Published online Nov 25, 2014. doi: 10.5495/wjcid.v4.i4.27
Revised: July 26, 2014
Accepted: September 4, 2014
Published online: November 25, 2014
Processing time: 162 Days and 12.4 Hours
Cellular stress responses are powerful mechanisms that prevent and cope with the accumulation of macromolecular damage in the cells and also boost host defenses against pathogens. Cells can initiate either protective or destructive stress responses depending, to a large extent, on the nature and duration of the stressing stimulus as well as the cell type. The productive replication of a virus within a given cell places inordinate stress on the metabolism machinery of the host and, to assure the continuity of its replication, many viruses have developed ways to modulate the cell stress responses. Poxviruses are among the viruses that have evolved a large number of strategies to manipulate host stress responses in order to control cell fate and enhance their replicative success. Remarkably, nearly every step of the stress responses that is mounted during infection can be targeted by virally encoded functions. The fine-tuned interactions between poxviruses and the host stress responses has aided virologists to understand specific aspects of viral replication; has helped cell biologists to evaluate the role of stress signaling in the uninfected cell; and has tipped immunologists on how these signals contribute to alert the cells against pathogen invasion and boost subsequent immune responses. This review discusses the diverse strategies that poxviruses use to subvert host cell stress responses.
Core tip: Poxviruses are known to encode a plethora of proteins that interact with cell biology processes in order to achieve replicative success. In this article, we review how poxviruses cope with cellular stress signals that are usually triggered upon infection to tentatively block virus replication. The understanding of mechanisms by which poxviruses and other complex viruses interfere with stress responses can further illuminate the web of pathways regulating cell homeostasis, as well as how viruses intertwine their own biochemical needs into this intricate scenario.
- Citation: Leão TL, da Fonseca FG. Subversion of cellular stress responses by poxviruses. World J Clin Infect Dis 2014; 4(4): 27-40
- URL: https://www.wjgnet.com/2220-3176/full/v4/i4/27.htm
- DOI: https://dx.doi.org/10.5495/wjcid.v4.i4.27
The Poxviridae family is taxonomically divided into two subfamilies of double-stranded DNA (dsDNA) viruses that are able to infect insects (Entomopoxvirinae) and a wide spectrum of vertebrate hosts (Chordopoxvirinae). The Chordopoxvirinae subfamily currently contains ten genera (Avipoxvirus, Capripoxvirus, Cervidpoxvirus, Crocodylidpoxvirus, Leporipoxvirus, Molluscipoxvirus, Orthopoxvirus, Parapoxvirus, Suinopoxvirus, Yatapoxvirus) and one unassigned species (Squirrelpox virus), whereas the Entomopoxvirinae subfamily comprises three genera (Alphaentomopoxvirus, Betaentomopoxvirus, Gammaentomopoxvirus) and two unclassified species (Diachasmimorpha entomopoxvirus and Melanoplus sanguinipes entomopoxvirus “O”)[1]. Members of the Poxviridae family are large viruses (approximately 350 nm × 250 nm × 200 nm) with a linear genome ranging from 130 to 300 kb, each often encoding approximately 200 proteins. Virions are brick-shaped, multi-enveloped particles and, unlike other DNA viruses, replicate exclusively in the cytoplasm of the infected host cell. Most poxviral biosynthetic pathways occur in distinct sites of the cytoplasm called viral factories: large masses of electron dense material, the viroplasm, that are frequently surrounded by membranes from the endoplasmic reticulum (ER) and/or membranes from the ER-Golgi intermediate compartments[2-6].
Different viruses have evolved two very distinct general strategies to compete with the host cell for biochemical resources and successfully replicate within them. One such strategy is a “hit and run” type of approach, in which viruses rapidly replicate and generate a progeny that spreads quickly to other cells. In order to be effective, these viruses have invested in replication speed by keeping small genomes which code for few essential proteins - the faster they replicate, the more efficiently they can escape antiviral responses by the host. A second strategy, however, is based on a “stay and fight” approach. Viruses that adopted this strategy tend to endure within the host cell and, therefore, may be susceptible to antiviral responses that are gradually elicited against them. Thus, in order to achieve replicative success, these viruses have to cope with the host attempts to get rid of them and, as a way to counteract antiviral responses, many evolved processes to either block or delay such responses. Because most viral strategies to evade host responses are based in virus-coded proteins, this led inevitably to an increase in genome sizes. There are obvious exceptions to this rather simplistic classification of virus replication strategies, as in the case of hepadnaviruses (like hepatitis B virus) for instance. Nonetheless, most viruses can still fit one of the two aforementioned models. Poxviruses are one of the best examples of viruses that have developed ways to either counteract host strategies to hamper viral replication or boost their biosynthetic pathways to the detriment of the host’s. Indeed, most poxviruses (especially chordopoxviruses) spare up to 50% of their genomes to code for immune evasion-related and host-interaction genes[7].
As soon as these viruses enter the host cell, they set in motion a number of biochemical strategies to usurp cellular resources. One such strategy is to hijack the host translation apparatus to selectively produce large quantities of viral proteins. To this end, poxviruses produce proteins that are able to cleave host messenger RNAs (mRNAs)[4,8-10] early in infection, shutting down the host protein synthesis almost completely during the first hours of the viral cycle[11]. Furthermore, viruses are devoid of molecular chaperones, such as heat shock proteins (HSPs) with few exceptions and rely almost completely on chaperones of the host to adequately process viral proteins, avoiding misfolding or aggregation[12-14]. In parallel, viral double-stranded RNA intermediates, DNA and proteins are sensed by pattern recognition receptors in the cell, leading to the generation of innate immune responses potentially able to control the viral infection[15,16].
All the above mentioned virus-driven interferences within the cell may lead to the transduction of cell stress signals and consequent cell stress responses. The cell may respond to stress in a variety of ways, including the activation of pathways that promote survival or the elimination of damaged cells through programmed cell death (apoptosis, necrosis and/or autophagy). There is a multitude of pathways that may be elicited upon different types of stress and the resulting signal transduction cascades are often shared by other cell processes, such as the activation of innate immunity, cell cycling and so on. Nevertheless, the most common stress responses include those elicited against heat shock, ER stress (the unfolded protein response, UPR), DNA damage, hypoxia and oxidative stress. Some of these responses may limit or inhibit viral replication and/or induce cell death and others can promote cell survival and restore homeostasis. To cope with stress responses, poxviruses have evolved complex molecular strategies to counteract innate host cell defense signaling pathways while facilitating biological events that promote adaptation and survival of the host cell, all essential to a productive infection. This review summarizes the main cellular stress responses used or subverted by poxviruses to ensure completion of viral life cycle.
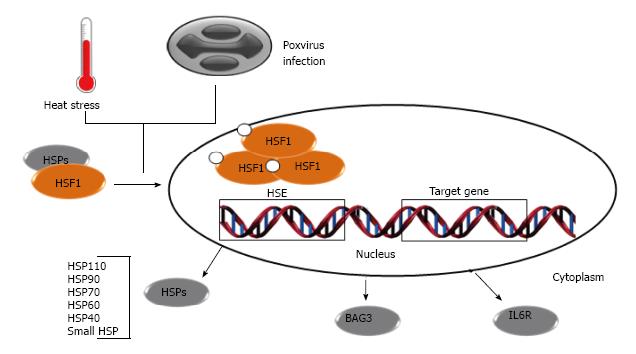
In the early 1960s, the discovery of the heat shock response (HSR) led to the elucidation of some aspects of the cell stress responses and the discovery of heat shock genes[17] and proteins (HSPs)[18,19]. Many HSPs are constitutively present in cells while some are expressed only after stress. HSPs and other molecular chaperones (e.g., co-chaperones and folding enzymes) are active in a myriad of biological essential processes that include: (1) the normal folding of polypeptides; (2) assisting misfolded proteins to attain or regain their native states; (3) regulation of protein degradation; and (4) translocation of proteins across membranes to different cellular compartments[20,21]. Some of these proteins are conserved in all three superkingdoms and are encoded by genes that contain cis-acting regulatory sequences, termed heat shock elements (HSE), which are regulated by heat shock transcription factors (HSFs)[22,23]. Upon stress, one of the main regulators of the HSR, the HSF1, undergoes trimerization and subsequent translocation into the nucleus where these complexes bind to the HSE[23] (Figure 1). HSF1 is regulated by post-translational modifications such as phosphorylation, acetylation[24], sumoylation[25,26] and interactions with other proteins. HSF1 is constitutively expressed and is neither a stress-inducible protein nor is its expression correlated with the expression of heat shock genes[27] (Figure 1).
Recent studies, using different genome-scale approaches to identify host proteins used by poxviruses during infection, revealed that HSF1 is a crucial transcription factor for virus replication and some targets of HSF1 are induced upon infection[28]. At the early stages of poxvirus infections, a decrease in HSF1 mRNA synthesis is observed; however, this does not seem to affect the protein levels as its half-life is quite long. As the viral lifecycle progresses, an increase in HSF1 mRNA levels can be detected, although this is not followed by augmentation in this protein contents within the cell[29]. Infections by some poxviruses result in the phosphorylation of HSF1 and its translocations to nucleus, where they bind to HSE[28,29]. Several HSF1-regulated genes are upregulated during infection, including genes coding for the molecular chaperones BAG3, STIP1, all classes of HSPs (HSP10, HSP20, HSP40, HSP60, HSP70, HSP90 and HSP105/110) and other important proteins like IL6R, which has a role in cell growth and differentiation[8,28,30] (Figure 1).
