Copyright
©The Author(s) 2015.
World J Psychiatr. Mar 22, 2015; 5(1): 15-34
Published online Mar 22, 2015. doi: 10.5498/wjp.v5.i1.15
Published online Mar 22, 2015. doi: 10.5498/wjp.v5.i1.15
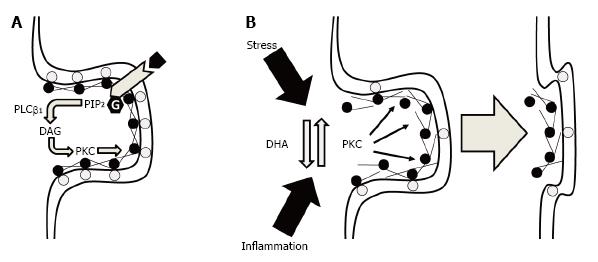
Figure 1 Potential ultrastructural mechanisms by which membrane DHA deficits could lead to a loss in synaptic connectivity.
A: Under normal physiological conditions, synaptic membrane phosphatidylserine (gray circles) bind MARCKS (black circles) at the membrane which also cross-links and tethers F-actin to support dendritic spine cytoskeletal structural stability. Binding of MARCKS with membrane phosphatidylserine also inhibits phospholipase Cβ1-mediated hydrolysis of phosphatidylinositol 4,5-bisphosphate (PIP2) into diacylglycerol (DAG) which increases PKC activity. PKC-mediated phosphorylation of MARCKS reduces the tensile strength of the F-actin cytoskeleton leading to the eventual collapse of the spine; B: Under conditions of membrane DHA deficits, reductions in membrane phosphatidylserine and membrane-bound MARCKS increase PKC activity and destabilizes the F-actin cytoskeleton leading to spine collapse. Elevated PKC activity secondary to membrane DHA deficits may also reduce the resilience of dendritic spines to other pathophysiological factors including chronic stress or inflammation. DHA: Docosahexaenoic acid; PKC: Protein kinase C; MARCKS: Myristoylated alanine-rich C kinase substrate.
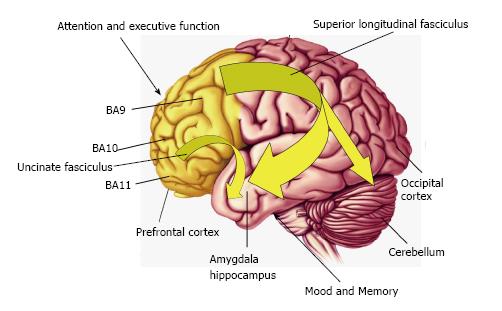
Figure 2 Diagram illustrating connectivity between frontal lobe regions, including the dorsolateral prefrontal cortex (Brodmann area 9, BA9,) and orbitofrontal cortex (BA11) which regulate attention and executive function, and temporal lobe structures including the amygdala and hippocampus which regulate mood and memory.
Frontal lobe connectivity with limbic structures is mediated in part by the uncinate fasciculus and superior longitudinal fasciculus which develop during gestation and undergo significant maturation during childhood and adolescence. Reduced frontal circuit connectivity is exhibited by DHA-deficient non-human primates, children and adolescents born preterm, and patients with psychiatric disorders including ADHD and bipolar disorder which are associated with DHA deficits. DHA: Docosahexaenoic acid; ADHD: Attention deficit/hyperactivity disorder.
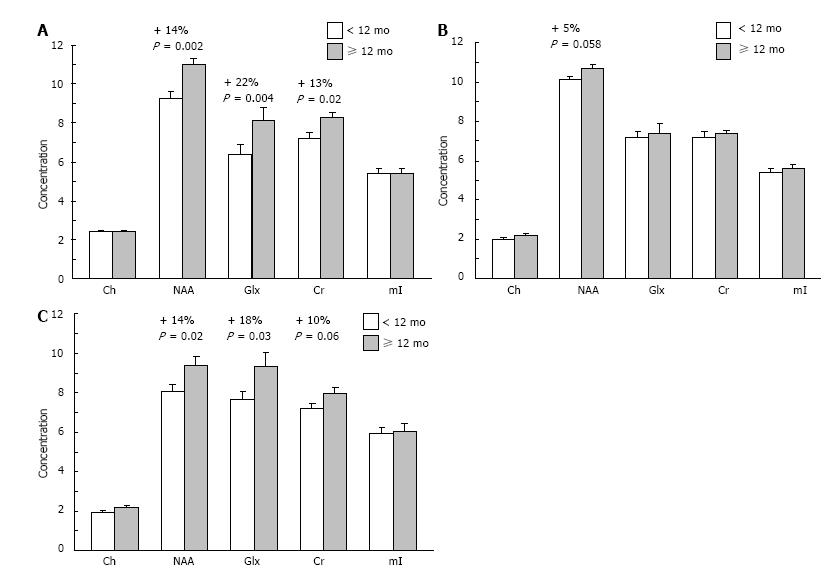
Figure 3 Mean concentrations of choline (Ch), N-acetyl aspartate (NAA), glutamate+ glutamine (Glx), creatine (Cr), and myo-inositol (mI) in the right dorsolateral PFC (A), left dorsolateral PFC (B), and anterior cingulate cortex (C) of children breastfed for < 12 mo (n = 22) or ≥ 12 mo (n = 16).
Note that NAA concentrations are significantly greater in the right dorsolateral PFC and anterior cingulate cortex of children breastfed for ≥ 12 mo vs < 12 mo. Values are group means ± SEM. PFC: Prefrontal cortex.
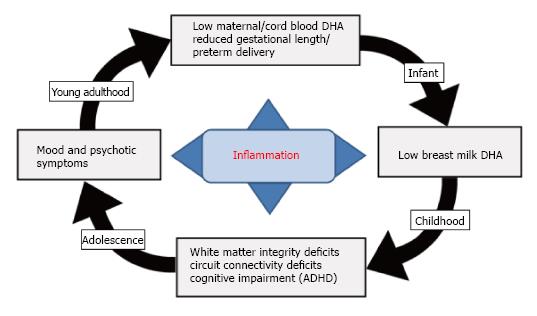
Figure 4 Diagram illustrating a hypothetical role of LCn-3 fatty acid deficiency in the familial transmission of psychopathology.
Adolescent and young adult females with mood disorders exhibit significant blood DHA deficits leading to reduced fetal (cord blood) DHA accrual during pregnancy. Low maternal DHA status during pregnancy also increases risk for preterm delivery due in part to elevated pro-inflammatory cytokine levels, as well as low breast milk DHA levels postpartum. Uncorrected deficits in fetal brain DHA accrual lead to long-standing deficits in white matter resilience and integrity and reduced functional connectivity in fronto-striatal circuits and increase risk of developing ADHD symptoms in childhood. Deficits in white matter integrity and reduced functional connectivity in fronto-limbic circuits during adolescent development is associated with the emergence of emotional and/or thought dysregulation and the onset of mood and/or psychotic symptoms. DHA: Docosahexaenoic acid; ADHD: Attention deficit/hyperactivity disorder.
- Citation: McNamara RK, Vannest JJ, Valentine CJ. Role of perinatal long-chain omega-3 fatty acids in cortical circuit maturation: Mechanisms and implications for psychopathology. World J Psychiatr 2015; 5(1): 15-34
- URL: https://www.wjgnet.com/2220-3206/full/v5/i1/15.htm
- DOI: https://dx.doi.org/10.5498/wjp.v5.i1.15