Published online Sep 20, 2025. doi: 10.5493/wjem.v15.i3.104678
Revised: February 26, 2025
Accepted: April 9, 2025
Published online: September 20, 2025
Processing time: 227 Days and 21.6 Hours
Vitex negundo (V. negundo), an Indian herb with a rich historical background for the handling of various complaints, is a member of the Verbenaceae family and is characterized by small trees with pale gray bark. This herb is widely used and has been recognized in traditional medicine for its pharmacological effects on a wide range of diseases. All sections of the plant, but particularly the leaves, contain a variety of secondary metabolites, including alkaloids, phenols, flavonoids, glycoside iridoids, tannins, and terpenes. The system is included in a number of store-bought herbal preparations and has the potential to function as an efficient bio-committee. Cancer continues to be a major cause of death and morbidity in spite of the intervention. One of the leading causes of death is cancer, and current therapies can have unpleasant side effects. Unhindered reactions, resistance to traditional cancer medications, radiation therapy, chemotherapy, and restricted access to tumor tissue are some of the reasons why treatment frequently fails. To reduce side effects, increase chemotherapy sensitivity, and slow the spread of cancer, new approaches are required. Small food molecules have been suggested in numerous research as supplemental therapies for cancer patients. The effectiveness of the flavonoid-rich V. negundo extract in treating cancer was assessed mechanically in this investigation. Total flavonoids have been isolated for qualitative phytochemical investigation using V. negundo ethanolic extract. This article highlights significant developments in this field and explores how flavonoids contribute to the targeted suppression of the phosphoinositide 3-kinase-protein kinase B-mammalian target of rapamycin pathway in different cancer types.
Core Tip: Vitex negundo (V. negundo) is one of the very crucial plants that have wide packages in traditional structures of medicines. As discuss above V. negundo is a very good supply of flavonoids. Flavonoids are recommended as anticancer retailers because of their natural assets, price-effectiveness, and ease of use. Their intrinsic properties, such as restricted bioavailability, rapid metabolism, untargeted transport, and cytotoxicity to normal cells, prevent their full clinical potential from being discovered. The efficient use of a combination of flavonoids has been suggested to boost their anticancer efficacy, taking into account the potential to concurrently impact unique signaling cascades.
- Citation: Garg G, Chaudhary S, Khatana K, Bharadwaj A. Flavonoids extract from Vitex negundo inhibit autophagy by targeting PI3K/AKT/mTOR/p70S6K/ULK signaling cascade in cancerous cells. World J Exp Med 2025; 15(3): 104678
- URL: https://www.wjgnet.com/2220-315X/full/v15/i3/104678.htm
- DOI: https://dx.doi.org/10.5493/wjem.v15.i3.104678
Vitex negundo (V. negundo) is a tall, fragrant shrub with dense, four-sided, white and Tomentos branches that belongs to the Lamiaceae family[1,2]. This woody shrub is commonly referred to as a horseshoe Vitex, a Chinese chaste tree, or a five folk chaste tree. Traditional medicine makes extensive use of it, particularly in South and Southeast Asia. Nochi, also known as Nirgundi or V. negundo, is indigenous to Eastern and Southern Africa as well as Asia. It can be easily reco
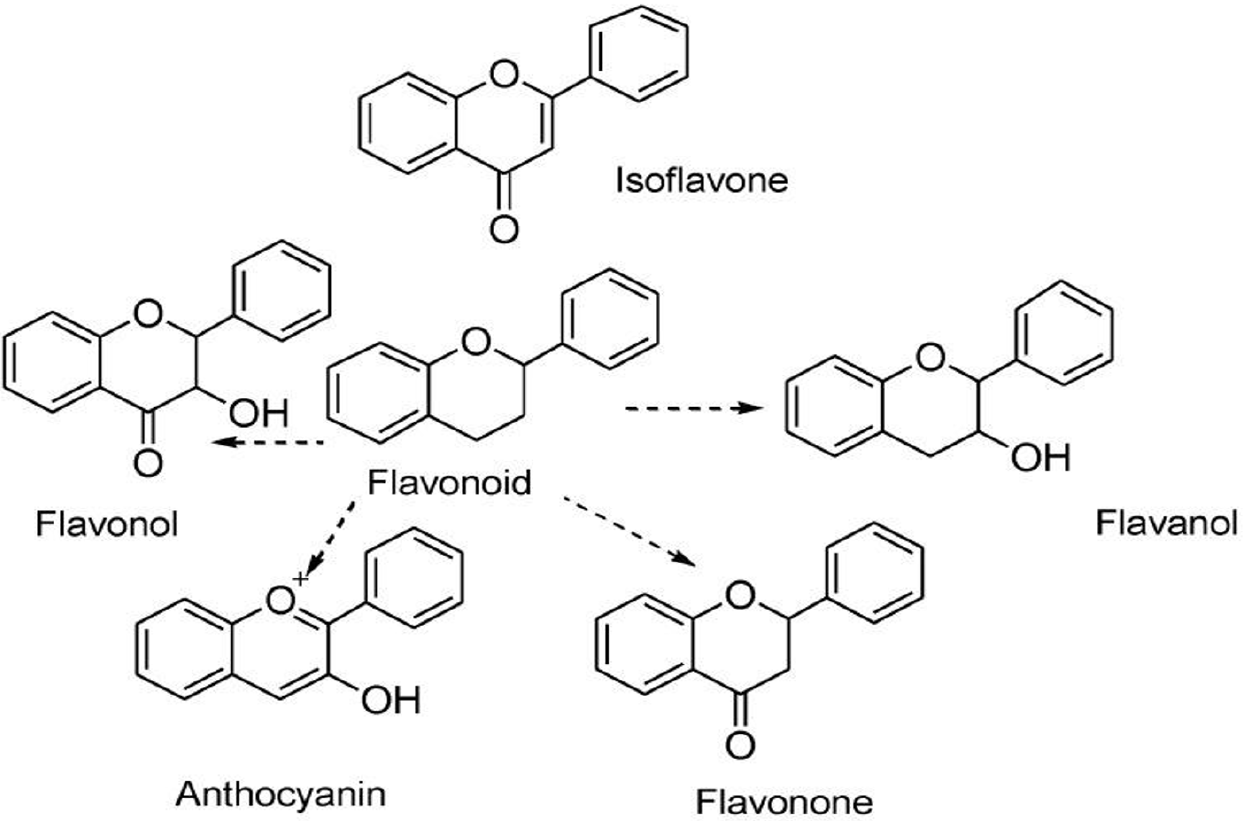
As shown in Figure 1, flavonoids, which are classified as secondary metabolites, mainly consist of a benzopyron circle with phenolic or polyphenolic groups arranged in various locations. Fruits, herbs, stalks, cereals, nuts, vegetables, flowers, and seeds are typically where they can be found[5]. These plant sections include bioactive phytochemical components that support their biological activity and therapeutic potential. Over 10000 flavonoid compounds have been identified and described to date. The majority of flavonoids are well known for their medicinal qualities. These substances are produced naturally via the phenylpropanoid pathway, and their biological availability and mode of absorption influence their bio-activity[6].
Flavonoids were utilized in skin care products, cosmetics, natural colors, and wrinkle-reducing treatments[7]. However, the primary use of these polyphenols is in the medical area. Flavonoids have been utilized extensively as anti-cancer, anti-microbe, anti-virus, anti-angiogenesis, anti-malarial, anti-oxidant, anti-neurodegenerative, anti-tumor, and anti-cell proliferation agents[8]. Apple peeled extracts, which are rich in flavonoids, have been shown to effectively decrease blood pressure and inhibit acetylcholinesterase in vitro. Because flavonoids are classified into several categories according to their chemical makeup, degree of non-mounting, and oxidation of carbon circles, they also aid in the prevention of cardio-metabolic illnesses and support the maintenance of cognitive function in people of all ages[9]. There are several subclasses of flavonoids, including flavanona, flavanonols, anthoxanthins (flavanon and flavanol), flavany, chalcones, antocyanidins, and isoflavonoids. In nature, these flavonoids are extensively distributed[10]. There are many health advantages to eating foods high in flavonoids. Interest in getting these chemicals from various plants is growing as a result of their beneficial effects on human health. Citrus fruits, for instance, are a rich source of flavonoids. Narigenin and hesperetin are two flavonoids found in oranges, lemons, and grapes. Quercetin glycoside flavonoids and an
Because flavonoids can prevent gene mutations, control oxidation, lower inflammation, and prevent the development of cancer, they are recognized to offer health advantages[11]. These characteristics help prevent cancer and decrease blood pressure, among other illnesses. High blood pressure raises the risk of heart disease, which is the primary cause of mortality globally[12]. Numerous plants, including fruits and vegetables, contain polyphenols and flavonoids, which have been shown to be effective in the treatment of high blood pressure and heart disease[13]. There are different types of flavonoids which extracts from different parts of V. negundo which have been shown in Table 1. Flavonoids' antioxidant properties can lower the risk of cardiovascular disease by improving lipid profiles and lowering oxidation in low-density lipoprotein, also known as the "bad" cholesterol. Flavonoids can also help in vasodilation, or the widening of blood vessels, and regulate the endothelium's (the inner lining of blood vessels) process of programmed cell death. Recent research has revealed that other signaling pathways are involved, suggesting that flavonoids may have alternative mechanisms of action, even though studies have demonstrated that their antioxidant properties are what cause these benefits[14].
| No. | Compound name (flavonoids) | Plant parts |
| 1 | 5-Hydroxy-7,4′-dimethoxy flavone | Leave |
| 2 | 5,7-Dihydroxy-6,4′-dimethoxy flavone | Leave |
| 3 | Luteolin | Leaves |
| 4 | Luteolin-7-O-β-D-glucoside | Leave |
| 5 | 7,8-Dimethyl herbacetin-3-rhamnoside | Leave |
| 6 | Vitegnoside | Leaves |
| 7 | Iso-orientin | Leaves |
| 8 | Chrysoplenetin | Seeds |
| 9 | Chrysosplenol D | Seeds |
| 10 | 4′,5-Dihydroxy-3,6,7-trimethoxyflavone | Seeds |
| 11 | 5,3′-Hydroxy-6,7,4′-trimethoxy flavone | Leave |
| 12 | 5,7,3′-Trihydroxy-6,8,4′-trimethoxyflavone | Leaves |
| 13 | Acerosin-5-O-glucoside | Leaves |
| 14 | Corymbosin | Leave, twigs |
| 15 | 15 5-Hydroxy-6,7,8,3′,4′-pentamethoxyflavone | Leave, twigs |
| 16 | 5,6,7,8,3′,4′,5′-Heptamethoxyflavone | Leave |
| 17 | Casticin | Seeds |
| 18 | 5,3′-Hydroxy-7,8,4′-trimethoxyflavone | Stem bark |
| 19 | 3,6,7,3′,4′-Pentamethoxyflavone-5-O-glucopyransylrhamnoside | Stem bark |
| 20 | 5-Hydroxy-6,7,8,4′-tetramethoxyflavone | Leave, twigs |
| 21 | Vitexin cafeate | Stem bark |
| 23 | Vitexicarpin | Leaves |
| 24 | Vitexin cafeate | Stem bark |
| 25 | 3,6,7,3′,4′-Pentamethoxy-5-O-glucopyranosyl-rhamnoside | Stem bark |
| 26 | 4′-O-methylmyricetin-3-O-[4′′-O-β-D-galactosyl]-β-D-galactopyranoside | Stem bark |
| 27 | 3,4,5,7,3′,4′,5′-Hexahydroxy-6,8-dimethoxyflavone | Leave, twigs |
| 28 | 4,5-Dihydroxy-3′,4′-dimethoxyflavone-6-O-rhamnoglucoside | Leave, twigs |
| 29 | 5,7-Dihydroxychromone Seeds | Seeds |
Elevated blood glucose levels or increased insulin resistance are hallmarks of diabetes mellitus. Consuming foods that contain flavonoids aids in controlling insulin signaling and secretion, glucose absorption, fat storage, and carbohydrate digestion[15]. Flavonoids act on specific molecules to raise the number of beta cells (the cells in the pancreas that make insulin), decrease apoptosis (programmed cell death), boost insulin production, and improve high blood sugar levels by controlling the liver's glucose metabolism[16]. In a study involving 200000 people, the relationship between dietary intake of flavonoids and their subtypes and type 2 diabetes was examined. According to the study, consuming more anthocyanins from pears, apples, and blueberries reduces the risk of developing diabetes[17].
Flavonoids have been shown to have anti-cancer effects and to fight off free radicals, which can damage big molecules like DNA. Other anti-cancer effects include of: Blocking enzymes involved in metabolizing various substances like drugs, toxins, procarcinogens, and steroid hormones, which could hinder their conversion into cancer-causing chemicals and promote their elimination from the body[18]. Repairing DNA or, in the case of irreparable DNA damage, starting processes that result in programmed cell death[19]. preventing tumor invasion and angiogenesis, the development of a tumor's blood supply. controlling cellular metabolism and avoiding oxidative stress-related illnesses[20].
