Published online May 20, 2015. doi: 10.5493/wjem.v5.i2.40
Peer-review started: December 6, 2014
First decision: January 20, 2015
Revised: March 18, 2015
Accepted: April 10, 2015
Article in press: April 12, 2015
Published online: May 20, 2015
Processing time: 166 Days and 2.1 Hours
Endothelial cells (ECs) are essential for pancreas differentiation, endocrine specification, and endocrine function. They are also involved in the physiopathology of type 1 and type 2 diabetes. During embryogenesis, aortic ECs provide specific factors that maintain the expression of key genes for pancreas development such as pancreatic and duodenal homeobox-1. Other unknown factors are also important for pancreatic endocrine specification and formation of insulin-producing beta cells. Endocrine precursors proliferate interspersed with ductal cells and exocrine precursors and, at some point of development, these endocrine precursors migrate to pancreatic mesenchyme and start forming the islets of Langerhans. By the end of the gestation and close to birth, these islets contain immature beta cells with the capacity to express vascular endothelial growth factor and therefore to recruit ECs from the surrounding microenvironment. ECs in turn produce factors that are essential to maintain insulin secretion in pancreatic beta cells. Once assembled, a cross talk between endocrine cells and ECs maintain the integrity of islets toward an adequate function during the whole life of the adult individual. This review will focus in the EC role in the differentiation and maturation of pancreatic beta cells during embryogenesis as well as the current knowledge about the involvement of endothelium to derive pancreatic beta cells in vitro from mouse or human pluripotent stem cells.
Core tip: Many studies have demonstrated that endothelial cells (ECs) have an important role in organogenesis. For instance, during embryogenesis, aortic ECs provide specific factors that maintain the expression of key genes for pancreas development. Other unknown factors are also important for pancreatic endocrine specification and formation of insulin-producing beta cells. In addition, by the end of the gestation and close to birth, pancreatic islets contain immature beta cells with the capacity to express factors that recruit ECs from the surrounding microenvironment and form a functional unit that will lasts for the whole life of the individual. In the present review, we will analyze the current endothelial-derived factors called angiocrine factors that are essential in organogenesis and we will focus the role of these factors in pancreas development and pancreatic beta cells.
- Citation: Talavera-Adame D, Dafoe DC. Endothelium-derived essential signals involved in pancreas organogenesis. World J Exp Med 2015; 5(2): 40-49
- URL: https://www.wjgnet.com/2220-315X/full/v5/i2/40.htm
- DOI: https://dx.doi.org/10.5493/wjem.v5.i2.40
At present, insulin producing cells have been derived from different sources[1-3]. With the emergence of embryonic stem cells (ESCs) and induced pluripotent stem cells (iPSCs), that can be plated in vitro, the potential production of several cell types including pancreas can be now achieved[2,4-11]. Endothelial cells (ECs) are present at early stages during embryogenesis[12-15]. These cells are the main component of most niches[16]. Therefore, ECs interact early with developing tissues during organogenesis even before they are able to form blood vessels and nourish specific regions[16-19]. It has been reported that the absence of ECs results in agenesis of some organs such as pancreas[20,21]. This fact points out the essential role of EC signaling during organogenesis. Apparently ECs are not only involved in pancreas differentiation, they are essential for endocrine differentiation as well[20]. Endocrine progenitors give rise to immature beta cells that recruit ECs after expression of vascular endothelial cell growth factor (VEGF)[18,20]. These ECs in turn provide factors to promote beta-cell maturation and stabilize β-cell function. In this review, we will be focusing in the role of ECs in the differentiation and maturation of beta cells in vivo and in vitro with the emerging technology of human PSCs that can be expanded in vitro.
Extensive studies with ECs have demonstrated that these cells play essential roles in immunity, inflammation, angiogenesis, and tumor metastasis. ECs do not only line the interior surface of blood vessels[22,23]. ECs are found at the interface between blood and other cell types. They not only maintain the blood fluid but also have a great plasticity that allows these cells to accomplish several essential functions to maintain homeostasis[18]. However, recent studies demonstrated that the endothelium is capable of releasing growth factors and cytokines that play an essential role in organogenesis[24] (Table 1). The term angiocrine has been proposed by Butler and collaborators to indicate the capacity of ECs to release growth factors and cytokines that may be involved in organogenesis[25]. For instance, bone marrow sinusoidal ECs (SECs) promote differentiation of hematopoietic stem cells (HSCs) through “angiocrine factors” such as hepatocyte growth factor (HGF), Wnt2, and Notch[25,26]. Additionally, Butler and colleagues reported that ECs promote self-renewal of hematopoietic stem cells when these cells are interacting in co-culture[25]. Apparently, Notch ligands expressed by ECs are associated with this response[25]. Another studies indicate that ECs from liver, called sinusoidal ECs (LSECs), express factors such as VE-cadherin, Factor VIII and vascular endothelial growth factors 2 and 3 (VEGF2, VEGF3) and these cells release angiocrine factors that may be involved in liver regeneration[26]. In another work it has been described that angiocrine factors are able to regulate tumor growth[27]. Other studies demonstrated that mice deficient in FLK-1 (VEGF receptor), die at embryonic day E9.5 or E10.5 because immaturity of blood cells and blood vessels[28]. Absence of embryonic liver budding is also present in these mice indicating that ECs play an important role during the early phases of organogenesis. In a similar work, it has been observed that ECs are also involved during the early stages of pancreas development[20]. Another research work indicated that signals from myocardium, such as those exerted by bone morphogenetic protein-2 (BMP-2), can promote epithelial-mesenchymal transformation mediated by ECs[24,29]. In the kidney, VEGF, bFGF, and PDGF coordinate cellular differentiation, proliferation, and migration[30]. It has been suggested that ECs promote the differentiation of endoderm cells toward liver or pancreas through secretion of HGF[17,19,24,28,31,32] (Table 1). At the same time, reciprocal interactions between tissue-specific cell types and ECs ensure coordinated growth and adequate tissue function. For instance, it is known that neurogenesis takes place close to blood vessels in adult brains[33]. Additionally, brain-derived neurotrophic factor (BDNF) secreted by ECs promotes neurogenesis and angiogenesis in the brain of song birds[34]. Another report indicates that pigment epithelium-derived factor (PEDF) is secreted by ECs and enhances self-renewal of neural stem cells (NSCs)[35]. Regarding the pancreas, it has been shown that pancreatic endoderm attract endothelial progenitor cells (EPCs) or angioblasts by expression of SDF-1/CXCL12[36]. Expression of pancreatic and duodenal homeobox 1 (PDX-1) appeared in endoderm cells in contact with angioblasts via LIM domain only 2 (LMO2) suggesting that angioblasts may induce expression of PDX-1[37]. Functional blood vessels may induce differentiation before they carry blood. However, some blood factors such as sphingosine-1-phosphate (SIP) are important for differentiation and maturation[38]. For instance, it has also been described that beta-cell differentiation can be regulated by oxygen tension via hypoxia-inducible factor 1 alpha (HIF-1α)[39]. This fact suggests that blood factors can also be involved in the complete differentiation and maturation of pancreatic endocrine cells.
