Published online Jul 28, 2016. doi: 10.5412/wjsp.v6.i2.19
Peer-review started: April 29, 2016
First decision: June 30, 2016
Revised: July 13, 2016
Accepted: July 20, 2016
Article in press: July 22, 2016
Published online: July 28, 2016
Processing time: 91 Days and 11.2 Hours
AIM: To evaluate the outcomes of surgery for lung cancer after induction therapy.
METHODS: Using the American College of Surgeons National Surgical Quality Improvement Program (ACS-NSQIP) database (2005-2012), we identified 4063 patients who underwent a pulmonary resection for lung cancer. Two hundred and thirty-six (5.8%) received neo-adjuvant therapy prior to surgery (64 chemo-radiation, 103 radiation alone, 69 chemotherapy alone). The outcomes were compared to 3827 patients (94.2%) treated with surgery alone. Primary outcome was 30-d mortality, and secondary outcomes included length of stay, operative time and NSQIP measured postoperative complications.
RESULTS: Lung cancer patients who received preoperative treatment were younger (66 vs 69, P < 0.001), were more likely to have experienced recent weight loss (6.8% vs 3.5%; P = 0.011), to be active smokers (48.3 vs 34.9, P < 0.001), and had lower preoperative hematological cell counts (abnormal white blood cell: 25.6 vs 13.4; P < 0.001; low hematocrit 53% vs 17.3%, P < 0.001). On unadjusted analysis, neo-adjuvant patients had significantly higher 30-d mortality, overall and serious morbidity (all P < 0.001). Adjusted analysis showed similar findings, while matched cohorts comparison confirmed higher morbidity, but not higher early mortality.
CONCLUSION: Our data suggest that patients who receive neo-adjuvant therapy for lung cancer have worse early surgical outcomes. Although NSQIP does not provide stage information, this analysis shows important findings that should be considered when selecting patients for induction treatment.
Core tip: The aim of this retrospective study was to evaluate the results of lung cancer patients undergoing surgery after induction treatment. Using the American College of Surgeons National Surgical Quality Improvement Program database, we identified 4063 patients who underwent lung resection for cancer. Two hundred and thirty-six (5.8%) underwent neo-adjuvant therapy. The results were compared to 3827 patients (94.2%) who underwent upfront surgery. On unadjusted and adjusted analysis, neo-adjuvant patients had significantly higher 30-d mortality, overall and serious morbidity than patient treated with surgery alone. Matched cohorts comparison confirmed higher morbidity, but not higher early mortality.
- Citation: Mungo B, Zogg CK, Schlottmann F, Barbetta A, Hooker CM, Molena D. Surgical outcomes of pulmonary resection for lung cancer after neo-adjuvant treatment. World J Surg Proced 2016; 6(2): 19-29
- URL: https://www.wjgnet.com/2219-2832/full/v6/i2/19.htm
- DOI: https://dx.doi.org/10.5412/wjsp.v6.i2.19
Lung cancer is among the highest reason of cancer-related mortality in the United States, including about 27% of all cancer deaths in 2014, and 224210 estimated new cases in the same year[1].
Surgery represents the mainstay of treatment for lung cancer and ongoing advancements in surgical techniques across the last two decades have led to a remarkable reduction in operative mortality[2]. Lung cancer-related mortality, however, remains disappointingly high, showing a 5-year relative survival of about 15%[3].
In an attempt to improve survival for this disease, several multimodality treatment approaches, including neo-adjuvant therapy protocols, have been developed through the years. Given its potential benefits, such as clearance of micrometastasis and tumor downstaging, the efficacy of induction treatment has been evaluated with many trials; unfortunately the results have been controversial, hence the use of neo-adjuvant therapy for locally advanced disease still represents the subject of an ongoing debate[4]. Reluctance towards the use of induction is in part due to a perceived increase in surgical risk for lung cancer patients undergoing neo-adjuvant treatment. It has in fact been reported that, in this population, induction may lead to non-negligible treatment-related mortality, and increased occurrence of post-operative adverse events such as air leaks and infectious complications[5-7]. The concern of developing life-threatening complications, prevalent and severe enough to offset the potential benefits of induction, can constitute a significant obstacle for the diffusion of neo-adjuvant protocols. In this regard, an analysis of the Society of Thoracic Surgeons (STS) General Thoracic Surgery Database has demonstrated that neo-adjuvant treatment is underutilized in the United States[8]. Only less than 10% of all major lung resections for primary lung cancer were reported to be preceded by induction treatment and, even for clinical stage IIIA-N2, the percentage barely topped 50%. We queried the American College of Surgeons National Surgical Quality Improvement Program (ACS-NSQIP) database, to evaluate the effects of neo-adjuvant treatment on 30-d outcomes of resection for lung cancer in the United States.
The study consisted of a retrospective research database review using the 2005-2012 ACS-NSQIP. The ACS-NSQIP is a large, nationally-validated, risk-adjusted, outcomes-based program used to measure and improve the quality of surgical care. It collects data from approximately 500 collaborating hospitals each year that vary in size and academic affiliation[9]. At participating institutions, trained surgical/clinical reviewers abstract data via a process of prospective systematic collection that includes information on 135 patient demographic, preoperative risk factor, laboratory value, and intraoperative factor variables in addition to 30-d measures of postoperative morbidity and mortality. Surgical cases from multiple specialties are sampled using an ACS-validated, systemic sampling protocol. Standardization of methods, data field definitions, and data collection are ensured by training and auditing protocols as well as regular assessment of inter-rater reliability. Details of the ACS-NSQIP are described elsewhere[10]. The study was deemed exempt from ethical review by the Johns Hopkins University School of Medicine Institutional Review Board.
The study population was restricted to include patients ≥ 18 years of age with a primary diagnosis of lung cancer (according to International Classification of Diseases, 9th revision, Clinical Modification), who underwent pneumonectomy [defined by Current Procedural Terminology (CPT) codes: 32440, 32442, 32445, 32488, 32671], lobectomy (32480, 32482, 32486, 32503, 32504, 32663, 32670), segmentectomy (32484, 32669), or wedge resection (32505, 32506, 32666, 32667). Patients were further excluded if they lacked reported information on administration of chemotherapy (defined by the ACS-NSQIP to be chemotherapy within 30 d pre-operation) or radiotherapy (defined by the ACS-NSQIP to be radiotherapy within 90 d pre-operation).
