Published online May 25, 2017. doi: 10.5495/wjcid.v7.i2.21
Peer-review started: September 26, 2016
First decision: November 2, 2016
Revised: January 11, 2017
Accepted: February 10, 2017
Article in press: February 13, 2017
Published online: May 25, 2017
Processing time: 242 Days and 7.5 Hours
To study the sensitivity and antioxidant enzyme response in two clinical isolates of Entamoeba histolytica (E. histolytica) during treatment with antiamoebic drugs, auranofin and metronidazole.
E. histolytica were isolated from stool samples and maintained in Robinson’s biphasic culture medium. Clinial isolates were maintained in xenic culture medium, and harvested for determination of minimum inhibitory concentrations to the two antiamoebic drugs, Metronidazole and Auranofin using microtiter plate tests. The percent survival of the two isolates were determined using the trypan blue cell count. Isolate 980 was treated with 70 μmol/L and 2 μmol/L while isolate 989 was treated with 20 μmol/L and 0.5 μmol/L of metronidazole and auranofin respectively for 24 h. Fifty thousand cells of each isolate were harvested after 24 h of treatment for analysis of the mRNA expressions of the antioxidant enzymes, thioredoxin reductase, peroxiredoxin and FeSOD using the specific primers. Cell lysate was used for determination of enzyme activity of thioredoxin reductase by measuring DTNB reduction spectrophotometrically at 412 nm.
Minimum inhibitory concentration of the clinical isolates 980 and 989 for auranofin was 3 μmol/L and 1 μmol/L respectively while that for metronidazole was 80 μmol/L and 30 μmol/L respectively. Thioredoxin reductase, peroxiredoxin and FeSOD expression levels were significantly reduced in the isolate 980 when treated with Auranofin. Metronidazole treatment showed a down regulation of thioredoxin reductase. Though not significant both at the mRNA and the enzyme activity levels. Peroxiredoxin and FeSOD however remained unchanged. Auranofin treatment of isolate 989, showed an upregulation in expression of thioredoxin reductase while Peroxiredoxin and FeSOD did not show any change in expression. Upon treatment with metronidazole, isolate 989 showed an increase in thioredoxin reductase expression. Peroxiredoxin and FeSOD expressions however remain unchanged both at mRNA and enzyme activity level.
Clinical isolates from New Delhi NCR region show different sensitivities to antiamoebic drugs. Auranofin is effective against isolate showing higher tolerance to metronidazole as shown by its inhibition in thioredoxin reductase activity.
Core tip: Due to overuse of the mainstay drug against amoebiasis in an endemic country like India, there are concerns regarding the development of resistance towards metronidazole by the parasite. When Entamoeba histolytica (E. histolytica) from stool samples of diarrheal patients were cultivated in xenic medium, two clinical isolates of E. histolytica showed differential tolerance to the commonly used drug metronidazole. A new drug Auranofin was found to be effective on the isolate with higher tolerance to metronidazole. This was shown by inhibition of the antioxidant enzyme thioredoxin reductase as monitored by mRNA expression of TrxR gene and its enzyme activity.
- Citation: Iyer LR, Banyal N, Naik S, Paul J. Antioxidant enzyme profile of two clinical isolates of Entamoeba histolytica varying in sensitivity to antiamoebic drugs. World J Clin Infect Dis 2017; 7(2): 21-31
- URL: https://www.wjgnet.com/2220-3176/full/v7/i2/21.htm
- DOI: https://dx.doi.org/10.5495/wjcid.v7.i2.21
Amoebiasis is a disease caused by Entamoeba histolytica (E. histolytica), a protozoan parasite. It has been classified as category B priority biodefence pathogen by the National Institutes of health. This pathogen is effective even at a low infectious dose, and it has a high potential for transmission through contaminated food and water[1]. The parasite invades and destroys human tissue. It survives and proliferates in the human gut in an atmosphere of reduced oxygen. When the microaerophilic E. histolytica invades tissue, it is exposed to reactive oxygen species. It overcomes oxygen stress using antioxidant enzymes. E. histolytica lacks glutathione reductase enzyme, therefore a thioredoxin-linked system plays the major role to counter oxidative stress. This system is made up of proteins like peroxiredoxin, rubrerythrin, Iron containing superoxide dismutase, NADPH: Flavin oxidoreductase, and amino acids like L-cysteine, S-methyl-L-cysteine, and thioprolines[2].
The thioredoxin reductase/thioredoxin system (TrxR/Trx) protects sensitive proteins like serine acetyltransferase-1 against oxidative stress in E. histolytica. Peroxiredoxin, a central redox regulatory and antioxidant protein in Eh is the terminal peroxidase reducing H2O2 and depends on electrons provided by the TrxR/Trx system[3]. The Eh TrxR is versatile, and can use NADPH or NADP as its reducing cofactor and there is evidence that it protects the parasite from reactive oxygen (ROS) and reactive nitrogen species. It is therefore an ideal drug target[4].
Metronidazole is a 5-nitroimidazole derivative and is used to treat infections by anaerobic bacteria and protozoans like amoeba and giardia. This drug shows selective toxicity to anaerobic organisms as they possess metabolic pathways of low redox potential. Metronidazole is converted to its active form when its nitro group is reduced to an anion radical in Entamoeba cell. The active form of the drug is highly reactive and known to form additives with proteins and DNA causing loss of their functions[5]. Other enzymes have also been reported to be metronidazole activating nitroreductases, out of which thioredoxin reductase is one such enzyme. Jeelani et al[6] identified two additional NADPH dependent nitroreductases having metronidazole reducing activity. The reduced form of active metronidazole is detoxified inside the cell by the action of Superoxide dismutase, forming hydrogen peroxide and oxygen. Peroxiredoxin scavenges the hydrogen peroxide converting it to water. E. histolytica with induced resistance to metronidazole have been reported in literature earlier. Induced resistance to metronidazole leads to increased activity of FeSOD and peroxiredoxin and reduced expression of ferredoxin and Flavin reductase[7,8].