The first observation of the interaction between poxvirus and HSPs was made by Jindal et al[10] (1992) who also showed that the infection led to a small increase in HSP90 and HSP60 mRNA contents and to a substantial increase in the HSP70 mRNA levels, suggesting that these proteins may play some role in viral protein folding. Opposed to this view, subsequent studies revealed that the overexpression of the 72 kDa HSP, the major inducible cytoplasmic HSP, did not affect virus replication[31,32]. Furthermore, during poxvirus infections, HSP70 accumulates predominantly in the nucleus where these proteins interact with poly (ADP-ribose) polymerase 1, PARP1 and XRCC1 and prevent single-stranded DNA break (SSB)[29,33]. Globally, these observations suggest that HSP70s are important for cell survival and death prevention but may have a lesser impact in the proper folding of poxviral proteins.
So far, the most likely HSP to have a role in the poxvirus life cycle is HSP90. This chaperone is the most abundant HSP in unstressed cells and many of its targets are either kinases or transcription factors such as Akt and HSF1, respectively[34,35]. The inhibition of HSP90 function during infection by the use of geldanamycin, a drug that blocks the ATPase activity of that chaperone, impairs viral multiplication by delaying viral DNA replication and intermediate transcription, and also by reducing expression of late genes[36].
It has been shown that HSP90 interacts directly with the 4a core protein (encoded by A10L orthologous genes), implicating this chaperone in conformational maturation of the poxvirus capsid. Nonetheless, HSP90 does not colocalize with capsid proteins at later stages of infection, suggesting a transient role for HSP90 in virion morphogenesis[36]. Other host chaperones (e.g., cyclophilin A and Hsc71) are found to be associated with intracellular mature virions (IMV) but the importance of these proteins in such a context needs be further investigated[37,38].
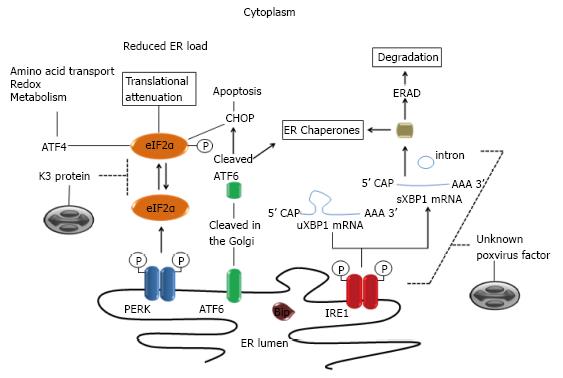
The endoplasmic reticulum (ER) is a multifunctional organelle that controls several critical aspects of cellular processes: it ensures the correct structure of most proteins; plays a key role in the synthesis of lipids and sterols; and helps in the maintenance of intracellular calcium levels and many other functions[39]. The protein homeostasis (proteostasis) surveillance in the ER is mediated by specific pathways generally called unfolded protein response (UPR), which is activated when the intrinsic protein folding capacity of the organelle is overwhelmed by a large input of unfolded proteins into the ER[40,41]. Such imbalance activates three signaling pathways through ER-resident transmembrane proteins [inositol-requiring protein 1 (IRE1), activating transcription factor 6 (ATF6) and protein kinase RNA-like ER kinase (PERK)], resulting either in recovery of proteostasis or in cell death[42,43] (Figure 2). In resting cells, these molecular sensors are maintained in inactive states through interactions with the major and most abundant ER-resident chaperone, the binding immunoglobulin protein (BiP) (Figure 2), also known as glucose regulated protein of 78 kDa (GRP78), encoded by the HSPA5 gene[44,45].
Because poxviruses replicate in close association with the ER, using components of this organelle to its own benefit, it was suggested that they might trigger ER stress and activate UPR signaling[46]. Indeed, many structural Vaccinia virus (the prototypic member of the family) proteins are known to closely interact with membranes of the ER during the formation of crescent membranes and immature virions[47-49]. Yet, no activation of IRE1-dependent stress pathways is usually detected[50] and how poxviruses evade and/or subvert this UPR signaling is still not known. During ER stress, IRE1 undergoes dissociation from BiP and BAX inhibitor 1[51,52], triggering its dimerization and the activation of its endonuclease activity[53-55]. The IRE1 nuclease domain has homology to RNase L and its activation causes splicing of a residual intron (26nt) in the XBP1 mRNA, resulting in a more stable and active form of the XBP1 protein (HAC1 in yeast)[56] (Figure 2). In some circumstances, activation of the IRE1 endonuclease domain mediates the cleavage and degradation of other cell mRNAs[57] and this feature complements other cellular mechanisms to control global protein translation[58].
Upon activation, the ATF6 transcription factor relocates to the Golgi where it is cleaved by S1P and S2P proteases[59], resulting in the release of an amino-terminal fragment that translocates to the nucleus where it promotes expression of chaperones, modifying enzymes and genes that code for transcription factors such as DNA damage-inducible transcript 3 [(DDIT3), also known as CCAAT/enhancer binding protein homologous protein (CHOP)] and X-box binding protein 1 (XBP1), which play an important role in ER stress induced apoptosis and proteostasis, respectively[60-62]. Although this was never fully investigated, it is tempting to speculate that poxviruses may somehow interact with IRE1/ATF6-dependent stress pathways as these are such central components during the unfolded protein response.
It is known that XBP1 can be activated by TLR-2 and TL4-4 stimulation in an IRE1 dependent manner; also known is the fact that Vaccinia virus and other chordopoxviruses are able to interfere with TLR signaling. Therefore, this seems to be a virus-driven indirect strategy to down-modulate XBP1 activation. Because XBP1 has been shown to be important for sustained production of cytokines by macrophages, it seems logical that poxvirus may interfere with XBP1 activation as a way to cope both with the host innate responses as well as with the ER stress.
Another component of the UPR, PERK (also known as eIF2αK3) shares homology to the IRE1 structure and activation pathways but lacks the RNase domain of IRE1[63]. Like the IRE1 activation, the release of BiP from PERK triggers dimerization of the later and its transphosphorylation (Figure 2). The activated PERK dimer is capable of recognizing and phosphorylating the alpha subunit of the translation initiation factor eIF2α at serine 51, reducing the translation of virus and cell mRNAs[64] (Figure 2). On the other hand, eIF2α phosphorylation upregulates the translation of ATF4, which induces expression of CHOP, GADD34, ATF3[65-67] and other genes involved in processes that are usurped and modulated during poxvirus replication, including amino acids transport[11], glutathione metabolism[68] and control of oxidative stress[69]. Not surprisingly, poxviruses encode proteins that mimic eIF2α and act as a pseudosubstrate for PERK, consequently suppressing phosphorylation of eIF2α and the shutoff of viral protein synthesis[70,71] (Figure 2).
Most viruses, as obligate intracellular parasites, lack most genes related to the transcriptional and translational machinery, including those coding for enzymes, transcriptional factors, ribosomal subunits, translation factors and transfer RNAs (tRNA). Poxviruses encode their own transcriptional machinery but, to ensure viral mRNA translation during productive infections, they must effectively govern the host translation apparatus while avoiding stress responses like the eIF2α phosphorylation mediated translation shutoff.
In addition to PERK, which is involved in responses to the proteostasis imbalance in the ER, three other stress-activated eIF2α kinases are capable of inducing a broad range of responses designed to protect the cell. Protein kinase R (PKR), heme-regulated inhibitor (HRI) and general control nonderepressible 2 (GCN2) respond to dsRNA, oxidative stress and nutrient deprivation, respectively[72-74]. PKR (also known as eIF2αK2) is activated in response to stress signals usually resulting from viral infections and, together with other sensing and responding pathways that lead to eIF2α inactivation, is part of the so called integrated stress response (ISR). Poxviruses evolved non-redundant strategies to suppress activation of ISR and collectively inhibit the host translational shutoff response. The best characterized poxvirus’ strategy to evade ISR is the expression of a pleiotropic viral protein, encoded by E3L orthologous genes, which is able to bind dsRNA and inhibit PKR activation. Nonetheless, other viral proteins also play critical roles in this process, including those encoded by K1L, C7L and CP77L orthologues[75-77]. Poxviruses lacking E3L orthologous genes induce the formation of host-protein dense antiviral granules (AVGs) that suppress translation of viral but not stress-induced host mRNAs and thus inhibit poxvirus replication[78].
