Published online Mar 19, 2023. doi: 10.5498/wjp.v13.i3.113
Peer-review started: December 27, 2022
First decision: January 31, 2023
Revised: February 4, 2023
Accepted: March 14, 2023
Article in press: March 14, 2023
Published online: March 19, 2023
Processing time: 79 Days and 14.8 Hours
Sleep breathing, one of the basic human needs, is a physiological need that affects cardiac functions, body temperature, daily vitality, muscle tone, hormone secretion, blood pressure, and many more. In the international literature, studies reported that patients have had sleep problems in the hospital since the 1990s, but no measurement tool has been developed to determine the causes of hospital-acquired insomnia in individuals. These findings suggest that sleep remains in the background compared to activities such as nutrition and breathing. Although patients generally experience hospital-acquired sleep problems, there is no measurement tool to determine hospital-acquired sleep problems. These features show the originality of the research.
To develop a measurement tool to determine the sleep problems experienced by patients in the hospital.
A personal information form, hospital-acquired insomnia scale (HAIS), and insomnia severity index (ISI) were used to collect research data. The study population consisted of patients hospitalized in the internal and surgical clinics of a research hospital in Turkey between December 2021 and March 2022. The sample consisted of 64 patients in the pilot application stage and 223 patients in the main application stage. Exploratory factor analysis and confirmatory factor analysis (CFA) analyses were performed using the SPSS 20 package program and the analysis of moment structure (AMOS) package program. Equivalent forms method used.
The HAIS consisted of 18 items and 5 subscales. The Cronbach alpha values of the subscales ranged between 0.672 and 0.842 and the Cronbach alpha value of the overall scale was 0.783. The scale explained 58.269% of the total variance. The items that constitute the factors were examined in terms of content integrity and named as physical environmental, psychological, safety, socioeconomic, and nutritional factors. CFA analysis of the 5-factor structure was performed in the AMOS package program. The fit indices of the obtained structure were examined. It was determined that the values obtained from the fit indices were sufficient. A significant correlation was determined between the HAIS and the ISI, which was used for the equivalent form method.
The HAIS is a valid and reliable measurement tool for determining patients’ level of hospital-acquired insomnia. It is recommended to use this measurement tool to determine the insomnia problems of patients and to adapt it in other countries.
Core Tip: Sleep, which is one of the basic human needs, is a physiological need that affects heart functions, hormone secretion, mood, psychological state and many more. Although there are studies in the international literature reporting that patients have sleep problems in the hospital since the 1990s, a measurement tool has not been developed to determine the causes of hospital-acquired insomnia in individuals. Determination of hospital-acquired insomnia causes of individuals; It will provide many benefits such as increasing the quality of care, improving mood, reducing stress levels, increasing the effectiveness of treatment, and increasing psychological resilience. In this research, a measurement tool was developed to identify the causes of hospital-acquired insomnia and to identify the causes of insomnia in hospitals or inpatient health institutions by focusing on the vital sleep activity. A measurement tool consisting of 18 items and 5 factors was developed. In addition, the literature on the factors that cause hospital-acquired insomnia was reviewed and some recommendations were made.
- Citation: Çiftçi B, Yıldız GN, Yıldız Ö. Hospital-acquired insomnia scale: A validity and reliability study. World J Psychiatry 2023; 13(3): 113-125
- URL: https://www.wjgnet.com/2220-3206/full/v13/i3/113.htm
- DOI: https://dx.doi.org/10.5498/wjp.v13.i3.113
Sleep breathing, one of the basic human needs, is a physiological need that affects cardiac functions, body temperature, daily vitality, muscle tone, hormone secretion, blood pressure, and many more. With these properties, sleep is the basic need for human survival[1-3]. Some conditions such as pregnancy, stress, menopause, physical diseases, and hospitalization increase the need for sleep[1,4]. Sleep plays an important role in maintaining health and recovering diseases[1,3]. Moreover, sleep is a need that people are most vulnerable to deprivation and must inevitably be replaced[2].
Insomnia can be defined as difficulty falling asleep, maintaining or ending sleep, and situations in which individuals do not feel rested or ready for the new day because they do not sleep enough or for a sufficient time[1,2]. Insomnia can occur independently or due to other problems. Temporary insomnia, which may arise from reasons such as stress, is a common problem, including in hospitals. Studies show that 33% to 69% of patients experience sleep deprivation in the hospital[5-7].
Insomnia is a common patient complaint during hospitalization[8-12]. While in the hospital, patients may experience sleep problems due to many reasons such as hospitalization, not getting used to the bed, being away from the family, a foreign environment, disease anxiety, surgical interventions, sound, light, noise, inability to meet the needs of daily living, and deterioration in body image[13,14]. In addition, it is stated that some factors such as the high number of patients in the room, room temperature, implementations made at sleep time, and the obligatory sleeping positions affect sleep patterns in the hospital[15]. It is stated that the reasons such as the stuffiness of the room, smell, reasons arising from the bedding, inconvenience of the bed and the pillow, pain, lack of information, drugs, tubes such as drains-catheters, hunger-fullness also cause sleep problems among hospitalized patients[16]. In another study, it was stated that in intensive care units, sleep is affected by factors originating from intensive care (noise, patient care, and treatment practices) and disease-related factors (pain, anxiety, disease severity, drugs, mechanical ventilation)[17].
Individuals resort to medication since insomnia creates stress and anxiety in individuals. However, in this process, it will be a more effective method to determine the cause of insomnia, take precautions, eliminate the cause, make necessary arrangements for sleep, plan the nursing care accordingly, and use medication as a last resort[2,18,19]. The ability of a nurse to solve this problem is associated with his/her ability to evaluate sleep and the appropriateness of the measurement tools used.