Auranofin is an oral gold salt and was first used to treat rheumatoid arthritis. The anti-parasitic activity of auranifin is due to the monovalent gold molecules which inhibit thioredoxin reductase. Auranofin was found to be active at nanomolar concentrations against various parasites, including E. histolytica, Giardia, Trypanosoma brucei, Leishmania infantum, L. major, and P. falciparum[4]. Auranofin was also found to be active against G.lamblia isolates pathogenic to humans in the 4-6 μmol/L range and against metronidazole resistant strains of giardia. Auranofin reportedly blocked the activity of giardial thioredoxin oxidoreductase. It was found effective in vivo in eradicating infections in different rodent models[9].
Auranofin has been recently been identified by a High Throughput Screening technique as active against trophozoites of E. histolytica and cysts of E. invadens. Auranofin inhibits E. histolytica thioredoxin reductase and it was shown that thioredoxin reductase protects trophozoites from oxidative attack and therefore in auranofin treated cells, thioredoxin was found in the oxidized state[10,11]. Auranofin has received orphan drug status and clinical trials are being carried out to treat amoeba and giardia infections. It shows promise as a broad-spectrum drug against Entamoeba, giardia and cryptosporidium, which are a major cause of diarrhea.
Both metronidazole and auranofin are combated by the thioredoxin reductase based enzyme system in Entamoeba. In this work, we therefore studied the sensitivity to auranofin of two isolates of Entamoeba from New Delhi, isolate 989 which is sensitive to metronidazole and isolate 980, which showed tolerance to metronidazole. We compared the activity and mRNA expression levels of thioredoxin reductase and the mRNA expression levels of peroxiredoxin and FeSOD in the above isolates, upon treatment with two antiamoebic drugs.
The clinical isolates were obtained from patient samples from Safdarjung Hospital, New Delhi, NCR region. They were isolated from stool specimens and cultured in Robinson’s biphasic culture medium with Escherichia coli[11,12]. They were subcultured every 48 h.
The E. histolytica isolates were grown in xenic culture and cells were harvested for DNA isolation and pelleted at 600 g at 4 °C. The pellets were stored in 70% ethanol at -20 °C till DNA isolation. QIA Amp DNA minikit was used for extracting genomic DNA (Qiagen catalog No. 51366). PCR amplification was used for strain identification from the genomic DNA using strain specific primers described by Srivastava et al[13]. The strains identified were either E. histolytica or E. dispar.
Microtitre plate tests to determine the minimum inhibitory concentration (MIC) of Auranofin to Indian isolate of E. histolytica were performed using a method modified from the one described by Upcroft et al[14]. Different drug dilutions were created in the liquid phase of the xenic Robinson’s medium in wells of microtiter plates and a fixed number of cells of the isolate was inoculated in the wells. A low oxygen environment was created using a sachet and bag system and cell growth was monitored in the wells under the microscope, without aerobic exposure at different time intervals. A score was given using a prefixed scoring system. For example, in case of isolate 980, in 24 h the control wells were fully confluent with live motile cells, so a score of ++++ indicated that.
The scores were as follows: (1) ++++ = (confluent well, covered with live motile cells); (2) +++ = 50%-70% (almost confluent well filled with live motile cells); (3) ++ = (30%-50% well coverage, few cells motile); (4) (+) ≤ 20% well coverage with rounded cells; and (5) (-) = dead and disintegrated cells. The MIC was the lowest concentration giving a + score, after 48 h.
To determine the percentage survival of the clinical isolates, each isolate was expanded in around 8 culture tube and harvested after 48 h to get a harvest of about approximately five lakh cells. Fifty thousand cells each were inoculated into twelve culture tubes, 6 tubes had the required drug concentration, while 6 tubes remained untreated to be used as controls.
The tubes were incubated at 35.5 °C for 15 h, 24 h and 48 h after inoculation. After each time period, the cells were harvested from two drug treated and two untreated controls and pelleted separately. Each pellet was dissolved in 1 mL medium and cell counting was done using a hemocytometer. Dilutions were made in PBS to obtain optimum count of up to 20 cells per quadrant in the hemocytometer. A 0.5 percent trypan blue solution was added in the ratio of 1:1 to the diluted cells and incubated for 1 min, before loading it to the hemocytometer and counting. Blue stained dead cells were not counted. Duplicate counts for each time period, in the treated cells and untreated controls were calculated. The mean and standard deviation values of the treated cells were plotted as percent of the untreated control and expressed as percent survival.
To give a short term treatment with the antiamoebic drugs, eight tubes each of the isolate 980 and isolate 989 were cultured in Robinson’s medium for 24 h. The cells were pelleted at 600 g for 5 min at 4 °C. The cells were counted and approximately 50000 cells each were suspended in the liquid phase of sixteen fresh tubes each per drug per isolate containing Robinsons medium, having the antiamoebic drugs in the required concentrations. This was 70 μmol/L and 20 μmol/L of metronidazole and 2 μmol/L and 0.5 μmol/L of Auranofin for isolate 980 and isolate 989 respectively. For each group of 16 tubes the complete medium was dispensed and they were incubated at 35.5 °C for 24 h. Subsequently cells were pelleted in the same way as above in RNAase free 50 mL conical centrifuge tubes. After decanting the supernatant, the cells pellets were stored at -80 °C in Trizol reagent for RNA isolation. Negative control RNAs were prepared for all the treated groups, which consisted of sixteen tubes of Robinson’s medium, without the inoculum (blank), harvested after incubation for 24 h at 35.5 °C and RNA was extracted following the same procedure as that for the test vials.
