Copyright
©2014 Baishideng Publishing Group Inc.
World J Clin Infect Dis. Nov 25, 2014; 4(4): 27-40
Published online Nov 25, 2014. doi: 10.5495/wjcid.v4.i4.27
Published online Nov 25, 2014. doi: 10.5495/wjcid.v4.i4.27
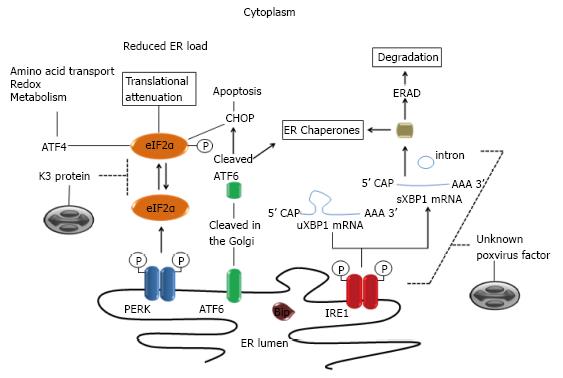
Figure 2 Modulation of mammalian unfolded protein response pathways by poxviruses.
ER stress is sensed by three ER-membrane bound sensors [PERK, ATF6 and Inositol-requiring protein 1 (IRE1)]. Under conditions of ER stress, unfolded proteins accumulate in the ER lumen causing the initiation of a coordinated signaling pathway, the unfolded protein response (UPR), to restore ER homeostasis. ATF6 traffics to the Golgi, where site-specific proteases (S1, S2) cleave it into an active transcription factor. Protein kinase PERK oligomerizes and is activated via trans-autophosphorylation. IRE1 is both a kinase and an endonuclease that splices 26bp from the X-box binding protein 1 (XBP1) mRNA. XBP1 is a transcription factor that regulates positively the expression of many essential UPR genes involved in folding and quality control of proteins. Poxviruses evade XBP1 splicing by an unknown mechanism. Activated PERK phosphorylates eIF2a, resulting in global translational attenuation. However, some mRNA such as ATF4 gains a selective advantage for translation via phosphorylated eIF2. ATF4 in turn contributes to cytoprotection. Expression of other UPR gene targets (e.g., CHOP) may result in cell death. Poxviruses K3L orthologous genes code for proteins that bind to PERK as a pseudosubstrate and thus inhibit eIF2a phosphorylation. ER: Endoplasmic reticulum; PERK: Protein kinase RNA-like ER kinase; eIF2a: Elongation initiation factor 2a; ATF: Activating transcription factor.
- Citation: Leão TL, da Fonseca FG. Subversion of cellular stress responses by poxviruses. World J Clin Infect Dis 2014; 4(4): 27-40
- URL: https://www.wjgnet.com/2220-3176/full/v4/i4/27.htm
- DOI: https://dx.doi.org/10.5495/wjcid.v4.i4.27