According to the literature, scales such as the Epworth sleepiness scale[20], Pittsburg sleep quality index[21], DSM-5 sleep-wake disorders module[22], Richard-Campbell sleep questionnaire[23], postpartum sleep quality scale[24], sleep quality scale[25], Jenkins sleep scale[26], sleep hygiene index[27], sleep assessment scale for children with cancer[28] are used to determine sleep-related problems in our country. In addition, the form of factors affecting sleep[29] is used to evaluate sleep in terms of quantity and quality. As can be understood from the names of the scales, there is no measurement tool to measure the causes of hospital-acquired insomnia. In the international literature, studies reported that patients have had sleep problems in the hospital since the 1990s, but no measurement tool has been developed to determine the causes of hospital-acquired insomnia in individuals. These findings suggest that sleep remains in the background compared to activities such as nutrition and breathing. This research focuses on the activity of sleep, which is vital and aimed to develop a measurement tool to reveal the causes of hospital-acquired insomnia and determine the causes of insomnia in hospitals or inpatient health institutions. In addition, literature on the factors that cause hospital-acquired insomnia was reviewed and some recommendations were made. These features show the originality of the research.
The research was designed as a methodological study.
The study was conducted with patients hospitalized in surgical and internal medicine clinics in a research hospital in Turkey between December 2021 and March 2022.
The population of the study consisted of patients hospitalized in Turkey. During the pilot application of the study, 64 patients were reached. It is stated that the sample size should be at least 5 times the number of scale items (10 times if possible) in scale adaptation studies[30]. The main application stage started with 42 items. For this reason, it was predicted that the sample size should have been at least 210 or 420. Therefore, 223 patients were reached during the main application stage. The data were filled in using the face-to-face interview method. It took 20-30 min to fill in the data of each patient. The study was completed with a total of 287 patients.
(1) Not having any sleep problems before hospitalization; and (2) Being hospitalized for at least three days.
The data were collected using a personal information form and the hospital-acquired insomnia scale (HAIS)-draft form.
Personal information form: This form consists of questions about gender, age, education level, social security, and previous hospitalization of the participants.
Insomnia severity index: The index was developed by Bastien et al[31] in 2001 and was validated in Turkish by Boysan et al[32] in 2010. The 7-item index was developed in a 5-point Likert type in order to measure the degree of insomnia of individuals. Items are scored between 0-4; the lowest score obtainable from the scale is 0 and the highest score is 28. In scoring, a score of 0-7 indicates clinically insignificant insomnia; 8-14 indicates insomnia lower threshold; 15-21 indicates clinical insomnia (moderately severe); 22-28 indicates clinical insomnia (insomnia) (severe). Insomnia severity index (ISI) was used to determine the concurrent validity of the scale. Concurrent validity is the comparison of the findings obtained from a measurement tool that is desired to be developed with a scale, whose validity and reliability has been proven, on a similar subject. A correlation is expected between the data obtained from both measurement tools[33].
HAIS: First stage: The relevant literature was examined in depth. Interviews were held with 2 patients, 2 patient relatives, and 4 nurses about the causes of hospital-acquired insomnia. After the literature review and interviews, a conceptual infrastructure on the subject was created. An item pool of 150 items considered suitable for the scale was prepared. The item pool was reviewed 4 times and some items were eliminated. The item pool was finalized with 66 items for expert opinion.
Second stage: The item pool consisting of 66 items was presented to 11 experts (2 Turkish language experts, 9 field experts) and the content validity was evaluated. The items in the scale were revised in line with expert opinions. Then, the suitability of the scale items was evaluated, the Turkish language validity was established, and the revisions were completed. 10 items were removed from the scale. The scale was finalized for the pilot application with 56 items. The Cronbach alpha value of the data of 56 items was determined as 0.882. In the pilot application stage, 14 items (i4, i6, i9, i12, i14, i19, i22, i26, i29, i30, i32, i35, i36, i43) with an item correlation value of less than 0.20 were removed from the item pool. The Cronbach alpha value was determined to be 0.913 with 42 items. Main application stage started with 42 items.
Third stage: Validity and reliability analysis of the HAIS was performed. The sample of the study consisted of 223 patients. In the analyses, it was determined that the scale is a valid and reliable measurement tool consisting of 18 items and 5 subscales. The scale was developed as a 5-point Likert scale (1: Strongly disagree - 5: Strongly agree). Scoring for the subscales and the overall scale was based on the item score average. The lowest score obtainable from the scale and its subscales is 1 and the highest score is 5. There is no reverse item on the scale. As the scale score increases, hospital-acquired insomnia increases. There is no cut-off point in the scale. The Cronbach alpha value for the overall scale was determined to be 0.783.
In scale adaptation studies, it is necessary to reach a sample size of more than 50; the internal consistency of the scale should be 0.70 and above; it should be checked whether the item-total correlation value is below 0.30[34]. In this study, the pilot application was carried out with 64 patients. In the pilot application stage, 14 items with an item correlation value below 0.20 were removed from the item pool. The Cronbach alpha value was determined to be 0.913 with 42 items and the main application stage was initiated.
The patients were visited in their rooms and their informed consent was obtained. After their consent was taken, the data of the study were collected by the face-to-face method. The scale items were asked to the patients one by one by the researcher (BÇ) and their answers were marked. At this stage, the HAIS and the ISI, which is thought to be a parallel form, were used. In the main application, 223 patients were reached. At this stage, validity and reliability analyses, two criteria that a scale should have, were performed[35].
SPSS 20 package program and analysis of moment structure (AMOS) 20 package program” were used for data analysis. Frequency and percentage were used to indicate the sociodemographic characteristics of the patients. Exploratory factor analysis (EFA) and confirmatory factor analysis (CFA) were used to ensure scale validity. In the EFA, item score averages and standard deviations, Kaiser-Meyer-Olkin (KMO), Bartlett’s test of sphericity, item-total correlation value was used. Scree plots chart and eigenvalue were used to determine the factor structures of the scale[34,36,37].