Primers used for RT-PCR: The primers sequences for the RT-PCR amplification of thioredoxin reductase, peroxiredoxin and FeSOD and 18S rRNA are listed in Table 1.
| S.No. | Primer | Sequence | Tm | Amplicon size | Ref. |
| 1 | Thioredoxin reductase (TrxR) | F-5’GTAATATTCATGATGTTGT3’ | 48 °C | 204 bp | [4] |
| Accession no (EHI_155440) | R-5’CATCATTAATTCATTTTCCA3’ | 48 °C | |||
| 2 | Eh Peroxiredoxin (Prx) | F 5’AAATCAATTGTGAAGTTATTGG3’ | 53.6 °C | 100 bp | [16] |
| R 5’TCCTACTCCTCCTTTACTTTTA3’ | 56.8 °C | ||||
| 3 | FeSOD | F 5’ACAATTACCTTATGCTTATAA3’ | 52 °C | 240 bp | [16] |
| Accession number (XM_643735.2) | F 5’TCCACATCCACACATACAAT3’ | 54 °C | |||
| 4 | Entamoeba histolytica 18s ribosomal RNA gene | F 5’TCAGCCTTGTGACCATACTC3’ | 61.7 °C | 200 bp | [16] |
| F 5’AAGACGATCAGATACCGTCG3’ | 68.9 °C |
Isolation of mRNA for semiquantitative RT-PCR: The total RNA was isolated from the untreated and treated harvested cells using Trizol reagent (Invitrogen) and treated with DNase (Roche), following the manufacturers protocol. Total RNA from uninoculated culture medium, incubated at 35.5 °C for 24 h served as blank for RT-PCR and gel electrophoresis.
Semiquantitative RT-PCR: The total RNA isolated from the drug treated and untreated E. histolytica cells were used for RT-PCR. Promega random hexamer and MMLV RT enzyme was used for the reverse transcriptase reaction. 18S rRNA was used for normalization. RT-PCR amplification of peroxiredoxin and 18S rRNA was carried out together as annealing temperatures were similar and their product sizes varied by 100 base pairs and could be easily separated in gel chromatography while RT-PCR of thioredoxin reductase and of FeSOD were carried out separately. The PCR reactions had an initial denaturation at 94 °C for 5 min, each targeted gene was subjected to 30 cycles of amplification followed by 1 min annealing. The annealing temperature was 50 °C for 18SrRNA as well as peroxiredoxin and 48 °C for FeSOD as well as TrxR. Extension temperature was 72 °C for 1 min and final extension was for 5 min at the same temperature.
A 1.2% agarose gel was used to run the amplified products and stained with ethidium bromide and quantified using the Alpha Imager gel documentation. Three repeats of the experiment for each of untreated and treated isolate was performed.
Spot densitometry: Alpha Ease FC software was used to quantify the bands obtained during electrophoresis for the amplified mRNAs. The densitometric values of the bands obtained for thioredoxin reductase, Peroxiredoxin and FeSOD were expressed as percents of the 18S rRNA band density.
Statistical analysis: The mean, standard error and paired t-test of treated as well as untreated groups were determined for the metronidazole and auranofin treated isolates.
Preparation of cell extracts: Short term metronidazole and Auranofin stress was given to the clinical isolate 980 and 989, and the cells were harvested after 24 h, as described above. The pellet was washed in PBS, supernatant was discarded, pellet volume measured and pellet transferred to a Dounce homogenizer after suspending it in Tris buffer [100 mmol/L Tris/HCl (pH 7.5)]. A protease inhibitor cocktail in the ratio 1:100 was added at this step to prevent breakdown of proteins. The homogenizer was placed in ice. The cells were then disrupted with 25 strokes of the pestle of the dounce homogenizer. The lysates were then transferred to an Eppendorf tube and pelleted at 20000 g for 10 min at 4 °C. The supernatant was stored at -80 °C until the protein and thioredoxin reductase assay was performed.
Determination of Thioredoxin reductase activity: Thioredoxin reductase activity was determined in the cell extracts by the spectrophotometric measurement of DTNB reduction at 412 λ by the action of E. histolytica thioredoxin reductase. The assay was carried out in microtitre plates. The assay mixture consisted of 100 mmol/L potassium phosphate (pH 7.0), 10 mmol/L EDTA and 0.24 mmol/L NADPH and 3 mmol/L DTNB. Measurements were made under aerobic conditions at 25 °C in a Thermoscientific Multiscango plate reader, using an enzymatic kinetic program. Ten readings per sample were taken at an interval of 30 s each. The data was expressed as units of enzymes per mg protein, where each unit causes an increase in λ 412. Each assay was performed thrice, and the data was expressed as mean ± SD for three independent experiments.
Protein estimation was carried out in the cell extracts using the colorimetric Bichinchonic acid assay in 96 well microtiter plates, where the absorbance was measured at 562 nm. The linear range of this assay was 200-100 μg/mL.
Statistical analysis: Mean ± SE of of all the data was determined and compared between untreated E. histolytica and cells treated with auranofin and metronidazole. A paired student’s t test was used for comparison between the groups.
Ethical consideration: Informed consent for obtaining stool samples and ethical permission was taken from participants for the study.