Research indicates that flavonoids may be used therapeutically to treat conditions like cardiovascular illnesses, osteoarthritis, Parkinson's disease, colitis, cancer pain, arthritis, and neuropathic pain because of their potent anti-inflammatory, pain-relieving, and antioxidative properties[21]. Chronic pain and the cellular inflammatory response can be reduced by flavonoids' ability to inhibit several cellular regulatory proteins, including cytokines and transcription factors[22].
Flavonoids have been acknowledged for their effectiveness as antiviral agents, capable of targeting various stages of viral infection at the molecular level to inhibit viral replication[23].
These antiviral flavonoids can be categorized further as follows: Certain extracellular components of the virus, like viral proteins on the virus's protein coat, to which flavonoids bind[24]. Flavonoids that stop the virus from attaching or entering host cells; in certain cases, flavonoids can connect to virions, which are the virus's infectious form outside of a host cell; they can also change the structure of the virus and make it more difficult for the virus to uncoat. Flavonoids that prevent viral infections by altering the immune system to lower the viral load or by interfering with host components required for a successful infection[25].
Researchers believe flavonoids may benefit the brain by protecting brain cells due to their anti-inflammatory properties and ability to fight free radicals[26]. According to research on animals, flavonoids can prevent the buildup of beta-amyloid plaque, a key indicator of Alzheimer's disease, in the brain. Additionally, flavonoids may increase blood flow to the brain, which would benefit the cardiovascular system and brain at the same time[27].
Although research on humans is still in its infancy, preliminary results are encouraging. A study that was published in The American Journal of Clinical Nutrition found that a high flavonoid consumption was associated with a decreased incidence of dementia and Alzheimer's disease. Over a 20-year period, researchers looked at the dietary habits of almost 3000 individuals without dementia symptoms and with an average age of 59 using data from the Framingham Heart Study. They discovered that people who consumed the most flavonoids (about 297 mg) were less likely to develop dementia or Alzheimer's than people who consumed about 123 mg) [28].
For decades, traditional medicine has utilized V. negundo, commonly referred to as the "five-leaved chaste tree", to cure a variety of illnesses, including cancer. The plant is rich in flavonoids, which are a class of compounds that have been shown to have anti-cancer properties.
Anti-proliferative activity: Flavonoids-rich V. negundo extract has been shown to inhibit the growth of cancer cells in vitro and in vivo. The extract has been found to induce apoptosis (cell death) in cancer cells, which can help to prevent the spread of cancer.
Antioxidant activity: Flavonoids-rich V. negundo extract has been found to have antioxidant activity, which can help to protect cells from damage caused by free radicals. This can help to prevent the development of cancer and other diseases.
Anti-inflammatory activity: The flavonoid-rich extract of V. negundo has been found to have anti-inflammatory qualities that may help reduce the incidence of ailments like cancer.
Anti-angiogenic activity: It has been found that V. negundo's flavonoid-rich extract prevents the formation of new blood vessels, which may stop cancer from growing and spreading.
The mechanism of action of flavonoids-rich V. negundo extract in cancerous diseases is complex and involves multiple pathways. Some of the key mechanisms include:
Inhibition of phosphoinositide 3-kinase/protein kinase B pathway: One important signaling system involved in cell growth and survival is the phosphoinositide 3-kinase (PI3K)/protein kinase B (AKT) pathway. It has been discovered that the flavonoid-rich extract of V. negundo inhibits this mechanism, potentially halting the development and spread of cancer.
Nuclear factor kappa-light-chain-enhancer of activated B cells pathway inhibition: Inflammation and cell survival are two important signaling pathways that are influenced by the nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) pathway. It has been found that V. negundo's flavonoid-rich extract inhibits this pathway, which may reduce the risk of cancer and other diseases.
Apoptosis induction: It has been discovered that a flavonoid-rich V. negundo extract causes cancer cells to undergo apoptosis, which may help stop the cancer from spreading.
Angiogenesis inhibition: It has been discovered that the flavonoid-rich extract of V. negundo inhibits the development of new blood vessels, potentially halting the growth and spread of cancer.
Uncontrolled cell development and the spread of aberrant cells through the circulation and lymphatic system are hallmarks of a variety of diseases collectively referred to as cancer. In 2019, cancer was the second most deadly disease, according to the World Health Organization (WHO)[29]. According to a recent GLOBOCAN assessment, there will likely be 19.3 million new cases of cancer and 10 million deaths from the disease in 2020. Furthermore, a WHO report released on February 4, 2020, warned that a 60% increase in cancer cases might occur in the next 20 years if the current upward trend continues. The aberrant regulation of several cell signaling pathways as a result of genetic and epigenetic changes is one of the many variables that contribute to cancer[30]. The mammalian target of rapamycin (mTOR)-PI3K-Akt pathway is one example of such a mechanism. Numerous studies have shown that the PI3K/Akt/mTOR pathway is dysregulated in a variety of cancers, including those of the breast, liver, colon, prostate, and stomach. Consequently, the PI3K/Akt/mTOR pathway has been the focus of molecular biomarker-based/targeted therapy for numerous cancers[31].
Natural substances obtained from plants have drawn interest lately because of their broad availability, low toxicity, affordability, and capacity to alter several pathways. Among these natural compounds, flavonoids have been found to have anticancer properties and have demonstrated potential against various forms of cancer. Flavonoids are low-molecular-weight polyphenolic compounds that fall into six classes: Isoflavonoids, flavanones, flavanols, flavones, and anthocyanidins[32]. The primary sources of these flavonoids in the human diet are fruits, vegetables, cereals, bark, roots, stems, flowers, and plant-based beverages including wine, green tea, and products made from cocoa.
Flavonoids have demonstrated a number of actions, including reversing multidrug resistance, causing cell cycle arrest and death, and preventing angiogenesis and cell proliferation. Additionally, they may interact with other medicinal drugs during biotransformation and have been reported to act as pro-oxidants in some situations. The true pharmacological potential of dietary flavonoids is, however, constrained by their quick metabolism, poor solubility, and inadequate absorption in the gastrointestinal system[20].
The downregulation of the PI3K/AKT/mTOR pathway, a critical signaling system involved in cell growth, proliferation, and survival, in malignant cells has been discovered to be significantly influenced by the flavonoid extract from V. negundo. Breast, lung, and colon cancer are among the cancer types that frequently exhibit dysregulation of the PI3K/AKT/mTOR pathway, which results in unchecked cell proliferation and tumor development. A study by Tewari et al[33] in 2022 found that the flavonoid extract from V. negundo inhibits the activation of this pathway, which causes apoptosis and stops breast cancer cells from proliferating. In a similar way, Sharma et al[34], in 2023 discovered that the extract reduced lung cancer cells' ability to proliferate and increased their apoptosis by blocking the PI3K/AKT/mTOR pathway. The flavonoids extract from V. negundo inhibited the PI3K/AKT/mTOR pathway in colon cancer cells, resulting in a decrease in cell proliferation and an increase in apoptosis, according to another study by Gill et al[1] in 2018. Furthermore, a review article by Liu et al[35] in 2018 highlighted the potential of flavonoids from V. negundo as a therapeutic agent in the treatment of cancer, due to their ability to downregulate the PI3K/AKT/mTOR pathway. Though further research is required to properly investigate its potential as a therapeutic agent in the treatment of cancer, the flavonoid extract from V. negundo has generally demonstrated encouraging findings in the downregulation of the PI3K/AKT/mTOR pathway in malignant cells.
Recent research has focused on the many flavonoids found in V. negundo and their ability to inhibit the PI3K/AKT/mTOR pathway. The flavonoid quercetin, found in V. negundo, has been demonstrated to inhibit the PI3K/AKT/mTOR pathway in breast cancer cells, resulting in a decrease in cell proliferation and an increase in apoptosis, per a study by Tewari et al[33], in 2022. The flavonoid kaempferol, which is also found in V. negundo, was discovered in another study by Sharma et al[34] in 2023 to inhibit the PI3K/AKT/mTOR pathway in lung cancer cells, which led to a decrease in cell proliferation and an increase in apoptosis. Furthermore, the flavonoid luteolin, found in V. negundo, blocked the PI3K/AKT/mTOR pathway in colon cancer cells, resulting in a decrease in cell proliferation and an increase in apoptosis, according to a study by Gill et al[1] in 2018. Additionally, a review paper by Liu et al[35], 2018 emphasized the potential of flavonoids found in V. negundo, such as apigenin, naringenin, and vitexin, to inhibit the PI3K/AKT/mTOR pathway and cause cancer cells to undergo apoptosis. All things considered, the fact that several flavonoids found in V. negundo block the PI3K/AKT/mTOR pathway raises the possibility that these substances could be used to prevent and treat cancer.
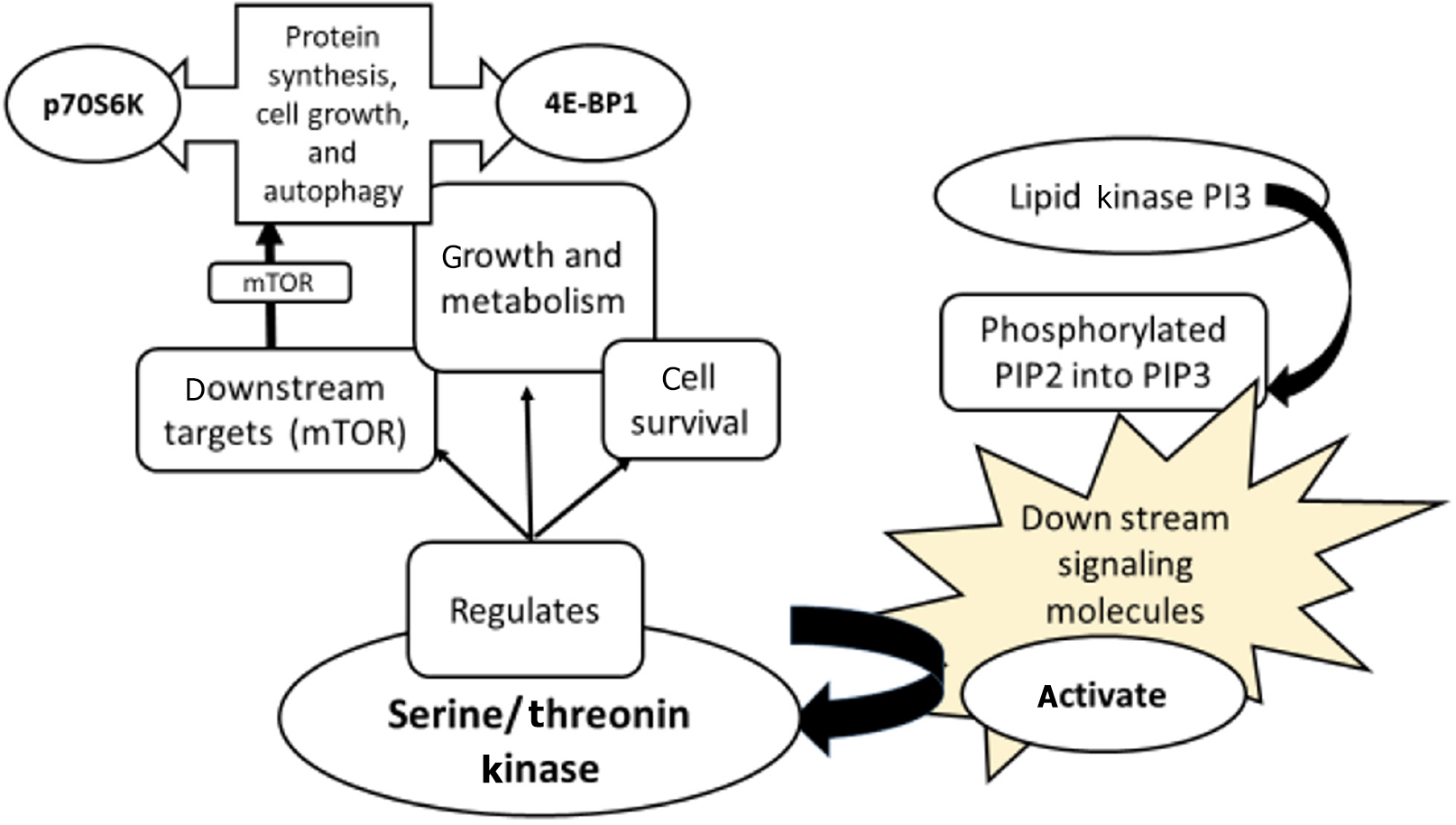
A complicated signaling cascade, the PI3K/AKT/mTOR/ribosomal protein S6 kinase (p70S6K)/Unc-51 like autophagy activating kinase (ULK) signaling pathway is essential for controlling a number of cellular functions, including as autophagy, cell growth, proliferation, and survival (Figure 2).