| Angiocrine factors | Source | Target | Ref |
| Notch ligands (1 and 2) | Bone marrow ECs; brain ECs | Neural progenitors and HSCs | [40,41] |
| VEGF | ECs | ECs and several tissues such as islets | [12,42,43] |
| bFGF | ECs | ECs and several tissues such as islets | [43] |
| PDGF | Brain capillaries | ECs | [42] |
| HGF | Lunga capillaries, SECs, | Lung epithelium, hepatocytes, islet beta cells | [26,31,44,45] |
| islet capillaries | |||
| Endothelins | ECs | Lung, neural cells | [46] |
| EG-VEGF | ECs | Endocrine glands | [32] |
| Brain-derived neurotrophic factor | Brain microvascular endothelium | Neuronal precursors, | [33] |
| islet endothelium | |||
| Pigment epithelium-derived factor | Brain capillaries | Neural stem cells | [35,47] |
| Vessel-derived stromal-derived factor 1 (SDF-1/CXCL12) | Microvascular endothelium | Endoderm and pancreatic beta cells | [48] |
| Wnt2 | Sinusoidal ECs | Hepatocytes | [26] |
| S1P | Plasma (plateles) | Pancreatic multipotent progenitor cells | [38] |
| CTGF | Pancreas capillaries | Pancreatic endocrine cells | [49] |
| Laminin | Islet capillaries | Islet endocrine cells | [50] |
| Collagen IV | Islet capillaries | Islet beta-cells | [51] |
| BMP-2 | ECs/MSCs | Islet beta-cells | [52,53] |
| BMP-4 | ECs/MSCs | Islet beta-cells; hepatocytes; cardiomyocytes | [52,53] |
| BMPR1A | ECs/MSCs | Islet beta-cells; hepatocytes; cardiomyocytes | [52,53] |
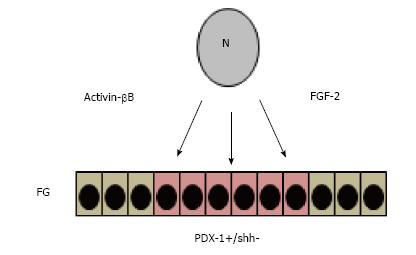
The pancreas originates from ventral and dorsal buds formed in the foregut at 8.5 d post-coitum (d.p.c) of gestation in mice and Carnegie stage 12 (CS12) in humans[54-56]. The cells that composed these buds express transcription factors such as PDX-1 which is a key regulator of pancreas development[57-59]. However, before these cells express these genes, the cells interact with other surrounding cells such as those that compose the notochord and factors such as fibroblast growth factor-2 (FGF-2) and activin-βB suppress the expression of sonic hedgehog (SHH) locally and promotes expression of PDX-1 in the subjacent endoderm[60-63] (Figure 1).
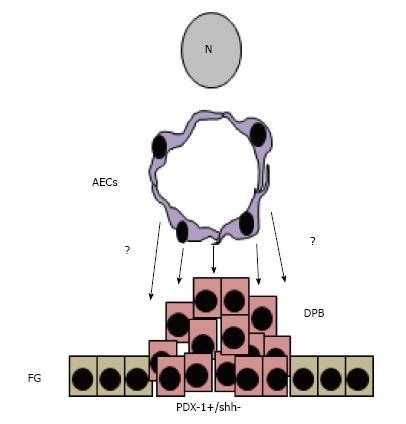
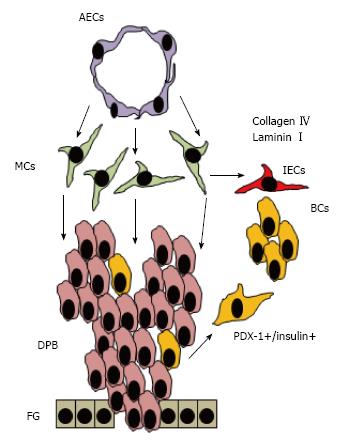
Once the mesoderm layer starts proliferating, other signals from aortic endothelial cells (AECs) and mesenchyme (MCs) continue interacting with these PDX-1 expressing cells that give rise to acinar cells that harbor pancreatic exocrine and endocrine progenitors[64] (Figure 2). As these progenitors continue receiving more surrounding instructive signals, definition of cell function is established and the exocrine cells form acini while the endocrine cells form islets of Langerhans[54] (Figure 3). However, these islets contain immature beta cells that will become more mature after islet vascularization that allows closer interaction between beta cells and ECs.
The vascular system is one of the first tissues that develop during embryogenesis. Mesodermal progenitors coalesce in the yolk sac and give rise to endothelium and blood cells[15]. Endothelial cells exert inductive effects on specific points were they are in contact with pre-patterned definitive endoderm (DE) cells of the FG[20]. DE forms during gastrulation and Nodal, a member of transforming growth factor β (TGFβ) family, plays a central role in DE formation[65]. At these points of effective cell-cell interactions, the gut endoderm has to be competent to respond to EC-derived signals. Competence of these cells takes place during gastrulation when the mesoderm germ layer invades the middle area between primitive ectoderm and endoderm[66]. Apparently mesoderm-derived cells are required to maintain the phenotype of posterior endoderm that includes the site where pancreas and duodenum will be formed. Therefore, anterior-posterior (A-P) endoderm axis will be sustained by the presence of mesoderm-derived factors such as Wingless-type MMTV integration site family (Wnt), fibroblast growth factor (FGF), bone morphogenetic proteins (BMPs), and retinoic acid (RA)[63,67]. For instance, high signaling of the canonical WNT/β-catenin pathway promotes endoderm posterior pattern with foregut-derived structures such as pancreas and liver[68]. Interestingly it has been found that some of these factors are produced by ECs[13,42,53,69-71].
