Copyright
©2014 Baishideng Publishing Group Inc.
World J Clin Infect Dis. Nov 25, 2014; 4(4): 27-40
Published online Nov 25, 2014. doi: 10.5495/wjcid.v4.i4.27
Published online Nov 25, 2014. doi: 10.5495/wjcid.v4.i4.27
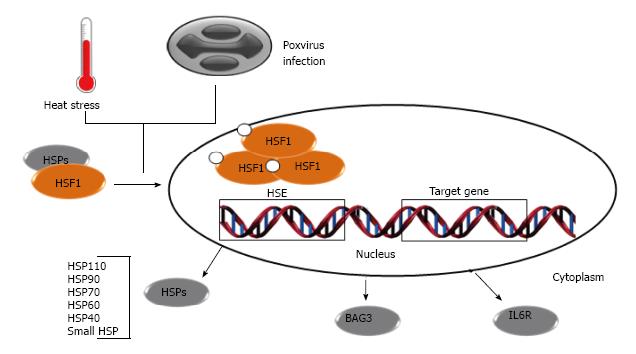
Figure 1 Heat shock responses induced by poxviruses.
Under normal conditions, HSFs interact with HSPs or exist as a monomer in the cytosol. Upon exposure to stress conditions such as heat shock, oxidative stress or poxvirus infection, HSF1 undergoes post-translational modifications, such as phosphorylation, trimerizes and migrates to the nucleus. In the nucleus, HSF1 trimer binds to the HSE, leading to induction of all classes of HSPs and other chaperones. HSE: Heat shock elements; HSFs: Heat shock transcription factors; HSPs: Heat shock proteins.
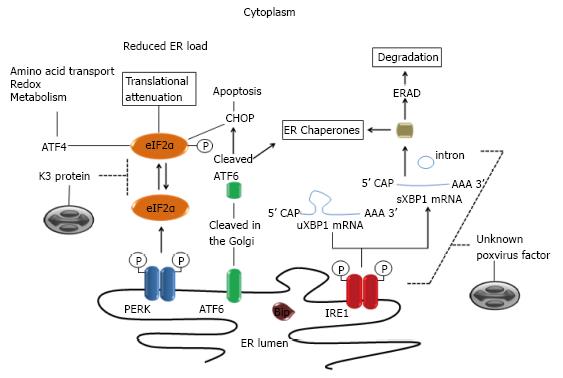
Figure 2 Modulation of mammalian unfolded protein response pathways by poxviruses.
ER stress is sensed by three ER-membrane bound sensors [PERK, ATF6 and Inositol-requiring protein 1 (IRE1)]. Under conditions of ER stress, unfolded proteins accumulate in the ER lumen causing the initiation of a coordinated signaling pathway, the unfolded protein response (UPR), to restore ER homeostasis. ATF6 traffics to the Golgi, where site-specific proteases (S1, S2) cleave it into an active transcription factor. Protein kinase PERK oligomerizes and is activated via trans-autophosphorylation. IRE1 is both a kinase and an endonuclease that splices 26bp from the X-box binding protein 1 (XBP1) mRNA. XBP1 is a transcription factor that regulates positively the expression of many essential UPR genes involved in folding and quality control of proteins. Poxviruses evade XBP1 splicing by an unknown mechanism. Activated PERK phosphorylates eIF2a, resulting in global translational attenuation. However, some mRNA such as ATF4 gains a selective advantage for translation via phosphorylated eIF2. ATF4 in turn contributes to cytoprotection. Expression of other UPR gene targets (e.g., CHOP) may result in cell death. Poxviruses K3L orthologous genes code for proteins that bind to PERK as a pseudosubstrate and thus inhibit eIF2a phosphorylation. ER: Endoplasmic reticulum; PERK: Protein kinase RNA-like ER kinase; eIF2a: Elongation initiation factor 2a; ATF: Activating transcription factor.
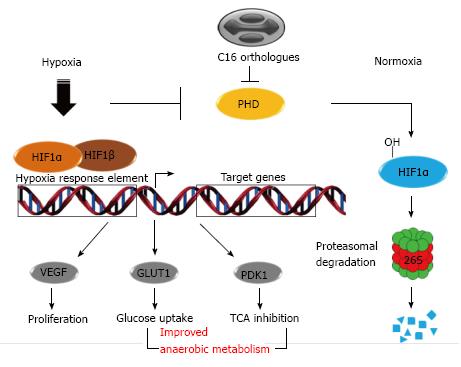
Figure 3 Hypoxic responses in poxviruses infected cells.