Collected baseline demographic and clinical characteristics are reported in Table 1. They include: Age (years), gender, race (White, Black, Latino/a, other/unknown), American Society of Anesthesiology (ASA) classification (1/2 no-mild disturbance, 3 serious disturbance, 4/5 life-threatening/moribund), functional status (independent vs partially/totally dependent), obesity (defined as a BMI > 29 kg/m2), diagnosis of diabetes, current smoker within 1 year, alcohol consumption (defined as > 2 drinks/d in the 2 wk prior to admission), dyspnea, history of chronic obstructive pulmonary disease, history of heart disease (congestive heart failure and/or myocardial infarction), hypertension requiring medication, previous cardiac surgery, > 10% loss of body weight in the last 6 mo, steroid use for a chronic condition, year of operation (2005-2008, 2009-2010, 2011-2012), preoperative white blood cell (WBC) count [normal (4.5-11.0 × 109/L) vs abnormal (< 4.5 or > 11.0 × 109/L)], [preoperative hematocrit (normal) ≥ 36 mg/dL vs abnormal (< 36 mg/dL)], and surgery type [video-assisted thoracic surgery (VATS) vs open]. Baseline demographic and clinical characteristics were compared between neo-adjuvant patients (receipt of chemotherapy and/or radiotherapy) and surgery-only patients (receipt of neither chemotherapy nor radiotherapy) using χ2 tests (Fisher’s exact test in cell counts less than five) for categorical variables. Two-sided P-values < 0.05 were considered statistically significant. To account for non-normal age distributions within the study population, Wilcoxon rank-sum tests were used to compare rank sum differences in age.
| Characteristic | Total n = 4063 | Neoadjuvant patients n = 236 (5.81%) | Surgery-only patients n = 3827 (94.19%) | P-value1 | |
| Age (yr), median (IQR) | 68 (61-75) | 66 (55-72) | 69 (61-75) | < 0.0012 | |
| Male (%) | 2003 (49.30) | 125 (52.97) | 1878 (49.07) | 0.246 | |
| Race (%) | |||||
| White | 3019 (74.30) | 189 (80.08) | 2830 (73.95) | 0.173 | |
| Black | 222 (5.46) | 12 (5.08) | 210 (5.49) | ||
| Latino(a) | 432 (10.63) | 17 (7.20) | 415 (10.84) | ||
| Other or unknown | 390 (9.60) | 18 (7.63) | 372 (9.72) | ||
| ASA classification (%) | |||||
| 1-2 no-mild disturbance | 769 (18.97) | 38 (16.24) | 731 (19.14) | 0.523 | |
| 3 serious disturbance | 2888 (71.24) | 171 (73.08) | 2717 (71.13) | ||
| 4-5 life threatening/moribund | 397 (9.79) | 25 (10.68) | 372 (9.74) | ||
| Functional status | |||||
| Independent | 3985 (98.08) | 229 (97.03) | 3756 (98.14) | 0.227 | |
| Partially/totally dependent | 78 (1.92) | 7 (2.97) | 71 (1.86) | ||
| Obese, BMI ≥ 30 (%) | 1155 (28.65) | 58 (24.79) | 1097 (28.88) | 0.178 | |
| Diabetes (%) | 598 (14.72) | 27 (11.44) | 571 (14.92) | 0.143 | |
| Current smoker (%) | 1448 (35.64) | 114 (48.31) | 1334 (34.86) | < 0.0012 | |
| Alcohol consumption (%) | 221 (5.44) | 15 (6.36) | 206 (5.38) | 0.522 | |
| Dyspnea (%) | 1106 (27.22) | 57 (24.15) | 1049 (27.41) | 0.275 | |
| History of COPD (%) | 1082 (26.63) | 54 (22.88) | 1028 (26.86) | 0.179 | |
| History of heart disease (%) | 42 (1.03) | 3 (1.27) | 39 (1.02) | 0.734 | |
| Hypertension (%) | 2375 (58.45) | 111 (47.03) | 2264 (59.16) | < 0.0012 | |
| Previous cardiac surgery (%) | 308 (7.58) | 11 (4.66) | 297 (7.76) | 0.081 | |
| Weight loss (%) | 152 (3.74) | 16 (6.78) | 136 (3.55) | 0.0112 | |
| Steroid use (%) | 161 (3.96) | 22 (9.32) | 139 (3.63) | < 0.0012 | |
| Year of operation (%) | |||||
| 2005-2008 | 834 (20.53) | 53 (22.46) | 781 (20.41) | 0.662 | |
| 2009-2010 | 1794 (44.15) | 105 (44.49) | 1689 (44.13) | ||
| 2011-2012 | 1435 (35.32) | 78 (33.05) | 1357 (35.46) | ||
| Preoperative WBC (%) | |||||
| Normal (4.5-11.0 × 109/L) | 3364 (85.88) | 174 (74.36) | 3190 (86.61) | < 0.0012 | |
| Abnormal (< 4.5 or > 11.0 × 109/L) | 553 (14.12) | 60 (25.64) | 493 (13.39) | ||
| Preoperative hematocrit (%) | |||||
| Normal (≥ 36) | 3147 (80.53) | 110 (47.01) | 3037 (82.66) | < 0.0012 | |
| Abnormal (< 36) | 761 (19.47) | 124 (52.99) | 637 (17.34) | ||
| Surgery type (%) | |||||
| VATS | 1203 (29.61) | 34 (14.41) | 1169 (30.55) | < 0.0012 | |
| Open | 2860 (70.39) | 202 (85.59) | 2658 (69.45) |
The primary outcome measure considered was 30-d postoperative mortality. Secondary intraoperative and postoperative outcomes measures included overall morbidity, serious morbidity, length of stay (LOS, days), prolonged LOS (defined as a LOS longer than the 75th percentile), operative time (min), and prolonged operative time (defined as an operative time longer than the 75th percentile). Overall morbidity was defined by presence of at least one of the following ACS-NSQIP complications: Wound infection [superficial or deep incisional surgical site infection (SSI), wound dehiscence], pneumonia, urinary tract infection (UTI), return to operating room (OR), venous thromboembolic event (VTE) (deep vein thrombosis/thrombophlebitis, pulmonary embolism), cardiac complication (cardiac arrest, myocardial infarction), shock/sepsis, unplanned intubation, bleeding requiring transfusion, renal complication (postoperative renal failure, progressive renal insufficiency), ventilator dependency > 48 h, or organ space SSI. Serious morbidity included occurrence of at least one of the following complications: Return to OR, cardiac complication, shock/sepsis, unplanned intubation, ventilator dependency for > 48 h, or organ space SSI. As with baseline characteristics, outcome measures (Table 2) were compared between neo-adjuvant and surgery-only patients using χ2 tests (Fisher’s exact test in cell counts less than five) for categorical variables and Wilcoxon rank-sum tests to compare non-normal distributions of LOS and operative times.