PI3K: Phosphorylinositol 4,5-bisphosphate (PIP2) is phosphorylated by the lipid kinase PI3K to yield phosphatidylinositol 3,4,5-trisphosphate (PIP3). PIP3 recruits and activates downstream signaling molecules by acting as a second messenger.
AKT: PIP3 activates a serine/threonine kinase called AKT. AKT is essential for controlling cell growth, survival, and metabolism. mTOR is one of the many downstream targets that AKT phosphorylates and activates.
mTOR: mTOR is a serine/threonine kinase that integrates inputs from multiple upstream pathways, including PI3K/AKT and amino acid availability. mTOR regulates protein synthesis, cell growth, and autophagy by phosphorylating and activating downstream targets, such as p70S6K and 4E-BP1.
p70S6K: mTOR activates the serine/threonine kinase P70S6K. The ribosomal protein S6, which is involved in cell proliferation and protein synthesis, is phosphorylated and activated by p70S6K.
ULK: mTOR activates a serine/threonine kinase called ULK. ULK is essential for controlling autophagy, a mechanism by which cells recycle proteins and organelles that are damaged or malfunctioning.
The PI3K/AKT/mTOR/p70S6K/ULK signaling pathway is regulated by various feedback mechanisms and is influenced by multiple upstream signals.
Growth factors: Insulin, IGF-1, and other growth factors activate PI3K/AKT signaling.
Amino acid availability: Amino acids, such as leucine, activate mTOR signaling.
Energy status: ATP and other energy-rich molecules regulate mTOR signaling.
Stress signals: Stress signals, such as DNA damage and oxidative stress, activate ULK and induce autophagy.
Neurodegenerative illnesses, diabetes, and cancer have all been linked to dysregulation of the PI3K/AKT/mTOR/p70S6K/ULK signaling pathway. For instance, in cancer, PI3K/AKT/mTOR pathway hyperactivation can enhance tumor growth and survival, whereas pathway inhibition can cause apoptosis and suppress tumor growth.
The PI3K/Akt/mTOR pathway is a highly dysregulated signaling cascade that is crucial to the development of many human cancer types, with each central node being highly activated in the majority of tumors[36]. These central nodes include receptor tyrosine kinase (RTK) class I [which includes the epidermal growth factor receptor (EGFR), human epidermal growth factor receptor 2 (HER2), etc.], Akt, phosphatase and tensin homolog deleted on chromosome 10 (PTEN), and phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha (PIK3CA)[37]. The majority of cancer types have mutations in the PIK3CA gene, which codes for the p110α catalytic subunit of PI3K. Furthermore, cancer can be brought on by PIK3CA mutations and independent PI3K pathway activation without Akt[38]. EGFR gene mutations, on the other hand, act as PI3K activators and aid in the development of non-small cell lung cancer. Similarly, glioblastoma frequently exhibits EGFR gene overexpression and amplification[39]. Although its overexpression is less common in other cancer types, such as ovarian, colon, salivary, biliary, and lung cancer, HER2, another member of the EGFR family, is overexpressed and amplified in invasive gastric and breast cancers. Numerous malignancies, including those of the breast, ovaries, colon, and pancreas, have been linked to somatic mutations and amplification in the pleckstrin homology (PH) domain (E17K) of Akt1.
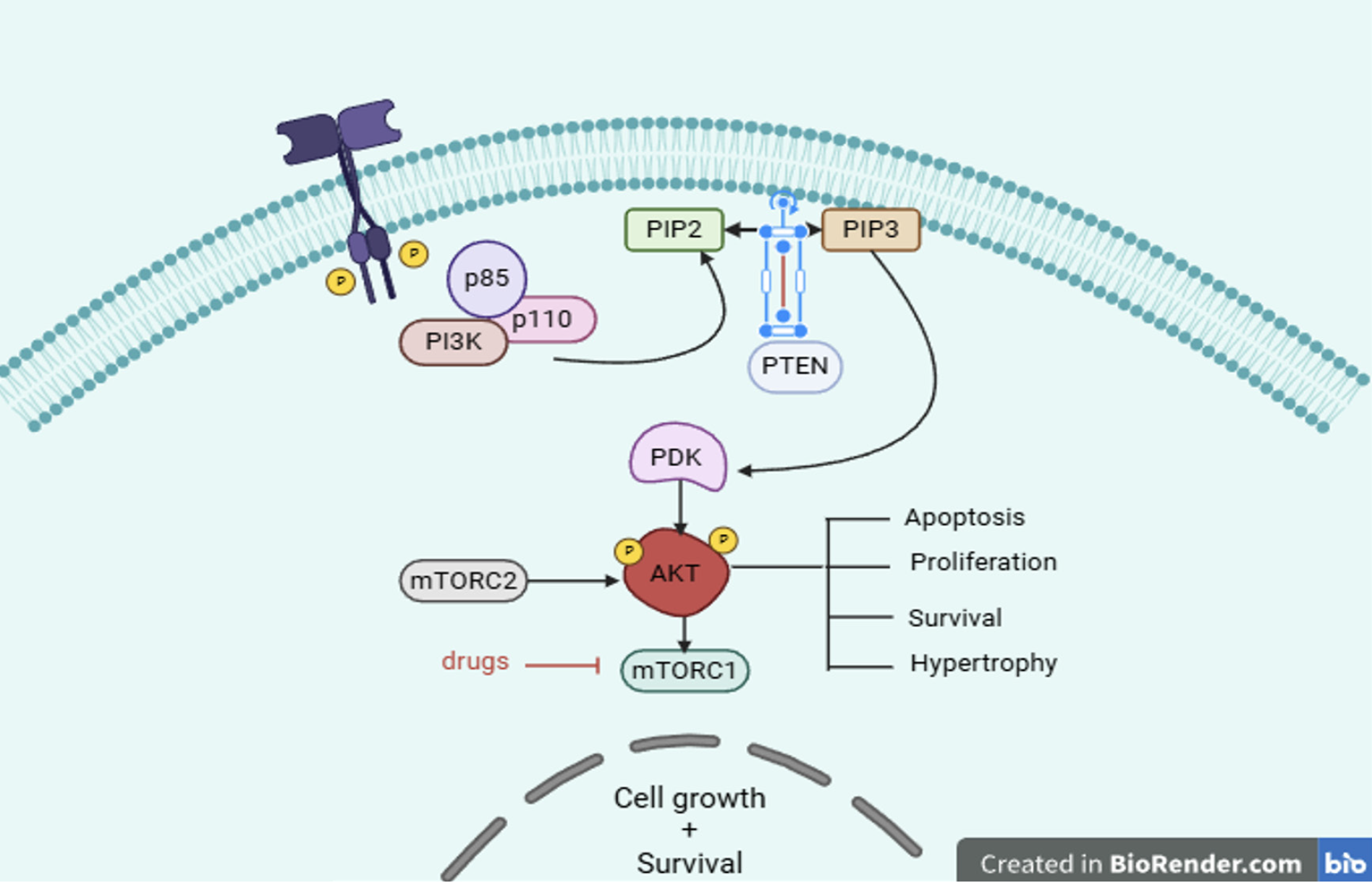
One of the most important therapeutic targets is the PI3K/Akt/mTOR pathway, which is a master regulator of cancer growth[40]. PIP2 is phosphorylated by the PI3Ks to PIP3, which phosphorylates Akt and affects the cell cycle, the growth and survival of cancer cells, and the proliferation of cancer cells. PTEN concurrently dephosphorylate PIP3 into PIP2 as PI3K antagonists. It may be possible to control the growth of some cancer types by completely blocking PI3K signaling[41].
Akt has a major impact on the control of tumor-associated cell processes, such as angiogenesis, migration, proliferation, cell cycle progression, cell survival, and epithelial-mesenchymal transition (EMT). Apoptosis is triggered and the proliferation of Akt-associated tumor cells is inhibited when the Akt pathway is blocked[42]. Tyrosine kinase receptors, integrin receptors, cytokine receptors, B and T cell receptors, and G-protein-coupled receptors (GPCRs) all utilize PIP3 produced by PI3Ks to start the Akt pathway. PIP3 attracts Akt to the plasma membrane and binds to its PH domain to change its conformation instead of directly activating Akt. As a result, the kinase domain at the Thr308 site can be phosphorylated by phosphoinositide-dependent kinase-1 (PDK1). Among other biological effects, the activated Akt promotes cell growth and survival by phosphorylating a number of downstream proteins in the cytosol, plasma membrane, and nucleus[43]. However, protein phosphatase 2A dephosphorylates Akt at Thr308 and Ser473 residues, inhibiting Akt and potentially increasing fibroblast proliferation, vasodilatation, cell cycle arrest, blockage of the fork head box O1 protein, activation of B-cell lymphoma 2 (Bcl-2) associated agonist of cell death (BAD), which reduces autophagy and apoptosis, and GLUT4 translocation. A variety of cancer types have been found to have aberrant Akt signaling pathways in several scientific investigations, which can occasionally make the tumor more aggressive. While Akt2 amplification has been shown in head and neck squamous cell carcinoma, pancreatic, ovarian, and breast cancers, Akt gene abnormalities are present in several human malignancies, including as gastric carcinoma, glioma, and gliosarcoma[37].
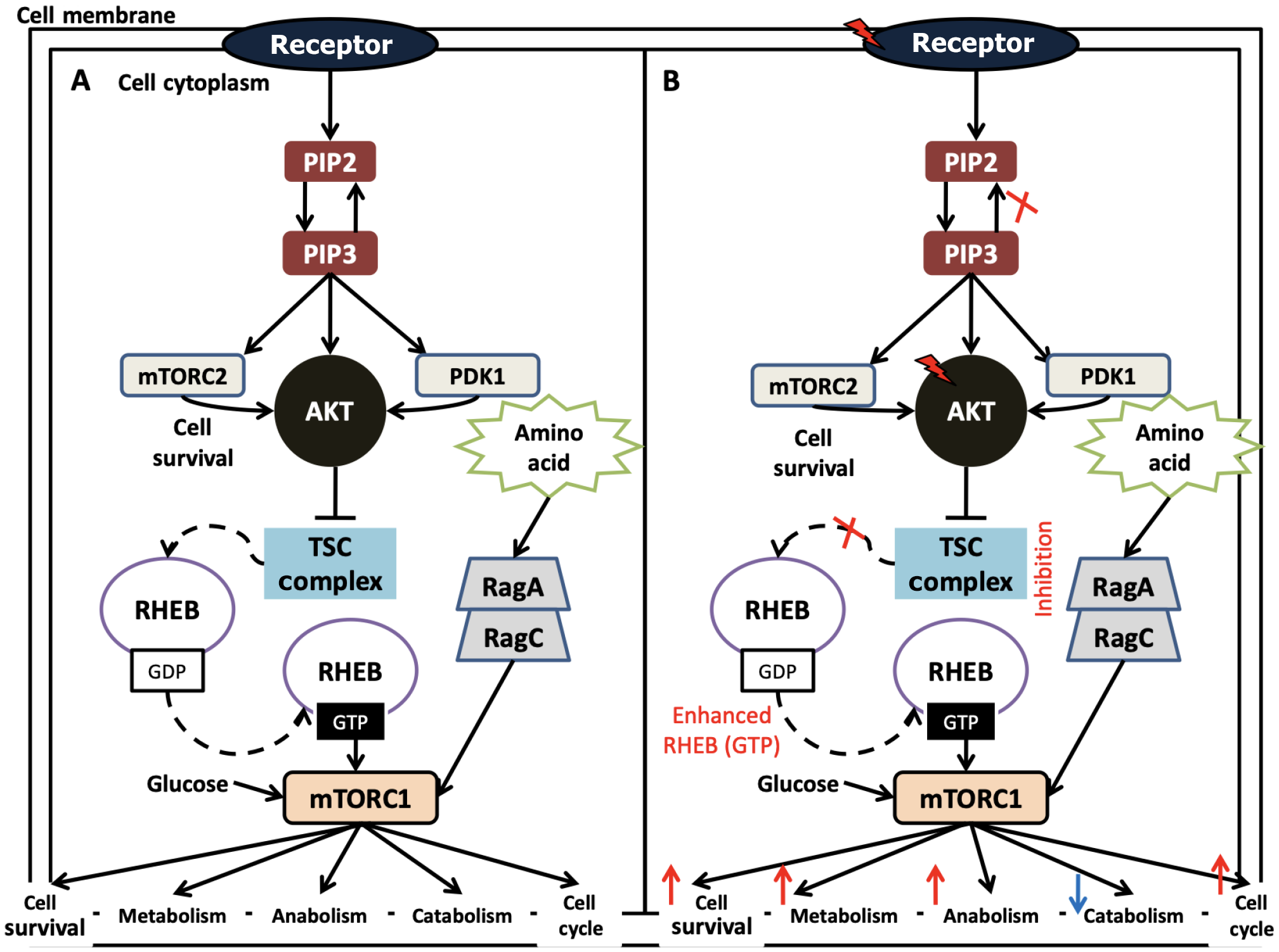
In response to upstream stimuli, the mTOR pathway is also essential for controlling other processes, such as protein synthesis, metabolism, cell growth, and survival[44]. Two separate complexes, mTORC1 and mTORC2, make up this pathway, which is a downstream substrate of PI3K and Akt. Either directly via phosphorylating mTOR at Ser2448 or indirectly by phosphorylating and blocking tuberous sclerosis complex 2 (TSC2), Akt stimulates mTOR activity[45]. Akt increases mTOR activity and strengthens its mTOR inhibitory impact by directly phosphorylating TSC2 at S939 and T1462. TSC2 functions as a negative regulator of GTPase-activating protein activity and forms a heterodimeric complex with TSC1. TSC2 suppression by Akt increases mTORC1 by inhibiting the activity of the Ras-related GTPase Rheb, an important activator of mTORC1[46]. Through a number of pathways, including increased growth factor receptor signaling, decreased autophagy, lipid metabolism, glycolytic metabolism, angiogenesis, and cancer cell motility, hyperactivation of this cascade can accelerate the growth and progression of tumors (Figure 3). The mTOR signaling activity is regulated by a variety of growth factors, such as transforming growth factor, insulin-like growth factor 1, platelet-derived growth factor, vascular endothelial growth factor (VEGF), hepatocyte growth factor, and epidermal growth factor[47].