KMO and Bartlett’s sphericity tests were performed to check the suitability of the sample size and the suitability of the data set for analysis. The fact that the KMO test was greater than 0.60 and the P value of the Barlett's sphericity test was significant showed the adequacy of the sample and the suitability of the data set[34,38,39].
EFA was performed to ensure construct validity. During the EFA, there should be no overlapping items. In addition, the item eigenvalue of the scale items should be at least 1 and the item load should be at least 0.30[34,40]. Varimax rotation, which is a vertical rotation method preferred in scale development studies, was used to ensure construct validity[34,36]. The presence of multicollinearity problem was tested with linear regression [variance inflation factors (VIF) < 10, C-index (CI) < 30, tolerance > 10][38]. If an item has a loading value of more than 0.32 in more than one dimension and the difference between factor loadings in these dimensions is less than 0.10, these items were accepted as overlapping items[34].
In CFA, fit indices and path diagram were used. The Cronbach alpha value of the items, factors and total scale was used to determine the reliability level of the scale. In addition, split half analysis was performed. Retest method was used to determine the invariance of the scale against time. The following criteria were taken as basis: a minimum KMO value of 0.60, a Bartlett’s test of sphericity P value being significant, an eigenvalue of at least 1, a Cronbach alpha value of at least 0.7 for factors and the scale, a split half Cronbach alpha value of at least 0.7. The values suggested by Bae et al[41], Woo et al[42] were taken into account in the fit indices [χ2/SD value, goodness of fit index (GFI), adjusted GFI, comparative fit index, root mean square error of approximation, standardized root mean square residual fit indices][41,42].
In the analyzes related to reliability, a Cronbach alpha value of 0.70 and above was taken as the basis[43-45].
Ethics committee approval dated 16.08.2021 and numbered 20028 was obtained from the Muş Alparslan University Scientific Research and Publication Ethics Committee. Institutional permission was obtained from Atatürk University Research Hospital in order to carry out the study. Patients were informed about the purpose and method of the study and that the research was based on volunteerism in detail. Consent was taken from the patients participating in the study. The individuals participating in the research were informed that their data would not be shared.
Of the patients, 52% were famale; 47.1% were aged between 51-70; 49% were primary school graduates; 69.5% had social security; 30.9% had a history of 1-2 wk-hospitalization; the sleep quality of 31.8% was between 7 and 8 (Table 1).
| Characteristics | Variables | n | % |
| Sex | Famale | 116 | 52 |
| Male | 107 | 48 | |
| Age | 18-30 | 16 | 7.2 |
| 31-50 | 50 | 22.4 | |
| 51-70 | 105 | 47.1 | |
| 71 and above | 52 | 23.3 | |
| Educational status | Illiterate | 45 | 20.2 |
| Primary school | 110 | 49.3 | |
| Middle school | 20 | 9 | |
| High school and above | 48 | 21.5 | |
| Social security | Yes | 155 | 69.5 |
| No | 68 | 30.5 | |
| Hospitalization | No | 44 | 19.7 |
| Less than 1 wk | 57 | 25.6 | |
| 1-2 wk | 69 | 30.9 | |
| 2-3 wk | 23 | 10.3 | |
| 1 mo and more | 15 | 13.4 | |
| Sleep quality with 1 lowest 10 highest | 1-2 | 21 | 9.4 |
| 3-4 | 38 | 17.1 | |
| 5-6 | 49 | 22 | |
| 7-8 | 71 | 31.8 | |
| 9-10 | 44 | 19.8 |
EFA: Reliability analysis was performed to determine whether the items had appropriate values. Therefore, 11 items were removed before starting the EFA. EFA was performed to ensure construct validity. In order to determine the items to be included in the scale, it was taken into account that there was no overlapping item, the item eigenvalue was 1, and the item load value was at least 0.30. The Cronbach alpha value of the remaining 31 items was determined to be 0.783. The analysis was continued with 31 items. Varimax axis rotation, a vertical rotation method preferred in scale development studies, was used in order to ensure construct validity.
Before performing exploratory and CFA for the scale, KMO and Bartlett’s sphericity tests were performed to check the suitability of the sample size and the suitability of the data set for analysis. In addition, the anti-image test, which is one of the sampling criteria, was applied. The KMO value was determined as 0.663. Bartlett’s sphericity test was found to be significant (χ2 = 3019.015; P = 0.001) (Table 2). As a result of the anti-image test, it was determined that there was no value below 0.50. These results showed that the sample size and data set were suitable for analysis.
