Revised: December 12, 2013
Accepted: January 15, 2014
Published online: February 23, 2014
Processing time: 229 Days and 23.2 Hours
AIM: To investigate the effects of nicotine and nicotine plus angiotensin II receptor blocker (ARB) on the gene expression profile of human coronary artery endothelial cells (HCAECs).
METHODS: The changes in gene expression profiles in HCAECs treated with nicotine and nicotine plus ARB olmesartan were analyzed by DNA microarray. In nicotine-treated HCAECs, 432 genes selected by P < 0.01 were greater than 1.5-fold compared with the untreated cells. Data were analyzed using IPA (Ingenuity® Systems, www.ingenuity.com).
RESULTS: The gene expression levels of tumor necrosis factor-α, collagen type 1, matrix metalloproteinase-10, and disintegrin and metalloprotease domain 8, which are related to “cardiovascular function and disease”, were significantly increased. In canonical pathway analyses using IPA, “atherosclerosis signaling” was strongly affected by nicotine treatment and this effect was reduced by co-incubation with ARB olmesartan. These data indicate that the deleterious cardiovascular consequences of cigarette smoking may, at least in part, be due to the nicotine-induced gene expression profile related to “atherosclerosis signaling”.
CONCLUSION: The inhibitory effect of ARB against the nicotine-induced gene expression profile may possibly induce anti-atherosclerotic effects that are independent of those from lowering the blood pressure.
Core tip: Tobacco smoking is well known as one of the major risk factors for coronary heart disease and nicotine is recognized as the main addictive component. We investigate the effects of nicotine and nicotine plus angiotensin II receptor blocker (ARB) on the gene expression profile of human coronary artery endothelial cells. Nicotine induced changes in the gene expression profile related to “atherosclerosis signaling” may increase the risk for the initiation and progression of atherosclerosis. The inhibitory effect of ARB against the nicotine-induced gene expression profile may possibly induce anti-atherosclerotic effects against tobacco smoking independently of the lowering of the blood pressure.
- Citation: Kudo M, Matsuda K, Sugawara K, Iki Y, Kogure N, Saito-Ito T, Shimizu K, Sato I, Yoshikawa T, Uruno A, Ito R, Yokoyama A, Saito-Hakoda A, Ito S, Sugawara A. ARB affects nicotine-induced gene expression profile in human coronary artery endothelial cells. World J Hypertens 2014; 4(1): 7-14
- URL: https://www.wjgnet.com/2220-3168/full/v4/i1/7.htm
- DOI: https://dx.doi.org/10.5494/wjh.v4.i1.7
Tobacco smoking is well known as one of the major risk factors for coronary heart disease[1-6]. Tobacco smoke is composed of a mixture of thousands of different molecules and nicotine is recognized as the main addictive component[7-10]. Although nicotine is known to induce vascular endothelial dysfunction/atherosclerosis[11,12], its molecular mechanism(s) in terms of gene transcription regulation remain unknown. In 2001, the effects of nicotine on the gene expression in human coronary artery endothelial cells (HCAECs) were examined by DNA microarray analyses[13]. However, due to technical limitations at that time, only 4000 genes were analyzed. We therefore re-examined the effects of nicotine on the gene expression profile of HCAECs using DNA microarray analyses that cover the whole human genome and observed that nicotine strongly affected the gene expression profiles related to “atherosclerosis signaling”. Moreover, we also examined the effects of an angiotensin II receptor blocker (ARB), olmesartan, on the nicotine-induced gene expression profile and observed its inhibitory effects against nicotine in terms of “atherosclerosis signaling”.
Nicotine was purchased from Sigma (St. Louis, MO). Olmesartan medoxomil (olmesartan) was purchased from Toronto Research Chemicals (North York, Canada).
HCAECs were obtained from Lonza (Walkersville, MD) and were grown in EGM-2MV medium. Cells were passaged in a 1:3 dilution and the cells from passages 3-4 were used for experiments.