Under normal O2 disponibility (normoxia), HIF1α is hydroxylated on proline residues by PHDs. After that, HIF1α is recognized and ubiquitinated by E3 ubiquitin ligase and undergoes proteasomal degradation. Upon an insufficient O2 availability (hypoxia), PHD become inactive and HIF1α forms heterodimers with HIF1β and triggers expression of regulators of TCA, cell proliferation and glucose metabolism. Poxviruses C16L orthologous genes code for proteins that inhibit PHD activities and result in expression of hypoxia target genes under normoxia conditions. HIF1: Hypoxia-inducible factor-1; PHD: Prolyl-hydroxylase domain-containing enzyme; TCA: Tricarboxylic acid cycle; VEGF: Endothelial growth factor; GLUT1: Glucose transporter-1; PDK1: Pyruvate dehydrogenase kinase-1.
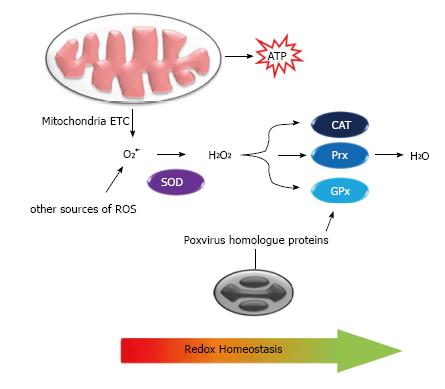
Figure 4 Role of poxvirus proteins in cell redox homeostasis.
ROS are produced during physiological and stress conditions, for instance, during energetic metabolism in the mitochondria, and are detoxified by cellular enzymes (SOD, CAT, Prx, GPx) into water and oxygen. Poxviruses code proteins with homology to SOD, inhibiting the conversion of superoxide into hydrogen peroxide. Furthermore, MC066L gene product is homologous to the human GPx and can protect host cells of peroxide accumulation. ROS: Reactive oxygen species; SOD: Superoxide dismutase; CAT: Catalase; Prx: Peroxiredoxin; GPx: Glutathione peroxidase.
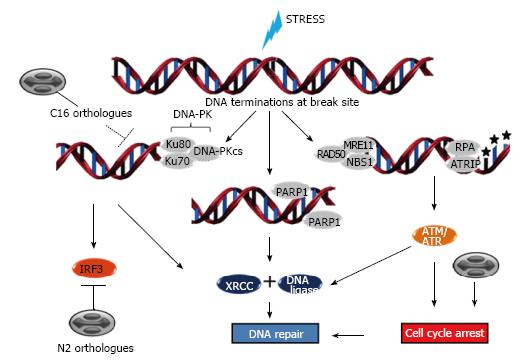
Figure 5 DNA damage responses and poxvirus infections.
DNA breaks may be caused by many different sources. At sites of DNA double strand breaks (DSBs), DNA-PK is recruited by Ku proteins and induces DNA repair through XRCC4 and DNA ligase 4; DSBs also lead to the activation of the major interferon regulatory factor, IRF3. Upon DSBs occurrence, ataxia telangiectasia mutated (ATM) is recruited by the MRE-11-Rad50-NBS1 (MRN) complex to sites of broken DNA where they induce repair by XRCC2/3 and DNA ligase 1. ATM also controls cell cycle arrest which facilitates proper function of the DNA repair mechanisms. Upon single strand breaks, ataxia telangiectasia and Rad3 related (ATR) or poly (ADP-ribose) polymerase 1 (PARP1) are recruited to lesions sites and are activated, resulting in phosphorylation of downstream substrates, control of cell cycle arrest and/or repair of DNA lesions by XRCC1 and DNA ligase 3. Poxvirus infections affect cell cycle progression arresting cells in G2 phase. They also encode C16 orthologues that bind to Ku70, blocking DNA-PK recruitment to broken DNA sites, and N2 orthologues, that inhibit IRF3-dependent innate immune responses. DNA-PK: DNA-dependent protein kinase; DNA-PKcs: DNA-dependent protein kinase catalytic subunit; RPA: Replication protein A.
- Citation: Leão TL, da Fonseca FG. Subversion of cellular stress responses by poxviruses. World J Clin Infect Dis 2014; 4(4): 27-40
- URL: https://www.wjgnet.com/2220-3176/full/v4/i4/27.htm
- DOI: https://dx.doi.org/10.5495/wjcid.v4.i4.27