| Tests | Test results | |
| Kaiser-Meyer-Olkin sampling adequacy | 0.663 | |
| Bartlett’s test of sphericity | χ2 | 3019.015 |
| Standard error | 465 | |
| P value | 0.001 | |
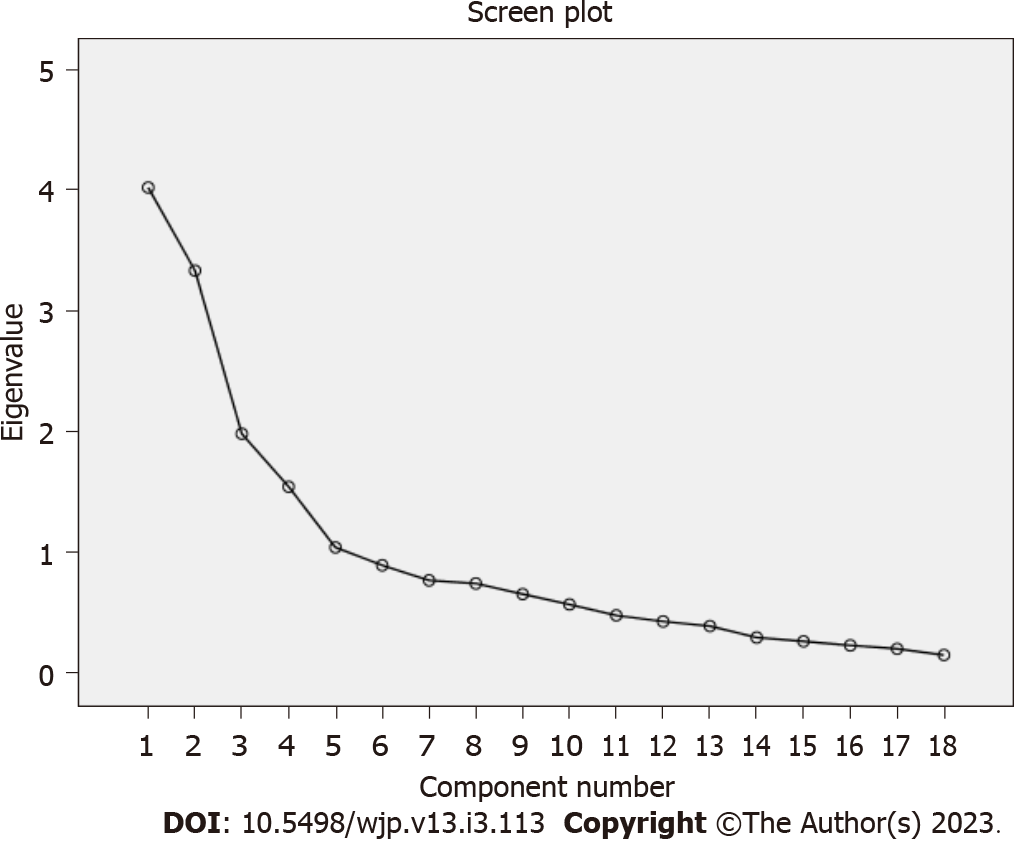
Items i4, i5, i6, i13, i18, i19, i20, i23, i24, i28, i30, i39, and i40 were excluded from the scale since they were overlapping items. In the EFA performed with 18 items, it was determined that the KMO value was 0.741 and that Bartlett’s sphericity test was significant (χ2 = 1704.892, P = 0.001). The anti-image test was examined and it was found that there was no value below 0.50. These findings showed that the data were suitable for factor analysis. As a result of the Varimax analysis, it was determined that the scale items were gathered under 5 factors. The scree plot also confirmed that the scale has a 5-factor structure. In light of this information, CFA was performed with 5 factors and 18 items (Figure 1).
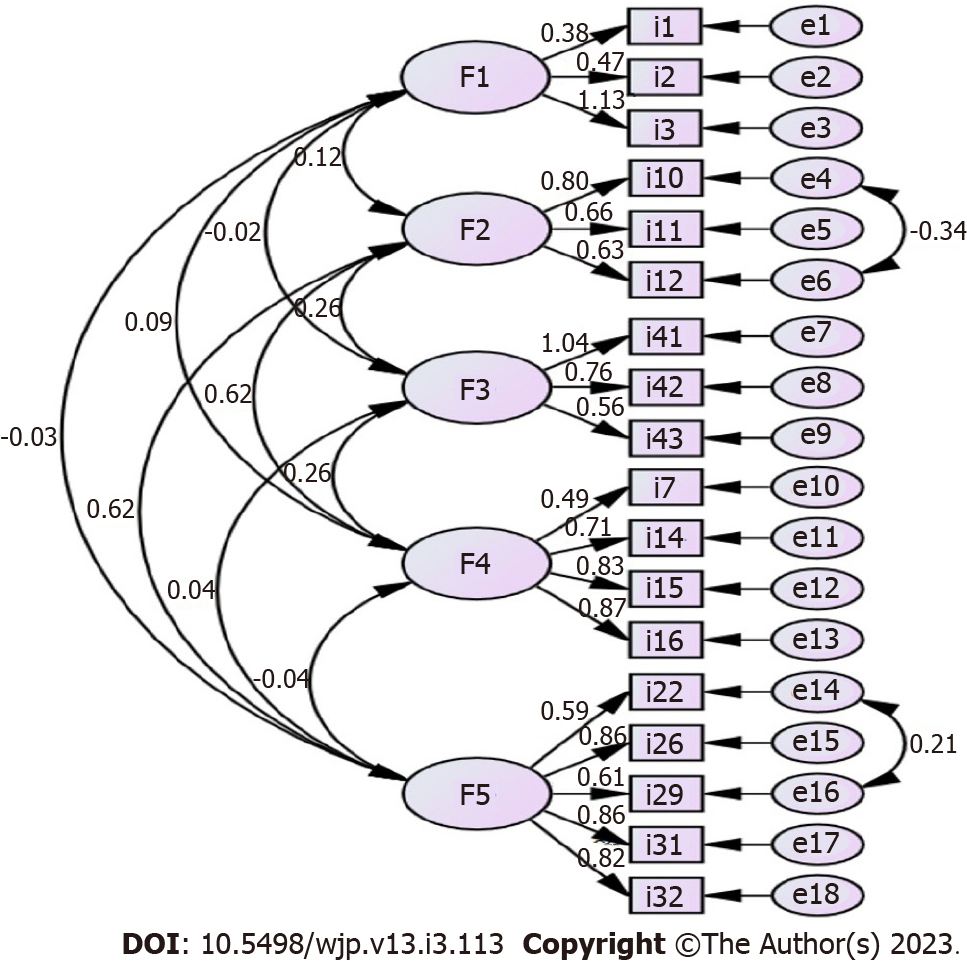
When the analysis results were examined, it was found that factor 1 consists of 5 items, i22, i26, i29, i31, i32, and explained 22.345% of the total variance. This factor was named physical environmental factors.