When HCAECs were grown to approximately 90% confluence (during the exponential phase), the cells were treated either with 100 μmol/L nicotine alone or with 100 μmol/L nicotine plus 10 μmol/L olmesartan for 24 h.
Total RNA of HCAECs was extracted using TaKaRa FastPure RNA Kit (Takara Bio, Ohtsu, Japan) according to the manufacturer’s instructions. Trace genomic DNA in the crude total RNA samples was removed by incubation with 4-10 units DNase I per 100 μg total RNA (Takara Bio, Ohtsu, Japan) at 37 °C for 15 min. The concentration of the total RNA was determined with a NanoDrop ND-1000 spectrophotometer (NanoDrop Technologies, Rockland, DE) and the RNA integrity was verified with a Bioanalyzer 1000 (Agilent, Palo Alto, CA).
The generated cDNA was fragmented and biotin labeled using a Low Input Quick Amp Labeling kit (Agilent). Fragmented biotin-labeled cDNA was hybridized to an individual Human Gene Expression 4 × 44K v2 Microarray Kit (Agilent, Palo Alto, CA). The arrays were thereafter washed, stained and scanned under the low photo multiplier tube setting. Quality metric parameters including noise level, background and the efficiency of transcription were ascertained for all hybridizations. The array results were normalized by GeneSpring 12 (Agilent) to identify differentially expressed genes and associated fold changes. Data cleansing was performed to remove the values for which the fluorescent intensity was too low compared with negative controls. In each (nicotine, nicotine plus olmesartan) group (n = 4), the genes that demonstrated significant differences compared with an untreated group were selected by the Welch t test. To limit the number of false positives, only genes whose P value was less than 0.01 and whose change was greater than 1.5 fold were selected.
Data were analyzed using IPA (Ingenuity® Systems, http://www.ingenuity.com). A data set containing gene identifiers and corresponding expression values was uploaded into the application. Each gene identifier was mapped to its corresponding gene object in the Ingenuity Pathways Knowledge Base. These genes, called Focus Genes, were overlaid onto a global molecular network developed from information contained in the Ingenuity Pathways Knowledge Base. Networks of these Focus Genes were then algorithmically generated based on their connectivity.
A network pathway is a graphical representation of the molecular relationships between genes/gene products. Genes or gene products are represented as nodes and the biological relationship between two nodes is represented as an edge (line). All edges are supported by at least one reference from the literature, from a textbook, or from canonical information stored in the Ingenuity Pathways Knowledge Base. The intensity of the node color indicates the degree of up- (red) or down- (green) regulation.
Analyses of canonical pathways identified the pathways from the IPA library of canonical pathways that were most significant to the data set. Genes from the data set that were associated with a canonical pathway in the Ingenuity Pathways Knowledge Base were considered for the analysis. The significance of the association between the data set and the canonical pathway was measured in two ways: (1) The ratio of the number of genes from the data set that map to the pathway divided by the total number of genes that map to the canonical pathway is displayed; and (2) Fischer’s exact test was used to calculate the P value determining the probability that the association between the genes in the data set and the canonical pathway can be explained by chance alone.
After normalizing and cleansing the data, there were 34183 genes to be analyzed in the HCAECs. In the nicotine treated HCAECs, 432 genes satisfying the criteria P < 0.01 and greater than 1.5 fold compared with untreated cells were selected. There were 287 genes up-regulated and 145 genes down-regulated. Among these selected genes, the top ten genes up-regulated or down-regulated by nicotine treatment in HCAECs were detected by IPA and are listed in Table 1.