| Characteristic | Total n = 4063 | Neoadjuvant patients n = 236 (5.81%) | Surgery-only patients n = 3827 (94.19%) | P-value1 |
| 30-d mortality (%) | 107 (2.63) | 19 (8.05) | 88 (2.30) | < 0.0012 |
| Overall morbidity (%) | 646 (15.90) | 63 (26.69) | 583 (15.23) | < 0.0012 |
| Wound infection | 4 (0.10) | 0 (0.00) | 4 (0.10) | 0.787 |
| Pneumonia | 5 (0.73) | 0 (0.00) | 5 (0.77) | 0.774 |
| Urinary tract infection | 1 (0.15) | 0 (0.00) | 1 (0.16) | 0.956 |
| Return to OR | 201 (4.95) | 22 (9.32) | 179 (4.68) | < 0.0012 |
| Venous thromboembolism | 80 (1.97) | 7 (2.97) | 73 (1.91) | 0.256 |
| Cardiac complication | 60 (1.48) | 10 (4.24) | 50 (1.31) | < 0.0012 |
| Shock/sepsis | 107 (2.63) | 11 (4.66) | 96 (2.51) | 0.0452 |
| Unplanned intubation | 207 (5.09) | 27 (11.44) | 180 (4.70) | < 0.0012 |
| Bleeding transfusion | 178 (4.38) | 28 (11.86) | 150 (3.92) | <0.0012 |
| Renal complication | 22 (0.54) | 0 (0.00) | 22 (0.57) | 0.636 |
| On ventilator > 48 h | 3 (0.07) | 1 (0.42) | 2 (0.05) | 0.164 |
| Organ space SSI | 44 (1.08) | 1 (0.42) | 43 (1.12) | 0.516 |
| Serious morbidity (%) | 469 (11.54) | 44 (18.64) | 425 (11.11) | < 0.0012 |
| Length of stay (d), median (IQR) | 6 (4-9) | 6 (4-9) | 6 (4-8) | 0.915 |
| Prolonged length of stay (%) | 816 (20.08) | 46 (19.49) | 770 (20.12) | 0.815 |
| Operative time (min), median (IQR) | 161 (123-216) | 160 (121-214) | 192 (147-250) | < 0.0012 |
| Prolonged operative time (%) | 1006 (24.76) | 91 (38.56) | 915 (23.91) | < 0.0012 |
Unadjusted and risk-adjusted odds ratios (and corresponding 95%CI) were calculated for differences in 30-d mortality, serious and overall morbidity, constituent morbidity measures, and prolonged LOS and operative time using (multivariable) logistic regression. Risk-adjusted models accounted for potential confounding due to significant differences in baseline factors: Age, smoking, hypertension, weight loss, steroid use, abnormal WBC count, abnormal hematocrit, and type of surgery performed. Colinearity/multicolinearity was assessed for adjusted models via calculation of variance inflation factors all well below a critical threshold of 10.0. For the continuous right-skewed distributions of LOS and operative time, modified Park tests were used to determine the most appropriate distribution (Poisson in both cases) to be used in a generalized linear model (link log). Average marginal effects were then used to calculate predicted differences in unadjusted and adjusted mean LOS (days) and operative time (min) in a manner analogous to that described for logistic regression (Table 3).
| Characteristic | Unadjusted (95%CI) | P-value1 | Risk-adjusted(95%CI) | P-value1 | Propensity-score matched cohort3 (95%CI) | P-value1 |
| 30-d mortality (%) | 3.72 (2.22-6.22) | < 0.0011 | 2.70 (1.54-4.72) | 0.0011 | 1.63 (0.77-3.45) | 0.197 |
| Overall morbidity (%) | 2.02 (1.50-2.74) | < 0.0011 | 1.53 (1.12-2.11) | 0.0101 | 1.68 (1.08-2.62) | 0.0211 |
| Return to OR | 2.09 (1.32-3.33) | 0.0011 | 1.77 (1.08-2.90) | 0.0231 | 3.37 (1.41-8.04) | 0.0061 |
| Cardiac complication | 3.34 (1.67-6.70) | 0.0011 | 3.11 (1.47-6.57) | 0.0031 | 2.57 (0.79-8.30) | 0.116 |
| Shock/sepsis | 1.90 (1.00-3.60) | 0.0491 | 1.53 (0.78-3.02) | 0.217 | 3.80 (1.05-13.80) | 0.0431 |
| Unplanned intubation | 2.62 (1.71-4.02) | < 0.0011 | 2.03 (1.28-3.22) | 0.0021 | 1.66 (0.88-3.14) | 0.116 |
| Bleeding transfusion | 3.30 (3.15-5.06) | < 0.0011 | 1.72 (1.08-2.73) | 0.0231 | 2.31 (1.17-4.58) | 0.0161 |
| Serious morbidity (%) | 1.83 (1.30-2.58) | 0.0011 | 1.55 (1.08-2.23) | 0.0181 | 1.70 (1.02-2.85) | 0.0421 |
| Length of stay (d)2 | ||||||
| Predicted difference in means | -0.02 (-0.38 to 0.35) | 0.927 | -0.93 (-1.32 to -0.55) | < 0.0011 | -1.02 (-1.54 to -0.50) | < 0.0011 |
| Prolonged length of stay (%) | 0.96 (0.87-1.34) | 0.815 | 0.67 (0.47-0.96) | 0.0301 | 0.63 (0.41-0.97) | 0.0371 |
| Operative time (min)2 | ||||||
| Predicted difference in means | 30.5 (28.8-32.1) | < 0.0011 | 26.4 (24.7-28.1) | < 0.0011 | 29.0 (26.5-31.6) | < 0.0011 |
| Prolonged operative time (%) | 2.00 (1.52-2.62) | < 0.0011 | 1.81 (1.36-2.41) | < 0.0011 | 1.73 (1.17-2.57) | 0.0061 |
Finally, to more robustly corroborate the findings presented in Table 3 and to bolster the weight of the low percentage of neo-adjuvant patients observed (5.81%), rates of intraoperative and postoperative complications and corresponding adjusted odds ratios were calculated among separate cohorts generated for each outcome using propensity-score-based 1:1 nearest-neighboring matching without replacement, accounting for baseline differences in demographic and clinical factors. Within the calculated cohorts, logistic regression and modified Park tests/Poisson regression with average marginal effects were used as previously described.