MIC of isolate 980 to Auranofin and Metronidazole: Table 2 shows a representative plate test to determine the MIC of isolate 980 to Auranofin. MIC of Auranofin for isolate 980 was 3 μmol/L, the lowest concentration where live cells were present at 48 h. This experiment was carried out five times in triplicate wells and in all attempts the MIC was found to be 3 μmol/L. Table 2 shows a representative plate test to determine the MIC of isolate 980 to metronidazole. MIC was 80 μmol/L, the lowest concentration where live cells were present at 48 h. This experiment was carried out ten times in triplicate wells. The MIC was found to be 80 μmol/L in most attempts, while in a couple of attempts the MIC was 100 μmol/L.
| Concentration | 15 h | 24 h | 48 h | ||||||
| W-1 | W-2 | W-3 | W-1 | W-2 | W-3 | W-1 | W-2 | W-3 | |
| MIC of Entamoeba histolytica clinical isolate 980 to auranofin = 3 μmol/L | |||||||||
| Control | +++ | ++++ | +++ | +++ | +++ | +++ | +++ | ++ | ++ |
| DMSO control | +++ | +++ | ++++ | +++ | +++ | +++ | +++ | ++ | ++ |
| 1 μmol/L | ++ | +++ | ++ | ++ | ++ | ++ | ++ | ++ | ++ |
| 2 μmol/L | + | ++ | ++ | ++ | ++ | ++ | ++ | + | + |
| 3 μmol/L | + | + | + | + | + | - | + | - | + |
| 4 μmol/L | + | + | + | - | - | - | - | - | - |
| MIC of of Entamoeba histolytica clinical isolate 980 to metronidazole = 80 μmol/L | |||||||||
| Control | +++ | ++++ | +++ | +++ | +++ | +++ | +++ | ++ | ++ |
| DMSO control | +++ | +++ | ++++ | +++ | +++ | +++ | +++ | ++ | ++ |
| 50 μmol/L | ++ | +++ | +++ | ++ | ++ | ++ | ++ | ++ | ++ |
| 60 μmol/L | ++ | ++ | ++ | ++ | ++ | ++ | ++ | ++ | + |
| 70 μmol/L | + | ++ | ++ | ++ | ++ | ++ | ++ | + | + |
| 80 μmol/L | ++ | + | ++ | ++ | + | + | + | + | - |
| 90 μmol/L | + | + | + | + | - | - | - | - | - |
| MIC of Entamoeba histolytica clinical isolate 989 to auranofin = 1 μmol/L (MIC determined to be 2 μmol/L) | |||||||||
| Control | +++ | ++++ | +++ | +++ | +++ | +++ | +++ | ++ | ++ |
| DMSO control | +++ | +++ | +++ | +++ | +++ | +++ | +++ | ++ | ++ |
| 1 μmol/L | ++ | +++ | +++ | ++ | + | + | + | + | + |
| 2 μmol/L | ++ | ++ | + | + | + | + | + | - | - |
| 3 μmol/L | + | + | + | - | - | - | - | - | - |
| 4 μmol/L | - | - | - | - | - | - | - | - | - |
| MIC of of Entamoeba histolytica clinical isolate 989 to metronidazole = 30 μmol/L | |||||||||
| Control | +++ | ++++ | +++ | +++ | +++ | +++ | +++ | ++ | ++ |
| DMSO control | +++ | +++ | +++ | +++ | +++ | +++ | +++ | ++ | ++ |
| 10 μmol/L | ++ | +++ | +++ | ++ | ++ | ++ | ++ | ++ | ++ |
| 20 μmol/L | ++ | +++ | ++ | + | ++ | ++ | ++ | ++ | + |
| 30 μmol/L | ++ | ++ | + | + | + | + | + | + | - |
| 40 μmol/L | + | + | + | - | - | - | - | - | - |
MIC of isolate 989 to auranofin and metronidazole: Table 2 shows a representative plate test to determine the MIC of isolate 989 to Auranofin. MIC of Auranofin in isolate 989 was found to be 1 μmol/L, the lowest concentration where live cells were present at 48 h. Table 2 shows a representative plate test to determine the MIC of metronidazole in isolate 989. The MIC was found to be 30 μmol/L, the lowest concentration with live cells at 48 h. This experiment was carried out in triplicate wells and in 5 attempts, and each time the same MIC was observed.
MIC of metronidazole and auranofin in other clinical isolates: Using the same microtiter plate test method described for 980 and 989, we performed a pilot study on other clinical isolates of E. histolytica, cultured in our laboratory to assess their MICs (Table 3). MIC’s for metronidazole ranged from 30 μmol/L to 50 μmol/L, amongst these, isolate 980 showed a high tolerance to metronidazole with an MIC of 80 μmol/L and isolate 989 was more sensitive compared to the rest of the clinical isolates. MIC’s for auranofin ranged from 1-3 μmol/L in the five clinical isolates studied. From the clinical samples maintained in xenic culture, two isolates, 989 and 980, representing a sensitive and tolerant population respectively to antiamoebic drug, metronidazole were selected for expression studies. These isolates were further tested for expression analysis using auranofin drug also.