ISR activation often promotes the formation of ribonucleoprotein aggregates called stress granules (SGs) at random sites throughout the cytoplasm. These SGs function as a protection zone for host RNAs where they can be stored when intracellular conditions are harmful[79]. SGs are distinct from AVGs in function and composition but share some components, like mRNA and RNA binding proteins [including Ras GTPase-activating protein-binding protein 1, Caprin-1, TIA1 and mRNA poly(A) binding protein, PABP] and other translation initiation components [including eIF3H and eIF4A/E/G (eIF4F complex) with the exception of 40S ribosomal subunits and eIF3B which only localize to SGs][80,81]; both granules, nevertheless, are dependent on translation repression. In productive poxviral infections, some of these granule components (as well as eIF4E and eIF4G) are sequestered to viral factories where they assemble and form eIF4F complexes that act together with PABP to promote activation of mRNAs harboring 7-methyl GTP caps and poly(A) tails[82]. Poxvirus mRNAs are capped on their 5’ ends by the action of a viral methyl transferase enzyme complex[83-85] and are also polyadenylated by a complex mechanism involving repetitive transcription of thymidylates in the sequence 3’-ATTTA-5’ often present at the sites of transcriptional initiation[86,87]. By sequestering molecules that activate capped and polyadenylated mRNAs to the viral factories, poxviruses are able to vigorously boost the translation of their own mRNAs.
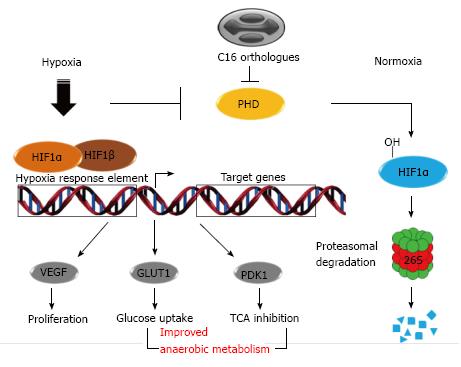
Molecular oxygen (O2) is an essential element to aerobic organisms that serves as a key substrate in cellular metabolism and bioenergetics. Hypoxic stress response is the process by which cells react and adapt to an insufficient O2 availability (or hypoxia)[88]. During hypoxic conditions, cells activate a number of adaptive responses to match O2 supply with metabolic, bioenergetic and redox demands. The hypoxia-inducible factor-1 (HIF-1) is the key regulator of the cell resilience in response to O2 deprivation and it is regulated by prolyl hydroxylase domain-containing enzymes (PHDs)[89,90]. HIFs are obligate heterodimers, consisting of an O2-destructible α-subunit and O2-indestructible β subunit, and under physiologically normal O2 levels (normoxia), PHDs mediate hydroxylation of proline residues in the HIFα subunit, triggering their recognition and labeling by E3 ubiquitin ligases, which leads in turn to their proteasomal degradation[91,92]. PHD activities are regulated by O2 availability and by cellular metabolites such as tricarboxylic acid cycle (TCA) intermediates[93]. Due to the lack of sufficient O2 upon hypoxia, PHDs become inactive and HIFα is consequently stabilized, causing the HIFs translocation to the nucleus where they bind to hypoxic responsive elements present in genes, such as HSPA5, Fos, CXCR, among other genes related to signal transduction, cell metabolism, apoptosis, etc[94-96] (Figure 3).
There are three PHD isoforms but PHD2 is believed to be the primary regulator of the HIF transcription factors[88]. The Vaccinia virus C16 protein is non-essential for virus replication but seems to play an important role in the down-modulation of the host immune responses[97]. Further studies showed that this protein can inhibit HIF1α hydroxylation through direct interaction with the PHD2 enzyme even when ectopically expressed[98]. Consequently, HIF1α is not ubiquitinated and degraded by proteasome, leading to the stabilization of this factor and up-regulation of HIF-responsive genes [endothelial growth factor (VEGF), glucose transporter-1 (GLUT1) and pyruvate dehydrogenase kinase-1 (PDK1)], improving cell metabolism and creating conditions that favor virus replication (Figure 3).
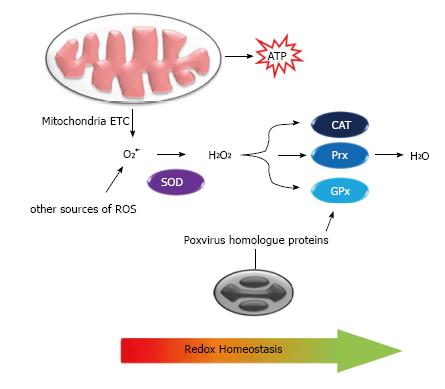
Poxviruses exploit the de novo fatty acid biosynthesis in the cell and especially the production of palmitates. These molecules undergo β-oxidation in mitochondria and, together with the glutamine catabolism, generate acetyl-CoA and α-ketoglutarate, respectively. Both molecules enter in the TCA cycle and are used as major energy sources instead of glucose in infected cells[68,99,100]. In this metabolic pathway, O2 plays a pivotal role as the final electron acceptor of oxidative phosphorylation coupled to the electron transfer chain, resulting in the production of water (H2O), but also superoxide anion (O2•-) and hydrogen peroxide (H2O2), as well as other reactive oxygen species (ROS)[101,102] (Figure 4). ROS can significantly damage cell structures, causing lipoperoxidation, protein denaturation and DNA degradation; but on the other hand, ROS acts as a second messenger in mediating inflammation, stimulating cell proliferation and regulating apoptosis to maintain cell homeostasis[103]. Due to their cytotoxicity activity, cellular ROS levels are tightly limited by multiple detoxification processes such as antioxidant enzymes and vitamins whose functions are collectively appointed as an oxidative stress response[102].
ROS are usually controlled by antioxidant enzymes such as cooper/zinc-dependent superoxide dismutase (SOD) (cytoplasm), manganese-dependent SOD (mitochondria) and extracellular-SOD (also utilizes Cu/Zn as cofactor), which dismutate O2•- into H2O2. Hydrogen peroxides are in turn decomposed by catalase (CAT) and peroxidases such as glutathione peroxidase (GPx)[104] (Figure 4).
It has been shown that Myxoma virus and Shope fibroma virus increase intracellular ROS accumulation to promote growth of infected cells and immune evasion. This is achieved via inhibition of Cu/Zn-SOD1 activity through the expression of catalytically inactive homologs of cellular SOD1 that cannot bind Cu, which is essential for dismutase activity but retains the Zn-binding properties and, similarly to their cellular homologs, forms stable heterodimeric complexes with cellular Cu-dependent chaperones that are essential for SOD1 function[69,105] (Figure 4). It is likely that other poxviruses cause a similar effect during their multiplication cycle as some encode SOD-1 like genes; one such example is the A45R SOD-1-like gene from Vaccinia virus. Besides the SOD1 homologues, another known poxvirus gene product that can alter the redox state in infected cells is the Molluscum contagiosum virus MC066L gene product, which is homologous to the human GPx[12], an enzyme able to protect cells from the proapoptotic peroxides generated by ultraviolet (UV) light[106] (Figure 4).
Cellular peroxiredoxins and thioredoxins, among other host proteins that are not essential to the cellular redox state (e.g., 60S ribosomal proteins, HGM1 and Rab10), can be detected in IMV particles. It has been speculated that those redox regulation proteins may play some role in virion maturation[38]. Indeed, redox conditions seem to be so important for poxviruses that many of them encode their own redox machinery in order to mediate disulfide bond formation in newly made viral proteins[107-109].
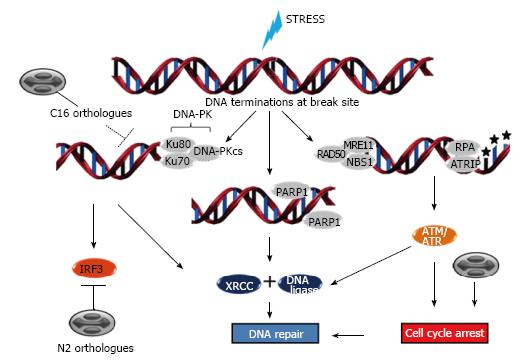
Several reports correlate stressful conditions with DNA damage responses (DDR). Hypoxia, ROS accumulation, ER stress, heat shock and mainly UV light exposure are conditions that either result or are resultant from DNA damage and whose sensing by the cell might contribute to the global stress adaptation response fostering cell resilience[96,110-114]. DDR events operate in diverse biological settings such as telomere homeostasis and generation of immune-receptor diversity[115] and include cell cycle checkpoint control, transcription, activation of DNA repair pathways, senescence and/or apoptosis. DNA damage can be subdivided into a few major types, including DNA double-strand breaks (DSB), DNA nucleotide adduct formation and base modification, DNA base pairing mismatches and single-strand breaks (SSB) which are caused by exposure to chemotherapeutic agents or environmental genotoxic agents such as polycyclic hydrocarbons and UV radiation. Accordingly, the major classes of DNA repair are DNA dsb repair by homologous recombination (HR) or nonhomologous end-joining (NHEJ), nucleotide excision repair, base-excision repair (BER), the Fanconi anemia/BRCA pathway and nucleotide mismatch repair[116]. The central sensor proteins in the DDR signal transduction cascade (ataxia telangiectasia mutated-ATM, ataxia telangiectasia and Rad3 related-ATR, DNA-dependent protein kinase-DNA-PKcs) belong to the phosphoinositide-3-kinase-related kinase (PIKK) family, with the exception of proteins from the PARP family which also respond to DNA lesions[117] (Figure 5).