Factor 2 consists of 4 items, i7, i14, i15, i16, and explained 18% of the total variance. This factor was named psychological factors.
Factor 3 consists of 3 items, i41, i42, i43, and explained 11.016% of the total variance. This factor was named safety factor.
Factor 4 consists of 3 items, i10, i11, i12, and explained 8.580% of the total variance. This factor was named socioeconomic factors.
Factor 5 consists of 3 items, i1, i2, i3, and explained 5.788% of the total variance. This factor was named nutritional factors.
When the 18-item scale was analyzed as a whole, it was determined that it has a 5-factor structure and that the common factor loads of 18 items varied between 0.407 and 0.886. It was determined that 5 factors explained 66.25% of the total variance. These values showed that the scale is sufficient to explain the hospital-acquired insomnia problems of patients (Table 3).
| Common load value | Faktors | |||||
| 1 | 2 | 3 | 4 | 5 | ||
| i31 | 0.781 | 0.869 | ||||
| i26 | 0.758 | 0.865 | ||||
| i32 | 0.713 | 0.843 | ||||
| i22 | 0.567 | 0.733 | ||||
| i29 | 0.554 | 0.729 | ||||
| i16 | 0.781 | 0.855 | ||||
| i15 | 0.727 | 0.815 | ||||
| i14 | 0.662 | 0.666 | 0.339 | |||
| i7 | 0.485 | 0.647 | ||||
| i41 | 0.886 | 0.924 | ||||
| i42 | 0.758 | 0.835 | ||||
| i43 | 0.572 | 0.696 | ||||
| i11 | 0.704 | 0.783 | ||||
| i12 | 0.552 | 0.705 | ||||
| i10 | 0.666 | 0.392 | 0.680 | |||
| i3 | 0.747 | 0.855 | ||||
| i2 | 0.604 | 0.774 | ||||
| i1 | 0.407 | 0.631 | ||||
| Eigenvalue (total = 11.925) | 4.022 | 3.334 | 1.983 | 1.544 | 1.042 | |
| %Total explained variance = 66.250% | 22.345 | 18.521 | 11.016 | 8.580 | 5.788 | |
CFA: The structure obtained from EFA was tested with CFA. In line with the modification recommendations made with CFA, it was deemed appropriate to make modifications between items i10-i12 and i22-i29. After modification, improvements were observed in the χ2 value. Information on the fit indices obtained as a result of CFA is given in the table. Fit values were evaluated considering the reference values stated (Table 4). The items were considered important for the factors in which they were included. The path diagram was examined and it was determined that the obtained values were appropriate in terms of the item-factor agreement (Figure 2).
| Index | Normal value | Acceptable value | Measurement | Result |
| χ2/P Value | > 0.05 | - | 0.001 | Perfect fit |
| χ2/SD (CMIN/DF) | < 2 | < 5 | 1.874 | Perfect fit |
| GFI | > 0.95 | > 0.90 | 0.900 | Acceptable fit |
| AGFI | > 0.95 | > 0.85 | 0.861 | Acceptable fit |
| CFI | >0.95 | > 0.90 | 0.933 | Acceptable fit |
| RMSEA | < 0.05 | < 0.08 | 0.0.070 | Acceptable fit |
| RMR | < 0.05 | < 0.08 | 0.039 | Perfect fit |
| SRMR | < 0.05 | < 0.08 | 0.0600 | Acceptable fit |
| NFI | > 0.95 | > 0.80 | 0.869 | Acceptable fit |
| TLI | 0.95 < TLI < 1 | 0.90 < TLI < 0.94 | 0.917 | Acceptable fit |
| IFI | > 0.90 | - | 0.934 | Perfect fit |
| PGFI | > 0.89 | > 0.50 | 0.648 | Acceptable fit |
| PNFI | > 0.89 | > 0.50 | 0.699 | Acceptable fit |
Internal consistency (Cronbach alpha) coefficients: The Cronbach alpha coefficient was calculated to determine the reliability analysis of the 18 items of the finalized scale. The Cronbach alpha values were 0.672 for the nutritional factors subscale including items i1, i2, and i3; 0.677 for the socioeconomic factors including items i10, i11, i12; 0.804 for the psychological factors subscale including items i7, i14, i15, and i16; 0.842 for the physical environmental factors subscale including items i22, i26, i29, i31, i32; 0.786 for the safety factors subscale including i41, i42, and i43. The Cronbach alpha value for the overall scale was 0.783. These values show that the scale is reliable.
Split half confidence coefficient: As a result of the split half reliability analyses of the 18-item final version of the scale, it can be said that the Spearman-Brown correlation value (r = 0.670) and the Guttman split half coefficient value (r = 0.60) of the scale have sufficient values and that the Cronbach alpha reliability coefficients for the split halves are sufficient. The split half reliability coefficients are given in the table (Table 5). The results of the split half reliability analyses showed that the scale is reliable (Table 5).
| Two half | Cronbach alpha |
| Part 1 | 0.738 |
| Part 2 | 0.606 |
The item score means were determined to be 1.06 ± 032 for F1, 1.57 ± 0.90 for F2, 1.12 ± 0.46 for F3, 1.41 ± 0.77 for F4, and 1.08 ± 0.27 for F5. The item score mean of HAIS was 1.25 ± 0.35. The mean total ISI score was determined to be 10.35 ± 7.76 (Table 6).
| Average | SD | Min | Max | |
| F1 | 1.06 | 0.32 | 1.00 | 5.00 |
| F2 | 1.57 | 0.90 | 1.00 | 5.00 |
| F3 | 1.12 | 0.46 | 1.00 | 4.00 |
| F4 | 1.41 | 0.77 | 1.00 | 5.00 |
| F5 | 1.08 | 0.27 | 1.00 | 3.00 |
| Total hospital-acquired insomnia scale | 1.25 | 0.35 | 1.00 | 3.06 |
| Insomnia severity index | 10.35 | 7.76 | 0 | 28 |
It was determined that there was a positive and significant correlation between the mean ISI score and the mean scores on F2, F3, F4, and HAIS. A statistically significant correlation was found between HAIS and F1, F2, F3, F4, and F5. These findings show that the scale is an adequate tool to measure the hospital-acquired insomnia levels of patients (Table 7).