Endothelial signaling is required to induce insulin gene expression during pancreas development[20]. Cell-cell interactions between definitive endoderm and aortic endothelial cells take place at about 9-10 d.p.c. in mice and give rise to PDX-1 expressing cells[18,20]. Apparently, these interactions are also essential to promote insulin expression in pre-patterned endoderm[20]. However, signals from the developing pancreas to embryonic endothelium also promote endothelium-specific phenotype and these interactions are crucial for adequate organ function in adulthood[32]. As mentioned above, the first signals to promote expression of PDX-1 come from the notochord that produce factors such as activin-βB and FGF-2[60]. These cross-talk take place between cells from notochord and cells from the subjacent endoderm leading to inhibition of factors such as SHH and promotion of PDX-1 expression[60]. However, these permissive signals are apparently replaced by instructive signals from the growing mesoderm and AECs that become in close proximity with pre-patterned endoderm. Interestingly, ECs not only exert these signals directly to the pre-patterned endoderm but also promote the survival of adjacent mesodermal cells that produced essential factors such as Islet-1 (Isl1)[64]. Homozygous mice lacking expression of Flk-1 (flk-1-/-) had absence of aorta with no formation of dorsal mesenchyme that led to diminished expression of PDX-1 in the subjacent endoderm[64]. However, lateral and ventral mesenchyme were not affected and PDX-1 positive cells also appeared in the ventral endoderm[64]. These findings indicate that aortic EC signaling is essential to maintain the dorsal mesenchyme and therefore to direct differentiation of dorsal pancreatic endoderm. Additionally, at later embryo stage, flk-1-/- mice showed dorsal mesenchyme that does not express Isl-1 in the absence of aortic ECs suggesting that endothelial-cell signaling promotes Isl-1 cell expression from dorsal mesenchymal cells[64]. In addition, it has been reported that mesenchymal cells also express bone morphogenetic proteins (BMPs) and that these proteins have a pivotal role in pancreas development[71,72].
It is well known that once the mesenchymal cells proliferate between aortic ECs and the foregut endoderm, the aorta is pulled out from the subjacent gut (compare Figure 2 with Figure 3)[20,55]. This fact implies that the subjacent foregut starts receiving signals from the mesenchyme and that a gradient can be formed with diluted signals from aortic ECs. However, it has been demonstrated that ECs maintain the integrity of the subjacent mesenchyme and that absence of aorta promotes apoptosis of the mesenchymal cells and avoids the formation of the dorsal pancreas[64]. As mentioned before, mesenchymal cells between aorta and foregut maintain the expression of PDX-1 in pancreatic endoderm cells through the expression of the transcription factor Isl-1[54]. In addition, signals from mesenchyme such as Fgf-10 are essential to promote proliferation of pancreatic buds that already received signals from ECs[64]. Apparently, the specification of pancreatic fate is determined by permissive signals from notochord and instructive signals by ECs that are maintained by mesenchyme after the aorta is pulled out from the gut. It has been suggested recently that specification of endocrine phenotype also takes place during the close interaction between aortic ECs and pancreatic progenitors that express PDX-1 within the pancreatic bud[54]. Previous experiments indicated that insulin is expressing in foregut explants only after co-culturing with aortic endothelial cells[20]. However, the signals from ECs that promote such specification in the foregut endoderm toward the formation of pancreatic endocrine progenitors are still under investigation. In the same way more characterization is required to identify the signals from ECs that promote survival and adequate function of mesenchyme. Therefore, functional mesenchyme is crucial for appropriate signaling to the subjacent foregut endoderm toward maintenance of the pancreatic phenotype and branching[43].
Pancreatic endocrine progenitors receive signals from aortic ECs and mesenchyme that determined their fate. However, they remain interspersed with the ductal cells that form the epithelium of the growing branches. At a certain time of development, these cells receive still unknown instructions to migrate from the ductal area to the mesenchyme region. Once in the mesenchyme, these pancreatic progenitors that are apart from ductal cells form the islets of Langerhans[73]. This fact raises two questions: (1) Are there signals from ECs that promote islet neogenesis? and (2) Once the blood vessels are formed are there factors in the blood stream that promote the final maturation of beta cells? The answer to these questions is still unknown. The cells that migrate are pancreatic endocrine progenitors that give rise islets of Langerhans composed of alpha, beta, delta, and PP cells that will produce glucagon, insulin, somatostatin, and pancreatic polypeptide respectively. These islets will be distributed differently into the pancreas and apparently will be subjected to different stimuli[74,75]. Although endocrine specification takes place before migration, maturation of endocrine cells occurs at islet level and coincides with islet vascularization[54,76]. There is a significant growth of islet cells that correlates with islet endothelial-cell proliferation in rats the first week after birth[76]. For instance, endocrine cells with higher proliferative capacity closer to blood vessels[76]. Furthermore, it is known that ECs are able to produce HGF which is a potent mitogen for beta cells and ECs[31,77]. Therefore, the endothelial signaling is essential for beta-cell maturation. For instance, immature beta cells are formed some days before birth and maturation occurs several days after birth[76]. During this period, immature beta cells express VEGF and start recruiting ECs to the vicinity of the islet to provide signals for further differentiation and maturation[32,78]. However, along with EC stimuli, another signals should also be considered. For example, hormones that can reach beta cells through the blood stream once the vascular network is established may have a role in cell maturation[79]. Apparently, endogenous insulin has a minor role for the glucose homeostasis before birth[80]. In this condition, insulin provided by the mother regulates glucose in the fetus[80]. This fact suggests that fetal beta cells are not mature enough at birth to maintain the glucose homeostasis and that further maturation can be promoted by ECs after birth.