(1) PI3K/AKT pathway and tumor cell survival: One important signaling mechanism that controls cell survival and death is the PI3K/AKT pathway. This pathway is frequently overactivated in tumor cells, which increases cell survival and apoptosis resistance. Pro-apoptotic proteins like BAD and BAX are phosphorylated and rendered inactive by AKT activation, while anti-apoptotic proteins like BCL-2 and BCL-XL are activated; (2) mTOR and tumor cell growth: One important modulator of cell growth and proliferation is the mTOR pathway. mTOR is frequently overactivated in tumor cells, which promotes protein synthesis and cell division. Protein synthesis and cell proliferation are regulated by downstream targets like p70S6K and 4E-BP1, which are phosphorylated and activated when mTOR is activated; (3) p70S6K and tumor cell proliferation: A downstream target of mTOR, p70S6K is essential for controlling cell division and protein synthesis. p70S6K is frequently overactivated in tumor cells, which promotes protein production and cell division; (4) ULK and tumor cell autophagy: ULK is a key regulator of autophagy, a process by which cells recycle damaged or dysfunctional organelles and proteins. In tumor cells, ULK is often downregulated, leading to decreased autophagy and increased cell survival; (5) PTEN and tumor cell suppression: PTEN is a tumor suppressor gene that regulates the PI3K/AKT pathway. In tumor cells, PTEN is often downregulated or mutated, leading to hyperactivation of thePI3K/AKT pathway and increased cell survival and proliferation; and (6) AMPK and tumor cell metabolism: AMPK isa key regulator of cellular metabolism and energy homeostasis. In tumor cells, AMPK is often downregulated, leading to increased glycolysis and glucose uptake, which promotes tumor cell growth and survival.
Some key relationships between the components and signalling pathways in tumor cells include: (1) PI3K/AKT/mTOR pathway: This pathway is often hyperactivated in tumor cells, leading to increased cell survival, proliferation, and metabolism; (2) PTEN/PI3K/AKT pathway: PTEN is a tumor suppressor gene that regulates the PI3K/AKT pathway. Downregulation or mutation of PTEN leads to hyperactivation of the PI3K/AKT pathway and increased tumor cell growth and survival; (3) mTOR/p70S6K pathway: This pathway is often hyperactivated in tumor cells, leading to increased protein synthesis and cell growth; (4) ULK/mTOR pathway: ULK is a key regulator of autophagy, and its downregulation leads to decreased autophagy and increased tumor cell survival; and (5) AMPK/mTOR pathway: AMPK is a key regulator of cellular metabolism and energy homeostasis. Downregulation of AMPK leads to increased glycolysis and glucose uptake, which promotes tumor cell growth and survival.
These connections show how intricately signaling pathways and tumor cell components interact, and how crucial it is to comprehend these connections in order to design successful cancer treatments.
The process by which different signaling pathways contribute to the development of distinct malignancies is intricate and multifaceted. The following are some instances of signaling pathways that have been linked to the onset and spread of various cancer types: (1) PI3K/AKT pathway: Numerous cancer forms, including breast, lung, and colon cancer, frequently exhibit dysregulation of this pathway, which is important in cell survival, proliferation, and metabolism; (2) MAPK/ERK pathway: Numerous cancer forms, such as melanoma, lung, and colon cancer, frequently exhibit dysregulation of this pathway, which is important in cell proliferation, differentiation, and survival; (3) NF-κB pathway: Numerous cancer types, including breast, lung, and colon cancer, frequently exhibit dysregulation of this pathway, which is implicated in inflammation, immune response, and cell survival; (4) WNT/β-catenin pathway: This pathway, which is implicated in cell survival, differentiation, and proliferation, is frequently dysregulated in a variety of cancer types, including as lung, breast, and colon cancer; (5) Notch pathway: This pathway, which plays a role in cell survival, differentiation, and proliferation, is frequently dysregulated in cancers such as breast, lung, and colon cancer; (6) Hedgehog pathway: Skin, lung, and colon cancer are among the cancer types that frequently exhibit dysregulation of this pathway, which is implicated in cell proliferation, differentiation, and survival; and (7) mTOR pathway: In many cancer types, such as breast, lung, and colon cancer, this pathway-which is involved in cell development, proliferation, and metabolism-is dysregulated.
Some specific examples of signaling pathways involved in different types of cancer include: (1) Breast cancer: PI3K/AKT, MAPK/ERK,NF-κB, and WNT/β-catenin pathways; (2) Lung cancer: PI3K/AKT, MAPK/ERK, NF-κB,and WNT/β-catenin pathways; (3) Colon cancer: PI3K/AKT, MAPK/ERK,NF-κB, and WNT/β-catenin pathways; (4) Melanoma: MAPK/ERK, NF-κB, and WNT/β-catenin pathways; and (5) Leukemia: PI3K/AKT, MAPK/ERK, and NF-κB pathways
There are several reasons why these signaling pathways may be involved in cancer, including: (1) Genetic mutations: These pathways may become dysregulated as a result of mutations in genes that encode their constituent parts; (2) Epigenetic changes: These pathways may also become dysregulated as a result of epigenetic changes such DNA methylation and histone modification; and (3) Environmental influences: The deregulation of these pathways can also be caused by environmental factors, such as exposure to carcinogens.
Gaining knowledge about how these signaling pathways contribute to cancer can help create novel treatments for the disease.
RTK over activation: AKT activation, plasma membrane localization, and the expression of one or more class I PI3K family isoforms are typically the outcomes of the traditional route being activated when cancer growth factors stimulate RTKs or GPCRs[48]. Changes in the genes of the insulin receptor, fibroblast growth factor receptor (FGFR), platelet-derived growth factor receptor, VEGF receptor, hepatocyte growth factor, leukocyte RTK, and EGFR are largely responsible for treatment failure by activating the PAM pathway[49].
Only class I of the several PI3K forms in cancer are capable of lipid phosphorylation in response to growth stimulation. PI3K (class I) is made up of two different subunits: The catalytic subunit p110 and the regulatory subunit p85[50]. Through its Src homology 2 domain, the PI3K regulatory subunit p85 binds specifically to phosphorylated tyrosine residues on activated RTKs. As a result, a fully functional PI3K enzyme is formed by the catalytic subunit 110. The most commonly mutated oncogene across various tumor types is the activating mutation in PIK3CA, which encodes the p110α catalytic subunit of PI3K[51]. Human cancers such as colorectal cancer (CRC), breast cancer, lung cancer, gastric cancer, prostate cancer, and cervical cancer frequently include mutations or amplified forms of PIK3CA. Most mutations take place in the vicinity of exons 9 and 20. While H1047, which is next to the end of the catalytic domain, improves the interaction of p110α with the lipid membrane, hotspots in the helical phosphatidylinositol (inositol phospholipid) kinase homology domain, specifically E542K and E545K, lessen the inhibition of p110α by the p85regulatory subunit[52].
Mutations in the p110β, p110γ, and p110δ subunits are extremely rare, but when they are overexpressed, they can dramatically increase oncogenicity in cultured cells. PIK3CA mutations are found in melanoma, head and neck squamous cell carcinoma, gallbladder cancer, and gastric cancer. Additionally, it is known that mutations in PIK3CA E542K and PIK3CA E545K promote the formation and growth of cervical cancer[53]. Furthermore, the PIK3CA mutation promotes prostate cancer in mice and is associated with a poor outcome for prostate cancer. Importantly, the coexistence of PTEN loss and PIK3CA mutation in prostate cancer patients may accelerate carcinogenesis and cancer progression by hyperactivating the PAM pathway. FGFR3 mutations are frequently linked to PIK3CA mutations in metastatic non-muscle invasive bladder cancer. It has been repeatedly shown that PAM pathway mutations are present in 25% of osteosarcoma patients[53]. Actually, the survival and proliferation of osteosarcoma cells depend on mTOR and PIK3CA. Glioblastoma multiforme, testicular germ cell cancers, and Ewing's sarcoma are frequently associated with mutations in the PI3K family of genes, including PIK3CA and PIK3R1. The PAM pathway pattern is altered as a result of these alterations. Hepatocellular cancer, small-cell lung cancer (SCLC), non-SCLC (NSCLC), and ovarian serous cystadeno-carcinoma have all been linked to mutations in PIK3CA, PIK3R1, and PIK3R2[44].
Similarly, mutations in PIK3CA, and to a lesser extent, PIK3R1 and PIK3R2, are the primary cause of the PAM pathway's dysregulation in CRC. Furthermore, KRAS mutations are typically linked to CRC PIK3CA mutations. Mutations in PIK3CA, PIK3R1, and PIK3R2 are also linked to genetic changes in the PAM pathway in renal cancer. Furthermore, via the AKT/glycogen synthase kinase 3 beta (GSK-3β)/β-catenin signaling pathway, PIK3R1 can control the EMT and the stem-like phenotype of renal cell carcinoma cells. Genetic changes in PI3K family genes and PTEN, such as somatic mutations of PIK3CA, PIK3CG, PIK3C2A, and PIK3C2G, are linked to oesophageal squamous cell carcinoma[50]. Additionally, poorly differentiated thyroid carcinoma and anaplastic thyroid cancer frequently exhibit mutations in PAM pathway genes, including PIK3CA, PIK3CG, PIK3C2G, PIK3C3, PIK3R1, and PIK3R2. Therefore, aberrant signaling activity may be caused by mutations in the PAM pathway that affect Ras and PI3K p110 subunits, RTKs, and growth factors[54].
The tumor suppressor PTEN, which dephosphorylates PIP3 and transforms it back into PIP2, is the primary mechanism by which the PI3K/PIP3 signal is terminated[55]. PTEN affects cell growth and survival by acting as a critical negative regulator of the PAM pathway. These intracellular signals are produced continuously when PTEN is lost. Many cancers, including primary and metastatic breast cancer, have loss-of-function mutations in PTEN, which cause the PAM pathway to become hyperactivated and promote cell proliferation. Anti-estrogen treatment resistance is linked to PTEN downregulation and PAM pathway activation[56]. PTEN mutations are commonly found in gastric cancer. Furthermore, PRL-3 overexpression and/or amplification dephosphorylates PTEN, which in turn increases signals via the PAM pathway in human gastric cancer. CRC also exhibits PTEN mutations. Crucially, in primary CRC, loss of TGF-β signaling promotes EMT and tumor aggressiveness by activating the PAM pathway and upregulating PRL-3. PTEN mutations have also been reported in bladder cancer, where the loss of PTEN combined with TP53 changes has a negative impact that speeds up the tumor's growth[57]. Because the PAM pathway can act as a pro-survival factor in leukemia stem cells, genetic defects in PTEN are likely to be present in leukemia-dependent mechanisms, such as leukemogenesis and hematopoiesis. PTEN regulates hematopoiesis, leukemogenesis, and hematopoietic stem cell activity via a niche-dependent mechanism. Brain cancer, glioblastoma multiforme, anaplastic/poorly differentiated thyroid cancer, SCLC, NSCLC, melanoma, esophageal cancer, gallbladder cancer, pancreatic cancer, renal cell carcinoma, prostate cancer, testicular germ cell tumors, cervical cancer, ovarian cancer, and various types of sarcoma are also associated with loss-of-function alterations, specifically deletions, in PTEN. These changes result in the typical pathogenic effects of the PAM pathway.