In order to create the conceptual structure of the scale and prepare the item pool, the literature was reviewed and in-depth interviews were conducted with 2 patients, 2 patient relatives, and 4 nurses. An item pool of 150 items was created. The item pool was reviewed 4 times and an item pool of 66 items was created to be presented to experts. Two Turkish language experts and 9 field experts were consulted. In line with the recommendations of 11 experts, 11 items were removed from the item pool and the pilot application started with 56 items. During the pilot application stage, 64 patients were reached. In line with the data obtained from 64 patients, 14 items were excluded since the total correlation value was low and the main application stage was initiated with 42 items.
During the main application stage, 223 individuals were reached. Reliability analyses were performed in line with the data obtained from 223 individuals and 11 items with low item correlation values were eliminated from the analysis. As a result of the EFA performed with 31 items, 13 items were excluded from the analysis one by one since they were overlapping. While each item was removed from the analysis one by one, KMO, Bartlett’s sphericity test, and anti-image test were checked. The overlapping status was checked and items were removed accordingly. Bartlett’s sphericity test, KMO value, and anti-image test were checked with the remaining 18 items. Bartlett’s sphericity test (P < 0.05), KMO value (0.741), and anti-image test for 18 items were found to be suitable for analysis[33-35]. As a result of the EFA of 18 items, a 5-factor structure was created. Scree plot also showed that the structure consisted of 5 factors. The items that constitute the factors were examined in terms of content integrity and named as physical environmental, psychological, safety, socioeconomic, and nutritional factors. CFA analysis of the 5-factor structure was performed in the AMOS package program. The fit indices of the obtained structure were examined. It was determined that the values obtained from the fit indices were sufficient. These results revealed that the scale is valid and reliable.
With the keywords “sleep” and “insomnia”, national and international literature was first searched on the Turkish measurement tools index and Google scholar. 20 relevant scales were reached. Eight scales were found to be regarding adult patients or associated with the hospital. The names of these scales were as follows; Pittsburgh sleep quality index[21], Epworth sleepiness scale[20], insomnia severity index[31], Richard-Campbell sleep questionnaire[23], DSM 5 sleep-wake disorders scale[22], Thought control questionnaire-insomnia revised[46], insomnia catastrophizing scale[47]. All scales were examined one by one and it was determined that there was no tool to determine hospital-acquired sleep problems. In addition, the subscales of the scales in question and the subscales of the hospital-acquired insomnia scale, which is the subject of the research, were examined and no similarity was detected. This shows the originality of the scale. It was determined that the Cronbach alpha values of the subscales ranged between 0.672 and 0.842 and the Cronbach alpha value for the overall scale was 0.783. It was determined that the scale explained 58.269% of the total variance.
It is stated that patients who do not have sleep problems in their daily routine at home have problems after hospitalization, such as waking up frequently, having difficulty falling asleep, decreased sleep time, not feeling rested when they wake up, and inability to sleep. A new environment, the severity of the underlying disease, underlying psychiatric conditions, pain, room light, sharing a room with others, being away from the family, changes in sleep-wake-up times, pain, bad condition of the roommate patient, worrying about the disease, inadequate physical activity, frequent entries to the room, medications, and infections.
Hospital-acquired insomnia is usually treated symptomatically at the time of hospitalization. However, misuse or overuse of sedatives/hypnotics is thought to be associated with complications in this population of acute patients. The use of a scale to determine the causes and severity of insomnia before resorting to pharmacological methods is one of the indicators of quality nursing care. Prevention of hospital-acquired insomnia, which is evaluated with the scale, with simple and applicable nursing interventions will contribute to the prevention of unnecessary use of medications.
In conclusion, the scale consists of 18 items and 5 subscales: Physical environmental factors, psychological factors, safety factors, socioeconomic factors, and nutritional factors. The subscale of physical environmental factors consists of 5 items; psychological factors consist of 4 items; safety factors, socioeconomic factors, and nutritional factors subscales consist of 3 items. There is no reverse item on the scale. The item score average is used in the calculation of the scale score. The lowest score obtainable from the scale and 5 subscales is 1 and the highest score is 5. There is no cut-off point in the scale. As the scale score increases, the hospital-acquired insomnia level increases. The scale explains 66.250% of the total variance. In the light of this information, it can be said that the hospital-acquired insomnia scale is a valid and reliable measurement tool.
Insomnia is a major problem for people. Many causes of insomnia have been reported. One of these reasons is hospital-acquired insomnia. Although it has been stated in many studies that patients experience hospital-acquired insomnia, it has been determined that there is no valid and reliable measurement tool that measures hospital-acquired insomnia problems of individuals.
Insomnia, which affects individuals physiologically, psychologically and therapeutically, is a serious problem in the hospital. Hospital-induced insomnia affects the quality of treatment and care. For this reason, healthcare professionals need a measurement tool that can measure the hospital-acquired insomnia levels of patients.