Finally, in order to explore potential variations in outcomes between different neo-adjuvant regimens, a sub group analysis was performed (Tables 4-6). More specifically, outcomes of patients treated with surgery alone were compared to outcomes of patients who underwent surgical resection after neo-adjuvant chemotherapy alone, neo-adjuvant radiotherapy alone and neo-adjuvant chemo-radiotherapy. The methodology of this sub-group analysis closely reflects that of the primary analysis of the study, except for the fact that to account for non-normal age distributions within the study population, Kruskal-Wallis non-parametric one-way analysis of variance tests were used to compare rank sum differences in age. Moreover, additional variables, such as preoperative albumin level [normal (≥ 3.5 g/dL) vs abnormal (< 3.5 g/dL)] and managing surgical specialty (thoracic, general, other speciality) were accounted for.
| Characteristic | Surgery only n = 3593 (94.21%) | Chemotherapy n = 64 (1.68%) | Radiotherapy n = 100 (2.62%) | Chemo and radio n = 57 (1.49%) | P-value1 |
| Age (yr), median (IQR) | 69 (61-75) | 66 (62-73) | 66 (53-69) | 62 (52-70) | < 0.0012 |
| Male (%) | 1774 (49.59) | 35 (54.69) | 45 (45.45) | 28 (49.12) | 0.662 |
| Race (%) | |||||
| Non-Hispanic White | 2678 (78.58) | 48 (78.69) | 83 (86.46) | 46 (85.19) | 0.508 |
| Non-Hispanic Black | 194 (5.69) | 3 (4.92) | 5 (5.21) | 4 (7.41) | |
| Hispanic | 393 (11.53) | 7 (11.48) | 4 (4.17) | 3 (5.56) | |
| Other or unknown | 143 (4.20) | 3 (4.92) | 4 (4.17) | 1 (1.85) | |
| ASA classification (%) | |||||
| 1-2 no-mild disturbance | 2573 (71.61) | 51 (79.69) | 71 (71.00) | 41 (71.93) | 0.445 |
| 3 serious disturbance | 665 (18.51) | 10 (15.63) | 19 (19.00) | 7 (12.28) | |
| 4-5 life threatening/moribund | 355 (9.88) | 3 (4.69) | 10 (10.00) | 9 (15.79) | |
| Functional status | |||||
| Independent | 3528 (98.19) | 61 (95.31) | 98 (98.00) | 56 (98.25) | 0.413 |
| Partially/totally dependent | 65 (1.81) | 3 (4.69) | 2 (2.00) | 1 (1.75) | |
| Obese, BMI ≥ 30 (%) | 1093 (30.42) | 20 (31.25) | 22 (22.00) | 14 (24.56) | 0.245 |
| Diabetes (%) | 546 (15.20) | 11 (17.19) | 9 (9.00) | 5 (8.77) | 0.178 |
| Current smoker (%) | 1223 (34.04) | 29 (45.31) | 48 (48.00) | 30 (52.63) | < 0.0012 |
| Alcohol consumption (%) | 193 (5.37) | 5 (7.81) | 6 (6.00) | 4 (7.02) | 0.785 |
| Dyspnea (%) | 975 (27.14) | 14 (21.88) | 24 (24.00) | 16 (28.07) | 0.711 |
| History of COPD (%) | 962 (26.77) | 19 (29.69) | 21 (21.00) | 11 (19.30) | 0.317 |
| History of heart disease (%) | 38 (1.06) | 0 (0.00) | 3 (3.00) | 0 (0.00) | 0.186 |
| Hypertension (%) | 2134 (59.39) | 31 (48.44) | 48 (48.00) | 25 (43.86) | 0.0042 |
| Previous cardiac surgery (%) | 280 (7.79) | 2 (3.13) | 6 (6.00) | 3 (5.26) | 0.422 |
| Weight loss (%) | 115 (3.20) | 0 (0.00) | 8 (8.00) | 5 (8.77) | 0.0032 |
| Steroid use (%) | 132 (3.67) | 6 (9.38) | 10 (10.00) | 3 (5.26) | 0.0012 |
| Year of operation (%) | |||||
| 2005-2008 | 728 (20.26) | 6 (9.38) | 34 (34.00) | 9 (15.79) | < 0.0012 |
| 2009-2010 | 1577 (43.89) | 21 (32.81) | 61 (61.00) | 18 (31.58) | |
| 2011-2012 | 1288 (35.85) | 37 (57.81) | 5 (5.00) | 30 (52.63) | |
| Preoperative WBC (%) | |||||
| Normal (4.5-11.0 × 109/L) | 2988 (83.16) | 54 (84.38) | 73 (73.00) | 38 (66.67) | < 0.0012 |
| Abnormal (< 4.5 or > 11.0 × 109/L) | 468 (13.03) | 10 (15.63) | 27 (27.00) | 17 (29.82) | |
| Preoperative hematocrit (%) | |||||
| Normal (≥ 36) | 2860 (79.60) | 32 (50.00) | 52 (52.00) | 23 (40.35) | < 0.0012 |
| Abnormal (< 36) | 588 (16.37) | 32 (50.00) | 48 (48.00) | 32 (56.14) | |
| Preoperative albumin (%) | |||||
| Normal (≥ 3.5 g/dL) | 3345 (93.10) | 58 (90.63) | 82 (82.00) | 55 (96.49) | < 0.0012 |
| Abnormal (< 3.5 g/dL) | 248 (6.90) | 6 (9.38) | 18 (18.00) | 2 (3.51) | |
| Managing specialty (%) | |||||
| Thoracic | 2243 (62.43) | 48 (75.00) | 48 (48.00) | 40 (70.18) | 0.0082 |
| General | 991 (27.58) | 11 (17.19) | 42 (42.00) | 11 (19.30) | |
| Other specialty | 359 (9.99) | 5 (7.81) | 10 (10.00) | 6 (10.53) | |
| Surgery type (%) | |||||
| Open | 2545 (70.83) | 51 (79.69) | 86 (86.00) | 53 (92.28) | < 0.0012 |
| VATS | 1048 (29.17) | 13 (20.31) | 14 (14.00) | 4 (7.02) |
| Characteristic | Surgery only n = 3593 (94.21%) | Chemotherapy n = 64 (1.68%) | Radiotherapy n = 100 (2.62%) | Chemo and radio n = 57 (1.49%) | P-value1 |
| 30-d mortality (%) | 83 (2.31) | 3 (4.69) | 9 (9.00) | 5 (8.77) | < 0.0012 |