| Isolate | MIC metronidazole | Range (µmol/L) | MIC auranofin | Range (µmol/L) | No. of attempts |
| 654 | 50 μmol/L | 50-60 | 2 μmol/L | 2-3 | 3 |
| 812 | 40 μmol/L | 30-40 | 2 μmol/L | 2-3 | 3 |
| 980 | 80 μmol/L | 80-100 | 3 μmol/L | 80-100 | 5 (auranofin) |
| 10 (metronidazole) | |||||
| 989 | 30 μmol/L | 30-40 | 1 μmol/L | 1-2 | 5 |
| 5132 | 50 μmol/L | 50-60 | 2 μmol/L | 2-3 | 3 |
| MS-96:3382 | 24 μmol/L | 20-30 | 5 μmol/L | 4-5 | 4 |
The percent survival of the clinical isolate 980 after treatment with metronidazole, for different time periods is shown in Table 4. The survival of the treated isolate was expressed as percent of the untreated controls. A concentration where the effect of metronidazole was seen and yet enough viable cells could be harvested was 70 μmol/L and this concentration was used for further experiments on metronidazole stress. The percent survival of clinical isolate 980 with different concentrations of auranofin is shown in Table 4. The viability was expressed as percent of the untreated controls. At a concentration of 2 μmol/L of Auranofin there were 27% viable cells at 24 h. This concentration was chosen for further experiments. Percent survival of clinical isolate 989 on treatment with different concentrations of metronidazole is shown in Table 4. The survival of the treated isolate was compared with untreated control. It was observed that at 20 μmol/L metronidazole, 65% of cells survived in 48 h. This concentration was chosen for further experiments. Percent survival of isolate 989 during auranofin treatment, is shown in Table 4. Zero point five μmol/L auranofin treatment gave 45% survival in 24 h and 43% in 48 h. Treatment with 1 μmol/L auranofin reduced cell survival to 19% in 48 h in this isolate. Therefore 0.5 μmol/L treatment was given to the cells for further experiments in this isolate in order to harvest sufficient number of cells.
| 15 h | 24 h | 48 h | |
| Percent viability of clinical isolate 980 after treatment with metronidazole | |||
| 50 μmol/L metronidazole | 61.8 ± 0.13 | 74.12 ± 14.1 | 70.23 ± 3.66 |
| 70 μmol/L metronidazole | 53.06 ± 14.1 | 69.38 ± 3.13 | 60.68 ± 6.74 |
| 90 μmol/L metronidazole | 47.31 ± 6.2 | 26.39 ± 10.7 | 24.3 ± 14.75 |
| Percent viability of clinical isolate 980 after treatment with auranofin | |||
| 1 μmol/L auranofin | 91.5 ± 0.26 | 25.17 ± 5.85 | 12.88 ± 1.63 |
| 2 μmol/L auranofin | 56.6 ± 5.81 | 27.92 ± 5.84 | 0 |
| 3 μmol/L auranofin | 47.74 ± 7.67 | 22.41 ± 4.62 | 0 |
| Percent viability of clinical isolate 989 after treatment with metronidazole | |||
| 20 μmol/L | - | 92.51 ± 2.79 | 65.22 ± 18.5 |
| 30 μmol/L | - | 76.11 ± 17.13 | 25.39 ± 5.33 |
| 40 μmol/L | - | 54.81 ± 0.57 | 0 |
| Percent viability of clinical isolate 989 after treatment with auranofin | |||
| 0.5 μmol/L | - | 45.47 ± 0.26 | 43.01 ± 2.33 |
| 1 μmol/L | - | 36.63 ± 3.00 | 19.4 ± 2.95 |
| 2 μmol/L | - | 29.16 ± 2.95 | 0 |
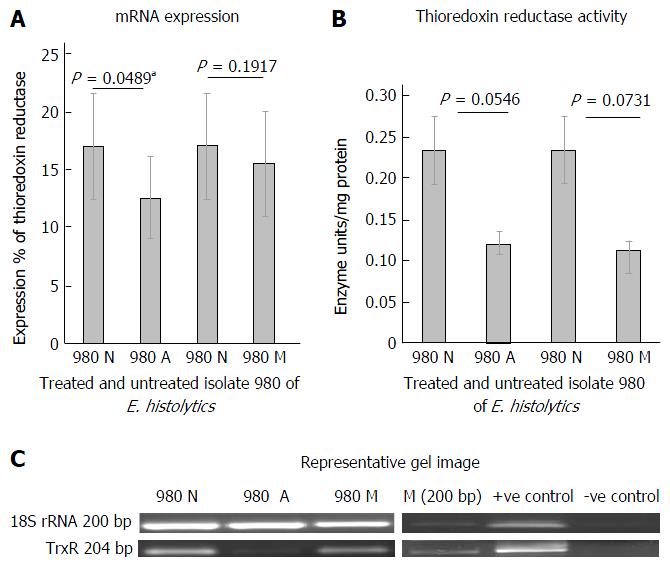
Figure 1A shows the mRNA expression percent using semiquantitative RT-PCR of thioredoxin reductase in clinical isolate 980 after treatment with metronidazole and Auranofin. The paired columns compare the untreated and the treated isolate. The columns represent the spot densitometry data of thioredoxin reductase mRNA expression graphically. It is expressed as a percent of 18SrRNA, an internal control. TrxR expression in isolate 980 showed a downregulation on treatment with anti amoebic drugs, auranofin and metronidazole. This was statistically significant in case of auranofin treatment (P < 0.05). Figure 1B is a representative gel picture of the thioredoxin reductase mRNA expression in the treated and untreated isolate. Figure 1C shows the thioredoxin reductase enzyme activity in units/mg protein. The activity shows a decreasing trend on treatment with antiamoebic drugs in comparison to control, this decrease was however not statistically significant.