Intracellular proteins with PH or FYVE zinc finger domains can interact directly with phosphorylated phosphatidylinositol lipids found on the inner side of the cell membrane[58]. AKT and PDK1 are selectively bound by PIP3, which causes them to gather close to the membrane. AKT can target a wide range of substrates after it is activated and travels from the cell membrane to the cytoplasm and nucleus. Human cancers seldom have mutations in the AKT gene. However, gain-of-function missense mutations and amplification have been found in genes encoding one of the three isoforms of the oncogenic protein AKT, which regulates cell survival, proliferation, growth, apoptosis, and glycogen metabolism. In the PH domain, the most prevalent mutation is the AKT1 point mutation. It happens when glutamic acid at position 17 is replaced with lysine (E17K), which causes AKT1 to continuously relocate to the cell membrane and increase its activity[59]. Additional activating mutations include the E49K substitution in the PH domain of AKT1 and the G171R alteration in the kinase domain of AKT3.
These point mutations cause cancer cell lines to have markedly higher levels of active p-AKT, and p-AKT levels are linked to susceptibility to AKT inhibition. Additional somatic variations in the AKT PH domain have also been discovered by high-resolution sequencing studies in breast cancer. However, their function as oncogenesis drivers has not been fully elucidated because of the very low prevalence of AKT mutations. Actually, activating mutations or amplifications upstream of AKT, like those in PIK3CA or PTEN, growth factor or cytokine receptors, and intracellular oncogenes like Ras, usually cause alterations in AKT activity by increasing the expression and activity of any or all three AKT isoforms. AKT gene amplifications are more prevalent than AKT mutations and have been seen in the thyroid, esophagus, pancreas, colon, gastric, ovarian, breast[60], and gastric cancer. AKT2 isoforms are typically implicated in molecular malignancies, which are tumors with significant amplifications. Furthermore, even when normal PI3K and PTEN activity is still present, post-translational changes of AKT, including lysine alterations, tyrosine phosphorylation, O-GlcNAcylation, acetylation, and sumoylation, are essential for sustaining AKT hyperactivation in malignancies. A fraction of premalignant breast lesions had increased AKT. In particular, 38% of invasive breast cancers and 33% of ductal carcinoma in-situ lesions overexpress p-AKT, while the majority of tumors (79%) express the estrogen receptor (Figure 4).
The PI3K/Akt/mTOR signaling pathways are critical for many aspects of cell growth and survival in both healthy and pathological conditions, including cancer[56]. When cells are triggered externally, the interaction between p85 and insulin receptor substrate attracts class IA PI3K to the cell membrane by activating RTKs or GPCRs. PIP2 is converted to PIP3, a secondary messenger that controls cell growth, survival, and proliferation, by the class IA PI3Ks phosphorylating it at position 3 of the inositol ring[60]. PDK-1 phosphorylates Akt when PIP3 binds to its PH domain and transports it to the cell membrane. Once activated, Akt goes on to phosphorylate a number of proteins that are crucial for regulating the cell cycle, growth, proliferation, apoptosis, and cell survival. The phosphatase PTEN inhibits signaling through the PI3K-Akt pathway and negatively controls the mTOR cascade by interacting with TSC1/2. Deregulated mTOR pathway components, such as PI3Kamplification/mutation, loss of PTEN function, overexpression of Akt, ribosomal protein S6 kinase beta-1, eukaryotic translation initiation factor 4Ebinding protein 1, and overexpression of eukaryotic translation initiation factor 4E, have been identified in a number of cancers, particularly melanoma. Changes in important mTOR signaling components significantly affect the growth of tumors in melanoma.
Natural compounds and herbs like resveratrol, diosgenin, timosaponin III,3,3'-diindolylmethane, epigallocatechin gallate, pomegranate, curcumin, gallic acid, and genistein may either directly or indirectly inhibit the mTOR pathway, according to a study[61]. The section that follows provides a list of well-known flavonoids that have been demonstrated to function as anticancer agents in a range of cancer models.
Flavonoids have demonstrated a number of actions, including reversing multidrug resistance with regard to tumor cells and preventing angiogenesis and cell proliferation, including cell cycle arrest and apoptosis.
Numerous activities that can aid in the prevention or treatment of cancer have been demonstrated by flavonoids, which have been thoroughly investigated for their possible anti-cancer qualities. The following are some of the main ways that flavonoids affect the growth and development of tumors:
Inhibiting cell proliferation: It has been demonstrated that flavonoids prevent cancer cells from growing and proliferating, which may assist to halt or decrease the disease's course.
Inhibiting angiogenesis: Flavonoids have been shown to inhibit the formation of new blood vessels, which is a critical step in the growth and development of tumors.
Inducing cell cycle arrest: It has been demonstrated that flavonoids cause cell cycle arrest, which may help stop cancer cells from proliferating and dividing.
Inducing apoptosis: It has been demonstrated that flavonoids cause cancer cells to undergo apoptosis, or programmed cell death, which may aid in the body's removal of cancerous cells.
Reversing multidrug resistance: It has been demonstrated that flavonoids can reverse multidrug resistance in cancer cells, increasing the cells' susceptibility to chemotherapy and other cancer treatments.
Complex and multifaceted, the processes by which flavonoids display these effects may entail the manipulation of many signaling pathways, such as the NF-κB system, the MAPK pathway, and the PI3K/AKT circuit.
Onions, scallions, kale, broccoli, apples, berries, and teas are just a few of the fruits and vegetables that contain quercetin, which is a member of the flavonol subclass of flavonoids[62]. According to studies, quercetin can prevent the phosphorylation of ribosomal protein S6K and 4E-BP1, two of mTOR's main downstream targets. In many animal models and cancer cell lines, its dose-dependent anticancer effects have been reported[63]. Quercetin has been demonstrated to be more cytotoxic than ellagic acid and to inhibit the advancement of the cell cycle in the S phase of breast cancer and leukemia cells. The life span of tumor-bearing mice has also been shown to be around five times longer than that of untreated animals[64].
Oranges, berries, almonds, tea, red wine, and vegetables (tomatoes) are among the foods and beverages that frequently include myricetin, a plant-based flavonoid. By disrupting the PI3K/Akt/mTOR signaling pathway, it has been demonstrated to have anticancer effects, preventing cell cycle progression and proliferation and inducing apoptosis and autophagy in human colon cancer cells[65]. In addition, myricetin protects UVB-induced skin cancer and inhibits the proliferation of breast cancer cells. According to a study, myricetin inhibits tube formation, cell migration, and the PI3K/Akt/mTOR signaling pathway in human umbilical vascular endothelial cells, and it causes death by producing reactive oxygen species (ROS)[66].
It has been shown that kaempferol, a naturally occurring flavonol that is frequently found in foods and vegetables such as broccoli, Brussels sprouts, spinach, apples, grapefruit, kale, beans, and green tea, possesses anticancer and antioxidant qualities[67]. According to studies, it causes cell cycle arrest at the G2/M phase, inhibits cell migration, induces apoptosis, and lowers the levels of mTOR, pm-TOR, PI3K, p-PI3K, and Akt proteins in the human malignant melanoma A375 cell line, among other powerful anticancer actions[68]. Additionally, by activating mitogen-activated protein kinase (MAPK) signaling, it exhibits anti-proliferative effects on human endothelial cells and lung cancer[69]. Recent studies have also demonstrated its strong anticancer and anti-proliferation properties in liver cancer, where it suppresses the invasion, migration, and proliferation of HepG2 cells and triggers apoptosis via controlling PTEN and microRNA-21, which in turn suppresses the PI3K/Akt/mTOR pathway[70].
Isorhamnetin, a flavonoid extracted from the healing herb Hippophae rhamnoides L., has demonstrated anti-cancer properties in CRC[71]. By inhibiting the PI3K/Akt/mTOR pathway, it has been shown to prevent cell proliferation and induce G2/M phase cell cycle arrest in both colorectal and breast cancer[72].
Green tea, a popular beverage around the world, includes catechins such epicatechin and epigallocatechin-3-gallate[73]. In a number of cancer models, epigallocatechin-3-gallate has shown notable anti-cancer benefits. According to recent studies, epicatechin inhibits cell development by interacting with and neutralizing ROS inside the cell and controlling the MAP kinase pathway[74]. Additionally, in HCT-116 and renal tubular cancer, it has demonstrated inhibitory effects on Akt and NF-κB when paired with panaxadiol or cisplatin[75]. According to some research, it inhibits the PI3K/Akt and mitogen-activated protein kinase kinase/extracellular signal-regulated kinase (MEK/ERK) signaling pathways, which in turn lowers the overexpression of P-glycoprotein caused by doxorubicin. Additionally, it causes apoptosis in human breast cancer T47D cells and downregulates the MEK/ERK and PI3K/Akt signaling pathways[66].
Like other plant polyphenols, fisetin, a flavonol found in a variety of fruits and plants, including kale, strawberries, grapes, apples, persimmons, onions, and kiwis, has antioxidant qualities[63]. A dietary tetrahydroxy flavone called fisetin has been shown in a study to reduce the PI3K/Akt/mTOR signaling cascade, thereby inhibiting human non-small cell lung cancer cells[65]. Fisetin has been shown to lower PTEN protein levels in A549 lung cancer and multiple myeloma U266 cells[76]. Additionally, it has been shown to reduce, in a dosage-dependent way, the phosphorylation of Akt, mTOR, microphthalmia-associated transcription factor, and p70S6K proteins in human melanoma 451 Lu cells[77].
Glycyrrhiza glabra, Lupinus sp., and Lotus pedunculatus are among the medicinal plants that contain the isoflavone lupiwighteone[63]. Numerous cancer cells, including neuroblastoma, prostate, and breast cancer, have shown evidence of its anticancer properties. Furthermore, by blocking the PI3K/Akt/mTOR pathway, it has been demonstrated to cause both caspase-dependent and independent apoptosis in breast cancer cells[78].
Parsley, celery, and chamomile are plants that contain the active component apigenin, a flavone[79]. It has been demonstrated to prevent the formation and progression of cancer by blocking the PI3K/Akt/FoxO pathway and inhibitory-κB kinase alpha activation in a TRAMP mouse model. Additionally, it inhibits the PI3K/Akt/mTOR pathway in liver cancer cells, which results in the induction of autophagy and the reduction of cell growth.
Citrus fruits contain nobiletin, commonly referred to as 5,6,7,8,3′,4′-hexamethoxyflavone, a substance that is a member of the polymethoxy flavonoid group[80]. It has antioxidative, anti-inflammatory, anticancer, cardio/neuro-protective, and anti-metabolic actions, among other pharmacological characteristics[81]. By inhibiting the release of important angiogenesis regulators such as Akt, VEGF, NF-κB, and hypoxia-inducible factor 1-alpha, studies have shown that it can prevent the growth of ovarian cancer cells. Furthermore, it has been noted that normal ovarian epithelial cells remain viable at doses below 40 μmol/L[82].
It has been discovered that galangin, a naturally occurring flavonoid obtained from honey and the Chinese herbal remedy Alpinia officinarum Hance (Zingiberaceae), has a number of advantageous qualities, such as anti-cancer, antiviral, antibacterial, and antidiabetic actions, without causing any problems. It has been demonstrated that galangin prevents kidney cancer cells from proliferating, migrating, and invading[83]. Furthermore, it can inhibit the PI3K/Akt/mTOR signaling pathway and cause apoptosis[84].
Numerous studies have been conducted on hesperidin, a dietary flavanone present in citrus fruits such as oranges, lemons, and limes[85]. Numerous biological effects, such as its anti-inflammatory, antioxidant, and carcinogenic qualities, have been linked to hesperidin in both in vitro and in vivo investigations. According to scientific research, hesperidin can interact with a variety of cellular targets to cause apoptosis and cell cycle arrest as well as prevent the growth of cancer cells[86]. It has also been shown to suppress angiogenesis, chemoresistance, and tumor metastasis. By blocking the aurora-a driven PI3K/Akt/mTOR and GSK-3β pathway, hesperidin administration was shown to cause apoptosis and autophagy in a study using a colon cancer mice model.
Cherries, berries, and grapes are among the many fruits and vegetables that contain anthocyanins, a type of flavonoid, in the form of glycosides bonded to different sugars[87]. Cyanidin, a member of the anthocyanin family, has been demonstrated to deactivate PI3K/Akt signaling in hepatocellular carcinoma, thereby blocking cell migration and reversing oxaliplatin-induced EMT biomarker alterations[88]. In human osteosarcoma cells, pelargonidin, another anthocyanin group member, exhibits anticancer effects. This particular anthocyanin causes cell cycle arrest in the G2/M phase, autophagy, and a reduction in mitochondrial membrane potential brought on by ROS. Furthermore, it suppresses p-PI3K and p-Aktina production in a dosage-dependent manner[89].