The role of ECs in β-cell function and pathology has been previously described[47,81-82]. It has been found that ECs from islets correspond to fenestrated endothelium[83,84]. Apparently the characteristics of islet endothelium differ from pancreatic exocrine endothelium and endothelium from other regions[47,83,85]. For instance, pancreatic endocrine capillaries have higher diameter than exocrine capillaries and endothelium from endocrine capillaries have 10 times more fenestrae that endothelium from exocrine capillaries[83]. These facts suggest that cell-cell interactions and signaling between endothelium and the surrounding cells are different even in the same organ. Pancreatic beta cells have polarity with an apical and basolateral membrane and insulin vesicles are more dense in the apical region close to ECs[47,86]. This aspect is very important when considering the ability of beta cells to release insulin into the blood stream. After islet transplantation, suitable EC-signals for polarization are crucial for appropriate insulin release into the capillaries. Islet ECs express common markers of ECs but one specific marker called the proteinase inhibitor and angiostatin factor α1-antitrypsin[70,84]. This marker of specific islet ECs can be absent in surrogate vasculature with deregulation of islet function. Apparently EPCs or islet ECs are important for islet revascularization after transplantation[87-89]. In this sense, any islet injury leads to islet restoration through recruitment of EPCs toward islet re-vascularization with specific islet ECs and beta cell function[87]. For instance, normoglycemia is improved in streptozotocin-treated animals after co-transplantation of EPCs and islets[87].
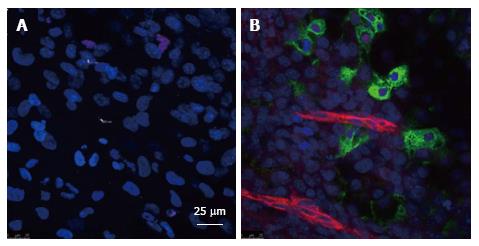
As a first approach to investigate the role of endothelium in β-cell differentiation, we studied the role of in vivo surrogate vasculature in mouse embryoid body (EB) differentiation using quail chorioallantoic membranes (CAM)[90]. We found that some cells expressed cardiotin, myosin heavy chain, collagen IV, CD34, CD31, and neurofilament. Although some epithelial cells appeared, no cells derived from endoderm were identified[90]. Then, studies using co-cultures between human microvascular ECs (HMECs) and mouse EBs were performed[52,53,91]. In these studies, ECs from human dermis were able to induce differentiation of mouse EBs to pancreatic progenitors and insulin-producing cells[52]. Furthermore, BMP-2/-4 were involved in this differentiation process as evaluated by the effects of agonists (recombinant BMPs), and specific antagonists of BMP bioactivities (Noggin, Chordin). BMPs are members of the TGF-β superfamily[92]. In addition to the effects of BMP antagonists, we explored the levels of phosphorylation of SMAD1, 5, 8 in cells that expressed proinsulin[52,53]. The role of BMPs in pancreas development has also been explored previously[72,93]. We demonstrated that HMECs or mouse dermis as well as mouse AECs expressed BMPs and that BMP-2 and BMP-4 increased the phosphorylation levels of SMAD1,5,8 in pancreatic progenitors and beta-like cells derived from mouse ESCs[52,53,94]. These findings together with previous works pointed out the important role of ECs in beta-cell differentiation in vitro. We recently have observed that when human ESCs (cell line H9) are co-cultured with HMECs, the formation of proinsulin positive cells takes place in about twenty days in close proximity to internal ECs without the use of additional growth factors (Figure 4).
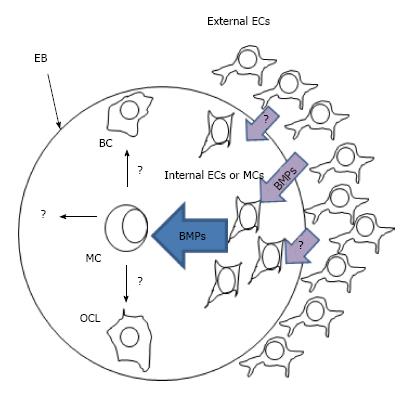
In the model using mouse EBs, we observed that ECs promote up-regulation of BMPs within EBs[53]. However, the target cells that produce these BMPs are still unknown. One good candidate for these cells are mesenchymal cells since it has been demonstrated that ECs are essential to maintain dorsal pancreatic mesenchyme during pancreas morphogenesis that may promote pancreas differentiation within EBs[64] (Figure 5). However, further studies should be done to demonstrate that internal ECs are able to trigger beta cell differentiation through signaling to mesenchymal stem cells. At present, ECs can be generated in vitro from human induced pluripotential stem cells (hiPSCs) or human embryonic stem cells (hESCs) and this studies can be important to answer this question[95,96].
In this model, an excess of human microvascular ECs (HMECs) surround a human embryoid body which is composed of endoderm, ectoderm, and mesoderm cells. External ECs produce factors such as BMPs and other EC-derived factors that promote upregulation of endogenous BMPs in still unknown target cells (possibly mesenchymal or internal ECs). These BMPs together with other unknown factors may promote differentiation of multipotent cells (MC) toward beta-like cells (BC) and other cell lineages (OCL)[52,53].
ECs play an important role for the pathogenesis of type 1 (T1DM) and type 2 diabetes mellitus (T2DM). ECs involvement in cellular diapedesis, inflammation, and Vessel fibrosis, which leads to islet dysfunction, has been demonstrated. In T1DM, Ecs allow infiltration of leucocytes[47]. Interestingly multiple diabetic complications relate to endothelial-cell function. The key to prevention or reversal of diabetes may rest in the recently emphasized role of endothelium in beta-cell differentiation and maturation in vitro[52,53].
In addition, it has been suggested that EC pathology can lead to islet dysfunction suggesting that ECs are essential to maintain islet function in adults[97].
In this review we focused in the essential role of endothelium for pancreatic endocrine differentiation, functional maturation, and islet dysfunction. ECs play a key role during the differentiation of the dorsal pancreas by maintaining the expression of transcription factors necessary for pancreas development including endocrine progenitors. Before birth, immature beta cells recruit ECs close to their microenvironment and these ECs provide signals for further maturation and function of pancreatic beta cells. In addition, ECs co-transplanted with islets have demonstrated to improve the engraftment of human islets. Finally, ECs are able to provide signals in vitro for derivation of functional beta-like cells from human pluripotent stem cells. Therefore, the study of interactions between EC and beta cells is relevant for future clinical applications in regenerative medicine.