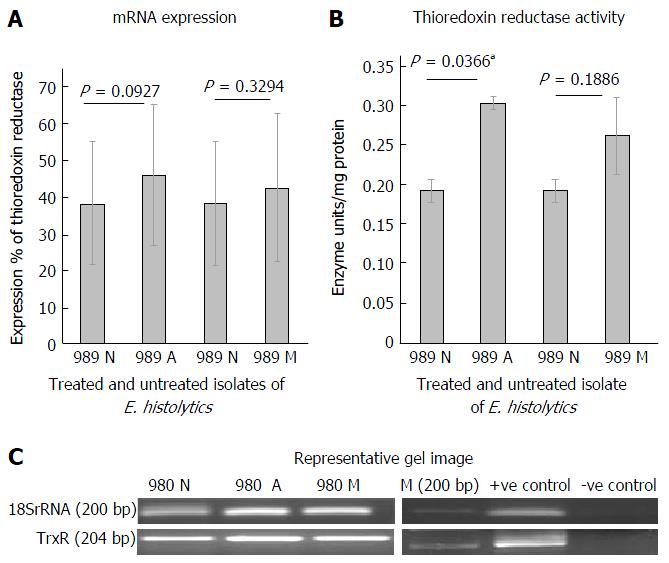
Figure 2A shows the mRNA expression percent of thioredoxin reductase in clinical isolate 989 after treatment with metronidazole and auranofin, using semi quantitative RT-PCR. At mRNA level, an increase in expression (not significant) was observed when cells were treated with 0.5 μmol/L of auranofin. A similar increase in mRNA level was seen when treated with 20 μmol/L of metronidazole. Figure 2B shows a representative gel picture of the TrxR in treated and untreated isolate at mRNA level. 18S rRNA was used as an internal control. Figure 2C shows the Thioredoxin reductase activity on treatment of 989 with auranofin and metronidazole for 24 h. We observed a significant increase (P = 0.036) in TrxR activity when cells were treated with 0.5 μmol/L of auranofin. Similar increase was seen in case of metronidazole treatment however it was not significant.
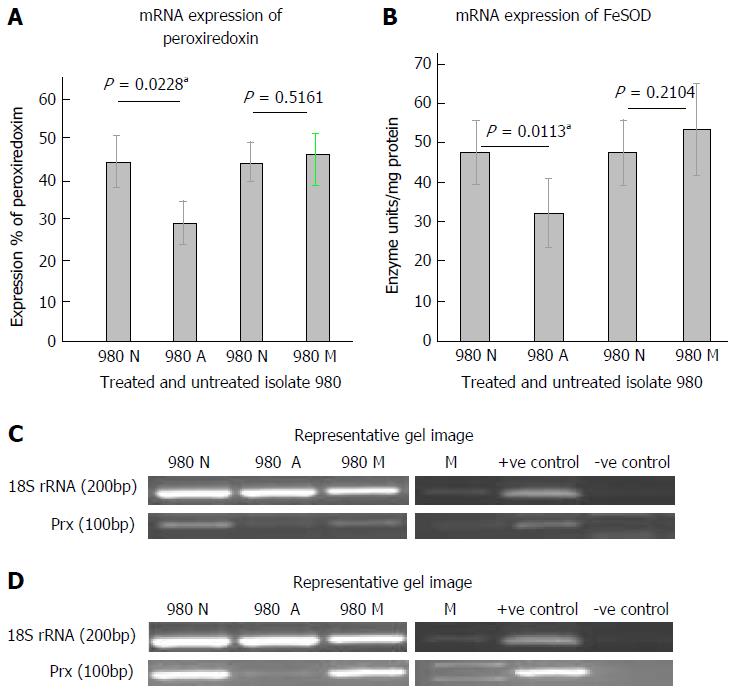
The mRNA expression of peroxiredoxin in the isolate when treated with Auranofin and Metronidazole is shown in Figure 3A. It was observed that peroxiredoxin expression in isolate 980 was significantly down regulated at the mRNA level after treatment with auranofin. This decrease was significant when the data was analyzed using paired t-test (P = 0.0228). However, in case of metronidazole treatment no significant change was observed. Figure 3B shows a representative gel picture of the peroxiredoxin expression. Figure 3C shows the mRNA expression of FeSOD in isolate 980 after treatment with auranofin and metronidazole. There was a decreased FeSOD expression on treatment with auranofin. The decrease was significant with a P value of 0.0113. However, no significant change was observed when treated with metronidazole. Figure 3D shows a representative gel picture of FeSOD expression in 980. Internal control in both the experiments was 18S rRNA.
No significant change could be seen in the mRNA expression of either peroxiredoxin or FeSOD when the isolate 989 was treated with both antiamebic drugs, auranofin and metronidazole. Data not shown.
MICs of clinical isolates maintained in xenic cultures from New Delhi, NCR region, using this method ranged from 30-50 μmol/L for metronidazole, and 1-3 μmol/L for auranofin. In case of the axenic strains HM-1: IMSS the reported MICs using this method ranged from 12.5-25 μmol/L[14]. We report here for the first time on the MIC of auranofin in clinical isolates.
The clinical isolates showing different sensitivities to auranofin and metronidazole were further tested for their antioxidant activities upon treatment. It was observed in isolate 980, at a concentration of 2 μmol/L auranofin, the thioredoxin reductase mRNA expression was down regulated. This was further confirmed by a significant decrease in thioredoxin reductase enzyme activity at the protein level. Auranofin has been shown to inhibit E. histolytica HM-1: IMSS thioredoxin reductase[11]. Treatment of isolate 980 with 2 μmol/L auranofin also downregulated its peroxiredoxin expression and superoxide dismutase expression at the mRNA level.