| Symbol | Gene name | Expression change |
| KCNQ1 | Potassium voltage-gated channel, KQT-like subfamily, member 1 | 6.09 |
| MYCN | V-myc myelocytomatosis viral related oncogene, neuroblastoma derived | 5.761 |
| RRAGD | Ras-related GTP binding D | 4.56 |
| TNNI1 | Troponin I type 1 | 3.933 |
| FCN1 | Ficolin 1 (collagen/fibrinogen domain containing) | 3.866 |
| C1orf204 | Chromosome 1 open reading frame 204 | 3.792 |
| PCDHGC4 | Protocadherin gamma subfamily C, 4 | 3.657 |
| C14orf165 | Chromosome 14 open reading frame 165 | 3.461 |
| TLR2 | Toll-like receptor 2 | 3.443 |
| RCAN2 | Regulator of calcineurin 2 | 3.431 |
| LAIR1 | Leukocyte-associated immunoglobulin-like receptor 1 | -5.014 |
| SIX3 | SIX homeobox 3 | -4.926 |
| MYO1A | Myosin IA | -4.824 |
| PRTN3 | Proteinase 3 | -4.173 |
| MRGPRE | MAS-related GPR, member E | -3.914 |
| EGR2 | Early growth response 2 | -3.766 |
| CASQ1 | Calsequestrin 1 | -3.587 |
| CLEC12B | C-type lectin domain family 12, member B | -3.338 |
| ALPI | Alkaline phosphatase, intestinal | -3.033 |
| SLC7A14 | Solute carrier family 7, member 14 | -2.941 |
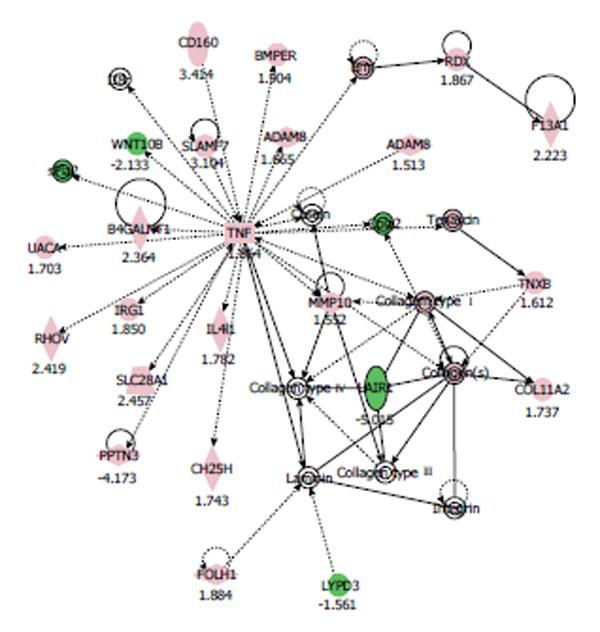
In the nicotine-treated HCAECs, IPA generated the networks listed in Table 2. Among these networks, we focused on the ‘‘tissue development, cardiovascular system development and function, cell-to-cell signaling and interaction” network, since its score was the highest (40 score). The pathway network of gene expression in HCAECs treated with 100 μmol/L nicotine is shown in Figure 1. In this network, genes involved in cardiovascular diseases, including tumor necrosis factor (TNF)-α, collagen type 1, and matrix metalloproteinase (MMP)-10, and a disintegrin and metalloprotease domain 8 (ADAM8) were up-regulated.
| Associated network functions | Score |
| Tissue development, cardiovascular system development and function, cell-to-cell signaling and interaction | 40 |
| Cellular movement, immune cell trafficking, inflammatory response | 32 |
| Cellular growth and proliferation, cellular movement, cellular development | 30 |
| Cellular growth and proliferation, carbohydrate metabolism, molecular transport | 27 |
| Antimicrobial response, humoral immune response, cell-to-cell signaling and interaction | 24 |
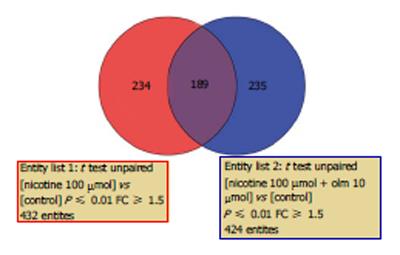
In HCAECs treated with nicotine plus olmesartan, 424 genes were selected. When the two data sets were filtered and compared, there were a total of 667 genes in the two data sets for comparison; 189 genes were common in the two sets, 243 genes were unique in the nicotine-treated HCAECs, and 235 genes were unique in nicotine plus olmesartan-treated HCAECs (Figure 2).
