Published online Sep 19, 2020. doi: 10.5409/wjcp.v9.i2.29
Peer-review started: May 28, 2020
First decision: June 15, 2020
Revised: June 28, 2020
Accepted: September 1, 2020
Article in press: September 1, 2020
Published online: September 19, 2020
Processing time: 111 Days and 22.8 Hours
Esophageal atresia (EA) is the most common congenital anomaly of the gastrointestinal tract. Gastroesophageal reflux disease (GERD) is a frequent and lifelong problem in these patients. GERD can be asymptomatic and the incidence of esophageal gastric and intestinal metaplasia (Barrett’s esophagus) is increased in adults with EA compared with the general population. Timely and accurate diagnosis of GERD is important to reduce long-term problems and this may be achieved by pH-impedance testing.
To assess symptoms and pH-impedance data in children after EA, in order to identify their specific features of GERD.
This study was conducted from November 2017 to February 2020 and involved 37 children who had undergone EA via open surgical repair (51.35% boys, 48.65% girls; age range: 1-14 years, median: 4.99 years). GERD diagnosis was made based on multichannel intraluminal impedance/pH study and two groups were established: EA without GERD, n = 17; EA with GERD, n = 20. A control group was established with 66 children with proven GERD (68.18% boys, 31.82% girls; median age: 7.21 years), composed of a nonerosive reflux disease (referred to as NERD) group (n = 41) and a reflux esophagitis group (n = 25). Upper gastrointestinal endoscopy with a mucosal esophageal biopsy was performed on all patients.
The most frequently observed symptom in EA patients with GERD and without GERD was cough (70% and 76.5% respectively). The number of patients with positive symptom association probability in the EA groups was significantly larger in the EA without GERD group (P = 0.03). In the control reflux esophagitis group, prevalence of gastrointestinal symptoms was significantly higher than in the NERD group (P = 0.017). For both EA groups, there was strong correlation with index of proximal events (IPE) and total proximal events (EA with GERD: 0.96, P < 0.001; EA without GERD: 0.97, P < 0.001) but level of IPE was significantly lower than in GERD patients without any surgical treatment (P < 0.001). Data on distal mean nocturnal baseline impedance were significantly different between the EA with GERD group (P < 0.001) and the two control groups but not between EA without GERD and the two control groups.
Mean nocturnal baseline impedance may have diagnostic value for GERD in EA children after open surgical repair. IPE might be an additional parameter of pH-impedance monitoring.
Core Tip: Esophageal atresia (EA) is the most common congenital anomaly of the gastrointestinal tract. Gastroesophageal reflux disease (GERD) is a frequent and lifelong problem after EA repair. pH-impedance testing makes assessment of pH and other parameters of GERD possible, aiding disease diagnosis and management. Even asymptomatic patients should undergo monitoring of GERD to confirm the absence or the persistence of reflux, and the need to continue treatment. We analyzed data of children with EA open surgical repair to determine the features of GERD among them and propose some important issues for consideration in the follow-up program for these patients.
- Citation: Aksionchyk M, Marakhouski K, Svirsky A. Gastroesophageal reflux disease in pediatric esophageal atresia: Assessment of clinical symptoms and pH-impedance data. World J Clin Pediatr 2020; 9(2): 29-43
- URL: https://www.wjgnet.com/2219-2808/full/v9/i2/29.htm
- DOI: https://dx.doi.org/10.5409/wjcp.v9.i2.29
Esophageal atresia (EA), with or without trachea-esophageal fistula, is the most common congenital anomaly of the gastrointestinal tract and, given the increasingly successful surgical outcomes, it currently represents a lifelong issue[1-3]. Other than respiratory problems, nutritional and gastrointestinal issues are prevalent, not only in the first years of life but also in adolescence and adulthood. Gastroesophageal reflux disease (GERD), peptic esophagitis, esophageal gastric and intestinal metaplasia (known as Barrett’s esophagus), anastomotic strictures, feeding disorders, dysphagia, and esophageal dysmotility are the most frequent gastrointestinal short-term and long-term complications encountered in children and adolescents with EA[4]. The incidence of esophagitis and Barrett’s esophagus is increased in adults with EA compared with the general population[4].
The current gold-standard tests for the diagnosis of GERD are pH probe testing and pH-impedance testing, both of which measure the esophageal reflux burden[5]. Multichannel intraluminal impedance (MII) is an additional procedure for measuring the movement of fluids, solids and air in the esophagus. When combined with MII, pH recording is able to detect liquid reflux, independent of its pH, and gas episodes[6]. Twenty-four-hour measurement of esophageal MII combined with pH-metry (known collectively as MII/pH) makes possible the assessment of pH and other parameters of gastroesophageal reflux together with disease symptoms and the diagnosis of GERD[6-7].
There remains a critical need for an effective way to diagnose and monitor reflux. pH-metry is able to quantify acid burden, ensure that acid suppression is adequate during long-term follow-up, and correlate acid reflux to symptoms. pH with impedance is additionally able to detect non-acid reflux as well as volume clearance, both of which correlate with patient symptoms. It is also able to correlate extra-gastrointestinal symptoms to reflux, which may help guide treatment. pH-impedance is also useful in quantifying the proportion of reflux reaching up to the proximal esophagus, referred to as “high reflux.” EA patients frequently experience extraesophageal symptoms, and pH-MII has the unique ability to determine if these symptoms correlate with reflux episodes, regardless of whether they involve acid or non-acid[8].