By regulating a variety of cell signal transduction pathways, delphinidin has a crucial role in preventing oxidative stress, inflammation, angiogenesis, metastasis, and carcinogenesis in a number of malignancies, including breast, prostate, lungs, liver, colon, and fibrosarcoma[90]. By inhibiting the PI3K/Akt and ERK1/2 MAPK signaling pathways, delphinidin has shown anti-proliferative effects in ovarian cancer cells[91]. By blocking the PI3K/Akt and ERK1/2 mitogen-activated protein kinase signaling pathways, delphinidin treatment has been demonstrated to reduce SKOV3 cell proliferation in a dose-dependent manner[92].
Commonly present in cruciferous vegetables, sulforaphane is an isothiocyanate with anticancer qualities and the ability to naturally alter the PI3K/Akt signaling pathway[93]. According to a study, sulforaphane stops lung cancer cells from growing by preventing Akt phosphorylation and lowering PTEN expression in mouse lung cancer xenografts. According to these results, sulforaphane may be a useful anticancer therapy for lung cancer[94].
A complex network of molecules called the PI3K/AKT/mTOR/p70S6K/ULK signaling pathway sends signals internally to control a number of cellular functions, such as autophagy, growth, proliferation, and survival. PI3K is activated at the start of the route, phosphorylating PIP2 to create PIP3. AKT, an aserine/threonine kinase that is essential for controlling cell survival and metabolism, is subsequently recruited and activated by PIP3. mTOR, a crucial modulator of protein synthesis and cell proliferation, is phosphorylated and activated by AKT. In turn, p70S6K, a kinase that controls protein synthesis and cell division, is phosphorylated and activated by mTOR. Furthermore, ULK, a kinase that controls autophagy-the process by which cells recycle damaged or malfunctioning organelles and proteins-is phosphorylated and inhibited by mTOR. Signals that encourage cell growth, proliferation, and survival while preventing autophagy are transmitted when this pathway is activated. On the other hand, when this pathway is blocked, signals are sent that promote autophagy while preventing cell growth, proliferation, and survival. Numerous feedback mechanisms tightly regulate the PI3K/AKT/mTOR/p70S6K/ULK signaling pathway, which is impacted by a number of upstream signals such as growth factors, hormones, and energy status. Numerous illnesses, such as diabetes, cancer, and neurodegenerative diseases, have been linked to the dysregulation of this system.
Several molecules and pathways work in concert to transmit signals internally inside the PI3K/AKT/mTOR/p70S6K/ULK signaling pathway. For instance, PIP3 is produced when PI3K is activated, and PIP3 recruits and activates AKT. Following AKT's phosphorylation and activation of mTOR, p70S6K is phosphorylated and activated. Autophagy is inhibited when ULK is inhibited, whereas protein synthesis and cell proliferation are regulated when p70S6K is activated. A number of feedback mechanisms also control the system, such as PTEN, a tumor suppressor gene that controls cell growth and survival, inhibiting PI3K. Numerous upstream cues, such as growth factors, hormones, and energy status, also affect the route and have the ability to modify its activity and control cellular functions.
Cell growth, proliferation, survival, and autophagy are all crucially regulated by the PI3K/AKT/mTOR/p70S6K/ULK signaling pathway. The route is impacted by several upstream signals, such as growth factors, hormones, and energy status, and is strictly controlled by a number of feedback mechanisms. Numerous illnesses, such as diabetes, cancer, and neurodegenerative diseases, have been linked to the dysregulation of this system. The development of innovative therapeutic approaches for the treatment of various illnesses depends on an understanding of the internal signal transmission within this route.
In recent years, there have been substantial changes in the modern development and use of traditional medicine. For ages, people have employed traditional medicine, which includes techniques like acupuncture, herbalism, and Ayurveda, to prevent and treat a variety of illnesses. However, traditional medicine has frequently been perceived as inferior or unscientific due to the development of modern medicine. However, efforts have been made to incorporate traditional medicine into contemporary healthcare systems as awareness of its potential advantages has grown in recent years.
The growing use of evidence-based research to confirm the efficacy of traditional medicine is one of the major advancements in its contemporary application. In order to assess the safety and effectiveness of traditional medicine procedures and products, clinical trials and research have been carried out. For instance, research has demonstrated that acupuncture can help people with chronic illnesses including depression and arthritis by lowering pain and enhancing sensations.
The increasing understanding of the value of traditional medicine in tackling global health issues is another development. The potential of traditional medicine to help accomplish the Sustainable Development Goals, especially those pertaining to universal health coverage and health security, has been acknowledged by the WHO. In order to facilitate the incorporation of traditional medicine into national health systems, the WHO has created frameworks and guidelines.
The modernization of traditional medical procedures and supplies is also becoming more and more popular. In order to comprehend the mechanisms of action of goods used in traditional medicine and to create novel products and cures, this has involved the use of contemporary technologies like proteomics and genomics. For instance, scientists have employed genomics to find the active ingredients in plants used in traditional medicine and to create novel medications and treatments.
The increasing desire for complementary and alternative therapies has also contributed to the modern development and application of traditional medicine. Traditional medicine has been successful in meeting the needs of patients who are looking for more patient-centered, holistic alternative approaches to healthcare. Because of this, the market for goods and services related to traditional medicine is expanding, and numerous businesses are making investments in the creation of novel treatments and products.
But even with these advancements, there are still issues with using traditional medicine in the modern era. The absence of regulation and standardization of traditional medicine procedures and goods is one of the main issues. This might hinder the integration of traditional medicine into contemporary healthcare systems and make it challenging to guarantee the quality and safety of its goods and services.
The requirement for increased knowledge and comprehension of traditional medicine among the public and medical professionals is another difficulty. More education and awareness-raising initiatives are required to promote the advantages and possibilities of traditional medicine, as many people remain doubtful about its efficacy.
Since ancient times, flavonoids have been a part of the human diet, and now they are frequently taken as dietary supplements or in food[63]. However, because of their low or restricted bioavailability, these bioactive flavonoids have very little practical application as anticancer medicines. This is explained by the fact that phase II enzymes break them down, producing hydrophilic excretable conjugates[95]. Increased exposure to flavonoids may result from ineffective excretion of these metabolites, which could also interfere with general cellular metabolism. The scientific community is focusing on studies that try to lower metabolism or target the delivery of these chemicals in order to address this problem. If these strategies are successfully applied, flavonoids' anticancer properties may be improved[78].
Research on how V. negundo flavonoid extracts suppress the PI3K/AKT/mTOR/p70S6K/ULK signaling pathway in malignant cells is a promising field. This study may go in the following paths in the future.
Although in vitro research has yielded encouraging findings, in vivo investigations are required to validate the safety and effectiveness of V. negundo flavonoid extract in the treatment of cancer.
To ascertain the ideal dosage and course of treatment, as well as to assess the efficacy of V. negundo flavonoid extract in cancer patients, clinical trials are required.
Examining how the anti-cancer benefits of V. negundo flavonoid extract might be strengthened by combining it with other cancer treatments like chemotherapy or radiation therapy.
To completely comprehend the processes by which V. negundo flavonoid extract inhibits the PI3K/AKT/mTOR/p70S6K/ULK signaling pathway and to find possible biomarkers for its anti-cancer activities, more research is required.
To guarantee the consistency and effectiveness of V. negundo flavonoid extract in the treatment of cancer, standardization and quality control are essential.
Determining which flavonoids in V. negundo extract are in charge of the anti-cancer properties and looking into how they work.
Although the majority of recent study has been on breast, lung, and colon cancer, it is still important to investigate the extract's potential in other cancer types, such as liver, ovarian, and prostate cancer.
Creating innovative delivery methods, including liposomes or nanoparticles, can improve the bioavailability and target of V. negundo flavonoid extract to cancer cells.
Examining V. negundo extract's potential for cancer prevention, especially in high-risk patients.
To ascertain the effectiveness and safety of V. negundo flavonoid extract in cancer treatment, comparative studies with other anti-cancer drugs are being conducted.
All things considered, research on how flavonoid extracts from V. negundo affect the PI3K/AKT/mTOR/p70S6K/ULK signaling pathway in malignant cells is headed in a positive route that could result in the creation of innovative and potent cancer treatments.
We attempt to emphasize in this scientific literature how different signaling pathways contribute to the development of distinct malignancies. Anticancer drugs are known to target the PI3K/Akt/mTOR pathway. One of the most significant plants with numerous uses in conventional medical systems is V. negundo. Numerous phytochemicals are secondary metabolites found in every part of the plant, from the root to the fruit. V. negundo is an excellent source of flavonoids, as was previously discussed. Because of their natural sources, affordability, and simplicity of use, flavonoids are recommended as anticancer agents. However, because of intrinsic characteristics such poor bioavailability, fast metabolism, untargeted administration, and damage to healthy cells, their full clinical potential remains unrealized. It has been suggested that a mixture of flavonoids could be used to increase their anticancer efficacy, taking into account the potential for simultaneous impact on various signaling cascades. To lessen the aforementioned disadvantages, it is also recommended to employ cutting-edge methods, such as different nanotechnology-based strategies. Combining them with currently available chemotherapeutic medications may help lower the dosages required, which would ultimately result in fewer adverse effects.
The authors express their gratitude to all members of Department of Biotechnology Gautam Buddha University, Greater Noida for conducting this work.