When comparing two data sets using Global Canonical Pathway analyses, the significance calculated for each canonical pathway is a measurement of the likelihood that the pathway is associated with the dysregulated genes by random chance. Figure 3 shows the top 10 canonical pathways that were significantly regulated by nicotine treatment and a comparison with nicotine plus olmesartan treatment is shown side by side. Nicotine thus induced a significant change in the gene expression profile related to the signaling pathway of atherosclerosis and cell proliferation, while ARB olmesartan appeared to significantly antagonize the cell response to nicotine treatment.
We therefore analyzed their effects on the “atherosclerosis signaling”. Graphical representation of “atherosclerosis signaling” is shown in Figures 4A and 4B for comparison. In this pathway, the genes that play central roles in response to nicotine treatment are TNF-α and collagen type 1. These factors were significantly up-regulated when HCAECs were treated with nicotine (Figure 4A). However, when the cells were treated with nicotine plus olmesartan, their up-regulation was abrogated (Figure 4B). Olmesartan thus altered the response of HCAECs to nicotine in terms of gene expression regulation.
Our study using DNA microarray analyses revealed that the gene expression levels of TNF-α, collagen type 1, MMP-10 and ADAM8, which are related to “cardiovascular function and disease”, were significantly increased in HCAECs treated with nicotine. In canonical pathway analyses using IPA, “atherosclerosis signaling” was most strongly affected by nicotine treatment and this effect was reduced by co-incubation with olmesartan.
The most well-established receptors for nicotine are nicotinic acetylcholine receptors (nAChRs) that belong to a family of neurotransmitter-gated ion channels. These receptors are ubiquitously expressed in almost all cell types in blood vessels[14,15]. Nicotine stimulation is thought to activate nAChRs, resulting in the induction of angiogenesis and atherogenesis[16-19]. It has also been reported that nicotine induces the gene expression of cell-cell adhesion molecules such as monocyte chemotactic protein-1 and MMPs in different cell types[20-24]. In the present study, we have newly identified that various genes associated with “cellular growth and proliferation, cellular movement and cellular development” are regulated by nicotine stimulation. In addition, we have also observed that ARB (olmesartan) inhibits the gene expression profile regulated by nicotine stimulation. It has previously been reported that the renin-angiotensin system does not contribute to smoking-induced endothelial dysfunction in vivo[25]. However, it has also been reported that perinatal nicotine exposure epigenetically represses type 2 angiotensin II receptor gene in the developing brain[26]. Our present data therefore suggest that ARB may play an important role in reducing the deleterious effects of cigarette smoking on endothelial function, independently of its blood pressure lowering effect.
In summary, we have here indicated that the deleterious cardiovascular consequences of cigarette smoking may, at least in part, be due to the nicotine-induced changes in the gene expression profile related to “atherosclerosis signaling” that may increase the risk for the initiation and progression of atherosclerosis. The inhibitory effect of ARB against the nicotine-induced gene expression profile may possibly induce anti-atherosclerotic effects against tobacco smoking independently of the lowering of the blood pressure. Future studies will be needed to confirm whether ARB might help decrease the incidence of cardiovascular events in smokers.
Tobacco smoking is well known as one of the major risk factors for coronary heart disease. Nicotine is recognized as the main addictive component and it is known to induce vascular endothelial dysfunction/atherosclerosis.
Nicotine stimulation is thought to activate nicotinic acetylcholine receptors (nAChRs), resulting in the induction of angiogenesis and atherogenesis. It has also been reported that nicotine induces the gene expression of cell-cell adhesion molecules such as monocyte chemotactic protein-1 and matrix metalloproteinases in different cell types. Nevertheless, its molecular mechanism(s) in terms of gene transcription regulation remain unknown.
The effects of nicotine on the gene expression in human coronary artery endothelial cells (HCAECs) were previously examined by DNA microarray analyses. However, due to technical limitations at that time, only 4000 genes were analyzed. The authors therefore re-examined the effects of nicotine on the gene expression profile of HCAECs using DNA microarray analyses that cover the whole human genome and, moreover, they also examined the effects of an angiotensin II receptor blocker (ARB). They newly determined that “atherosclerosis signaling” was strongly affected by nicotine treatment and this effect was reduced by co-incubation with ARB using pathway analysis.