| Overall morbidity (%) | 415 (11.55) | 13 (20.31) | 21 (21.00) | 17 (29.82) | < 0.0012 |
| Wound infection | 3 (0.08) | 0 (0.00) | 0 (0.00) | 0 (0.00) | -- |
| Pneumonia | 4 (0.11) | 0 (0.00) | 0 (0.00) | 0 (0.00) | -- |
| Urinary tract infection | 1 (0.03) | 0 (0.00) | 0 (0.00) | 0 (0.00) | -- |
| Return to OR | 170 (4.73) | 7 (10.94) | 10 (10.00) | 4 (7.02) | 0.0122 |
| Venous thromboembolism | 70 (1.95) | 0 (0.00) | 2 (2.00) | 3 (5.26) | 0.212 |
| Cardiac complication | 47 (1.31) | 2 (3.13) | 2 (2.00) | 4 (7.02) | 0.0022 |
| Shock/sepsis | 92 (2.62) | 2 (3.13) | 5 (5.00) | 2 (3.51) | 0.513 |
| Unplanned intubation | 169 (4.70) | 5 (7.81) | 12 (12.00) | 7 (12.28) | < 0.0012 |
| Bleeding transfusion | 133 (3.70) | 8 (12.50) | 7 (7.00) | 10 (17.54) | < 0.0012 |
| Renal complication | 22 (0.61) | 0 (0.00) | 0 (0.00) | 0 (0.00) | -- |
| On ventilator > 48 h | 2 (0.06) | 1 (1.56) | 0 (0.00) | 0 (0.00) | -- |
| Organ space SSI | 38 (1.06) | 0 (0.00) | 1 (1.00) | 0 (0.00) | -- |
| Serious morbidity (%) | 372 (10.35) | 12 (18.75) | 19 (19.00) | 10 (17.54) | 0.0022 |
| Length of stay (d), median (IQR) | 6 (4-8) | 5 (4-7) | 6 (5-9) | 5 (4-8) | 0.134 |
| Prolonged length of stay (%) | 899 (25.02) | 10 (15.63) | 28 (28.00) | 13 (22.81) | 0.306 |
| Operative time (min), median (IQR) | 160 (121-214) | 187 (140-246) | 202 (151-253) | 182 (142-249) | < 0.0012 |
| Prolonged operative time (%) | 858 (23.44) | 23 (35.94) | 41 (41.00) | 20 (35.09) | < 0.0012 |
| Characteristic | Surgery only varied size with cohort | Chemotherapy n = 64 matched patients | Radiotherapy n = 100 matched patients | Chemo and radio n = 57 matched patients |
| 30-d mortality (%) | 1.00 (reference) | 0.74 (0.16-3.43) | 1.24 (0.73-2.13) | 1.38 (0.79-2.43) |
| Overall morbidity (%) | 1.00 (reference) | 1.10 (0.46-2.65) | 1.14 (0.80-1.62) | 1.38 (1.01-1.89)1 |
| Serious morbidity (%) | 1.00 (reference) | 1.25 (0.50-3.13) | 1.00 (0.70-1.42) | 1.00 (0.72-1.38) |
| Length of stay (d)2 | ||||
| Predicted mean difference | 0.00 (reference) | -1.43 (-2.19 to -0.68)1 | 0.34 (0.08-0.60)1 | -0.04 (-0.28-0.21) |
| Prolonged length of stay (%) | 1.00 (reference) | 0.56 (0.21-1.46) | 0.95 (0.68-1.31) | 0.78 (0.57-1.06) |
| Operative time (min)2 | ||||
| Predicted mean difference | 0.00 (reference) | 13.8 (10.7-17.0)1 | 19.9 (18.7-21.1)1 | 9.49 (8.41-10.6)1 |
| Prolonged operative time (%) | 1.00 (reference) | 2.20 (1.00-4.87)1 | 1.56 (1.15-2.11)1 | 1.03 (0.79-1.34) |
All data analyses and management were performed using Stata/MP version 12 (StataCorp LP, College Station, TX, United States). The statistical review of the study was performed by a biomedical statistician.
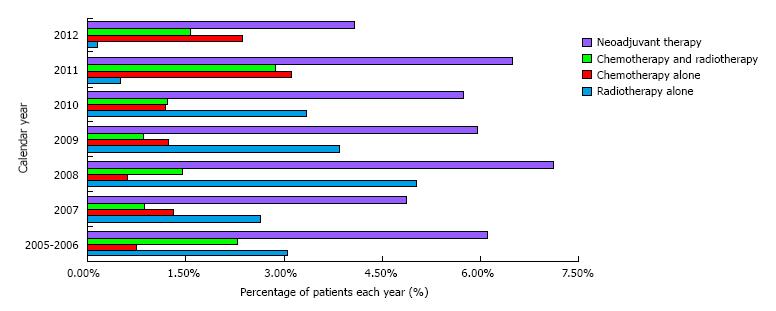
We identified 4063 patients who had lung surgery from 2005 to 2012, and had information on pre-operative treatment. Induction treatment was given to 236 (5.8%) patients; of those, 64 underwent chemo-radiation, 103 radiation alone, 69 chemotherapy alone. The percentages of patients receiving induction, and the type of neo-adjuvant treatment used across the study years are shown in Figure 1. We compared the results to 3827 patients (94.2%) treated with upfront surgery. Demographic characteristics were significantly different between the two groups (Table 1). Patients who underwent induction treatment were younger (66 vs 69, P < 0.001), reported higher recent weight loss (6.8% vs 3.5%; P = 0.011), were active smokers (48.3 vs 34.9, P < 0.001), and had lower preoperative cell counts (abnormal WBC: 25.6 vs 13.4; P < 0.001; low hematocrit 53% vs 17.3%, P < 0.001). Furthermore, we observed significantly lower rates of VATS resections (14.41% vs 13.55%, P < 0.001) among neo-adjuvant patients. On unadjusted analysis, patients who received induction therapy had significantly higher 30-d mortality, overall and serious morbidity (Table 2). Odds of experiencing prolonged operative time and reoperation rates were also higher among patients in the neo-adjuvant group. Adjusted analysis showed similar findings: Patients who underwent induction had significantly higher mortality [odds ratio (OR), 2.70; 95%CI: 1.54-4.72; P = 0.001], overall (OR, 1.53; 95%CI: 1.12-2.11; P = 0.010) and serious (OR, 1.55; 95%CI: 1.08-2.23; P = 0.018) morbidity and higher odds of experiencing prolonged operative time (OR, 1.81; 95%CI: 1.36-2.41; P < 0.001) (Table 3). Interestingly, patients treated with surgery alone had higher LOS and prolonged LOS.