| 1. | Gill BS, Mehra R, Navgeet, Kumar S. Vitex negundo and its medicinal value. Mol Biol Rep. 2018;45:2925-2934. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 31] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 2. | Husain SA, Perveen S, Khan MA, Parveen R, Insaf A, Parveen B, Ahmad S. An Updated Review on Traditional and Modern Aspects of Vitex negundo. Curr Tradit Med. 2023;9:e270822208079. [RCA] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 3. | Singh Y, Mishra P, Kannojia P. Morphology, Phytochemistry and Pharmacological Activity of Vitex negundo: An Overview. J Drug Delivery Ther. 2020;10:280-285. [DOI] [Full Text] |
| 4. | Huang Y, Ding GY, Hu P. Vitexnegheteroin M, a new phenolic glycoside from Vitex negundo var. heterophylla. Nat Prod Res. 2021;35:1518-1524. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 5. | Roy A, Khan A, Ahmad I, Alghamdi S, Rajab BS, Babalghith AO, Alshahrani MY, Islam S, Islam MR. Flavonoids a Bioactive Compound from Medicinal Plants and Its Therapeutic Applications. Biomed Res Int. 2022;2022:5445291. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 129] [Article Influence: 43.0] [Reference Citation Analysis (0)] |
| 6. | Shahrajabian MH, Sun W, Cheng Q. The Importance of Flavonoids and Phytochemicals of Medicinal Plants with Antiviral Activities. Mini-Rev Org Chem. 2022;19:293-318. [RCA] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 25] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 7. | Carrillo-Martinez EJ, Flores-Hernández FY, Salazar-Montes AM, Nario-Chaidez HF, Hernández-Ortega LD. Quercetin, a Flavonoid with Great Pharmacological Capacity. Molecules. 2024;29:1000. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 54] [Reference Citation Analysis (0)] |
| 8. | Trivedi A, Hasan A, Ahmad R, Siddiqui S, Srivastava A, Misra A, Mir SS. Flavonoid Myricetin as Potent Anticancer Agent: A Possibility towards Development of Potential Anticancer Nutraceuticals. Chin J Integr Med. 2024;30:75-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 9. | Yang L, Gao Y, Bajpai VK, El-Kammar HA, Simal-Gandara J, Cao H, Cheng KW, Wang M, Arroo RRJ, Zou L, Farag MA, Zhao Y, Xiao J. Advance toward isolation, extraction, metabolism and health benefits of kaempferol, a major dietary flavonoid with future perspectives. Crit Rev Food Sci Nutr. 2023;63:2773-2789. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 32] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 10. | Bangar SP, Chaudhary V, Sharma N, Bansal V, Ozogul F, Lorenzo JM. Kaempferol: A flavonoid with wider biological activities and its applications. Crit Rev Food Sci Nutr. 2023;63:9580-9604. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 102] [Article Influence: 34.0] [Reference Citation Analysis (1)] |
| 11. | Ahammed GJ, Wu Y, Wang Y, Guo T, Shamsy R, Li X. Epigallocatechin-3-Gallate (EGCG): A unique secondary metabolite with diverse roles in plant-environment interaction. Environ Exp Bot. 2023;209:105299. [DOI] [Full Text] |
| 12. | James A, Wang K, Wang Y. Therapeutic Activity of Green Tea Epigallocatechin-3-Gallate on Metabolic Diseases and Non-Alcoholic Fatty Liver Diseases: The Current Updates. Nutrients. 2023;15:3022. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 36] [Reference Citation Analysis (0)] |
| 13. | Chang PR, Liou JW, Chen PY, Gao WY, Wu CL, Wu MJ, Yen JH. The Neuroprotective Effects of Flavonoid Fisetin against Corticosterone-Induced Cell Death through Modulation of ERK, p38, and PI3K/Akt/FOXO3a-Dependent Pathways in PC12 Cells. Pharmaceutics. 2023;15:2376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 14. | Rauf A, Abu-Izneid T, Imran M, Hemeg HA, Bashir K, Aljohani ASM, Aljohani MSM, Alhumaydhi FA, Khan IN, Bin Emran T, Gondal TA, Nath N, Ahmad I, Thiruvengadam M. Therapeutic Potential and Molecular Mechanisms of the Multitargeted Flavonoid Fisetin. Curr Top Med Chem. 2023;23:2075-2096. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 8] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 15. | Lotfi MS, Rassouli FB. Natural Flavonoid Apigenin, an Effective Agent Against Nervous System Cancers. Mol Neurobiol. 2024;61:5572-5583. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 13] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 16. | Zhou Y, Xu P, Qin S, Zhu Y, Gu K. The associations between dietary flavonoid intake and the prevalence of diabetes mellitus: Data from the National Health and Nutrition Examination Survey 2007-2010 and 2017-2018. Front Endocrinol (Lausanne). 2023;14:1250410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 17. | Murata T, Ishiwa S, Lin X, Nakazawa Y, Tago K, Funakoshi-Tago M. The citrus flavonoid, nobiletin inhibits neuronal inflammation by preventing the activation of NF-κB. Neurochem Int. 2023;171:105613. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 18. | Wang D, Chen J, Pu L, Yu L, Xiong F, Sun L, Yu Q, Cao X, Chen Y, Peng F, Peng C. Galangin: A food-derived flavonoid with therapeutic potential against a wide spectrum of diseases. Phytother Res. 2023;37:5700-5723. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 26] [Reference Citation Analysis (0)] |
| 19. | Tiwari P, Mishra KP. Role of Plant-Derived Flavonoids in Cancer Treatment. Nutr Cancer. 2023;75:430-449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 16] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 20. | Rahmani AH, Babiker AY, Anwar S. Hesperidin, a Bioflavonoid in Cancer Therapy: A Review for a Mechanism of Action through the Modulation of Cell Signaling Pathways. Molecules. 2023;28:5152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 37] [Reference Citation Analysis (0)] |
| 21. | Wahnou H, Limami Y, Oudghiri M. Flavonoids and Flavonoid-Based Nanoparticles for Osteoarthritis and Rheumatoid Arthritis Management. BioChem. 2024;4:38-61. [DOI] [Full Text] |
| 22. | Giusti MM, Miyagusuku‐cruzado G, Wallace TC. Flavonoids as Natural Pigments. In: Stevens C, Bechtold T, Manian A, Pham T, editors. Handbook of Natural Colorants. NJ: John Wiley & Sons, Ltd., 2023. [DOI] [Full Text] |
| 23. | Han S, Luo Y, Liu B, Guo T, Qin D, Luo F. Dietary flavonoids prevent diabetes through epigenetic regulation: advance and challenge. Crit Rev Food Sci Nutr. 2023;63:11925-11941. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 11] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 24. | Asif Ali M, Khan N, Kaleem N, Ahmad W, Alharethi SH, Alharbi B, Alhassan HH, Al-Enazi MM, Razis AFA, Modu B, Calina D, Sharifi-Rad J. Anticancer properties of sulforaphane: current insights at the molecular level. Front Oncol. 2023;13:1168321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 25] [Reference Citation Analysis (0)] |
| 25. | Bhatt P, Kumar V, Malik MK, Kumar T. Citrus Flavonoids: Recent Advances and Future Perspectives on Preventing Cardiovascular Diseases. In: The Flavonoids. New York: Apple Academic Press, 2023. [DOI] [Full Text] |
| 26. | Moriwaki M, Tominaga E, Kito K, Nakagawa R, Kapoor MP, Matsumiya Y, Fukuhara T, Kobayashi J, Satomoto K, Yamagata H, Kuroiwa Y. Bioavailability of Flavonoids in Ginkgo biloba Extract-γ-Cyclodextrin Complex. Nat Prod Commun. 2023;18. [DOI] [Full Text] |
| 27. | Yuan D, Guo Y, Pu F, Yang C, Xiao X, Du H, He J, Lu S. Opportunities and challenges in enhancing the bioavailability and bioactivity of dietary flavonoids: A novel delivery system perspective. Food Chem. 2024;430:137115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 31] [Reference Citation Analysis (0)] |
| 28. | Liu T, Gong J, Lai G, Yang Y, Wu X, Wu X. Flavonoid extract Kushenol a exhibits anti-proliferative activity in breast cancer cells via suppression of PI3K/AKT/mTOR pathway. Cancer Med. 2023;12:1643-1654. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 29. | Finley LWS. What is cancer metabolism? Cell. 2023;186:1670-1688. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 160] [Article Influence: 80.0] [Reference Citation Analysis (0)] |
| 30. | Zhu JW, Charkhchi P, Adekunte S, Akbari MR. What Is Known about Breast Cancer in Young Women? Cancers (Basel). 2023;15:1917. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 52] [Reference Citation Analysis (0)] |
| 31. | Zaman S, Yaqoob HSA, Ullah A, Sheikh M. QSPR Analysis of Some Novel Drugs Used in Blood Cancer Treatment Via Degree Based Topological Indices and Regression Models. Polycycl Aromat Compd. 2024;44:2458-2474. [DOI] [Full Text] |
| 32. | Derevianko A, Pizzoli SFM, Pesapane F, Rotili A, Monzani D, Grasso R, Cassano E, Pravettoni G. The Use of Artificial Intelligence (AI) in the Radiology Field: What Is the State of Doctor-Patient Communication in Cancer Diagnosis? Cancers (Basel). 2023;15:470. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 28] [Reference Citation Analysis (0)] |
| 33. | Tewari M, Mahawer SK, Kumar R, Prakash O. A comparative study of selected vitex species for phenolics estimation along with their antioxidant and herbicidal activities. J Indian Chem Soc. 2022;99:100723. [RCA] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 34. | Sharma D, Radha, Kumar M, Andrade-Cetto A, Puri S, Kumar A, Thakur M, Chandran D, Pundir A, Prakash S, Pandiselvam R, Sandhu S, Khosla A, Kumar S, Lorenzo JM. Chemical Diversity and Medicinal Potential of Vitex negundo L.: From Traditional Knowledge to Modern Clinical Trials. Chem Biodivers. 2023;20:e202301086. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 35. | Liu N, Wang KS, Qi M, Zhou YJ, Zeng GY, Tao J, Zhou JD, Zhang JL, Chen X, Peng C. Vitexin compound 1, a novel extraction from a Chinese herb, suppresses melanoma cell growth through DNA damage by increasing ROS levels. J Exp Clin Cancer Res. 2018;37:269. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 55] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 36. | Zhong J, Ding S, Zhang X, Di W, Wang X, Zhang H, Chen Y, Zhang Y, Hu Y. To Investigate the Occurrence and Development of Colorectal Cancer Based on the PI3K/AKT/mTOR Signaling Pathway. Front Biosci (Landmark Ed). 2023;28:37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 25] [Reference Citation Analysis (0)] |
| 37. | Alves CL, Ditzel HJ. Drugging the PI3K/AKT/mTOR Pathway in ER+ Breast Cancer. Int J Mol Sci. 2023;24:4522. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 52] [Article Influence: 26.0] [Reference Citation Analysis (0)] |
| 38. | Ahmad I, Hoque M, Alam SSM, Zughaibi TA, Tabrez S. Curcumin and Plumbagin Synergistically Target the PI3K/Akt/mTOR Pathway: A Prospective Role in Cancer Treatment. Int J Mol Sci. 2023;24:6651. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 43] [Reference Citation Analysis (0)] |
| 39. | Ghoneum A, Said N. PI3K-AKT-mTOR and NFκB Pathways in Ovarian Cancer: Implications for Targeted Therapeutics. Cancers (Basel). 2019;11:949. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 113] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 40. | Roudsari NM, Lashgari NA, Momtaz S, Abaft S, Jamali F, Safaiepour P, Narimisa K, Jackson G, Bishayee A, Rezaei N, Abdolghaffari AH, Bishayee A. Inhibitors of the PI3K/Akt/mTOR Pathway in Prostate Cancer Chemoprevention and Intervention. Pharmaceutics. 2021;13:1195. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 42] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 41. | Quan Z, Yang Y, Zheng H, Zhan Y, Luo J, Ning Y, Fan S. Clinical implications of the interaction between PD-1/PD-L1 and PI3K/AKT/mTOR pathway in progression and treatment of non-small cell lung cancer. J Cancer. 2022;13:3434-3443. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 50] [Reference Citation Analysis (0)] |
| 42. | Lang L, Chen F, Li Y, Shay C, Yang F, Dan H, Chen ZG, Saba NF, Teng Y. Supplementary Figure S4 from Adaptive c-Met-PLXDC2 Signaling Axis Mediates Cancer Stem Cell Plasticity to Confer Radioresistance-associated Aggressiveness in Head and Neck Cancer. Cancer Res Commun. 2023. [DOI] [Full Text] |
| 43. | Jayathirtha M, Jayaweera T, Whitham D, Sullivan I, Petre BA, Darie CC, Neagu AN. Two-Dimensional-PAGE Coupled with nLC-MS/MS-Based Identification of Differentially Expressed Proteins and Tumorigenic Pathways in MCF7 Breast Cancer Cells Transfected for JTB Protein Silencing. Molecules. 2023;28:7501. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 44. | Tian LY, Smit DJ, Jücker M. The Role of PI3K/AKT/mTOR Signaling in Hepatocellular Carcinoma Metabolism. Int J Mol Sci. 2023;24:2652. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 130] [Reference Citation Analysis (0)] |
| 45. | Mir SA, Dar A, Alshehri SA, Wahab S, Hamid L, Almoyad MAA, Ali T, Bader GN. Exploring the mTOR Signalling Pathway and Its Inhibitory Scope in Cancer. Pharmaceuticals (Basel). 2023;16:1004. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |
| 46. | Khalil MI, Ali MM, Holail J, Houssein M. Growth or death? Control of cell destiny by mTOR and autophagy pathways. Prog Biophys Mol Biol. 2023;185:39-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 15] [Reference Citation Analysis (0)] |
| 47. | Kuzilková D, Bugarin C, Rejlova K, Schulz AR, Mei HE, Paganin M, Biffi A, Biondi A, Kalina T, Gaipa G. Either IL-7 activation of JAK-STAT or BEZ inhibition of PI3K-AKT-mTOR pathways dominates the single-cell phosphosignature of ex vivo treated pediatric T-cell acute lymphoblastic leukemia cells. Haematologica. 2022;107:1293-1310. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 48. | Lei Y, Zhang R, Cai F. Role of MARK2 in the nervous system and cancer. Cancer Gene Ther. 2024;31:497-506. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 49. | Zhang Y, Yan Z, Wu H, Yang X, Yang K, Song W. Low-Temperature Plasma-Activated Medium Inhibits the Migration of Non-Small Cell Lung Cancer Cells via the Wnt/β-Catenin Pathway. Biomolecules. 2023;13:1073. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 50. | Shi Q, Xuhong J, Luo T, Ge J, Liu F, Lan Y, Chen Q, Tang P, Fan L, Chen L, Liang Y, Wang M, Hu Y, Zhang Y, Bian X, Qi X, Jiang J. PIK3CA mutations are associated with pathologic complete response rate to neoadjuvant pyrotinib and trastuzumab plus chemotherapy for HER2-positive breast cancer. Br J Cancer. 2023;128:121-129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 15] [Reference Citation Analysis (0)] |