The inhibitory effect of ARB against the nicotine-induced gene expression profile may possibly induce anti-atherosclerotic effects against tobacco smoking independently of the lowering of the blood pressure. ARB might help decrease the incidence of cardiovascular events in smokers.
ARB is an antihypertensive medication most frequently used. nAChRs is the most well-established receptors for nicotine that belong to a family of neurotransmitter-gated ion channels. These receptors are ubiquitously expressed in almost all cell types in blood vessels.
The authors demonstrate that the vascular endothelial dysfunction/atherosclerosis induced by smoking may be due to the nicotine-induced changes in the gene expression profile related to “atherosclerosis signaling” and that the inhibitory effect of ARB against the nicotine-induced gene expression profile may induce anti-atherosclerotic effects independently of blood pressure.
P- Reviewers: Peteiro J, Ueda H S- Editor: Gou SX L- Editor: Roemmele A E- Editor: Liu SQ
| 1. | Teo KK, Ounpuu S, Hawken S, Pandey MR, Valentin V, Hunt D, Diaz R, Rashed W, Freeman R, Jiang L. Tobacco use and risk of myocardial infarction in 52 countries in the INTERHEART study: a case-control study. Lancet. 2006;368:647-658. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 626] [Cited by in RCA: 614] [Article Influence: 32.3] [Reference Citation Analysis (0)] |
| 2. | Erhardt L. Cigarette smoking: an undertreated risk factor for cardiovascular disease. Atherosclerosis. 2009;205:23-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 183] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 3. | Benowitz NL. Nicotine and coronary heart disease. Trends Cardiovasc Med. 1991;1:315-321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 47] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 4. | Salahuddin S, Prabhakaran D, Roy A. Pathophysiological mechanisms of tobacco-related CVD. Global Heart. 2012;7:113-120. [RCA] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 49] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 5. | Piano MR, Benowitz NL, Fitzgerald GA, Corbridge S, Heath J, Hahn E, Pechacek TF, Howard G. Impact of smokeless tobacco products on cardiovascular disease: implications for policy, prevention, and treatment: a policy statement from the American Heart Association. Circulation. 2010;122:1520-1544. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 180] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 6. | Rudolph TK, Rudolph V, Baldus S. Contribution of myeloperoxidase to smoking-dependent vascular inflammation. Proc Am Thorac Soc. 2008;5:820-823. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 33] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 7. | Raupach T, Hoogsteder PH, Onno van Schayck CP. Nicotine vaccines to assist with smoking cessation: current status of research. Drugs. 2012;72:e1-16. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 55] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 8. | Landini L, Leone A. Smoking and hypertension: effects on clinical, biochemical and pathological variables due to isolated or combined action on cardiovascular system. Curr Pharm Des. 2011;17:2987-3001. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 20] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 9. | Balakumar P, Kaur J. Is nicotine a key player or spectator in the induction and progression of cardiovascular disorders. Pharmacol Res. 2009;60:361-368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 55] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 10. | Gaemperli O, Liga R, Bhamra-Ariza P, Rimoldi O. Nicotine addiction and coronary artery disease: impact of cessation interventions. Curr Pharm Des. 2010;16:2586-2597. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 11. | Adamopoulos D, van de Borne P, Argacha JF. New insights into the sympathetic, endothelial and coronary effects of nicotine. Clin Exp Pharmacol Physiol. 2008;35:458-463. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 46] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 12. | Toda N, Toda H. Nitric oxide-mediated blood flow regulation as affected by smoking and nicotine. Eur J Pharmacol. 2010;649:1-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 75] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 13. | Zhang S, Day IN, Ye S. Microarray analysis of nicotine-induced changes in gene expression in endothelial cells. Physiol Genomics. 2001;5:187-192. [PubMed] |