P- Reviewer: Sanal MG, Sakata N S- Editor: Ji FF L- Editor: A E- Editor: Lu YJ
| 1. | Liew CG. Generation of insulin-producing cells from pluripotent stem cells: from the selection of cell sources to the optimization of protocols. Rev Diabet Stud. 2010;7:82-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 2. | Pagliuca FW, Millman JR, Gürtler M, Segel M, Van Dervort A, Ryu JH, Peterson QP, Greiner D, Melton DA. Generation of functional human pancreatic β cells in vitro. Cell. 2014;159:428-439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1321] [Cited by in RCA: 1519] [Article Influence: 151.9] [Reference Citation Analysis (1)] |
| 3. | Lock LT, Tzanakakis ES. Stem/Progenitor cell sources of insulin-producing cells for the treatment of diabetes. Tissue Eng. 2007;13:1399-1412. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 60] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 4. | Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, Jones JM. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145-1147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11399] [Cited by in RCA: 10418] [Article Influence: 385.9] [Reference Citation Analysis (0)] |
| 5. | Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663-676. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17989] [Cited by in RCA: 18138] [Article Influence: 954.6] [Reference Citation Analysis (0)] |
| 6. | Segev H, Fishman B, Ziskind A, Shulman M, Itskovitz-Eldor J. Differentiation of human embryonic stem cells into insulin-producing clusters. Stem Cells. 2004;22:265-274. [PubMed] |
| 7. | Rezania A, Bruin JE, Riedel MJ, Mojibian M, Asadi A, Xu J, Gauvin R, Narayan K, Karanu F, O’Neil JJ. Maturation of human embryonic stem cell-derived pancreatic progenitors into functional islets capable of treating pre-existing diabetes in mice. Diabetes. 2012;61:2016-2029. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 408] [Cited by in RCA: 427] [Article Influence: 32.8] [Reference Citation Analysis (0)] |
| 8. | Assady S, Maor G, Amit M, Itskovitz-Eldor J, Skorecki KL, Tzukerman M. Insulin production by human embryonic stem cells. Diabetes. 2001;50:1691-1697. [PubMed] |
| 9. | Assady S. Challenges and prospects for stem cell-based therapy in diabetes mellitus. Isr Med Assoc J. 2009;11:212-215. [PubMed] |
| 10. | D’Amour KA, Bang AG, Eliazer S, Kelly OG, Agulnick AD, Smart NG, Moorman MA, Kroon E, Carpenter MK, Baetge EE. Production of pancreatic hormone-expressing endocrine cells from human embryonic stem cells. Nat Biotechnol. 2006;24:1392-1401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1447] [Cited by in RCA: 1410] [Article Influence: 74.2] [Reference Citation Analysis (0)] |
| 11. | Kroon E, Martinson LA, Kadoya K, Bang AG, Kelly OG, Eliazer S, Young H, Richardson M, Smart NG, Cunningham J. Pancreatic endoderm derived from human embryonic stem cells generates glucose-responsive insulin-secreting cells in vivo. Nat Biotechnol. 2008;26:443-452. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1331] [Cited by in RCA: 1303] [Article Influence: 76.6] [Reference Citation Analysis (0)] |
| 12. | Coultas L, Chawengsaksophak K, Rossant J. Endothelial cells and VEGF in vascular development. Nature. 2005;438:937-945. [PubMed] |
| 13. | Herbert SP, Stainier DY. Molecular control of endothelial cell behaviour during blood vessel morphogenesis. Nat Rev Mol Cell Biol. 2011;12:551-564. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 831] [Cited by in RCA: 797] [Article Influence: 56.9] [Reference Citation Analysis (0)] |
| 15. | Noden DM. Embryonic origins and assembly of blood vessels. Am Rev Respir Dis. 1989;140:1097-1103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 256] [Cited by in RCA: 228] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 16. | Nikolova G, Strilic B, Lammert E. The vascular niche and its basement membrane. Trends Cell Biol. 2007;17:19-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 124] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 17. | Matsumoto K, Yoshitomi H, Rossant J, Zaret KS. Liver organogenesis promoted by endothelial cells prior to vascular function. Science. 2001;294:559-563. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 676] [Cited by in RCA: 627] [Article Influence: 26.1] [Reference Citation Analysis (0)] |
| 18. | Nikolova G, Lammert E. Interdependent development of blood vessels and organs. Cell Tissue Res. 2003;314:33-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 59] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 19. | Crivellato E, Nico B, Ribatti D. Contribution of endothelial cells to organogenesis: a modern reappraisal of an old Aristotelian concept. J Anat. 2007;211:415-427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 32] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 20. | Lammert E, Cleaver O, Melton D. Induction of pancreatic differentiation by signals from blood vessels. Science. 2001;294:564-567. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 816] [Cited by in RCA: 779] [Article Influence: 32.5] [Reference Citation Analysis (0)] |
| 21. | Lammert E, Cleaver O, Melton D. Role of endothelial cells in early pancreas and liver development. Mech Dev. 2003;120:59-64. [PubMed] |
| 22. | Mantovani A, Bussolino F, Dejana E. Cytokine regulation of endothelial cell function. FASEB J. 1992;6:2591-2599. [PubMed] |
| 23. | Orr FW, Wang HH, Lafrenie RM, Scherbarth S, Nance DM. Interactions between cancer cells and the endothelium in metastasis. J Pathol. 2000;190:310-329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 24. | Crivellato E. The role of angiogenic growth factors in organogenesis. Int J Dev Biol. 2011;55:365-375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 44] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 25. | Butler JM, Nolan DJ, Vertes EL, Varnum-Finney B, Kobayashi H, Hooper AT, Seandel M, Shido K, White IA, Kobayashi M. Endothelial cells are essential for the self-renewal and repopulation of Notch-dependent hematopoietic stem cells. Cell Stem Cell. 2010;6:251-264. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 552] [Cited by in RCA: 510] [Article Influence: 34.0] [Reference Citation Analysis (0)] |