ATM is recruited by the MRE-11-Rad50-NBS1 (MRN) complex to sites of DSBs and phosphorylates downstream substrates such as checkpoint kinase 2 (Chk2) which, subsequently phosphorylates p53 that in turn signals through p21 to slow the cycling of cells in order to facilitate DNA repair[118] (Figure 5). If the damage is too severe to be repaired, the cascade leads to death signalization through pro-apoptotic proteins. In the case of SSBs, ATR is recruited to damage sites in association with ATR-interacting protein by replication protein A (RPA). Once activated, these complexes phosphorylate Chk1 which, in turn, phosphorylates and inhibits cdc25c to mediate G2/M arrest (or, alternatively, phosphorylates cdc25a to promote S-phase arrest). Most ATR substrates can also be phosphorylated by ATM and the major functions of ATR and ATM in cell cycle control are overlapping but non-redundant[119,120]. These signaling cascades appear to be the major repair pathways influenced by poxvirus infections (Figure 5) as they favor cell cycle progression to G1, S and G2 phases but arrest cells in the G2 phase. Indeed, there is a preferential accumulation of poxvirus infected cells in G2/M phases concurrent with a decrease in the number of cells in the G0/G1 ones[121,122].
The NHEJ repair pathway is initiated by association of Ku70/80 proteins to the DNA ends and the subsequent recruitment of the DNA-dependent protein kinase catalytic subunit (DNA-PKcs)[123,124]. These proteins localize both in the nucleus and the cytoplasm and are key factors in the immune response signaling, acting as viral dsDNA sensors leading to the induction of interferon regulatory factor 3 (IRF3) in a TANK-binding kinase 1-dependent manner[125]. Counteracting this immune signaling, the Vaccinia virus produces the C16 protein early in infection, which can bind to Ku70 blocking DNA-PK recruitment to DNA and the N2 protein, a virulence factor that presents with the ability to inhibit IRF3-dependent innate immune responses[126,127] (Figure 5).
Poxviruses exploit their own replication machinery in order to repair eventual lesions at the viral DNA[3], mainly through the action of virally encoded uracil DNA glycosylases (coded by D4R orthologous genes), which initiate BER by hydrolyzing the glycosylic bond linking uracil to a deoxyribose sugar, and also through the repair of nicked duplex DNA substrate by a viral DNA ligase, a product of the A50R ORF present in some chordopoxviruses[128-131]. Furthermore, the viral DNA polymerase (coded by E9L gene orthologues) which possess 3’ - 5’ proofreading exonuclease activity and the G5R gene product which belongs to FEN1-like nucleases appear to conjunctly play important roles in viral DNA recombination through HR[132-136]. The cellular DNA ligase I can compensate an eventual absence of the viral DNA ligase and is recruited from the nucleus to the cytoplasmic viral factories. However, in the absence of a G5 protein, the viral DNA is fragmented and cannot be packaged[136,137].
The phosphoinositide-3-kinase (PI3K) family of enzymes is grouped into three classes of proteins. PI3K is activated by G protein-coupled receptors and tyrosine kinase receptors to drive phosphorylation of inositol lipids at the 3’ position of the inositol ring, generating lipid second messengers [3-phosphoinositides PI(3)P, PI(3,4)P2 and PI(3,4,5)P3][138,139]. Class IA PI3K proteins were shown to play an important role in poxvirus infections, promoting Akt phosphorylation and downstream events leading to the suppression of apoptosis, cell growth, survival and proliferation[140,141]. The PI3K/Akt pathway seems to be a determinant for the replicative success of Vaccinia virus and Cowpox virus, as well as for the host cell survival during infection, as the pharmacological impairment of the pathway components leads to diminished virus multiplication and apoptosis[141].
Stress conditions (osmotic stress, ER stress, among others), growth factors and/or cytokines stimulate the activation of mitogen-activated protein kinases (MAPK)[142,143]. The MAPK family consists of a series of at least three main kinases active through distinct pathways: the extracellular signal-regulated protein kinases (ERKs), the c-Jun N-terminal kinases (JNKs) and the p38 family of kinases. These MAPK enzymes are activated by post-translational modifications induced by specific kinases, named MAPK kinases (MAP2K), which are activated by upstream MAPKK kinase (MAP3K) [Raf, MAPK/ERK kinase (MEKKs) and apoptosis signal-regulating kinase (ASK)][144] and which in turn respond either to external stimuli sensed by receptors on the cell surface or through interactions with GTP-binding proteins, among other kinases. Poxviruses have been shown to trigger mitogenic signals at early stages of infection, resulting in the expression of egr-1 and other genes, such as the proto-oncogene c-fos, through the activation of ERK1/2. This process is essential for multiplication of some members of this viral family as blocking of those kinases hampers normal virus multiplication[145-147]. Additionally, the JNK pathway is also important for normal virus morphogenesis and accumulation of enveloped infectious forms[148] as blocking of the pathway influences cell-to-cell virus spread.
The activation of cellular stress responses in infected cells is a complex process that promotes simultaneously both cell resilience and death mechanisms upon a viral infection. In order to achieve replicative success in such conditions, poxviruses must subvert these cell responses to their own benefit. Members of the Poxviridae family are fully geared up to interfere with and manipulate cell fate in a way that very few other animal viruses do. They have unique abilities to turn off and/or combat negative effects of stress responses while still fomenting mechanisms to support the completion of its life cycle. Overall, poxviruses modulate the activation of a network of protein kinases (PI3K, PIKKs, MAPKs) and other enzymatic post-translational modifiers, such as the ubiquitin ligases and proteins involved in cell reprogramming (including ATFs, HSFs, XBP1, HIFs), while selectively inhibiting the activation or expression of host proteins (DNA-PK, IRF3, PHDs, PKR, PERK among others). In parallel, they are able affect the cell metabolism and redox state, maintaining proteostasis (through HSPs and other hosts and viral chaperones) and controlling cell cycle and proliferation in order to establish a proper cell environment for virus replication. Many of these strategies are highly conserved among different poxviruses, while a few others are species-specific[149]. The evidence of horizontal gene transfer from host to virus, coupled with the proposed model of poxvirus genome evolution based on a simple mechanism of recombination-driven genomic expansions and contractions (which facilitates the rapid evolution of virus populations with otherwise low mutation rates), sheds light on how these viruses acquired this impressive number of strategies to wisely control their replication niche[150-152].
Over 50 years after the discovery of HSR by Ferruccio Ritossa[153], the cellular stress response knowledge is still growing (including specific organelle stress such as mitochondrial or peroxisomal UPR, Golgi stress response and so on) and the understanding of mechanisms by which poxviruses and other complex viruses interfere with stress responses can further illuminate the web of pathways regulating cell homeostasis, as well as how viruses intertwine their own biochemical needs into this intricate scenario.
We thank CAPES, CNPq and FAPEMIG for financial support. FGF is a CNPq-PQ fellowship recipient.