| 14. | Moccia F, Frost C, Berra-Romani R, Tanzi F, Adams DJ. Expression and function of neuronal nicotinic ACh receptors in rat microvascular endothelial cells. Am J Physiol Heart Circ Physiol. 2004;286:H486-H491. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 49] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 15. | Lam DC, Girard L, Ramirez R, Chau WS, Suen WS, Sheridan S, Tin VP, Chung LP, Wong MP, Shay JW. Expression of nicotinic acetylcholine receptor subunit genes in non-small-cell lung cancer reveals differences between smokers and nonsmokers. Cancer Res. 2007;67:4638-4647. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 169] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 16. | Arias HR, Richards VE, Ng D, Ghafoori ME, Le V, Mousa SA. Role of non-neuronal nicotinic acetylcholine receptors in angiogenesis. Int J Biochem Cell Biol. 2009;41:1441-1451. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 77] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 17. | Yu J, Huang NF, Wilson KD, Velotta JB, Huang M, Li Z, Lee A, Robbins RC, Cooke JP, Wu JC. nAChRs mediate human embryonic stem cell-derived endothelial cells: proliferation, apoptosis, and angiogenesis. PLoS One. 2009;4:e7040. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 49] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 18. | Santanam N, Thornhill BA, Lau JK, Crabtree CM, Cook CR, Brown KC, Dasgupta P. Nicotinic acetylcholine receptor signaling in atherogenesis. Atherosclerosis. 2012;225:264-273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 34] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 19. | Dasgupta P, Rastogi S, Pillai S, Ordonez-Ercan D, Morris M, Haura E, Chellappan S. Nicotine induces cell proliferation by beta-arrestin-mediated activation of Src and Rb-Raf-1 pathways. J Clin Invest. 2006;116:2208-2217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 221] [Cited by in RCA: 257] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 20. | Katono T, Kawato T, Tanabe N, Suzuki N, Yamanaka K, Oka H, Motohashi M, Maeno M. Nicotine treatment induces expression of matrix metalloproteinases in human osteoblastic Saos-2 cells. Acta Biochim Biophys Sin (Shanghai). 2006;38:874-882. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 29] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 21. | Patton GW, Powell DA, Hakki A, Friedman H, Pross S. Nicotine modulation of cytokine induction by LPS-stimulated human monocytes and coronary artery endothelial cells. Int Immunopharmacol. 2006;6:26-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 22] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 22. | Dom AM, Buckley AW, Brown KC, Egleton RD, Marcelo AJ, Proper NA, Weller DE, Shah YH, Lau JK, Dasgupta P. The α7-nicotinic acetylcholine receptor and MMP-2/-9 pathway mediate the proangiogenic effect of nicotine in human retinal endothelial cells. Invest Ophthalmol Vis Sci. 2011;52:4428-4438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 31] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 23. | Ko JK, Cho CH. The diverse actions of nicotine and different extracted fractions from tobacco smoke against hapten-induced colitis in rats. Toxicol Sci. 2005;87:285-295. [PubMed] |
| 24. | Suñer IJ, Espinosa-Heidmann DG, Marin-Castano ME, Hernandez EP, Pereira-Simon S, Cousins SW. Nicotine increases size and severity of experimental choroidal neovascularization. Invest Ophthalmol Vis Sci. 2004;45:311-317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 106] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 25. | Zhu BQ, Sievers RE, Browne AE, Hillman RT, Chair K, Lee RJ, Chatterjee K, Glantz SA, Parmley WW. The renin-angiotensin system does not contribute to the endothelial dysfunction and increased infarct size in rats exposed to second hand smoke. J Renin Angiotensin Aldosterone Syst. 2002;3:54-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 26. | Li Y, Xiao D, Dasgupta C, Xiong F, Tong W, Yang S, Zhang L. Perinatal nicotine exposure increases vulnerability of hypoxic-ischemic brain injury in neonatal rats: role of angiotensin II receptors. Stroke. 2012;43:2483-2490. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 60] [Article Influence: 4.6] [Reference Citation Analysis (0)] |