It is known that the reaction catalyzed by superoxide dismutase converts reactive oxygen to H2O2 and the peroxiredoxin further detoxifies it to H2O with the help of electrons provided by TrxR/Trx system[2]. TrxR enzyme is required for the reduction of thioredoxin which donates electrons to oxidized peroxiredoxin. It is likely that inhibition of TrxR leads to a general inhibition of the normal detoxification process in the parasite involving peroxiredoxin and superoxide dismutase. Debnath et.al have earlier shown by in vitro assays that auranofin treated E. histolytica cells were more susceptible to oxidative stress and accumulated more ROS[11]. Our data on clinical isolate 980 also showed that auranofin treatment significantly reduced expression of these antioxidant enzymes at the mRNA level and the enzyme activity of thioredoxin reductase at protein level was also reduced compared to untreated controls.
Treatment of isolate 980 with 70 μmol/L metronidazole, also reduced thioredoxin reductase expression at the mRNA level and also at the enzyme activity level though not significantly. Thioredoxin reductase enzyme plays a role in the activation of metronidazole by its nitro reductase activity[15]. This decrease in its expression may contribute to the higher metronidazole tolerance of isolate 980 compared to the other clinical isolate 989. The peroxiredoxin and superoxide dismutase expression of the isolate 980 at mRNA level did not show any significant change in expression after treatment with metronidazole for 24 h. This suggests that there is no increase in detoxification inside the cell. This further suggests that all metronidazole is not being converted to its active form, though the cells were exposed to a high metronidazole concentration due to downregulation of TrxR. We also observed similar results in two clinical isolates of E. histolytica 654 and MS96 (Dhaka) after treatment with metronidazole for 24 h[16].
When Auranofin (0.5 μmol/L) treatment was given for 24 h to isolate 989, it gave an increase in thioredoxin reductase expression at the mRNA level though not significant and a similar increase at the protein level, which was significant. The downregulation of TrxR expression was not observed in this isolate perhaps due to the low concentration of auranofin used. In case of mRNA expression of peroxiredoxin and superoxide dismutase there was no significant change on treatment of isolate 989 with 0.5 μmol/L auranofin for 24 h, compared to untreated controls. At this concentration of auranofin perhaps the toxic effects of the drug were not seen, and therefore the detoxifying enzymes were not upregulated.
On treatment of isolate 989 with 20 μmol/L metronidazole, the thioredoxin activity showed slight upregulation though not significant, at the mRNA level and a significant upregulation at the protein level. Thioredoxin reductase reduces metronidazole to its active form along with two other NADPH dependent oxidoreductases[6]. An upregulation of this enzyme could therefore explain the higher sensitivity of 989 to metronidazole. Tazreiter et al[17] also reported a modest upregulation of Thioredoxin reductase when an E. histolytica population was exposed for 8 h to a concentration of 50 μmol/L of metronidazole. On the other hand, TrxR, peroxiredoxin and FeSOD were shown to be downregulated at the protein level in E. histolytica when treated with 50 μmol/L metronidazole for up to 8 h[3]. However, our results with clinical isolates 989 did not show any significant change in peroxiredoxin or superoxide dismutase activity. We are yet to fully decipher the contribution of thioredoxin reductase in clinical isolates of Entamoeba during metronidazole stress.
Clinical isolates of E. histolytica from Delhi show different tolerance to antiamoebic drugs metronidazole and auranofin. Isolate 980 shows a higher tolerance to metronidazole with MIC’s of 80 μmol/L compared to other clinical isolates studied. In the isolate 980, mRNA expression levels of thioredoxin reductase, FeSOD and Peroxiredoxin were downregulated with auranofin treatment. TrxR enzyme activity also showed an inhibition at the protein level. Our results further confirm that in clinical isolates auranofin acts by inhibition of TrxR and perhaps the treated isolate has a lower capacity to combat oxidative stress as is evident by the downregulation of FeSOD and Peroxiredoxin. Metronidazole treatment also inhibited the mRNA expression of Thioredoxin reductase and the TrxR enzyme activity showing a higher tolerance to metronidazole. Lack of metronidazole activation could be the reason for the increase in tolerance of isolate 980 to metronidazole.
Isolate 989 showed a greater sensitivity to metronidazole compared to other clinical isolates, with MIC’s of 30 μmol/L. However, upon treatment with auranofin at 0.5 μmol/L, we could not observe the toxic effect the drug. Treatment of 989 with metronidazole, showed an upregulation of TrxR activity indicating higher rate of conversion of the drug to it active form.
We conclude that each clinical isolate responds differently to drug stress. Infections of E. histolytica which show a greater metronidazole tolerance can be effectively combated by treatment with auranofin.
The authors gratefully acknowledge Dr. Mukul Singh and Dr. Amit Dubey of Department of Pathology, Safdarjung Hospital, New Delhi for kindly providing the clinical samples and Professor Sudha Bhattacharya of School of Environmental Sciences, Jawaharlal Nehru University for the use of Entamoeba culturing facilities and her valuable inputs to the project.
Entamoeba histolytica (E. histolytica) infections are endemic to India and are associated with a high rate of morbidity and mortality. Metronidazole has been in use for around more than 40 years for treating amoebiasis. It has been used against both bacterial and protozoan infections.
Widespread use of this drug and short term exposure of the parasite to sub lethal doses has been the reason for the development of metronidazole tolerance by the parasite. This raises concerns on the treatment of amoebiasis. To combat this problem, auranofin a gold containing drug which is already in use against rheumatoid arthritis was tested for activity against entamoeba infections and found to be very effective.
The authors studied the effect of auranofin in two clinical isolates of Entamoeba from New Delhi which showed different tolerance to metronidazole. This research shows that on antioxidant profiling at mRNA level and at enzyme activity level, there is a difference in the expression of antioxidant enzymes between the isolates showing different tolerance. Till today no work has been done on the effect of Auranofin and the changes in antioxidant enzyme activities on clinical isolates of E. histolytica upon treatment with antiamoebic drugs.