| 51. | Guan J, Borenäs M, Xiong J, Lai WY, Palmer RH, Hallberg B. IGF1R Contributes to Cell Proliferation in ALK-Mutated Neuroblastoma with Preference for Activating the PI3K-AKT Signaling Pathway. Cancers (Basel). 2023;15:4252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 52. | Amato O, Buisseret L, Gebhart G, Plouznikoff N, Larsimont D, Awada A, Piccart M, Aftimos P. PIK3CA copy-number gain and inhibitors of the PI3K/AKT/mTOR pathway in triple-negative breast cancer. Cold Spring Harb Mol Case Stud. 2023;9:a006255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 53. | VanLandingham NK, Nazarenko A, Grandis JR, Johnson DE. The mutational profiles and corresponding therapeutic implications of PI3K mutations in cancer. Adv Biol Regul. 2023;87:100934. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 54. | Marques-Ramos A, Cervantes R. Expression of mTOR in normal and pathological conditions. Mol Cancer. 2023;22:112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 37] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 55. | Afify SM, Oo AKK, Hassan G, Seno A, Seno M. How can we turn the PI3K/AKT/mTOR pathway down? Insights into inhibition and treatment of cancer. Expert Rev Anticancer Ther. 2021;21:605-619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 27] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 56. | Ortega MA, Fraile-Martínez O, Asúnsolo Á, Buján J, García-Honduvilla N, Coca S. Signal Transduction Pathways in Breast Cancer: The Important Role of PI3K/Akt/mTOR. J Oncol. 2020;2020:9258396. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 106] [Cited by in RCA: 146] [Article Influence: 29.2] [Reference Citation Analysis (0)] |
| 57. | Saini KS, Loi S, de Azambuja E, Metzger-Filho O, Saini ML, Ignatiadis M, Dancey JE, Piccart-Gebhart MJ. Targeting the PI3K/AKT/mTOR and Raf/MEK/ERK pathways in the treatment of breast cancer. Cancer Treat Rev. 2013;39:935-946. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 245] [Cited by in RCA: 286] [Article Influence: 23.8] [Reference Citation Analysis (0)] |
| 58. | Ul Islam B, Suhail M, Khan MS, Ahmad A, Zughaibi TA, Husain FM, Rehman MT, Tabrez S. Flavonoids and PI3K/Akt/mTOR Signaling Cascade: A Potential Crosstalk in Anticancer Treatment. Curr Med Chem. 2021;28:8083-8097. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 33] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 59. | Zhai Y, Sun J, Sun C, Zhao H, Li X, Yao J, Su J, Xu X, Xu X, Hu J, Daglia M, Han B, Kai G. Total flavonoids from the dried root of Tetrastigma hemsleyanum Diels et Gilg inhibit colorectal cancer growth through PI3K/AKT/mTOR signaling pathway. Phytother Res. 2022;36:4263-4277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 15] [Reference Citation Analysis (0)] |
| 60. | Farghadani R, Naidu R. Curcumin: Modulator of Key Molecular Signaling Pathways in Hormone-Independent Breast Cancer. Cancers (Basel). 2021;13:3427. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 65] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 61. | Guan X, Pan Z, Xu Z, Zhang S, Tang H, Du G, Zhou J. Natural flavonoid luteolin promotes the differentiation of porcine myoblasts through activation of PI3K/Akt/mTOR signaling. Food Biosci. 2022;47:101766. [RCA] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 62. | Qamar M, Akhtar S, Ismail T, Sestili P, Tawab A, Ahmed N. Anticancer and anti-inflammatory perspectives of Pakistan’s indigenous berry Grewia asiatica Linn (Phalsa). J Berry Res. 2020;10:115-131. [DOI] [Full Text] |
| 63. | Zughaibi TA, Suhail M, Tarique M, Tabrez S. Targeting PI3K/Akt/mTOR Pathway by Different Flavonoids: A Cancer Chemopreventive Approach. Int J Mol Sci. 2021;22:12455. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 55] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 64. | Hafeez A, Khan Z, Armaghan M, Khan K, Sönmez Gürer E, Abdull Razis AF, Modu B, Almarhoon ZM, Setzer WN, Sharifi-Rad J. Exploring the therapeutic and anti-tumor properties of morusin: a review of recent advances. Front Mol Biosci. 2023;10:1168298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 65. | Badar Ul Islam, Khan MS, Husain FM, Rehman MT, Zughaibi TA, Abuzenadah AM, Urooj M, Kamal MA, Tabrez S. mTOR Targeted Cancer Chemoprevention by Flavonoids. Curr Med Chem. 2021;28:8068-8082. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 66. | Hussain Y, Khan H, Alam W, Aschner M, Abdullah, Alsharif KF, Saso L. Flavonoids Targeting the mTOR Signaling Cascades in Cancer: A Potential Crosstalk in Anti-Breast Cancer Therapy. Oxid Med Cell Longev. 2022;2022:4831833. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 67. | Wu Y, Wang H, Liu Y, Zhao L, Pei J. An efficient preparation and biocatalytic synthesis of novel C-glycosylflavonols kaempferol 8-C-glucoside and quercetin 8-C-glucoside through using resting cells and macroporous resins. Biotechnol Biofuels Bioprod. 2022;15:129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 9] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 68. | Oueslati MH, Tahar LB, Harrath AH. Catalytic, antioxidant and anticancer activities of gold nanoparticles synthesized by kaempferol glucoside from Lotus leguminosae. Arab J Chem. 2020;13:3112-3122. [RCA] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 62] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 69. | Jideani AIO, Silungwe H, Takalani T, Omolola AO, Udeh HO, Anyasi TA. Antioxidant-rich natural fruit and vegetable products and human health. Int J Food Prop. 2021;24:41-67. [RCA] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 103] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 70. | Egbuna C, Sawicka B, Khan J. Food and Agricultural Byproducts as Important Source of Valuable Nutraceuticals. 1st ed. Cham: Springer, 2022. [DOI] [Full Text] |
| 71. | Ge L, Li N, Hu Y, Zhou X. Qualitative and quantitative analyses of quercetin and isorhamnetin in Hippophae rhamnoides L. fruits hydrolysis products by high-performance thin-layer chromatography. JPC-J Planar Chromat. 2021;34:315-322. [RCA] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 72. | He N, Wang Q, Huang H, Chen J, Wu G, Zhu M, Shao F, Yan Z, Sang Z, Cao L, Wei R, Ma Q. A Comprehensive Review on Extraction, Structure, Detection, Bioactivity, and Metabolism of Flavonoids from Sea Buckthorn (Hippophae rhamnoides L.). J Food Biochem. 2023;2023:1-27. [RCA] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 73. | Pan SY, Nie Q, Tai HC, Song XL, Tong YF, Zhang LJ, Wu XW, Lin ZH, Zhang YY, Ye DY, Zhang Y, Wang XY, Zhu PL, Chu ZS, Yu ZL, Liang C. Tea and tea drinking: China's outstanding contributions to the mankind. Chin Med. 2022;17:27. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 93] [Article Influence: 31.0] [Reference Citation Analysis (0)] |
| 74. | Samanta S. Potential Bioactive Components and Health Promotional Benefits of Tea (Camellia sinensis). J Am Nutr Assoc. 2022;41:65-93. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 35] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 75. | Saeed M, Abd El-hac ME, Alagawany M, Naveed M, Arain MA, Arif M, Soomro RN, Kakar M, Manzoor R, Tiwari R, Khandia R, Munjal A, Karthik K, Dhama K, Iqbal HMN, Sun C. Phytochemistry, Modes of Action and Beneficial Health Applications of Green Tea (Camellia sinensis) in Humans and Animals. Internat J Pharmacol. 2017;13:698-708. [RCA] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 76. | Zhao J, Li P, Xia T, Wan X. Exploring plant metabolic genomics: chemical diversity, metabolic complexity in the biosynthesis and transport of specialized metabolites with the tea plant as a model. Crit Rev Biotechnol. 2020;40:667-688. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 85] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 77. | Maphutha J, Lall N. Synthetic Compounds vs. Phytochemicals for the Treatment of Human Cutaneous Malignant Melanoma. In: Lall N, editor. Medicinal Plants for Cosmetics, Health and Diseases. Boca Raton: CRC Press, 2022. [DOI] [Full Text] |
| 78. | Zuo Y, Zhang CZ, Ren Q, Chen Y, Li X, Yang JR, Li HX, Tang WT, Ho HM, Sun C, Li MM, Ren B, Deng Y, Wang ML, Lu J. Activation of mitochondrial-associated apoptosis signaling pathway and inhibition of PI3K/Akt/mTOR signaling pathway by voacamine suppress breast cancer progression. Phytomedicine. 2022;99:154015. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 29] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 79. | Dixit V, Joseph Kamal SW, Bajrang Chole P, Dayal D, Chaubey KK, Pal AK, Xavier J, Manjunath BT, Bachheti RK. Functional Foods: Exploring the Health Benefits of Bioactive Compounds from Plant and Animal Sources. J Food Qual. 2023;2023:1-22. [DOI] [Full Text] |
| 80. | Singh AP, Kandpal JB, Sharma RK, Chitme H. Nobiletin a Biologically Active Phytoconstituent: Systematic Review. J Biologically Active Prod Nat. 2021;11:204-211. [DOI] [Full Text] |
| 81. | Alam F, Mohammadin K, Shafique Z, Amjad ST, Asad MHHB. Citrus flavonoids as potential therapeutic agents: A review. Phytother Res. 2022;36:1417-1441. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 69] [Article Influence: 17.3] [Reference Citation Analysis (1)] |
| 82. | Seoka M, Ma G, Zhang L, Yahata M, Yamawaki K, Kan T, Kato M. Expression and functional analysis of the nobiletin biosynthesis-related gene CitOMT in citrus fruit. Sci Rep. 2020;10:15288. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 83. | Zou Y, Li R, Kuang D, Zuo M, Li W, Tong W, Jiang L, Zhou M, Chen Y, Gong W, Liu L, Tou F. Galangin Inhibits Cholangiocarcinoma Cell Growth and Metastasis through Downregulation of MicroRNA-21 Expression. Biomed Res Int. 2020;2020:5846938. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 84. | Aljobair MO. Chemical composition, antimicrobial properties, and antioxidant activity of galangal rhizome. Food Sci Technol. 2022;42. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 85. | Madureira MB, Concato VM, Cruz EMS, Bitencourt de Morais JM, Inoue FSR, Concimo Santos N, Gonçalves MD, Cremer de Souza M, Basso Scandolara T, Fontana Mezoni M, Galvani M, Rodrigues Ferreira Seiva F, Panis C, Miranda-Sapla MM, Pavanelli WR. Naringenin and Hesperidin as Promising Alternatives for Prevention and Co-Adjuvant Therapy for Breast Cancer. Antioxidants (Basel). 2023;12:586. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 27] [Reference Citation Analysis (0)] |
| 86. | Junedi S, Hermawan A, Fitriasari A, Setiawati A, Susidarti RA, Meiyanto E. The Doxorubicin-Induced G2/M Arrest in Breast Cancer Cells Modulated by Natural Compounds Naringenin and Hesperidin. Indones J Cancer Chemoprevent. 2021;12:83. [RCA] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 87. | Chen S, Wang X, Cheng Y, Gao H, Chen X. A Review of Classification, Biosynthesis, Biological Activities and Potential Applications of Flavonoids. Molecules. 2023;28:4982. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 111] [Reference Citation Analysis (0)] |
| 88. | Qi Q, Chu M, Yu X, Xie Y, Li Y, Du Y, Liu X, Zhang Z, Shi J, Yan N. Anthocyanins and Proanthocyanidins: Chemical Structures, Food Sources, Bioactivities, and Product Development. Food Rev Int. 2023;39:4581-4609. [RCA] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 84] [Article Influence: 28.0] [Reference Citation Analysis (0)] |
| 89. | Gonçalves AC, Nunes AR, Falcão A, Alves G, Silva LR. Dietary Effects of Anthocyanins in Human Health: A Comprehensive Review. Pharmaceuticals (Basel). 2021;14:690. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 108] [Article Influence: 27.0] [Reference Citation Analysis (0)] |
| 90. | Hazafa A, Rehman KU, Jahan N, Jabeen Z. The Role of Polyphenol (Flavonoids) Compounds in the Treatment of Cancer Cells. Nutr Cancer. 2020;72:386-397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 243] [Cited by in RCA: 195] [Article Influence: 32.5] [Reference Citation Analysis (0)] |
| 91. | Bhushan B, Jat BS, Dagla MC, Aggarwal SK, Rakshit S. Anthocyanins and Proanthocyanidins as Anticancer Agents. In: Malik S, editor. Exploring Plant Cells for the Production of Compounds of Interest. Cham: Springer, 2021. [DOI] [Full Text] |
| 92. | Sharma A, Choi HK, Kim YK, Lee HJ. Delphinidin and Its Glycosides' War on Cancer: Preclinical Perspectives. Int J Mol Sci. 2021;22:11500. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 26] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 93. | Wu G, Yan Y, Zhou Y, Duan Y, Zeng S, Wang X, Lin W, Ou C, Zhou J, Xu Z. Sulforaphane: Expected to Become a Novel Antitumor Compound. Oncol Res. 2020;28:439-446. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 30] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 94. | Rai R, Gong Essel K, Mangiaracina Benbrook D, Garland J, Daniel Zhao Y, Chandra V. Preclinical Efficacy and Involvement of AKT, mTOR, and ERK Kinases in the Mechanism of Sulforaphane against Endometrial Cancer. Cancers (Basel). 2020;12:1273. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 95. | Fernando W, Goralski KB, Hoskin DW, Rupasinghe HPV. Metabolism and pharmacokinetics of a novel polyphenol fatty acid ester phloridzin docosahexaenoate in Balb/c female mice. Sci Rep. 2020;10:21391. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |