent manner. Psychopharmacology (Berl). 2012;223:149-158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 82] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 125. | Wang SC, Lin CC, Chen CC, Tzeng NS, Liu YP. Effects of Oxytocin on Fear Memory and Neuroinflammation in a Rodent Model of Posttraumatic Stress Disorder. Int J Mol Sci. 2018;19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 53] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 126. | Hopkins DA, Holstege G. Amygdaloid projections to the mesencephalon, pons and medulla oblongata in the cat. Exp Brain Res. 1978;32:529-547. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 696] [Cited by in RCA: 678] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 127. | Schwaber JS, Kapp BS, Higgins GA, Rapp PR. Amygdaloid and basal forebrain direct connections with the nucleus of the solitary tract and the dorsal motor nucleus. J Neurosci. 1982;2:1424-1438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 321] [Cited by in RCA: 310] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 128. | Rizvi TA, Ennis M, Behbehani MM, Shipley MT. Connections between the central nucleus of the amygdala and the midbrain periaqueductal gray: topography and reciprocity. J Comp Neurol. 1991;303:121-131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 377] [Cited by in RCA: 388] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 129. | Neumann ID, Slattery DA. Oxytocin in General Anxiety and Social Fear: A Translational Approach. Biol Psychiatry. 2016;79:213-221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 309] [Cited by in RCA: 347] [Article Influence: 38.6] [Reference Citation Analysis (0)] |
| 130. | Pisansky MT, Hanson LR, Gottesman II, Gewirtz JC. Oxytocin enhances observational fear in mice. Nat Commun. 2017;8:2102. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 89] [Cited by in RCA: 125] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 131. | Byrd-Craven J, Criss MM, Calvi JL, Cui L, Baraldi A, Sheffield Morris A. Adrenocortical attunement, reactivity, and potential genetic correlates among parent-daughter dyads from low-income families. Dev Psychobiol. 2020;62:1035-1045. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 132. | Hostinar CE, Cicchetti D, Rogosch FA. Oxytocin receptor gene polymorphism, perceived social support, and psychological symptoms in maltreated adolescents. Dev Psychopathol. 2014;26:465-477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 65] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 133. | Kushner SC, Herzhoff K, Vrshek-Schallhorn S, Tackett JL. Depression in early adolescence: Contributions from relational aggression and variation in the oxytocin receptor gene. Aggress Behav. 2018;44:60-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 20] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 134. | Thompson RJ, Parker KJ, Hallmayer JF, Waugh CE, Gotlib IH. Oxytocin receptor gene polymorphism (rs2254298) interacts with familial risk for psychopathology to predict symptoms of depression and anxiety in adolescent girls. Psychoneuroendocrinology. 2011;36:144-147. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 175] [Cited by in RCA: 169] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 135. | Thompson SM, Hammen C, Starr LR, Najman JM. Oxytocin receptor gene polymorphism (rs53576) moderates the intergenerational transmission of depression. Psychoneuroendocrinology. 2014;43:11-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 52] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 136. | Cao C, Wang L, Wu J, Li G, Fang R, Liu P, Luo S, Elhai JD. Association between the OXTR rs53576 genotype and latent profiles of post-traumatic stress disorder and depression symptoms in a representative sample of earthquake survivors. Anxiety Stress Coping. 2020;33:140-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 137. | Cataldo I, Azhari A, Lepri B, Esposito G. Oxytocin receptors (OXTR) and early parental care: An interaction that modulates psychiatric disorders. Res Dev Disabil. 2018;82:27-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 49] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 138. | Dadds MR, Moul C, Cauchi A, Dobson-Stone C, Hawes DJ, Brennan J, Urwin R, Ebstein RE. Polymorphisms in the oxytocin receptor gene are associated with the development of psychopathy. Dev Psychopathol. 2014;26:21-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 57] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 139. | Tost H, Kolachana B, Hakimi S, Lemaitre H, Verchinski BA, Mattay VS, Weinberger DR, Meyer-Lindenberg A. A common allele in the oxytocin receptor gene (OXTR) impacts prosocial temperament and human hypothalamic-limbic structure and function. Proc Natl Acad Sci U S A. 2010;107:13936-13941. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 415] [Cited by in RCA: 406] [Article Influence: 27.1] [Reference Citation Analysis (0)] |
| 140. | Lancaster K, Goldbeck L, Puglia MH, Morris JP, Connelly JJ. DNA methylation of OXTR is associated with parasympathetic nervous system activity and amygdala morphology. Soc Cogn Affect Neurosci. 2018;13:1155-1162. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 141. | Coley EJL, Mayer EA, Osadchiy V, Chen Z, Subramanyam V, Zhang Y, Hsiao EY, Gao K, Bhatt R, Dong T, Vora P, Naliboff B, Jacobs JP, Gupta A. Early life adversity predicts brain-gut alterations associated with increased stress and mood. Neurobiol Stress. 2021;15:100348. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 31] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 142. | Brown AS, van Os J, Driessens C, Hoek HW, Susser ES. Further evidence of relation between prenatal famine and major affective disorder. Am J Psychiatry. 2000;157:190-195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 253] [Cited by in RCA: 238] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 143. | Painter RC, Roseboom TJ, Bleker OP. Prenatal exposure to the Dutch famine and disease in later life: an overview. Reprod Toxicol. 2005;20:345-352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 520] [Cited by in RCA: 455] [Article Influence: 22.8] [Reference Citation Analysis (0)] |
| 144. | Ye W, Pitlock MD, Javors MA, Thompson BJ, Lechleiter JD, Hensler JG. The long-term effect of maternal dietary protein restriction on 5-HT(1A) receptor function and behavioral responses to stress in adulthood. Behav Brain Res. 2018;349:116-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 145. | Nasrallah P, Haidar EA, Stephan JS, El Hayek L, Karnib N, Khalifeh M, Barmo N, Jabre V, Houbeika R, Ghanem A, Nasser J, Zeeni N, Bassil M, Sleiman SF. Branched-chain amino acids mediate resilience to chronic social defeat stress by activating BDNF/TRKB signaling. Neurobiol Stress. 2019;11:100170. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 36] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 146. | Reynolds CA, Gatz M, Berg S, Pedersen NL. Genotype-environment interactions: cognitive aging and social factors. Twin Res Hum Genet. 2007;10:241-254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 19] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 147. | Herrick K, Phillips DI, Haselden S, Shiell AW, Campbell-Brown M, Godfrey KM. Maternal consumption of a high-meat, low-carbohydrate diet in late pregnancy: relation to adult cortisol concentrations in the offspring. J Clin Endocrinol Metab. 2003;88:3554-3560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 42] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 148. | Drake AJ, McPherson RC, Godfrey KM, Cooper C, Lillycrop KA, Hanson MA, Meehan RR, Seckl JR, Reynolds RM. An unbalanced maternal diet in pregnancy associates with offspring epigenetic changes in genes controlling glucocorticoid action and foetal growth. Clin Endocrinol (Oxf). 2012;77:808-815. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 84] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 149. | Sudo N, Chida Y, Aiba Y, Sonoda J, Oyama N, Yu XN, Kubo C, Koga Y. Postnatal microbial colonization programs the hypothalamic-pituitary-adrenal system for stress response in mice. J Physiol. 2004;558:263-275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2097] [Cited by in RCA: 1861] [Article Influence: 88.6] [Reference Citation Analysis (1)] |
| 150. | Callaghan BL, Fields A, Gee DG, Gabard-Durnam L, Caldera C, Humphreys KL, Goff B, Flannery J, Telzer EH, Shapiro M, Tottenham N. Mind and gut: Associations between mood and gastrointestinal distress in children exposed to adversity. Dev Psychopathol. 2020;32:309-328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 53] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 151. | Dalile B, Vervliet B, Bergonzelli G, Verbeke K, Van Oudenhove L. Colon-delivered short-chain fatty acids attenuate the cortisol response to psychosocial stress in healthy men: a randomized, placebo-controlled trial. Neuropsychopharmacology. 2020;45:2257-2266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 118] [Article Influence: 23.6] [Reference Citation Analysis (0)] |
| 152. | Berding K, Vlckova K, Marx W, Schellekens H, Stanton C, Clarke G, Jacka F, Dinan TG, Cryan JF. Diet and the Microbiota-Gut-Brain Axis: Sowing the Seeds of Good Mental Health. Adv Nutr. 2021;12:1239-1285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 193] [Article Influence: 48.3] [Reference Citation Analysis (0)] |
| 153. | Morris G, Gamage E, Travica N, Berk M, Jacka FN, O'Neil A, Puri BK, Carvalho AF, Bortolasci CC, Walder K, Marx W. Polyphenols as adjunctive treatments in psychiatric and neurodegenerative disorders: Efficacy, mechanisms of action, and factors influencing inter-individual response. Free Radic Biol Med. 2021;172:101-122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 22] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 154. | Safadi JM, Quinton AMG, Lennox BR, Burnet PWJ, Minichino A. Gut dysbiosis in severe mental illness and chronic fatigue: a novel trans-diagnostic construct? Mol Psychiatry. 2022;27:141-153. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 65] [Article Influence: 21.7] [Reference Citation Analysis (0)] |
| 155. | Dong TS, Guan M, Mayer EA, Stains J, Liu C, Vora P, Jacobs JP, Lagishetty V, Chang L, Barry RL, Gupta A. Obesity is associated with a distinct brain-gut microbiome signature that connects Prevotella and Bacteroides to the brain's reward center. Gut Microbes. 2022;14:2051999. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 51] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 156. | Allen JM, Mailing LJ, Niemiro GM, Moore R, Cook MD, White BA, Holscher HD, Woods JA. Exercise Alters Gut Microbiota Composition and Function in Lean and Obese Humans. Med Sci Sports Exerc. 2018;50:747-757. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 370] [Cited by in RCA: 506] [Article Influence: 72.3] [Reference Citation Analysis (0)] |
| 157. | Cryan JF, O'Riordan KJ, Cowan CSM, Sandhu KV, Bastiaanssen TFS, Boehme M, Codagnone MG, Cussotto S, Fulling C, Golubeva AV, Guzzetta KE, Jaggar M, Long-Smith CM, Lyte JM, Martin JA, Molinero-Perez A, Moloney G, Morelli E, Morillas E, O'Connor R, Cruz-Pereira JS, Peterson VL, Rea K, Ritz NL, Sherwin E, Spichak S, Teichman EM, van de Wouw M, Ventura-Silva AP, Wallace-Fitzsimons SE, Hyland N, Clarke G, Dinan TG. The Microbiota-Gut-Brain Axis. Physiol Rev. 2019;99:1877-2013. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1156] [Cited by in RCA: 2772] [Article Influence: 462.0] [Reference Citation Analysis (2)] |
| 158. | van de Wouw M, Schellekens H, Dinan TG, Cryan JF. Microbiota-Gut-Brain Axis: Modulator of Host Metabolism and Appetite. J Nutr. 2017;147:727-745. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 277] [Article Influence: 34.6] [Reference Citation Analysis (0)] |
| 159. | Gupta A, Osadchiy V, Mayer EA. Brain-gut-microbiome interactions in obesity and food addiction. Nat Rev Gastroenterol Hepatol. 2020;17:655-672. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 147] [Article Influence: 29.4] [Reference Citation Analysis (0)] |
| 160. | Berding K, Cryan JF. Microbiota-targeted interventions for mental health. Curr Opin Psychiatry. 2022;35:3-9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 30] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 161. | Horn J, Mayer DE, Chen S, Mayer EA. Role of diet and its effects on the gut microbiome in the pathophysiology of mental disorders. Transl Psychiatry. 2022;12:164. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 78] [Cited by in RCA: 95] [Article Influence: 31.7] [Reference Citation Analysis (0)] |
| 162. | Maehata H, Kobayashi Y, Mitsuyama E, Kawase T, Kuhara T, Xiao JZ, Tsukahara T, Toyoda A. Heat-killed Lactobacillus helveticus strain MCC1848 confers resilience to anxiety or depression-like symptoms caused by subchronic social defeat stress in mice. Biosci Biotechnol Biochem. 2019;83:1239-1247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 57] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 163. | Natale NR, Kent M, Fox N, Vavra D, Lambert K. Neurobiological effects of a probiotic-supplemented diet in chronically stressed male Long-Evans rats: Evidence of enhanced resilience. IBRO Neurosci Rep. 2021;11:207-215. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 164. | O'Neil A, Berk M, Itsiopoulos C, Castle D, Opie R, Pizzinga J, Brazionis L, Hodge A, Mihalopoulos C, Chatterton ML, Dean OM, Jacka FN. A randomised, controlled trial of a dietary intervention for adults with major depression (the "SMILES" trial): study protocol. BMC Psychiatry. 2013;13:114. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 49] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 165. | Jacka FN, O'Neil A, Opie R, Itsiopoulos C, Cotton S, Mohebbi M, Castle D, Dash S, Mihalopoulos C, Chatterton ML, Brazionis L, Dean OM, Hodge AM, Berk M. A randomised controlled trial of dietary improvement for adults with major depression (the 'SMILES' trial). BMC Med. 2017;15:23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 538] [Cited by in RCA: 553] [Article Influence: 69.1] [Reference Citation Analysis (0)] |
| 166. | Bonaccio M, Di Castelnuovo A, Costanzo S, Pounis G, Persichillo M, Cerletti C, Donati MB, de Gaetano G, Iacoviello L. Mediterranean-type diet is associated with higher psychological resilience in a general adult population: findings from the Moli-sani study. Eur J Clin Nutr. 2018;72:154-160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 45] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 167. | Panossian A. Understanding adaptogenic activity: specificity of the pharmacological action of adaptogens and other phytochemicals. Ann N Y Acad Sci. 2017;1401:49-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 65] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 168. | Sato M, Okuno A, Suzuki K, Ohsawa N, Inoue E, Miyaguchi Y, Toyoda A. Dietary intake of the citrus flavonoid hesperidin affects stress-resilience and brain kynurenine levels in a subchronic and mild social defeat stress model in mice. Biosci Biotechnol Biochem. 2019;83:1756-1765. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 169. | Vinson JA. Intracellular Polyphenols: How Little We Know. J Agric Food Chem. 2019;67:3865-3870. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 170. | Yao W, Zhang JC, Ishima T, Dong C, Yang C, Ren Q, Ma M, Han M, Wu J, Suganuma H, Ushida Y, Yamamoto M, Hashimoto K. Role of Keap1-Nrf2 signaling in depression and dietary intake of glucoraphanin confers stress resilience in mice. Sci Rep. 2016;6:30659. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 90] [Cited by in RCA: 113] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 171. | Aubry AV, Khandaker H, Ravenelle R, Grunfeld IS, Bonnefil V, Chan KL, Cathomas F, Liu J, Schafe GE, Burghardt NS. A diet enriched with curcumin promotes resilience to chronic social defeat stress. Neuropsychopharmacology. 2019;44:733-742. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 35] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 172. | Cardona F, Andrés-Lacueva C, Tulipani S, Tinahones FJ, Queipo-Ortuño MI. Benefits of polyphenols on gut microbiota and implications in human health. J Nutr Biochem. 2013;24:1415-1422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 889] [Cited by in RCA: 1042] [Article Influence: 94.7] [Reference Citation Analysis (0)] |
| 173. | Dueñas M, Muñoz-González I, Cueva C, Jiménez-Girón A, Sánchez-Patán F, Santos-Buelga C, Moreno-Arribas MV, Bartolomé B. A survey of modulation of gut microbiota by dietary polyphenols. Biomed Res Int. 2015;2015:850902. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 225] [Cited by in RCA: 246] [Article Influence: 24.6] [Reference Citation Analysis (0)] |
| 174. | Ozdal T, Sela DA, Xiao J, Boyacioglu D, Chen F, Capanoglu E. The Reciprocal Interactions between Polyphenols and Gut Microbiota and Effects on Bioaccessibility. Nutrients. 2016;8:78. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 606] [Cited by in RCA: 524] [Article Influence: 58.2] [Reference Citation Analysis (0)] |
| 175. | Espín JC, González-Sarrías A, Tomás-Barberán FA. The gut microbiota: A key factor in the therapeutic effects of (poly)phenols. Biochem Pharmacol. 2017;139:82-93. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 331] [Cited by in RCA: 410] [Article Influence: 51.3] [Reference Citation Analysis (0)] |
| 176. | Chow LS, Gerszten RE, Taylor JM, Pedersen BK, van Praag H, Trappe S, Febbraio MA, Galis ZS, Gao Y, Haus JM, Lanza IR, Lavie CJ, Lee CH, Lucia A, Moro C, Pandey A, Robbins JM, Stanford KI, Thackray AE, Villeda S, Watt MJ, Xia A, Zierath JR, Goodpaster BH, Snyder MP. Exerkines in health, resilience and disease. Nat Rev Endocrinol. 2022;18:273-289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 361] [Cited by in RCA: 462] [Article Influence: 154.0] [Reference Citation Analysis (0)] |