Results after matching for baseline differences in demographic and clinical factors are shown in Table 7. While differences in mortality among the groups were non-significant, overall morbidity, serious morbidity and prolonged operative time remained higher in the neo-adjuvant group.
| Characteristic | Neoadjuvant patients n = 234 (50.00%)3 | Surgery-only patients n = 234 (50.00%)3 | Odds ratio (95%CI) | P-value1 |
| 30-d mortality (%) | 19 (8.12) | 12 (5.13) | 1.63 (0.77-3.45) | 0.197 |
| Overall morbidity (%) | 63 (26.92) | 42 (17.95) | 1.68 (1.08-2.62) | 0.0211 |
| Return to OR | 22 (9.40) | 7 (2.99) | 3.37 (1.41-8.04) | 0.0061 |
| Cardiac complication | 10 (4.27) | 4 (1.71) | 2.57 (0.79-8.30) | 0.116 |
| Shock/sepsis | 11 (4.70) | 3 (1.28) | 3.80 (1.05-13.80) | 0.0431 |
| Unplanned intubation | 27 (11.54) | 17 (7.26) | 1.66 (0.88-3.14) | 0.116 |
| Bleeding transfusion | 28 (11.97) | 13 (5.56) | 2.31 (1.17-4.58) | 0.0161 |
| Serious morbidity (%) | 44 (18.80) | 28 (11.97) | 1.70 (1.02-2.85) | 0.0421 |
| Length of stay (d)2 | ||||
| Predicted difference in means | -- | -- | -1.02 (-1.54 to -0.50) | < 0.0011 |
| Prolonged length of stay (%) | 44 (18.80) | 63 (26.92) | 0.63 (0.41-0.97) | 0.0371 |
| Operative time (min)2 | ||||
| Predicted difference in means | -- | -- | 29.0 (26.5-31.6) | < 0.0011 |
| Prolonged operative time (%) | 90 (38.46) | 62 (26.50) | 1.73 (1.17-2.57) | 0.0061 |
Results of the sub-group analysis comparing outcomes of surgery alone to those of different neo-adjuvant regimens did not show clinically meaningful differences between the neo-adjuvant sub groups (Tables 4-6).
The results from our analysis demonstrated globally worse postoperative outcomes in patients who received neo-adjuvant treatment before lung resection, when compared to those treated with surgery alone. Thirty-day overall and serious morbidity rates as well as operative times, were consistently higher in patients receiving induction treatment. Conversely, higher mortality in the neo-adjuvant group was statistically non-significant after matching.
The two groups showed several differences at baseline, some of which likely reflected the effects of neo-adjuvant administration. Patients in the induction group, in fact, demonstrated signs of malnutrition and myelosuppression, as shown by their weight loss and lower blood cell counts. Likewise, probably some patients’ characteristics such as more advanced age influenced the decision to avoid neo-adjuvant treatment.
In our population, a significantly higher percentage of patients in the neo-adjuvant group were current smokers, as defined by NSQIP (the patient has smoked cigarettes in the year prior to admission for surgery). It has been reported that continued smoking after cancer diagnosis was related to reduced treatment efficacy, increased treatment-related complications and reduced survival[11]. Even though this could have partially influenced the induction group’s worse results, it is worth stressing that most differences in post-operative adverse events persisted after adjusting for smoking habits. Chronic steroid use, which was more prevalent among patients who received neo-adjuvant therapy, is another factor that has previously been associated with worse surgical outcomes[12]. Of note, the higher rates of steroid use observed in the induction group could in part represent therapy for drug- and radiation-induced pulmonary toxicity, which is routinely treated with high dose of steroids[13,14]. Yet, only prolonged steroid treatment would meet the requirements to be collected by the NSQIP under the “steroid” variable.
The occurrence of some of the adverse events observed more frequently in the induction group can be directly related to neo-adjuvant therapy. Thrombocytopenia induced by myelotoxic drugs, for example, might worsen bleeding risk, regardless the chemotherapy used[15]. Similarly, lower leukocyte counts can certainly predispose to the development of sepsis. Moreover, some authors have expressed concern that induction therapy may promote pleural adhesion and vascular fragility, resulting in anatomic disruptions detrimental for surgical outcomes[5]. Analogously, radiation-induced fibrosis can result in a more complex-hence prone to structural damage-dissection between the anatomical planes, which can easily account for lengthier operative times and higher bleeding rates, as observed in the neo-adjuvant patients. Of note, in the NSQIP database the “postoperative bleeding” variable is recorded by using the number of transfusions given as a surrogate; since patients who received induction treatment had a higher chance for myelosuppression, they intuitively had higher probability of developing a significant postoperative anemia requiring transfusion.
On adjusted analysis, patients who underwent neo-adjuvant therapy appeared to have shorter LOS and reduced odds of experiencing prolonged LOS than patients treated with surgery alone; this is counterintuitive, give the globally worse outcomes of the induction group. Nevertheless it is worth recalling that the NSQIP variable “discharge destination” was included in 2011. Understanding the destination after discharge is important to evaluate if the LOS for neo-adjuvant patients was actually shorter due to early discharge home or an artifact attributable to a transfer to another facility.