Auranofin has been found to be effective in clinical isolates of E. histolytica, which was highly tolerant to metronidazole. This is a very important finding, and since auranofin is a drug already in use in humans, it can be safely used as an alternative therapy against amoebic infections.
Minimum inhibitory concentrations: In microbiology, minimum inhibitory concentration (MIC) is the lowest concentration of an antimicrobial drug that will inhibit the visible growth of a microorganism after overnight incubation. In medicine, culturing the organism infecting a patient with available antibiotic drugs and determining the MICs, is important for identifying the correct drug dosage to administer to the patient. Drug tolerance: The ability of an organism to persist despite the presence of high concentrations of drug which normally inhibit their growth.
It is interesting with regard to drug resistance.
Manuscript source: Invited manuscript
Specialty type: Infectious diseases
Country of origin: India
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: García-Elorriaga G, Sugawara I, Sundar S S- Editor: Ji FF L- Editor: A E- Editor: Li D
| 1. | Angelucci F, Sayed AA, Williams DL, Boumis G, Brunori M, Dimastrogiovanni D, Miele AE, Pauly F, Bellelli A. Inhibition of Schistosoma mansoni thioredoxin-glutathione reductase by auranofin: structural and kinetic aspects. J Biol Chem. 2009;284:28977-28985. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 163] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 2. | Jeelani G, Nozaki T. Entamoeba thiol-based redox metabolism: A potential target for drug development. Mol Biochem Parasitol. 2016;206:39-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 50] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 3. | Schlosser S, Leitsch D, Duchêne M. Entamoeba histolytica: identification of thioredoxin-targeted proteins and analysis of serine acetyltransferase-1 as a prototype example. Biochem J. 2013;451:277-288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 28] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 4. | Andrade RM, Reed SL. New drug target in protozoan parasites: the role of thioredoxin reductase. Front Microbiol. 2015;6:975. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 48] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 5. | Leitsch D, Kolarich D, Wilson IB, Altmann F, Duchêne M. Nitroimidazole action in Entamoeba histolytica: a central role for thioredoxin reductase. PLoS Biol. 2007;5:e211. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 107] [Cited by in RCA: 120] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 6. | Jeelani G, Husain A, Sato D, Ali V, Suematsu M, Soga T, Nozaki T. Two atypical L-cysteine-regulated NADPH-dependent oxidoreductases involved in redox maintenance, L-cystine and iron reduction, and metronidazole activation in the enteric protozoan Entamoeba histolytica. J Biol Chem. 2010;285:26889-26899. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 45] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 7. | Samarawickrema NA, Brown DM, Upcroft JA, Thammapalerd N, Upcroft P. Involvement of superoxide dismutase and pyruvate: ferredoxin oxidoreductase in mechanisms of metronidazole resistance in Entamoeba histolytica. J Antimicrob Chemother. 1997;40:833-840. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 109] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 8. | Wassmann C, Hellberg A, Tannich E, Bruchhaus I. Metronidazole resistance in the protozoan parasite Entamoeba histolytica is associated with increased expression of iron-containing superoxide dismutase and peroxiredoxin and decreased expression of ferredoxin 1 and flavin reductase. J Biol Chem. 1999;274:26051-26056. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 195] [Cited by in RCA: 187] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 9. | Tejman-Yarden N, Miyamoto Y, Leitsch D, Santini J, Debnath A, Gut J, McKerrow JH, Reed SL, Eckmann L. A reprofiled drug, auranofin, is effective against metronidazole-resistant Giardia lamblia. Antimicrob Agents Chemother. 2013;57:2029-2035. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 117] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 10. | Debnath A, Ndao M, Reed SL. Reprofiled drug targets ancient protozoans: drug discovery for parasitic diarrheal diseases. Gut Microbes. 2013;4:66-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 51] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 11. | Debnath A, Parsonage D, Andrade RM, He C, Cobo ER, Hirata K, Chen S, García-Rivera G, Orozco E, Martínez MB. A high-throughput drug screen for Entamoeba histolytica identifies a new lead and target. Nat Med. 2012;18:956-960. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 235] [Cited by in RCA: 237] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 12. | Clark CG, Diamond LS. Methods for cultivation of luminal parasitic protists of clinical importance. Clin Microbiol Rev. 2002;15:329-341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 319] [Cited by in RCA: 312] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 13. | Srivastava S, Bhattacharya S, Paul J. Species- and strain-specific probes derived from repetitive DNA for distinguishing Entamoeba histolytica and Entamoeba dispar. Exp Parasitol. 2005;110:303-308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 14. | Upcroft JA, Upcroft P. Drug susceptibility testing of anaerobic protozoa. Antimicrob Agents Chemother. 2001;45:1810-1814. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 92] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 15. | Robinson GL. The laboratory diagnosis of human parasitic amoebae. Trans R Soc Trop Med Hyg. 1968;62:285-294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 202] [Cited by in RCA: 190] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 16. | Iyer LR, Singh N, Verma AK, Paul J. Differential expression and immunolocalization of antioxidant enzymes in Entamoeba histolytica isolates during metronidazole stress. Biomed Res Int. 2014;2014:704937. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 17. | Tazreiter M, Leitsch D, Hatzenbichler E, Mair-Scorpio GE, Steinborn R, Schreiber M, Duchêne M. Entamoeba histolytica: response of the parasite to metronidazole challenge on the levels of mRNA and protein expression. Exp Parasitol. 2008;120:403-410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 18] [Article Influence: 1.1] [Reference Citation Analysis (0)] |