| 177. | Erickson KI, Voss MW, Prakash RS, Basak C, Szabo A, Chaddock L, Kim JS, Heo S, Alves H, White SM, Wojcicki TR, Mailey E, Vieira VJ, Martin SA, Pence BD, Woods JA, McAuley E, Kramer AF. Exercise training increases size of hippocampus and improves memory. Proc Natl Acad Sci U S A. 2011;108:3017-3022. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2601] [Cited by in RCA: 2957] [Article Influence: 211.2] [Reference Citation Analysis (0)] |
| 178. | Valenzuela PL, Castillo-García A, Morales JS, de la Villa P, Hampel H, Emanuele E, Lista S, Lucia A. Exercise benefits on Alzheimer's disease: State-of-the-science. Ageing Res Rev. 2020;62:101108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 204] [Article Influence: 40.8] [Reference Citation Analysis (0)] |
| 179. | Nicolini C, Michalski B, Toepp SL, Turco CV, D'Hoine T, Harasym D, Gibala MJ, Fahnestock M, Nelson AJ. A Single Bout of High-intensity Interval Exercise Increases Corticospinal Excitability, Brain-derived Neurotrophic Factor, and Uncarboxylated Osteolcalcin in Sedentary, Healthy Males. Neuroscience. 2020;437:242-255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 43] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 180. | Liu PZ, Nusslock R. Exercise-Mediated Neurogenesis in the Hippocampus via BDNF. Front Neurosci. 2018;12:52. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 263] [Cited by in RCA: 314] [Article Influence: 44.9] [Reference Citation Analysis (0)] |
| 181. | Wrann CD, White JP, Salogiannnis J, Laznik-Bogoslavski D, Wu J, Ma D, Lin JD, Greenberg ME, Spiegelman BM. Exercise induces hippocampal BDNF through a PGC-1α/FNDC5 pathway. Cell Metab. 2013;18:649-659. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 848] [Cited by in RCA: 976] [Article Influence: 81.3] [Reference Citation Analysis (0)] |
| 182. | Yau SY, Li A, Hoo RL, Ching YP, Christie BR, Lee TM, Xu A, So KF. Physical exercise-induced hippocampal neurogenesis and antidepressant effects are mediated by the adipocyte hormone adiponectin. Proc Natl Acad Sci U S A. 2014;111:15810-15815. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 252] [Article Influence: 22.9] [Reference Citation Analysis (0)] |
| 183. | Agudelo LZ, Femenía T, Orhan F, Porsmyr-Palmertz M, Goiny M, Martinez-Redondo V, Correia JC, Izadi M, Bhat M, Schuppe-Koistinen I, Pettersson AT, Ferreira DMS, Krook A, Barres R, Zierath JR, Erhardt S, Lindskog M, Ruas JL. Skeletal muscle PGC-1α1 modulates kynurenine metabolism and mediates resilience to stress-induced depression. Cell. 2014;159:33-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 422] [Cited by in RCA: 554] [Article Influence: 50.4] [Reference Citation Analysis (0)] |
| 184. | Steptoe A, Butler N. Sports participation and emotional wellbeing in adolescents. Lancet. 1996;347:1789-1792. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 239] [Cited by in RCA: 184] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 185. | Roth DL, Holmes DS. Influence of aerobic exercise training and relaxation training on physical and psychologic health following stressful life events. Psychosom Med. 1987;49:355-365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 92] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 186. | Harber VJ, Sutton JR. Endorphins and exercise. Sports Med. 1984;1:154-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 116] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 187. | Thorén P, Floras JS, Hoffmann P, Seals DR. Endorphins and exercise: physiological mechanisms and clinical implications. Med Sci Sports Exerc. 1990;22:417-428. [PubMed] |
| 188. | Morris M, Salmon P, Steinberg H, Sykes EA, Bouloux P, Newbould E, McLoughlin L, Besser GM, Grossman A. Endogenous opioids modulate the cardiovascular response to mental stress. Psychoneuroendocrinology. 1990;15:185-192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 38] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 189. | Janal MN, Colt EWD, Clark CW, Glusman M. Pain sensitivity, mood and plasma endocrine levels in man following long-distance running: effects of naloxone. Pain. 1984;19:13-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 285] [Cited by in RCA: 264] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 190. | Bethell C, Jones J, Gombojav N, Linkenbach J, Sege R. Positive Childhood Experiences and Adult Mental and Relational Health in a Statewide Sample: Associations Across Adverse Childhood Experiences Levels. JAMA Pediatr. 2019;173:e193007. [PubMed] |