Several different protocols of neo-adjuvant therapy have been designed and tested for lung cancer, and their overall benefit varies according to tumor stage and type of induction used. Results of a recent systematic review and meta-analysis of randomized controlled trials, showed that patients affected by non-small-cell lung cancer who underwent preoperative chemotherapy had significantly improved overall survival, time to distant recurrence, and recurrence-free survival in resectable non small cell lung cancer (NSCLC)[16]. Analysis of data from the National Cancer Database suggested that neo-adjuvant chemo-radiation followed by lobectomy, was associated with an improved survival in patients with advanced NSCLC[17]. A large randomized trial showed that the addition of pre-operative chemo-radiation to chemotherapy, in patients with resectable stage III NSCLC increases pathological response and mediastinal downstaging, without however affecting survival[18]. The same study showed a remarkable increased in treatment-related mortality in patients who underwent pneumonectomy after having received chemo-radiation, to the point that the risk outweighed the benefit of therapy. Shah et al[19] reported that the addition of induction radiotherapy to induction regimens granted no benefit in survival and discouraged its routinely use, given the potential harmful effects of radiation itself. On the other hand, Toyooka et al[20] indeed suggested that induction chemo-radiotherapy could be superior to induction chemotherapy alone in selected groups of patients, such as those with mediastinal lymph node metastasis. There are fewer studies on the use of neo-adjuvant therapy for early stage lung cancer; some data have suggested potential advantages of induction, showing a trend towards better survival, which, however, did not reach statistical significance[21]. Even though the NSQIP database does not allow us to study oncologic outcomes, it still provides valuable and reliable information about surgical outcomes. The assessment of mortality and morbidity in patients undergoing neo-adjuvant therapy for lung cancer is timely and relevant, given the concerns raised by the potential harms of induction protocols. Several authors have described increased post-operative adverse events after neo-adjuvant therapy, with global complication rates as high as 43.5% in patients who underwent chemo-radiotherapy[22]. Our results correlate well with the STS database analysis performed by Kozower et al[23]. These authors developed a large risk model for morbidity and mortality after lobectomy, sleeve lobectomy, bilobectomy, pneumonectomy, segmentectomy, and wedge resection for primary lung cancer, and observed that induction chemo-radiation therapy is an independent predictor of mortality and major morbidity. However, our work also showed some interesting differences from similar studies in the literature. Evans et al[8] queried the STS General Thoracic Surgery Database in order to examine outcomes of patients undergoing lung resections after neo-adjuvant treatment. According to their analysis, induction therapy did not increase the odds of discharge mortality, prolonged LOS, or major morbidity. Several differences, which may account for this discrepancy in results, are worth being stressed. First of all Evans et al[8] only focused on major resections, such as lobectomies and pneumonectomies. Secondarily, our two studies present some differences in the types of statistical analysis chosen, as well as in the morbidities selected as outcomes. Finally, it is important to recall that NSQIP has the potential of capturing more data from general surgery units than STS, which is more specialty-oriented. Our data, in fact, showed that almost 40% of the pulmonary resection in our study where not performed by thoracic surgeons (Table 4). It is indeed known that general surgeons perform the majority of lung resections in the United States (more than 50%), even though they have on average significantly lower median thoracic surgical procedure case volumes compared with general thoracic and cardiac surgeons[24]. In parallel, it has been reported that thoracic surgeons, in high-volume personal and hospital settings, achieve the best outcomes for lung resections[25]. As a consequence, it is reasonable to postulate that also differences in the distribution of surgeons’ specializations across the two datasets might be one of the underlying causes of the observed discrepancies in outcomes.
Our study has several limitations in part related to the type of dataset used. NSQIP in fact, collects data only for 30 and 90 d before surgery for chemotherapy and radiation therapy respectively. Therefore if a patient received any treatment before this period of time, the patient could have been mislabeled as never receiving treatment at all. Moreover all patients without information regarding induction therapy were excluded from the study. Also, patients who were not surgical candidate due to unexpected complications of induction therapy were not recorded in this dataset and therefore excluded from this study. Information about drugs type and dosage as well a radiation planning were not available to us to optimize our analysis. Furthermore, in order to achieve greater statistical power, we grouped different neo-adjuvant regimens together under the broader group of “surgery following neo-adjuvant therapy”. While this approach necessarily leads to some loss of insight within the single neo-adjuvant regimens, we believe that it was appropriate for the purpose of the present study; in fact it is worth stressing that our sub-group analysis did not show clinically meaningful differences among the various neo-adjuvant sub-groups. Patients’ baseline and tumor’s characteristics (including stage) might have influenced the decision to give induction therapy. NSQIP however does not provide this information and therefore we can’t comment on the indication for neo-adjuvant therapy. We found significantly more open cases in the induction group, yet, it is not possible to determine if those procedures started as open procedures or were conversions from VATS, since both these events are recorded as open in NSQIP. In addition only few hospitals voluntarily participate in the NSQIP database and therefore our results might not apply to all hospitals and the general population. Finally, this database records data only for 30 d after surgery and a longer follow-up cannot be evaluated, especially in regards to oncologic results.
Although with some limitations, our study shows important results to consider when treating a patient with lung cancer who underwent induction therapy.
This study shows that induction treatment for lung cancer leads to worse early post-operative outcomes after lung resection. Further research will be necessary in order to individuate subgroups of patients particularly susceptible to develop complications. With these assumptions, since the evidence in favor of neo-adjuvant therapy for lung cancer is not as compelling as for other cancers, we believe that the indication for induction should be weighted carefully for every patient against its possible downsides, in order to exploit its benefits while minimizing the potential harm.
Induction therapy for lung cancer has been reported to modestly improve survival in locally advanced disease. However, the impact of treatment on surgical outcomes has not been extensively studied.
In an attempt to improve survival for this disease, several multimodality treatment approaches, including neo-adjuvant therapy protocols, have been developed through the years. The concern of developing life-threatening complications, prevalent and severe enough to offset the potential benefits of induction, can constitute a significant obstacle for the diffusion of neo-adjuvant protocols.
In this study, results showed globally worse postoperative outcomes in patients who underwent neo-adjuvant therapy prior to lung resection, when compared to those treated with surgery alone.
Indication for induction therapy should be weighted carefully for every patient against its possible downsides, in order to exploit its benefits while minimizing the potential harm.
American College of Surgeons National Surgical Quality Improvement Project (ACS-NSQIP) is a large, nationally-validated, risk-adjusted, outcomes-based program used to measure and improve the quality of surgical care.
Well-prepared and discussed review on affects of treatment and/or surgery of lung cancer. While it is hard to believe that similar studies were not performed in past, particularly considering the wide occurrence of this type of tumor, the results are interesting and clinically important.