| 26. | Ding BS, Nolan DJ, Butler JM, James D, Babazadeh AO, Rosenwaks Z, Mittal V, Kobayashi H, Shido K, Lyden D. Inductive angiocrine signals from sinusoidal endothelium are required for liver regeneration. Nature. 2010;468:310-315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 27. | Brantley-Sieders DM, Dunaway CM, Rao M, Short S, Hwang Y, Gao Y, Li D, Jiang A, Shyr Y, Wu JY. Angiocrine factors modulate tumor proliferation and motility through EphA2 repression of Slit2 tumor suppressor function in endothelium. Cancer Res. 2011;71:976-987. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 51] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 28. | Bahary N, Zon LI. Development. Endothelium--chicken soup for the endoderm. Science. 2001;294:530-531. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 25] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 29. | Uchimura T, Komatsu Y, Tanaka M, McCann KL, Mishina Y. Bmp2 and Bmp4 genetically interact to support multiple aspects of mouse development including functional heart development. Genesis. 2009;47:374-384. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 48] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 30. | Takahashi T, Huynh-Do U, Daniel TO. Renal microvascular assembly and repair: power and promise of molecular definition. Kidney Int. 1998;53:826-835. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 51] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 31. | LeCouter J, Moritz DR, Li B, Phillips GL, Liang XH, Gerber HP, Hillan KJ, Ferrara N. Angiogenesis-independent endothelial protection of liver: role of VEGFR-1. Science. 2003;299:890-893. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 512] [Cited by in RCA: 514] [Article Influence: 23.4] [Reference Citation Analysis (0)] |
| 32. | Cleaver O, Melton DA. Endothelial signaling during development. Nat Med. 2003;9:661-668. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 376] [Cited by in RCA: 361] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 33. | Palmer TD, Willhoite AR, Gage FH. Vascular niche for adult hippocampal neurogenesis. J Comp Neurol. 2000;425:479-494. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 34. | Louissaint A, Rao S, Leventhal C, Goldman SA. Coordinated interaction of neurogenesis and angiogenesis in the adult songbird brain. Neuron. 2002;34:945-960. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 577] [Cited by in RCA: 563] [Article Influence: 24.5] [Reference Citation Analysis (0)] |
| 35. | Ramírez-Castillejo C, Sánchez-Sánchez F, Andreu-Agulló C, Ferrón SR, Aroca-Aguilar JD, Sánchez P, Mira H, Escribano J, Fariñas I. Pigment epithelium-derived factor is a niche signal for neural stem cell renewal. Nat Neurosci. 2006;9:331-339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 332] [Cited by in RCA: 331] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 36. | Katsumoto K, Kume S. The role of CXCL12-CXCR4 signaling pathway in pancreatic development. Theranostics. 2013;3:11-17. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 31] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 37. | Kumar M, Jordan N, Melton D, Grapin-Botton A. Signals from lateral plate mesoderm instruct endoderm toward a pancreatic fate. Dev Biol. 2003;259:109-122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 165] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 38. | Edsbagge J, Johansson JK, Esni F, Luo Y, Radice GL, Semb H. Vascular function and sphingosine-1-phosphate regulate development of the dorsal pancreatic mesenchyme. Development. 2005;132:1085-1092. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 61] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 39. | Heinis M, Simon MT, Ilc K, Mazure NM, Pouysségur J, Scharfmann R, Duvillié B. Oxygen tension regulates pancreatic beta-cell differentiation through hypoxia-inducible factor 1alpha. Diabetes. 2010;59:662-669. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 92] [Cited by in RCA: 93] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 40. | Kopp HG, Avecilla ST, Hooper AT, Rafii S. The bone marrow vascular niche: home of HSC differentiation and mobilization. Physiology (Bethesda). 2005;20:349-356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 297] [Cited by in RCA: 333] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 41. | Shen Q, Goderie SK, Jin L, Karanth N, Sun Y, Abramova N, Vincent P, Pumiglia K, Temple S. Endothelial cells stimulate self-renewal and expand neurogenesis of neural stem cells. Science. 2004;304:1338-1340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1137] [Cited by in RCA: 1142] [Article Influence: 54.4] [Reference Citation Analysis (0)] |
| 42. | Muñoz-Chápuli R, Quesada AR, Angel Medina M. Angiogenesis and signal transduction in endothelial cells. Cell Mol Life Sci. 2004;61:2224-2243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 236] [Cited by in RCA: 237] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 43. | Glienke J, Schmitt AO, Pilarsky C, Hinzmann B, Weiss B, Rosenthal A, Thierauch KH. Differential gene expression by endothelial cells in distinct angiogenic states. Eur J Biochem. 2000;267:2820-2830. [PubMed] |
| 44. | Yamamoto H, Yun EJ, Gerber HP, Ferrara N, Whitsett JA, Vu TH. Epithelial-vascular cross talk mediated by VEGF-A and HGF signaling directs primary septae formation during distal lung morphogenesis. Dev Biol. 2007;308:44-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 118] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 45. | LeCouter J, Lin R, Ferrara N. Endocrine gland-derived VEGF and the emerging hypothesis of organ-specific regulation of angiogenesis. Nat Med. 2002;8:913-917. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 73] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 46. | Masaki T. Historical review: Endothelin. Trends Pharmacol Sci. 2004;25:219-224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 171] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 47. | Olsson R, Carlsson PO. The pancreatic islet endothelial cell: emerging roles in islet function and disease. Int J Biochem Cell Biol. 2006;38:710-714. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 38] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 48. | Yano T, Liu Z, Donovan J, Thomas MK, Habener JF. Stromal cell derived factor-1 (SDF-1)/CXCL12 attenuates diabetes in mice and promotes pancreatic beta-cell survival by activation of the prosurvival kinase Akt. Diabetes. 2007;56:2946-2957. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 109] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 49. | Crawford LA, Guney MA, Oh YA, Deyoung RA, Valenzuela DM, Murphy AJ, Yancopoulos GD, Lyons KM, Brigstock DR, Economides A. Connective tissue growth factor (CTGF) inactivation leads to defects in islet cell lineage allocation and beta-cell proliferation during embryogenesis. Mol Endocrinol. 