In this study, it was aimed to develop a valid and reliable measurement tool that can determine the hospital-acquired insomnia levels of patients.
This research is scale development research consisting of a pilot application and a main application. First of all, an item pool was created for the scale and presented to expert opinion. After the expert opinion, the scale items were made ready for pre-application. In the preliminary application, data were obtained from 64 individuals. During the main application phase, 223 patients were reached. Exploratory factor analysis, confirmatory factor analysis and reliability analyzes were performed with the obtained data. Analyzes were performed using SPSS 20 package program and analysis of moment structure package program.
As a result of data analysis, it was determined that the scale consisted of 5 sub-dimensions and 18 items. The total Cronbach alpha value of the scale was determined to be 0.783.
It was determined that the scale, which consists of 5 sub-dimensions and 18 items, is a valid and reliable measurement tool in determining the hospital-acquired insomnia levels of the patients.
It is recommended to use this measurement tool to determine the insomnia problems of patients and to adapt it in other countries.
We thank the patients for taking part in the study.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Nursing
Country/Territory of origin: Turkey
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Bharara T, India; Taheri S, Iran S-Editor: Zhang H L-Editor: A P-Editor: Zhang H
| 1. | Demir D, Yurdanur. Sleep and Sleep Related Applications. In: Akça Ay F. Basic Concepts and Skills in Health Practice. İstanbul: Nobel Medical Bookstore, 2016: 772-789. |
| 2. | Şevik Erdöl H. Sleep. In: Akça Ay F. Basic Concepts and Skills in Health Practice. İstanbul: Academy Press and Publishing, 2019: 941-952. |
| 3. | Yalın H, Kürtüncü M. Evaluation of Sleep and Rest. In: Eti Aslan F. Evaluation of Health. İstanbul: Academician Publishing House, 2014: 95-105. |
| 4. | Baker FC, de Zambotti M, Colrain IM, Bei B. Sleep problems during the menopausal transition: prevalence, impact, and management challenges. Nat Sci Sleep. 2018;10:73-95. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 123] [Cited by in RCA: 168] [Article Influence: 24.0] [Reference Citation Analysis (0)] |
| 5. | Solverson KJ, Easton PA, Doig CJ. Assessment of sleep quality post-hospital discharge in survivors of critical illness. Respir Med. 2016;114:97-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 36] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 6. | Ho A, Raja B, Waldhorn R, Baez V, Mohammed I. New onset of insomnia in hospitalized patients in general medical wards: incidence, causes, and resolution rate. J Community Hosp Intern Med Perspect. 2017;7:309-313. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 40] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 7. | Manian FA, Manian CJ. Sleep quality in adult hospitalized patients with infection: an observational study. Am J Med Sci. 2015;349:56-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 22] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 8. | Ho A, Raja B, Baez Sosa V, Benno M, Waldhorn R. Acquired Insomnia in the Hospital, A Comprehensive Observational Study. Am J Respir Crit Care Med. 2020;201. [DOI] [Full Text] |
| 9. | Badr AF, Kurdi S, Alshehri S, McManus C, Lee J. Pharmacists' interventions to reduce sedative/hypnotic use for insomnia in hospitalized patients. Saudi Pharm J. 2018;26:1204-1207. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 10. | Lukoyanov AN, Fomenko IS, Gongola MI, Shul'pina LS, Ikonnikov NS, Shul'pin GB, Ketkov SY, Fukin GK, Rumyantcev RV, Novikov AS, Nadolinny VA, Sokolov MN, Gushchin AL. Novel Oxidovanadium Complexes with Redox-Active R-Mian and R-Bian Ligands: Synthesis, Structure, Redox and Catalytic Properties. Molecules. 2021;26. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 11. | Tan X, van Egmond L, Partinen M, Lange T, Benedict C. A narrative review of interventions for improving sleep and reducing circadian disruption in medical inpatients. Sleep Med. 2019;59:42-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 31] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 12. | Redberg RF. JAMA Internal Medicine-The Year in Review, 2018. JAMA Intern Med. 2019;179:612-613. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 13. | Caddick J, Green L, Stephenson J, Spyrou G. The psycho-social impact of facial skin cancers. J Plast Reconstr Aesthet Surg. 2012;65:e257-e259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 14. | Fındık Ü, Topçu S. Effect of the Way of Surgery on Preoperative Anxiety. J Hacettepe University Faculty Nurs. 2012;19:22-33. |
| 15. | Elitoğ N, Öztürk N, Menteş D, Zeytun B, Kahraman H, Kırdağ G. Evaluation of Factors Affecting Sleep and Changes in Daily Sleep Patterns in Post-Op Hospitalization Periods of Patients Who Have Had Cardiac Surgery Operation. Acıbedem Nursing. 2008; 1-4. Available from: http://www.acibademhemsirelik.com/edergi/yeni_tasarim/files/kalp%20_bil_2.pdf. |
| 16. | Karagözoğlu Ş, Çabuk S, Tahta Y, Temel F. Some factors influencing the sleep of hospitalized adult patients. Thorax. 2007;8:234-240. [DOI] [Full Text] |
| 17. | Uslu Y, Korkmaz FD. Sleep in intensive care patients: nursing care. J Educ Res Nurs. 2015;12:156-161. [DOI] [Full Text] |