P- Reviewer: Quarleri J, Roper RL S- Editor: Ji FF L- Editor: Roemmele A E- Editor: Lu YJ
| 1. | International Committee on Taxonomy of Viruses (ICTV). Virus Taxonomy: 2013 Release. Available from: http: //www.ictvonline.org/virusTaxonomy.asp. |
| 2. | Smith GL. Rapid spreading and immune evasion by vaccinia virus. Adv Exp Med Biol. 2014;808:65-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 3. | Moss B. Poxvirus DNA replication. Cold Spring Harb Perspect Biol. 2013;5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 243] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 4. | Katsafanas GC, Moss B. Colocalization of transcription and translation within cytoplasmic poxvirus factories coordinates viral expression and subjugates host functions. Cell Host Microbe. 2007;2:221-228. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 228] [Cited by in RCA: 211] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 5. | Tolonen N, Doglio L, Schleich S, Krijnse Locker J. Vaccinia virus DNA replication occurs in endoplasmic reticulum-enclosed cytoplasmic mini-nuclei. Mol Biol Cell. 2001;12:2031-2046. [PubMed] |
| 6. | Schramm B, Locker JK. Cytoplasmic organization of POXvirus DNA replication. Traffic. 2005;6:839-846. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 80] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 7. | Smith GL, Benfield CT, Maluquer de Motes C, Mazzon M, Ember SW, Ferguson BJ, Sumner RP. Vaccinia virus immune evasion: mechanisms, virulence and immunogenicity. J Gen Virol. 2013;94:2367-2392. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 245] [Cited by in RCA: 274] [Article Influence: 22.8] [Reference Citation Analysis (0)] |
| 8. | Yang Z, Bruno DP, Martens CA, Porcella SF, Moss B. Genome-wide analysis of the 5’ and 3’ ends of vaccinia virus early mRNAs delineates regulatory sequences of annotated and anomalous transcripts. J Virol. 2011;85:5897-5909. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 63] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 9. | Guerra S, López-Fernández LA, Pascual-Montano A, Muñoz M, Harshman K, Esteban M. Cellular gene expression survey of vaccinia virus infection of human HeLa cells. J Virol. 2003;77:6493-6506. [PubMed] |
| 10. | Jindal S, Young RA. Vaccinia virus infection induces a stress response that leads to association of Hsp70 with viral proteins. J Virol. 1992;66:5357-5362. [PubMed] |
| 11. | Pavon-Eternod M, David A, Dittmar K, Berglund P, Pan T, Bennink JR, Yewdell JW. Vaccinia and influenza A viruses select rather than adjust tRNAs to optimize translation. Nucleic Acids Res. 2013;41:1914-1921. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 39] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 12. | Senkevich TG, Bugert JJ, Sisler JR, Koonin EV, Darai G, Moss B. Genome sequence of a human tumorigenic poxvirus: prediction of specific host response-evasion genes. Science. 1996;273:813-816. [PubMed] |
| 13. | Xiao A, Wong J, Luo H. Viral interaction with molecular chaperones: role in regulating viral infection. Arch Virol. 2010;155:1021-1031. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 27] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 14. | Bisht H, Brown E, Moss B. Kinetics and intracellular location of intramolecular disulfide bond formation mediated by the cytoplasmic redox system encoded by vaccinia virus. Virology. 2010;398:187-193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 15. | Myskiw C, Arsenio J, Booy EP, Hammett C, Deschambault Y, Gibson SB, Cao J. RNA species generated in vaccinia virus infected cells activate cell type-specific MDA5 or RIG-I dependent interferon gene transcription and PKR dependent apoptosis. Virology. 2011;413:183-193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 30] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 16. | Willis KL, Langland JO, Shisler JL. Viral double-stranded RNAs from vaccinia virus early or intermediate gene transcripts possess PKR activating function, resulting in NF-kappaB activation, when the K1 protein is absent or mutated. J Biol Chem. 2011;286:7765-7778. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 42] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 17. | Ritossa F. A new puffing pattern induced by temperature shock and DNP in drosophila. Experientia. 1962;18:571-573. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1392] [Cited by in RCA: 1206] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 18. | Tissières A, Mitchell HK, Tracy UM. Protein synthesis in salivary glands of Drosophila melanogaster: relation to chromosome puffs. J Mol Biol. 1974;84:389-398. [PubMed] |
| 19. | Mirault ME, Goldschmidt-Clermont M, Moran L, Arrigo AP, Tissières A. The effect of heat shock on gene expression in Drosophila melanogaster. Cold Spring Harb Symp Quant Biol. 1978;42 Pt 2:819-827. [PubMed] |
| 20. | Kampinga HH, Craig EA. The HSP70 chaperone machinery: J proteins as drivers of functional specificity. Nat Rev Mol Cell Biol. 2010;11:579-592. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1420] [Cited by in RCA: 1278] [Article Influence: 85.2] [Reference Citation Analysis (0)] |
| 21. | Arya R, Mallik M, Lakhotia SC. Heat shock genes - integrating cell survival and death. J Biosci. 2007;32:595-610. [PubMed] |
| 22. | Pelham HR. A regulatory upstream promoter element in the Drosophila hsp 70 heat-shock gene. Cell. 1982;30:517-528. [PubMed] |
| 23. | Akerfelt M, Morimoto RI, Sistonen L. Heat shock factors: integrators of cell stress, development and lifespan. Nat Rev Mol Cell Biol. 2010;11:545-555. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1181] [Cited by in RCA: 1042] [Article Influence: 69.5] [Reference Citation Analysis (0)] |
| 24. | Westerheide SD, Anckar J, Stevens SM, Sistonen L, Morimoto RI. Stress-inducible regulation of heat shock factor 1 by the deacetylase SIRT1. Science. 2009;323:1063-1066. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 571] [Cited by in RCA: 554] [Article Influence: 34.6] [Reference Citation Analysis (0)] |
| 25. | Hietakangas V, Ahlskog JK, Jakobsson AM, Hellesuo M, Sahlberg NM, Holmberg CI, Mikhailov A, Palvimo JJ, Pirkkala L, Sistonen L. Phosphorylation of serine 303 is a prerequisite for the stress-inducible SUMO modification of heat shock factor 1. Mol Cell Biol. 2003;23:2953-2968. [PubMed] |
| 26. | Anckar J, Hietakangas V, Denessiouk K, Thiele DJ, Johnson MS, Sistonen L. Inhibition of DNA binding by differential sumoylation of heat shock factors. Mol Cell Biol. 2006;26:955-964. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 86] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 27. | Victor M, Benecke BJ. Expression levels of heat shock factors are not functionally coupled to the rate of expression of heat shock genes. Mol Biol Rep. 1998;25:135-141. [PubMed] |
| 28. | Filone CM, Caballero IS, Dower K, Mendillo ML, Cowley GS, Santagata S, Rozelle DK, Yen J, Rubins KH, Hacohen N. The master regulator of the cellular stress response (HSF1) is critical for orthopoxvirus infection. PLoS Pathog. 2014;10:e1003904. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 39] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 29. | Kowalczyk A, Guzik K, Slezak K, Dziedzic J, Rokita H. Heat shock protein and heat shock factor 1 expression and localization in vaccinia virus infected human monocyte derived macrophages. J Inflamm (Lond). 2005;2:12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 22] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 30. | Mihara M, Hashizume M, Yoshida H, Suzuki M, Shiina M. IL-6/IL-6 receptor system and its role in physiological and pathological conditions. Clin Sci (Lond). 2012;122:143-159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 461] [Cited by in RCA: 627] [Article Influence: 44.8] [Reference Citation Analysis (0)] |
| 31. | Sedger L, Ramshaw I, Condie A, Medveczky J, Braithwaite A, Ruby J. Vaccinia virus replication is independent of cellular HSP72 expression which is induced during virus infection. Virology. 1996;225:423-427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 32. | Sedger L, Ruby J. Heat shock response to vaccinia virus infection. J Virol. 1994;68:4685-4689. [PubMed] |
| 33. | Kotoglou P, Kalaitzakis A, Vezyraki P, Tzavaras T, Michalis LK, Dantzer F, Jung JU, Angelidis C. Hsp70 translocates to the nuclei and nucleoli, binds to XRCC1 and PARP-1, and protects HeLa cells from single-strand DNA breaks. Cell Stress Chaperones. 2009;14:391-406. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 84] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 34. | Dickey CA, Koren J, Zhang YJ, Xu YF, Jinwal UK, Birnbaum MJ, Monks B, Sun M, Cheng JQ, Patterson C. Akt and CHIP coregulate tau degradation through coordinated interactions. Proc Natl Acad Sci USA. 2008;105:3622-3627. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 181] [Cited by in RCA: 171] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 35. | Jacobs AT, Marnett LJ. Systems analysis of protein modification and cellular responses induced by electrophile stress. Acc Chem Res. 2010;43:673-683. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 174] [Cited by in RCA: 182] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 36. | Hung JJ, Chung CS, Chang W. Molecular chaperone Hsp90 is important for vaccinia virus growth in cells. J Virol. 2002;76:1379-1390. [PubMed] |
| 37. | Castro AP, Carvalho TM, Moussatché N, Damaso CR. Redistribution of cyclophilin A to viral factories during vaccinia virus infection and its incorporation into mature particles. J Virol. 2003;77:9052-9068. [PubMed] |
| 38. | Chung CS, Chen CH, Ho MY, Huang CY, Liao CL, Chang W. Vaccinia virus proteome: identification of proteins in vaccinia virus intracellular mature virion particles. J Virol. 2006;80:2127-2140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 207] [Cited by in RCA: 216] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 39. | Fagone P, Jackowski S. Membrane phospholipid synthesis and endoplasmic reticulum function. J Lipid Res. 2009;50 Suppl:S311-S316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 250] [Cited by in RCA: 329] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 40. | Taylor RC, Berendzen KM, Dillin A. Systemic stress signalling: understanding the cell non-autonomous control of proteostasis. Nat Rev Mol Cell Biol. 2014;15:211-217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 138] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 41. | Walter P, Ron D. The unfolded protein response: from stress pathway to homeostatic regulation. Science. 2011;334:1081-1086. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3842] [Cited by in RCA: 4538] [Article Influence: 349.1] [Reference Citation Analysis (0)] |