2009;23:324-336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 69] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 50. | Nikolova G, Jabs N, Konstantinova I, Domogatskaya A, Tryggvason K, Sorokin L, Fässler R, Gu G, Gerber HP, Ferrara N. The vascular basement membrane: a niche for insulin gene expression and Beta cell proliferation. Dev Cell. 2006;10:397-405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 390] [Cited by in RCA: 408] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 51. | Kaido T, Yebra M, Cirulli V, Montgomery AM. Regulation of human beta-cell adhesion, motility, and insulin secretion by collagen IV and its receptor alpha1beta1. J Biol Chem. 2004;279:53762-53769. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 157] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 52. | Talavera-Adame D, Wu G, He Y, Ng TT, Gupta A, Kurtovic S, Hwang JY, Farkas DL, Dafoe DC. Endothelial cells in co-culture enhance embryonic stem cell differentiation to pancreatic progenitors and insulin-producing cells through BMP signaling. Stem Cell Rev. 2011;7:532-543. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 41] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 53. | Talavera-Adame D, Gupta A, Kurtovic S, Chaiboonma KL, Arumugaswami V, Dafoe DC. Bone morphogenetic protein-2/-4 upregulation promoted by endothelial cells in coculture enhances mouse embryoid body differentiation. Stem Cells Dev. 2013;22:3252-3260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 54. | Habener JF, Kemp DM, Thomas MK. Minireview: transcriptional regulation in pancreatic development. Endocrinology. 2005;146:1025-1034. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 300] [Cited by in RCA: 288] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 55. | Jennings RE, Berry AA, Kirkwood-Wilson R, Roberts NA, Hearn T, Salisbury RJ, Blaylock J, Piper Hanley K, Hanley NA. Development of the human pancreas from foregut to endocrine commitment. Diabetes. 2013;62:3514-3522. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 185] [Cited by in RCA: 217] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 56. | Hill MA. Early human development. Clin Obstet Gynecol. 2007;50:2-9. [PubMed] |
| 57. | Grapin-Botton A. Antero-posterior patterning of the vertebrate digestive tract: 40 years after Nicole Le Douarin’s PhD thesis. Int J Dev Biol. 2005;49:335-347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 58] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 58. | Gao N, LeLay J, Vatamaniuk MZ, Rieck S, Friedman JR, Kaestner KH. Dynamic regulation of Pdx1 enhancers by Foxa1 and Foxa2 is essential for pancreas development. Genes Dev. 2008;22:3435-3448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 59. | Jensen J. Gene regulatory factors in pancreatic development. Dev Dyn. 2004;229:176-200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 293] [Cited by in RCA: 273] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 60. | Hebrok M, Kim SK, Melton DA. Notochord repression of endodermal Sonic hedgehog permits pancreas development. Genes Dev. 1998;12:1705-1713. [PubMed] |
| 61. | Stemple DL. Structure and function of the notochord: an essential organ for chordate development. Development. 2005;132:2503-2512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 321] [Cited by in RCA: 324] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 62. | Kim SK, Hebrok M. Intercellular signals regulating pancreas development and function. Genes Dev. 2001;15:111-127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 301] [Cited by in RCA: 289] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 63. | Ameri J, Ståhlberg A, Pedersen J, Johansson JK, Johannesson MM, Artner I, Semb H. FGF2 specifies hESC-derived definitive endoderm into foregut/midgut cell lineages in a concentration-dependent manner. Stem Cells. 2010;28:45-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 98] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 64. | Jacquemin P, Yoshitomi H, Kashima Y, Rousseau GG, Lemaigre FP, Zaret KS. An endothelial-mesenchymal relay pathway regulates early phases of pancreas development. Dev Biol. 2006;290:189-199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 104] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 65. | Vincent SD, Dunn NR, Hayashi S, Norris DP, Robertson EJ. Cell fate decisions within the mouse organizer are governed by graded Nodal signals. Genes Dev. 2003;17:1646-1662. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 257] [Cited by in RCA: 247] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 66. | McCracken KW, Wells JM. Molecular pathways controlling pancreas induction. Semin Cell Dev Biol. 2012;23:656-662. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 37] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 67. | Spence JR, Mayhew CN, Rankin SA, Kuhar MF, Vallance JE, Tolle K, Hoskins EE, Kalinichenko VV, Wells SI, Zorn AM. Directed differentiation of human pluripotent stem cells into intestinal tissue in vitro. Nature. 2011;470:105-109. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1668] [Cited by in RCA: 1426] [Article Influence: 101.9] [Reference Citation Analysis (0)] |
| 68. | McLin VA, Rankin SA, Zorn AM. Repression of Wnt/beta-catenin signaling in the anterior endoderm is essential for liver and pancreas development. Development. 2007;134:2207-2217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 307] [Cited by in RCA: 259] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 69. | Lai L, Bohnsack BL, Niederreither K, Hirschi KK. Retinoic acid regulates endothelial cell proliferation during vasculogenesis. Development. 2003;130:6465-6474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 112] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 70. | Linn T, Schneider K, Hammes HP, Preissner KT, Brandhorst H, Morgenstern E, Kiefer F, Bretzel RG. Angiogenic capacity of endothelial cells in islets of Langerhans. FASEB J. 2003;17:881-883. [PubMed] |