| 18. | Soong C, Burry L, Greco M, Tannenbaum C. Advise non-pharmacological therapy as first line treatment for chronic insomnia. BMJ. 2021;372:n680. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 20] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 19. | Stewart NH, Arora VM. Sleep in Hospitalized Older Adults. Sleep Med Clin. 2018;13:127-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 72] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 20. | Agargun M, Cilli A, Kara H, Bilici M, Telcioglu M, Semiz U. The Validity and Reliability of the Epworth Sleepiness Scale. Turk J Psychiatry. 1999;10:261-267. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 21. | Ağargün MY, Kara H, Anlar Ö. The validity and reliability of the Pittsburgh Sleep Quality Index. Turk J Psychiatry. 1996;7:107-115. |
| 22. | Yüzeren S, Herdem A, Aydemir Ö. Reliability and validity of Turkish Form of Sleep Disorder Scale. Anatol J Psychiatry. 2017;18:79-84. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 23. | Özlü ZK, Özer N. Richard-Campbell Sleep Scale Validity and Reliability Study. J Turk Sleep Med. 2015;2:29-32. [RCA] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 49] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 24. | Boz İ, Selvi N. Testing the Psychometric Properties of the Postpartum Sleep Quality Scale in Turkish Women. J Nurs Res. 2018;26:385-392. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 25. | Önder İ, Masal E, Demirhan E, Horzum MB, Beşoluk Ş. Psychometric properties of sleep quality scale and sleep variables questionnaire in Turkish student sample. Int J Psychol Educ Stud. 2016;3:9-21. [RCA] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 12] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 26. | Duruöz MT, Erdem D, Gencer K, Ulutatar F, Baklacıoğlu HŞ. Validity and reliability of the Turkish version of the Jenkins Sleep Scale in psoriatic arthritis. Rheumatol Int. 2018;38:261-265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 20] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 27. | Ozdemir PG, Boysan M, Selvi Y, Yildirim A, Yilmaz E. Psychometric properties of the Turkish version of the Sleep Hygiene Index in clinical and non-clinical samples. Compr Psychiatry. 2015;59:135-140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 39] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 28. | Arıcıoğlu A. Sleep Rating Scale for Children with Cancer: Adolescent Form. M.Sc. Thesis, Dokuz Eylul University. 2018. Available from: https://tez.yok.gov.tr/UlusalTezMerkezi/tezSorguSonucYeni.jsp. |
| 29. | Tosunoğlu A. Investigation of factors affecting sleep needs of hospitalized adult patients. M.Sc. Thesis, İzmir: University; 1997. Available from: https://tez.yok.gov.tr/UlusalTezMerkezi/tezSorguSonucYeni.jsp. |
| 30. | Alpar R. Applied statistics and validity reliability, with examples from sports health and educational sciences. 7th ed. Ankara: Detay Publishing; 2022: 1-672. |
| 31. | Bastien CH, Vallières A, Morin CM. Validation of the Insomnia Severity Index as an outcome measure for insomnia research. Sleep Med. 2001;2:297-307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4043] [Cited by in RCA: 5296] [Article Influence: 220.7] [Reference Citation Analysis (0)] |
| 32. | Boysan M, Gulec M, Besiroglu L, Kalafat T. Psychometric properties of the Insomnia Severity Index in Turkish sample. Anatol J Psychiatry. 2010;11:248-252. [RCA] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 33. | Tavşancıl E. Measuring attitudes and data analysis with SPSS. Ankara: Nobel Publishing; 2018: 1-174. |
| 34. | Correction: Alagoz F, Yildirim AE, Sahinoglu M, Korkmaz M, Secer M, Celik H, Yel C, Guvenc Y, Uckun OM, Narin F, Daglioglu E, Belen AD. Traumatic Acute Subdural Hematomas: Analysis of Outcomes and Predictive Factors at a Single Center. Turk Neurosurg 2017; 27(2):187-191. Turk Neurosurg. 2017;27:487. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 35. | Esin MN. Data collection methods and tools-reliability and validity of data collection tools. In: Erdoğan S, Nahcivan N, Esin MN. Research, process practice and critical in nursing. İstanbul: Nobel Medical Bookstores; 2021: 193-235. |
| 36. | DeVellis RF, Thorpe CT. Scale development: Theory and applications. Los Angeles: Sage publications; 2021: 1-103. |
| 37. | Pallant J. SPSS user manual: Step-by-step data analysis with SPSS. 3th ed. New York: McGraw Hill Hause; 2020: 1-211. |
| 38. | Aksu G, Eser MT, Güzeller CO. Exploratory and Confirmatory Factor Analysis and Structural Equation Model Applications. Ankara: Detail publishing; 2017: 1-247. |
| 39. | Ho RC, Hou Hong Ng A, Nourallah M. Impact of Globalization and Advanced Technologies on Online Business Models. IGI Global; 2021. 1-321. |
| 40. | Kim C. Misuse of exploratory factor analysis and its remedies. Survey Res. 2016;17:1-29. |
| 41. | Bae BR. Structural equation modeling with Amos 24. Seoul: Chenngram Books; 2017: 76-309. |
| 42. | Woo JP. The concept and understanding of structural equation model. Seoul: Hannarae, 2017: 230-236. |
| 43. | Kyriazos TA, Stalikas A. Applied psychometrics: The steps of scale development and standardization process. Psychol. 2018;9:2531. [RCA] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 41] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 44. | Noh G. The proper methods of statistical analysis for dissertation: SPSS & AMOS 21. Seoul: Hanbit Academy; 2019. |
| 45. | Taber KS. The use of Cronbach’s alpha when developing and reporting research instruments in science education. Res Sci Educ. 2018;48:1273-1296. [DOI] [Full Text] |
| 46. | Yıldırım A, Boysan M, Aktaş SA. Validation of the Turkish version of the Thought Control Questionnaire-Insomnia Revised (TCQI-R). Yeni Symposium. 2018;9-16. [DOI] [Full Text] |
| 47. | Uygur ÖF, Hursitoğlu O, Uygur H, Aydın EF, Orhan FÖ. Turkish adaptation of the Insomnia Catastrophizing Scale and its psychometric properties. J Clin Psy. 2022;25: 101-111. [DOI] [Full Text] |