| 42. | Marciniak SJ, Ron D. Endoplasmic reticulum stress signaling in disease. Physiol Rev. 2006;86:1133-1149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 687] [Cited by in RCA: 749] [Article Influence: 39.4] [Reference Citation Analysis (0)] |
| 43. | Hollien J. Evolution of the unfolded protein response. Biochim Biophys Acta. 2013;1833:2458-2463. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 111] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 44. | Shen J, Snapp EL, Lippincott-Schwartz J, Prywes R. Stable binding of ATF6 to BiP in the endoplasmic reticulum stress response. Mol Cell Biol. 2005;25:921-932. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 178] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 45. | Li J, Ni M, Lee B, Barron E, Hinton DR, Lee AS. The unfolded protein response regulator GRP78/BiP is required for endoplasmic reticulum integrity and stress-induced autophagy in mammalian cells. Cell Death Differ. 2008;15:1460-1471. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 366] [Cited by in RCA: 374] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 46. | Li G, Scull C, Ozcan L, Tabas I. NADPH oxidase links endoplasmic reticulum stress, oxidative stress, and PKR activation to induce apoptosis. J Cell Biol. 2010;191:1113-1125. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 66] [Cited by in RCA: 105] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 47. | Maruri-Avidal L, Weisberg AS, Bisht H, Moss B. Analysis of viral membranes formed in cells infected by a vaccinia virus L2-deletion mutant suggests their origin from the endoplasmic reticulum. J Virol. 2013;87:1861-1871. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 25] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 48. | Maruri-Avidal L, Weisberg AS, Moss B. Vaccinia virus L2 protein associates with the endoplasmic reticulum near the growing edge of crescent precursors of immature virions and stabilizes a subset of viral membrane proteins. J Virol. 2011;85:12431-12441. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 49. | Maruri-Avidal L, Weisberg AS, Moss B. Association of the vaccinia virus A11 protein with the endoplasmic reticulum and crescent precursors of immature virions. J Virol. 2013;87:10195-10206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 50. | Versteeg GA, van de Nes PS, Bredenbeek PJ, Spaan WJ. The coronavirus spike protein induces endoplasmic reticulum stress and upregulation of intracellular chemokine mRNA concentrations. J Virol. 2007;81:10981-10990. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 102] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 51. | Pincus D, Chevalier MW, Aragón T, van Anken E, Vidal SE, El-Samad H, Walter P. BiP binding to the ER-stress sensor Ire1 tunes the homeostatic behavior of the unfolded protein response. PLoS Biol. 2010;8:e1000415. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 300] [Cited by in RCA: 343] [Article Influence: 22.9] [Reference Citation Analysis (0)] |
| 52. | Lisbona F, Rojas-Rivera D, Thielen P, Zamorano S, Todd D, Martinon F, Glavic A, Kress C, Lin JH, Walter P. BAX inhibitor-1 is a negative regulator of the ER stress sensor IRE1alpha. Mol Cell. 2009;33:679-691. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 275] [Cited by in RCA: 275] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 53. | Korennykh AV, Egea PF, Korostelev AA, Finer-Moore J, Stroud RM, Zhang C, Shokat KM, Walter P. Cofactor-mediated conformational control in the bifunctional kinase/RNase Ire1. BMC Biol. 2011;9:48. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 45] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 54. | Korennykh AV, Korostelev AA, Egea PF, Finer-Moore J, Stroud RM, Zhang C, Shokat KM, Walter P. Structural and functional basis for RNA cleavage by Ire1. BMC Biol. 2011;9:47. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 59] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 55. | Hetz C. The biological meaning of the UPR. Nat Rev Mol Cell Biol. 2013;14:404. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 24] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 56. | Calfon M, Zeng H, Urano F, Till JH, Hubbard SR, Harding HP, Clark SG, Ron D. IRE1 couples endoplasmic reticulum load to secretory capacity by processing the XBP-1 mRNA. Nature. 2002;415:92-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2117] [Cited by in RCA: 2230] [Article Influence: 97.0] [Reference Citation Analysis (0)] |
| 57. | Hollien J, Lin JH, Li H, Stevens N, Walter P, Weissman JS. Regulated Ire1-dependent decay of messenger RNAs in mammalian cells. J Cell Biol. 2009;186:323-331. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 839] [Cited by in RCA: 782] [Article Influence: 48.9] [Reference Citation Analysis (0)] |
| 58. | Scheuner D, Kaufman RJ. The unfolded protein response: a pathway that links insulin demand with beta-cell failure and diabetes. Endocr Rev. 2008;29:317-333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 414] [Cited by in RCA: 443] [Article Influence: 26.1] [Reference Citation Analysis (0)] |
| 59. | Mori K. Signalling pathways in the unfolded protein response: development from yeast to mammals. J Biochem. 2009;146:743-750. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 272] [Cited by in RCA: 309] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 60. | Nishitoh H. CHOP is a multifunctional transcription factor in the ER stress response. J Biochem. 2012;151:217-219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 308] [Cited by in RCA: 373] [Article Influence: 26.6] [Reference Citation Analysis (0)] |
| 61. | Liou HC, Boothby MR, Finn PW, Davidon R, Nabavi N, Zeleznik-Le NJ, Ting JP, Glimcher LH. A new member of the leucine zipper class of proteins that binds to the HLA DR alpha promoter. Science. 1990;247:1581-1584. [PubMed] |
| 62. | Yoshida H, Matsui T, Yamamoto A, Okada T, Mori K. XBP1 mRNA is induced by ATF6 and spliced by IRE1 in response to ER stress to produce a highly active transcription factor. Cell. 2001;107:881-891. [PubMed] |
| 63. | Harding HP, Zhang Y, Ron D. Protein translation and folding are coupled by an endoplasmic-reticulum-resident kinase. Nature. 1999;397:271-274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2430] [Cited by in RCA: 2559] [Article Influence: 98.4] [Reference Citation Analysis (0)] |
| 64. | Jackson RJ, Hellen CU, Pestova TV. The mechanism of eukaryotic translation initiation and principles of its regulation. Nat Rev Mol Cell Biol. 2010;11:113-127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1766] [Cited by in RCA: 2033] [Article Influence: 135.5] [Reference Citation Analysis (0)] |
| 65. | Hussain SG, Ramaiah KV. Endoplasmic Reticulum: Stress, signalling and apoptosis. Current Science. 2007;91:1684-1696. |
| 66. | Hussain SG, Ramaiah KV. Reduced eIF2alpha phosphorylation and increased proapoptotic proteins in aging. Biochem Biophys Res Commun. 2007;355:365-370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 119] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 67. | Lee do Y, Lee KS, Lee HJ, Kim do H, Noh YH, Yu K, Jung HY, Lee SH, Lee JY, Youn YC. Activation of PERK signaling attenuates Abeta-mediated ER stress. PLoS One. 2010;5:e10489. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 116] [Cited by in RCA: 134] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 68. | Fontaine KA, Camarda R, Lagunoff M. Vaccinia virus requires glutamine but not glucose for efficient replication. J Virol. 2014;88:4366-4374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 134] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 69. | Teoh ML, Turner PV, Evans DH. Tumorigenic poxviruses up-regulate intracellular superoxide to inhibit apoptosis and promote cell proliferation. J Virol. 2005;79:5799-5811. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 70. | Kawagishi-Kobayashi M, Cao C, Lu J, Ozato K, Dever TE. Pseudosubstrate inhibition of protein kinase PKR by swine pox virus C8L gene product. Virology. 2000;276:424-434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 35] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 71. | Davies MV, Elroy-Stein O, Jagus R, Moss B, Kaufman RJ. The vaccinia virus K3L gene product potentiates translation by inhibiting double-stranded-RNA-activated protein kinase and phosphorylation of the alpha subunit of eukaryotic initiation factor 2. J Virol. 1992;66:1943-1950. [PubMed] |
| 72. | Williams BR. PKR; a sentinel kinase for cellular stress. Oncogene. 1999;18:6112-6120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 627] [Cited by in RCA: 641] [Article Influence: 24.7] [Reference Citation Analysis (0)] |
| 73. | Zhang P, McGrath BC, Reinert J, Olsen DS, Lei L, Gill S, Wek SA, Vattem KM, Wek RC, Kimball SR. The GCN2 eIF2alpha kinase is required for adaptation to amino acid deprivation in mice. Mol Cell Biol. 2002;22:6681-6688. [PubMed] |
| 74. | Miksanova M, Igarashi J, Minami M, Sagami I, Yamauchi S, Kurokawa H, Shimizu T. Characterization of heme-regulated eIF2alpha kinase: roles of the N-terminal domain in the oligomeric state, heme binding, catalysis, and inhibition. Biochemistry. 2006;45:9894-9905. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 51] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 75. | Backes S, Sperling KM, Zwilling J, Gasteiger G, Ludwig H, Kremmer E, Schwantes A, Staib C, Sutter G. Viral host-range factor C7 or K1 is essential for modified vaccinia virus Ankara late gene expression in human and murine cells, irrespective of their capacity to inhibit protein kinase R-mediated phosphorylation of eukaryotic translation initiation factor 2alpha. J Gen Virol. 2010;91:470-482. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 37] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 76. | Meng X, Schoggins J, Rose L, Cao J, Ploss A, Rice CM, Xiang Y. C7L family of poxvirus host range genes inhibits antiviral activities induced by type I interferons and interferon regulatory factor 1. J Virol. 2012;86:4538-4547. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 41] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 77. | Li G, Scull C, Ozcan L, Tabas I. NADPH oxidase links endoplasmic reticulum stress, oxidative stress, and PKR activation to induce apoptosis. J Cell Biol. 2010;191:1113-1125. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 227] [Cited by in RCA: 267] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 78. | Rozelle DK, Filone CM, Kedersha N, Connor JH. Activation of stress response pathways promotes formation of antiviral granules and restricts virus replication. Mol Cell Biol. 2014;34:2003-2016. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 43] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 79. | Nover L, Scharf KD, Neumann D. Cytoplasmic heat shock granules are formed from precursor particles and are associated with a specific set of mRNAs. Mol Cell Biol. 1989;9:1298-1308. [PubMed] |