| 71. | Talavera-Adame D, Xiong Y, Zhao T, Arias AE, Sierra-Honigmann MR, Farkas DL. Quantitative and morphometric evaluation of the angiogenic effects of leptin. J Biomed Opt. 2008;13:064017. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 19] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 72. | Ahnfelt-Rønne J, Ravassard P, Pardanaud-Glavieux C, Scharfmann R, Serup P. Mesenchymal bone morphogenetic protein signaling is required for normal pancreas development. Diabetes. 2010;59:1948-1956. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 48] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 73. | Paris M, Tourrel-Cuzin C, Plachot C, Ktorza A. Review: pancreatic beta-cell neogenesis revisited. Exp Diabesity Res. 2014;5:111-121. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 33] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 74. | Bosco D, Armanet M, Morel P, Niclauss N, Sgroi A, Muller YD, Giovannoni L, Parnaud G, Berney T. Unique arrangement of alpha- and beta-cells in human islets of Langerhans. Diabetes. 2010;59:1202-1210. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 317] [Cited by in RCA: 319] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 75. | Wang X, Misawa R, Zielinski MC, Cowen P, Jo J, Periwal V, Ricordi C, Khan A, Szust J, Shen J. Regional differences in islet distribution in the human pancreas--preferential beta-cell loss in the head region in patients with type 2 diabetes. PLoS One. 2013;8:e67454. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 98] [Cited by in RCA: 127] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 76. | Johansson M, Andersson A, Carlsson PO, Jansson L. Perinatal development of the pancreatic islet microvasculature in rats. J Anat. 2006;208:191-196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 31] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 77. | Otonkoski T, Cirulli V, Beattie M, Mally MI, Soto G, Rubin JS, Hayek A. A role for hepatocyte growth factor/scatter factor in fetal mesenchyme-induced pancreatic beta-cell growth. Endocrinology. 1996;137:3131-3139. [PubMed] |
| 78. | Inoue M, Hager JH, Ferrara N, Gerber HP, Hanahan D. VEGF-A has a critical, nonredundant role in angiogenic switching and pancreatic beta cell carcinogenesis. Cancer Cell. 2002;1:193-202. [PubMed] |
| 79. | Hardikar AA, Wang XY, Williams LJ, Kwok J, Wong R, Yao M, Tuch BE. Functional maturation of fetal porcine beta-cells by glucagon-like peptide 1 and cholecystokinin. Endocrinology. 2002;143:3505-3514. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 53] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 80. | Duvillié B, Cordonnier N, Deltour L, Dandoy-Dron F, Itier JM, Monthioux E, Jami J, Joshi RL, Bucchini D. Phenotypic alterations in insulin-deficient mutant mice. Proc Natl Acad Sci USA. 1997;94:5137-5140. [PubMed] |
| 81. | Richards OC, Raines SM, Attie AD. The role of blood vessels, endothelial cells, and vascular pericytes in insulin secretion and peripheral insulin action. Endocr Rev. 2010;31:343-363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 112] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 82. | Sabra G, Vermette P. A 3D cell culture system: separation distance between INS-1 cell and endothelial cell monolayers co-cultured in fibrin influences INS-1 cells insulin secretion. Biotechnol Bioeng. 2013;110:619-627. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 83. | Henderson JR, Moss MC. A morphometric study of the endocrine and exocrine capillaries of the pancreas. Q J Exp Physiol. 1985;70:347-356. [PubMed] |
| 84. | Lou J, Triponez F, Oberholzer J, Wang H, Yu D, Buhler L, Cretin N, Mentha G, Wollheim CB, Morel P. Expression of alpha-1 proteinase inhibitor in human islet microvascular endothelial cells. Diabetes. 1999;48:1773-1778. [PubMed] |
| 85. | Mattsson G, Danielsson A, Kriz V, Carlsson PO, Jansson L. Endothelial cells in endogenous and transplanted pancreatic islets: differences in the expression of angiogenic peptides and receptors. Pancreatology. 2006;6:86-95. [PubMed] |
| 86. | Bonner-Weir S. Morphological evidence for pancreatic polarity of beta-cell within islets of Langerhans. Diabetes. 1988;37:616-621. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 36] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 87. | Quaranta P, Antonini S, Spiga S, Mazzanti B, Curcio M, Mulas G, Diana M, Marzola P, Mosca F, Longoni B. Co-transplantation of endothelial progenitor cells and pancreatic islets to induce long-lasting normoglycemia in streptozotocin-treated diabetic rats. PLoS One. 2014;9:e94783. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 27] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 88. | Brissova M, Fowler M, Wiebe P, Shostak A, Shiota M, Radhika A, Lin PC, Gannon M, Powers AC. Intraislet endothelial cells contribute to revascularization of transplanted pancreatic islets. Diabetes. 2004;53:1318-1325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 195] [Cited by in RCA: 196] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 89. | Hess D, Li L, Martin M, Sakano S, Hill D, Strutt B, Thyssen S, Gray DA, Bhatia M. Bone marrow-derived stem cells initiate pancreatic regeneration. Nat Biotechnol. 2003;21:763-770. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 447] [Cited by in RCA: 419] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 90. | Talavera-Adame D, Dafoe DC, Ng TT, Wachsmann-Hogiu S, Castillo-Henkel C, Farkas DL. Enhancement of embryonic stem cell differentiation promoted by avian chorioallantoic membranes. Tissue Eng Part A. 2009;15:3193-3200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 91. | Banerjee I, Sharma N, Yarmush M. Impact of co-culture on pancreatic differentiation of embryonic stem cells. J Tissue Eng Regen Med. 2011;5:313-323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 92. | Bragdon B, Moseychuk O, Saldanha S, King D, Julian J, Nohe A. Bone morphogenetic proteins: a critical review. Cell Signal. 2011;23:609-620. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 475] [Cited by in RCA: 515] [Article Influence: 34.3] [Reference Citation Analysis (0)] |
| 93. | Goulley J, Dahl U, Baeza N, Mishina Y, Edlund H. BMP4-BMPR1A signaling in beta cells is required for and augments glucose-stimulated insulin secretion. Cell Metab. 2007;5:207-219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 109] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 94. | Talavera-Adame D, Ng TT, Gupta A, Kurtovic S, Wu GD, Dafoe DC. Characterization of microvascular endothelial cells isolated from the dermis of adult mouse tails. Microvasc Res. 2011;82:97-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 95. | Wang ZZ, Au P, Chen T, Shao Y, Daheron LM, Bai H, Arzigian M, Fukumura D, Jain RK, Scadden DT. Endothelial cells derived from human embryonic stem cells form durable blood vessels in vivo. Nat Biotechnol. 2007;25:317-318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 241] [Cited by in RCA: 224] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 96. | Kurtovic S, Ng TT, Gupta A, Arumugaswami V, Chaiboonma KL, Aminzadeh MA, Makkar R, Dafoe DC, Talavera-Adame D. Leptin enhances endothelial cell differentiation and angiogenesis in murine embryonic stem cells. Microvasc Res. 2015;97:65-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 97. | Almaça J, Molina J, Arrojo E Drigo R, Abdulreda MH, Jeon WB, Berggren PO, Caicedo A, Nam HG. Young capillary vessels rejuvenate aged pancreatic islets. Proc Natl Acad Sci USA. 2014;111:17612-17617. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 78] [Article Influence: 7.1] [Reference Citation Analysis (0)] |