Manuscript Source: Invited manuscript
Specialty Type: Surgery
Country of Origin: United States
Peer-Review Report Classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Sugawara I, Vetvicka V S- Editor: Ji FF L- Editor: A E- Editor: Lu YJ
| 1. | American Cancer Society. Cancer Facts and Figures 2014. Atlanta, USA. [accessed 2014 Oct 23]. Available from: http://www.cancer.org/acs/groups/content/@research/documents/webcontent/acspc-042151.pdf. |
| 2. | Sawabata N, Miyaoka E, Asamura H, Nakanishi Y, Eguchi K, Mori M, Nomori H, Fujii Y, Okumura M, Yokoi K. Japanese lung cancer registry study of 11,663 surgical cases in 2004: demographic and prognosis changes over decade. J Thorac Oncol. 2011;6:1229-1235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 236] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 3. | National Cancer Institute. Incidence and Mortality of Lung Cancer. Bethesda, USA. [accessed 2014 Oct 23]. Available from: http://www.cancer.gov/cancertopics/pdq/treatment/non-small-cell lung/healthprofessional #Section_12. |
| 4. | Ripley RT, Rusch VW. Role of induction therapy: surgical resection of non-small cell lung cancer after induction therapy. Thorac Surg Clin. 2013;23:273-285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 5. | Venuta F, Anile M, Diso D, Ibrahim M, De Giacomo T, Rolla M, Liparulo V, Coloni GF. Operative complications and early mortality after induction therapy for lung cancer. Eur J Cardiothorac Surg. 2007;31:714-717. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 6. | Roberts JR, Eustis C, Devore R, Carbone D, Choy H, Johnson D. Induction chemotherapy increases perioperative complications in patients undergoing resection for non-small cell lung cancer. Ann Thorac Surg. 2001;72:885-888. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 81] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 7. | Albain KS, Rusch VW, Crowley JJ, Rice TW, Turrisi AT, Weick JK, Lonchyna VA, Presant CA, McKenna RJ, Gandara DR. Concurrent cisplatin/etoposide plus chest radiotherapy followed by surgery for stages IIIA (N2) and IIIB non-small-cell lung cancer: mature results of Southwest Oncology Group phase II study 8805. J Clin Oncol. 1995;13:1880-1892. [PubMed] |
| 8. | Evans NR, Li S, Wright CD, Allen MS, Gaissert HA. The impact of induction therapy on morbidity and operative mortality after resection of primary lung cancer. J Thorac Cardiovasc Surg. 2010;139:991-6.e1-991-6.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 9. | American College of Surgeons National Surgical Quality Improvement Program. Participants. Chicago, USA. [accessed 2014 Oct 23]. Available from: http://site.acsnsqip.org/participants. |
| 10. | American College of Surgeons National Surgical Quality Improvement Program. ACS-NSQIP user guide for the 2012 Participant Data Use File. Chicago, USA. [accessed 2014 Oct 23]. Available from: http://site.acsnsqip.org/wp-content/uploads/2013/10/ACSNSQIP.PUF_.UserGuide.2012.pdf. |
| 11. | Florou AN, Gkiozos IC, Tsagouli SK, Souliotis KN, Syrigos KN. Clinical significance of smoking cessation in subjects with cancer: a 30-year review. Respir Care. 2014;59:1924-1936. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 54] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 12. | Ismael H, Horst M, Farooq M, Jordon J, Patton JH, Rubinfeld IS. Adverse effects of preoperative steroid use on surgical outcomes. Am J Surg. 2011;201:305-308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 150] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 13. | Vahid B, Marik PE. Pulmonary complications of novel antineoplastic agents for solid tumors. Chest. 2008;133:528-538. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 167] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 14. | Kim S, Oh IJ, Park SY, Song JH, Seon HJ, Kim YH, Yoon SH, Yu JY, Lee BR, Kim KS. Corticosteroid therapy against treatment-related pulmonary toxicities in patients with lung cancer. J Thorac Dis. 2014;6:1209-1217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 15. | Elting LS, Rubenstein EB, Martin CG, Kurtin D, Rodriguez S, Laiho E, Kanesan K, Cantor SB, Benjamin RS. Incidence, cost, and outcomes of bleeding and chemotherapy dose modification among solid tumor patients with chemotherapy-induced thrombocytopenia. J Clin Oncol. 2001;19:1137-1146. [PubMed] |
| 16. | NSCLC Meta-analysis Collaborative Group. Preoperative chemotherapy for non-small-cell lung cancer: a systematic review and meta-analysis of individual participant data. Lancet. 2014;383:1561-1571. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 654] [Cited by in RCA: 646] [Article Influence: 58.7] [Reference Citation Analysis (0)] |
| 17. | Koshy M, Fedewa SA, Malik R, Ferguson MK, Vigneswaran WT, Feldman L, Howard A, Abdelhady K, Weichselbaum RR, Virgo KS. Improved survival associated with neoadjuvant chemoradiation in patients with clinical stage IIIA(N2) non-small-cell lung cancer. J Thorac Oncol. 2013;8:915-922. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 62] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 18. | Thomas M, Rübe C, Hoffknecht P, Macha HN, Freitag L, Linder A, Willich N, Hamm M, Sybrecht GW, Ukena D. Effect of preoperative chemoradiation in addition to preoperative chemotherapy: a randomised trial in stage III non-small-cell lung cancer. Lancet Oncol. 2008;9:636-648. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 219] [Cited by in RCA: 245] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 19. | Shah AA, Berry MF, Tzao C, Gandhi M, Worni M, Pietrobon R, D’Amico TA. Induction chemoradiation is not superior to induction chemotherapy alone in stage IIIA lung cancer. Ann Thorac Surg. 2012;93:1807-1812. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 91] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 20. | Toyooka S, Kiura K, Shien K, Katsui K, Hotta K, Kanazawa S, Date H, Miyoshi S. Induction chemoradiotherapy is superior to induction chemotherapy for the survival of non-small-cell lung cancer patients with pathological mediastinal lymph node metastasis. Interact Cardiovasc Thorac Surg. 2012;15:954-960. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 31] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 21. | Pisters KM, Vallières E, Crowley JJ, Franklin WA, Bunn PA, Ginsberg RJ, Putnam JB, Chansky K, Gandara D. Surgery with or without preoperative paclitaxel and carboplatin in early-stage non-small-cell lung cancer: Southwest Oncology Group Trial S9900, an intergroup, randomized, phase III trial. J Clin Oncol. 2010;28:1843-1849. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 174] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 22. | Fujita S, Katakami N, Takahashi Y, Hirokawa K, Ikeda A, Tabata C, Mio T, Mishima M. Postoperative complications after induction chemoradiotherapy in patients with non-small-cell lung cancer. Eur J Cardiothorac Surg. 2006;29:896-901. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 35] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 23. | Kozower BD, Sheng S, O’Brien SM, Liptay MJ, Lau CL, Jones DR, Shahian DM, Wright CD. STS database risk models: predictors of mortality and major morbidity for lung cancer resection. Ann Thorac Surg. 2010;90:875-881; discussion 881-883. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 230] [Cited by in RCA: 267] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 24. | Ellis MC, Diggs BS, Vetto JT, Schipper PH. Intraoperative oncologic staging and outcomes for lung cancer resection vary by surgeon specialty. Ann Thorac Surg. 2011;92:1958-1963; discussion 1963-1964. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 39] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 25. | Goodney PP, Lucas FL, Stukel TA, Birkmeyer JD. Surgeon specialty and operative mortality with lung resection. Ann Surg. 2005;241:179-184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 147] [Article Influence: 7.4] [Reference Citation Analysis (0)] |