| 80. | Simpson-Holley M, Kedersha N, Dower K, Rubins KH, Anderson P, Hensley LE, Connor JH. Formation of antiviral cytoplasmic granules during orthopoxvirus infection. J Virol. 2011;85:1581-1593. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 79] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 81. | Anderson P, Kedersha N. RNA granules. J Cell Biol. 2006;172:803-808. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 829] [Cited by in RCA: 886] [Article Influence: 46.6] [Reference Citation Analysis (0)] |
| 82. | Walsh D, Arias C, Perez C, Halladin D, Escandon M, Ueda T, Watanabe-Fukunaga R, Fukunaga R, Mohr I. Eukaryotic translation initiation factor 4F architectural alterations accompany translation initiation factor redistribution in poxvirus-infected cells. Mol Cell Biol. 2008;28:2648-2658. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 90] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 83. | Boone RF, Moss B. Methylated 5’-terminal sequences of vaccinia virus mRNA species made in vivo at early and late times after infection. Virology. 1977;79:67-80. [PubMed] |
| 84. | Martin SA, Moss B. Modification of RNA by mRNA guanylyltransferase and mRNA (guanine-7-)methyltransferase from vaccinia virions. J Biol Chem. 1975;250:9330-9335. [PubMed] |
| 85. | Venkatesan S, Gershowitz A, Moss B. Modification of the 5’ end of mRNA. Association of RNA triphosphatase with the RNA guanylyltransferase-RNA (guanine-7-)methyltransferase complex from vaccinia virus. J Biol Chem. 1980;255:903-908. [PubMed] |
| 86. | Ink BS, Pickup DJ. Vaccinia virus directs the synthesis of early mRNAs containing 5’ poly(A) sequences. Proc Natl Acad Sci USA. 1990;87:1536-1540. [PubMed] |
| 87. | Ahn BY, Moss B. Capped poly(A) leaders of variable lengths at the 5’ ends of vaccinia virus late mRNAs. J Virol. 1989;63:226-232. [PubMed] |
| 88. | Bagnall J, Leedale J, Taylor SE, Spiller DG, White MR, Sharkey KJ, Bearon RN, Sée V. Tight control of hypoxia-inducible factor-α transient dynamics is essential for cell survival in hypoxia. J Biol Chem. 2014;289:5549-5564. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 52] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 89. | Karuppagounder SS, Ratan RR. Hypoxia-inducible factor prolyl hydroxylase inhibition: robust new target or another big bust for stroke therapeutics? J Cereb Blood Flow Metab. 2012;32:1347-1361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 88] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 90. | Rabinowitz MH. Inhibition of hypoxia-inducible factor prolyl hydroxylase domain oxygen sensors: tricking the body into mounting orchestrated survival and repair responses. J Med Chem. 2013;56:9369-9402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 131] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 91. | Buckley DL, Van Molle I, Gareiss PC, Tae HS, Michel J, Noblin DJ, Jorgensen WL, Ciulli A, Crews CM. Targeting the von Hippel-Lindau E3 ubiquitin ligase using small molecules to disrupt the VHL/HIF-1α interaction. J Am Chem Soc. 2012;134:4465-4468. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 278] [Cited by in RCA: 391] [Article Influence: 30.1] [Reference Citation Analysis (0)] |
| 92. | Essers PB, Klasson TD, Pereboom TC, Mans DA, Nicastro M, Boldt K, Giles RH, Macinnes AW. The von Hippel-Lindau tumor suppressor regulates programmed cell death 5-mediated degradation of Mdm2. Oncogene. 2014;Jan 27; Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 93. | Kaelin WG, Ratcliffe PJ. Oxygen sensing by metazoans: the central role of the HIF hydroxylase pathway. Mol Cell. 2008;30:393-402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2073] [Cited by in RCA: 2458] [Article Influence: 144.6] [Reference Citation Analysis (0)] |
| 94. | Simon MC, Keith B. The role of oxygen availability in embryonic development and stem cell function. Nat Rev Mol Cell Biol. 2008;9:285-296. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 770] [Cited by in RCA: 727] [Article Influence: 42.8] [Reference Citation Analysis (0)] |
| 95. | Wang WD, Chen ZT, Li R, Li DZ, Duan YZ, Cao ZH. Enhanced efficacy of radiation-induced gene therapy in mice bearing lung adenocarcinoma xenografts using hypoxia responsive elements. Cancer Sci. 2005;96:918-924. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 96. | Bouquet F, Ousset M, Biard D, Fallone F, Dauvillier S, Frit P, Salles B, Muller C. A DNA-dependent stress response involving DNA-PK occurs in hypoxic cells and contributes to cellular adaptation to hypoxia. J Cell Sci. 2011;124:1943-1951. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 67] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 97. | Fahy AS, Clark RH, Glyde EF, Smith GL. Vaccinia virus protein C16 acts intracellularly to modulate the host response and promote virulence. J Gen Virol. 2008;89:2377-2387. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 44] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 98. | Mazzon M, Peters NE, Loenarz C, Krysztofinska EM, Ember SW, Ferguson BJ, Smith GL. A mechanism for induction of a hypoxic response by vaccinia virus. Proc Natl Acad Sci USA. 2013;110:12444-12449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 68] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 99. | Greseth MD, Traktman P. De novo fatty acid biosynthesis contributes significantly to establishment of a bioenergetically favorable environment for vaccinia virus infection. PLoS Pathog. 2014;10:e1004021. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 78] [Cited by in RCA: 105] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 100. | Chang CW, Li HC, Hsu CF, Chang CY, Lo SY. Increased ATP generation in the host cell is required for efficient vaccinia virus production. J Biomed Sci. 2009;16:80. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 40] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 101. | Murphy MP. How mitochondria produce reactive oxygen species. Biochem J. 2009;417:1-13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6484] [Cited by in RCA: 6093] [Article Influence: 380.8] [Reference Citation Analysis (0)] |
| 102. | Scandalios JG. Oxidative stress: molecular perception and transduction of signals triggering antioxidant gene defenses. Braz J Med Biol Res. 2005;38:995-1014. [PubMed] |
| 103. | D’Autréaux B, Toledano MB. ROS as signalling molecules: mechanisms that generate specificity in ROS homeostasis. Nat Rev Mol Cell Biol. 2007;8:813-824. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2326] [Cited by in RCA: 2572] [Article Influence: 142.9] [Reference Citation Analysis (1)] |
| 104. | Liu N, Lin Z, Guan L, Gaughan G, Lin G. Antioxidant enzymes regulate reactive oxygen species during pod elongation in Pisum sativum and Brassica chinensis. PLoS One. 2014;9:e87588. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 45] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 105. | Teoh ML, Walasek PJ, Evans DH. Leporipoxvirus Cu,Zn-superoxide dismutase (SOD) homologs are catalytically inert decoy proteins that bind copper chaperone for SOD. J Biol Chem. 2003;278:33175-33184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 36] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 106. | Shisler JL, Senkevich TG, Berry MJ, Moss B. Ultraviolet-induced cell death blocked by a selenoprotein from a human dermatotropic poxvirus. Science. 1998;279:102-105. [PubMed] |
| 107. | Senkevich TG, White CL, Koonin EV, Moss B. Complete pathway for protein disulfide bond formation encoded by poxviruses. Proc Natl Acad Sci USA. 2002;99:6667-6672. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 161] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 108. | Senkevich TG, White CL, Weisberg A, Granek JA, Wolffe EJ, Koonin EV, Moss B. Expression of the vaccinia virus A2.5L redox protein is required for virion morphogenesis. Virology. 2002;300:296-303. [PubMed] |
| 109. | White CL, Senkevich TG, Moss B. Vaccinia virus G4L glutaredoxin is an essential intermediate of a cytoplasmic disulfide bond pathway required for virion assembly. J Virol. 2002;76:467-472. [PubMed] |
| 110. | Cadet J, Wagner JR. DNA base damage by reactive oxygen species, oxidizing agents, and UV radiation. Cold Spring Harb Perspect Biol. 2013;5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 506] [Cited by in RCA: 580] [Article Influence: 48.3] [Reference Citation Analysis (0)] |
| 111. | Jena NR. DNA damage by reactive species: Mechanisms, mutation and repair. J Biosci. 2012;37:503-517. [PubMed] |
| 112. | Yuzefovych LV, LeDoux SP, Wilson GL, Rachek LI. Mitochondrial DNA damage via augmented oxidative stress regulates endoplasmic reticulum stress and autophagy: crosstalk, links and signaling. PLoS One. 2013;8:e83349. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 49] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 113. | Yuzefovych LV, Musiyenko SI, Wilson GL, Rachek LI. Mitochondrial DNA damage and dysfunction, and oxidative stress are associated with endoplasmic reticulum stress, protein degradation and apoptosis in high fat diet-induced insulin resistance mice. PLoS One. 2013;8:e54059. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 171] [Cited by in RCA: 207] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 114. | Banerjee G, Gupta N, Kapoor A, Raman G. UV induced bystander signaling leading to apoptosis. Cancer Lett. 2005;223:275-284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 67] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 115. | Jackson SP, Bartek J. The DNA-damage response in human biology and disease. Nature. 2009;461:1071-1078. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4401] [Cited by in RCA: 4390] [Article Influence: 274.4] [Reference Citation Analysis (0)] |
| 116. | Ghosal G, Chen J. DNA damage tolerance: a double-edged sword guarding the genome. Transl Cancer Res. 2013;2:107-129. [PubMed] |
| 117. | Abraham RT. PI 3-kinase related kinases: ‘big’ players in stress-induced signaling pathways. DNA Repair (Amst). 2004;3:883-887. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 356] [Cited by in RCA: 353] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 118. | Lavin MF. Ataxia-telangiectasia: from a rare disorder to a paradigm for cell signalling and cancer. Nat Rev Mol Cell Biol. 2008;9:759-769. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 662] [Cited by in RCA: 670] [Article Influence: 39.4] [Reference Citation Analysis (0)] |
| 119. | Harper JW, Elledge SJ. The DNA damage response: ten years after. Mol Cell. 2007;28:739-745. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1222] [Cited by in RCA: 1284] [Article Influence: 75.5] [Reference Citation Analysis (0)] |
| 120. | Christmann M, Kaina B. Transcriptional regulation of human DNA repair genes following genotoxic stress: trigger mechanisms, inducible responses and genotoxic adaptation. Nucleic Acids Res. 2013;41:8403-8420. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 172] [Cited by in RCA: 184] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 121. | Alkhalil A, Hammamieh R, Hardick J, Ichou MA, Jett M, Ibrahim S. Gene expression profiling of monkeypox virus-infected cells reveals novel interfaces for host-virus interactions. Virol J. 2010;7:173. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 66] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 122. | Wali A, Strayer DS. Infection with vaccinia virus alters regulation of cell cycle progression. DNA Cell Biol. 1999;18:837-843. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 123. | Sancar A, Lindsey-Boltz LA, Unsal-Kaçmaz K, Linn S. Molecular mechanisms of mammalian DNA repair and the DNA damage checkpoints. Annu Rev Biochem. 2004;73:39-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2292] [Cited by in RCA: 2408] [Article Influence: 114.7] [Reference Citation Analysis (0)] |
| 124. | Davis AJ, Chen DJ. DNA double strand break repair via non-homologous end-joining. Transl Cancer Res. 2013;2:130-143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 257] [Reference Citation Analysis (0)] |
| 125. | Ferguson BJ, Mansur DS, Peters NE, Ren H, Smith GL. DNA-PK is a DNA sensor for IRF-3-dependent innate immunity. Elife (Cambridge). 2012;1:e00047. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 259] [Cited by in RCA: 335] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 126. | Peters NE, Ferguson BJ, Mazzon M, Fahy AS, Krysztofinska E, Arribas-Bosacoma R, Pearl LH, Ren H, Smith GL. A mechanism for the inhibition of DNA-PK-mediated DNA sensing by a virus. PLoS Pathog. 2013;9:e1003649. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 99] [Cited by in RCA: 91] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 127. | Ferguson BJ, Benfield CT, Ren H, Lee VH, Frazer GL, Strnadova P, Sumner RP, Smith GL. Vaccinia virus protein N2 is a nuclear IRF3 inhibitor that promotes virulence. J Gen Virol. 2013;94:2070-2081. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 72] [Cited by in RCA: 69] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 128. | Upton C, Stuart DT, McFadden G. Identification of a poxvirus gene encoding a uracil DNA glycosylase. Proc Natl Acad Sci USA. 1993;90:4518-4522. [PubMed] |
| 129. | Stuart DT, Upton C, Higman MA, Niles EG, McFadden G. A poxvirus-encoded uracil DNA glycosylase is essential for virus viability. J Virol. 1993;67:2503-2512. [PubMed] |
| 130. | Shuman S. Vaccinia virus DNA ligase: specificity, fidelity, and inhibition. Biochemistry. 1995;34:16138-16147. [PubMed] |
| 131. | Kerr SM, Smith GL. Vaccinia virus encodes a polypeptide with DNA ligase activity. Nucleic Acids Res. 1989;17:9039-9050. [PubMed] |
| 132. | Gammon DB, Evans DH. The 3’-to-5’ exonuclease activity of vaccinia virus DNA polymerase is essential and plays a role in promoting virus genetic recombination. J Virol. 2009;83:4236-4250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 64] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 133. | Hamilton MD, Evans DH. Enzymatic processing of replication and recombination intermediates by the vaccinia virus DNA polymerase. Nucleic Acids Res. 2005;33:2259-2268. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 22] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 134. | Hamilton MD, Nuara AA, Gammon DB, Buller RM, Evans DH. Duplex strand joining reactions catalyzed by vaccinia virus DNA polymerase. Nucleic Acids Res. 2007;35:143-151. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 55] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 135. | Da Silva M, Shen L, Tcherepanov V, Watson C, Upton C. Predicted function of the vaccinia virus G5R protein. Bioinformatics. 2006;22:2846-2850. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 136. | Senkevich TG, Koonin EV, Moss B. Predicted poxvirus FEN1-like nuclease required for homologous recombination, double-strand break repair and full-size genome formation. Proc Natl Acad Sci USA. 2009;106:17921-17926. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 46] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 137. | Paran N, De Silva FS, Senkevich TG, Moss B. Cellular DNA ligase I is recruited to cytoplasmic vaccinia virus factories and masks the role of the vaccinia ligase in viral DNA replication. Cell Host Microbe. 2009;6:563-569. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 31] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 138. | Leevers SJ, Vanhaesebroeck B, Waterfield MD. Signalling through phosphoinositide 3-kinases: the lipids take centre stage. Curr Opin Cell Biol. 1999;11:219-225. [PubMed] |
| 139. | Akinleye A, Avvaru P, Furqan M, Song Y, Liu D. Phosphatidylinositol 3-kinase (PI3K) inhibitors as cancer therapeutics. J Hematol Oncol. 2013;6:88. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 156] [Cited by in RCA: 203] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 140. | Wang G, Barrett JW, Stanford M, Werden SJ, Johnston JB, Gao X, Sun M, Cheng JQ, McFadden G. Infection of human cancer cells with myxoma virus requires Akt activation via interaction with a viral ankyrin-repeat host range factor. Proc Natl Acad Sci USA. 2006;103:4640-4645. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 156] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 141. | Soares JA, Leite FG, Andrade LG, Torres AA, De Sousa LP, Barcelos LS, Teixeira MM, Ferreira PC, Kroon EG, Souto-Padrón T. Activation of the PI3K/Akt pathway early during vaccinia and cowpox virus infections is required for both host survival and viral replication. J Virol. 2009;83:6883-6899. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 108] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 142. | Winter-Vann AM, Johnson GL. Integrated activation of MAP3Ks balances cell fate in response to stress. J Cell Biochem. 2007;102:848-858. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 49] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 143. | Yang SH, Sharrocks AD, Whitmarsh AJ. MAP kinase signalling cascades and transcriptional regulation. Gene. 2013;513:1-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 295] [Cited by in RCA: 329] [Article Influence: 25.3] [Reference Citation Analysis (0)] |
| 144. | Hirai S, Izawa M, Osada S, Spyrou G, Ohno S. Activation of the JNK pathway by distantly related protein kinases, MEKK and MUK. Oncogene. 1996;12:641-650. [PubMed] |
| 145. | de Magalhães JC, Andrade AA, Silva PN, Sousa LP, Ropert C, Ferreira PC, Kroon EG, Gazzinelli RT, Bonjardim CA. A mitogenic signal triggered at an early stage of vaccinia virus infection: implication of MEK/ERK and protein kinase A in virus multiplication. J Biol Chem. 2001;276:38353-38360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 87] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 146. | Andrade AA, Silva PN, Pereira AC, De Sousa LP, Ferreira PC, Gazzinelli RT, Kroon EG, Ropert C, Bonjardim CA. The vaccinia virus-stimulated mitogen-activated protein kinase (MAPK) pathway is required for virus multiplication. Biochem J. 2004;381:437-446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 121] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 147. | Silva PN, Soares JA, Brasil BS, Nogueira SV, Andrade AA, de Magalhães JC, Bonjardim MB, Ferreira PC, Kroon EG, Bruna-Romero O. Differential role played by the MEK/ERK/EGR-1 pathway in orthopoxviruses vaccinia and cowpox biology. Biochem J. 2006;398:83-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 31] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 148. | Pereira AC, Leite FG, Brasil BS, Soares-Martins JA, Torres AA, Pimenta PF, Souto-Padrón T, Traktman P, Ferreira PC, Kroon EG. A vaccinia virus-driven interplay between the MKK4/7-JNK1/2 pathway and cytoskeleton reorganization. J Virol. 2012;86:172-184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 24] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 149. | Bratke KA, McLysaght A, Rothenburg S. A survey of host range genes in poxvirus genomes. Infect Genet Evol. 2013;14:406-425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 106] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 150. | McLysaght A, Baldi PF, Gaut BS. Extensive gene gain associated with adaptive evolution of poxviruses. Proc Natl Acad Sci USA. 2003;100:15655-15660. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 136] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 151. | Hughes AL, Friedman R. Poxvirus genome evolution by gene gain and loss. Mol Phylogenet Evol. 2005;35:186-195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 95] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 152. | Elde NC, Child SJ, Eickbush MT, Kitzman JO, Rogers KS, Shendure J, Geballe AP, Malik HS. Poxviruses deploy genomic accordions to adapt rapidly against host antiviral defenses. Cell. 2012;150:831-841. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 272] [Cited by in RCA: 256] [Article Influence: 19.7] [Reference Citation Analysis (0)] |
| 153. | De Maio A, Santoro MG, Tanguay RM, Hightower LE. Ferruccio Ritossa’s scientific legacy 50 years after his discovery of the heat shock response: a new view of biology, a new society, and a new journal. Cell Stress Chaperones. 2012;17:139-143. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 43] [Article Influence: 3.3] [Reference Citation Analysis (0)] |