Many children with EA with chronic GER have no troublesome symptoms. Results from pH-impedance (pH-MII) studies as well as endoscopic evaluations in children with EA show that asymptomatic children can have severe abnormalities[7,9]. These data indicate that patients with EA should be evaluated regularly by a multidisciplinary team (pulmonology, gastroenterology and otolaryngology), even in the absence of GERD-related symptoms. Therefore, the European Society for Pediatric Gastroenterology, Hepatology, and Nutrition (known as ESPGHAN)-North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition (known as NASPGHAN) Guideline (2016) recommends that all EA patients (including asymptomatic patients) should undergo monitoring of GERD (impedance/pH-metry and/or endoscopy) at time of discontinuation of anti-acid treatment and during long-term follow-up[4].
One of the limitations of pH-impedance testing in patients with esophagitis or esophageal motility disorders (both of which are commonly found in patients with EA) is that baseline impedances are 75% lower than in control patients[7]. This is important to realize, and manual revision of pH-MII tracings should be considered for all EA patients, especially in cases of unexplained symptoms or persistent growth impairment[9].
We designed this study to assess the clinical symptoms and pH-impedance data in children who underwent EA open surgical repair, with comparison to a control group of children with proven GERD[10], to find specific features of GERD in the group of EA patients and to provide data that will aid in the development of an effective and efficient national follow-up program for the EA patients.
The study comprised 43 children with EA, ranging in age from 1 to 14 years (average: 5.09 years), treated within the first days of life via open surgical repair. All children were operated on in the Department of Pediatric Surgery of The National Centre of Pediatric Surgery. This study was approved by the ethics committee of the National Centre of Pediatric Surgery and registered in The National Centre of Pediatric Surgery Trial Registry. Written informed consent was obtained from the parent(s) or guardian of each patient on the day of the procedure. The National Centre of Pediatric Surgery is located in Minsk (Republic of Belarus) and serves a pediatric population (up to 18 years of age) of approximately 1865000, including treatment and follow-up of EA cases. The average number of children born with EA in Belarus is 15-17 per year. The total number of children with EA in Belarus over the last 5 years is 102.
All surgical repairs were carried out by thoracotomy in the early postnatal period (days 1-2), using primary direct anastomosis of the esophagus “end to end”. There were no cases of gastric/colonic pull-ups in the group of studied EA patients. All patients were treated with proton pump inhibitors (PPIs) for at least 6 mo after the open surgical EA repair.
This study was a retrospective chart review involving 43 children with EA who attended The National Centre of Pediatric Surgery. All EA open surgical repair patients (ages 1-18 years), who were bothered with troublesome symptoms and contacted our clinic, underwent combined impedance-pH testing and upper gastrointestinal endoscopy (with histological study of biopsied mucosa samples), and were considered eligible for study enrollment. For all, acid suppression therapy had been discontinued for at least 7 d before the impedance-pH testing. Between the enrollment dates of November 2017 and February 2020, the 43 children considered eligible included 23 boys (53.5%) and 20 girls (46.5%).
Patients were excluded according to MII/pH monitoring carried out < 18 h (n = 2), eosinophilic esophagitis diagnosis (n = 2), esophageal replacement therapy (gastric pull-up, jejunal/colonic interposition; n = 0), and receipt of fundoplication (n = 2). Thus, a total of 37 patients with EA were enrolled in the study, including 19 boys (51.4%) and 18 girls (48.6%).
Diagnosis of GERD was established based on the result of the MII/pH study and according the recommendation of guidelines on pediatric gastroesophageal reflux clinical practice[10-12]. Depending on the result of the pH-impedance testing, the EA patients were divided into groups of those with GERD (n = 20, 54.1%) and those without GERD (n = 17, 45.9%). The clinical and demographic features of both EA groups are listed in Table 1.
| Characteristic | EA with GERD, n = 20 | EA without GERD, n = 17 | P value |
| Age in yr, as median | 4.93 (95%CI: 2.78 to 7.08; SD: 4.59); P < 0.001 | 5.06 (95%CI: 3.49 to 6.62; SD: 3.05) | 0.444 |
| Male/female, n | 12/8 | 7/10 | 0.26 |
| Gross type of EA | Type C-20 (100) | Type C- 17 (100) | |
| Dysphagia | 6 (30) | 2 (11.8) | 0.186 |
| Vomiting | 2 (10) | 2 (11.8) | 0.862 |
| Heartburn | 1 (5) | 1 (5.9) | 0.905 |
| Cough | 14 (70) | 13 (76.5) | 0.662 |
| Recurrent pneumonia | 2 (10) | - | 0.186 |
| Recurrent bronchitis | 2 (10) | 2 (11.8) | 0.862 |
| Asymptomatic | 2 (10) | 1 (5.9) | 0.653 |
| History of atopy | 5 (25) | 2 (11.8) | 0.314 |
| Esophagitis | 9 (45) | 7 (41.2) | 0.819 |
| Previously treated with PPIs | 8 (40) | 9 (52.9) | 0.606 |
At the same time, we retrospectively evaluated 66 patients with proven GERD (acid exposure time > 7%, total number of retrograde bolus movement > 70), sex- and age-matched to the EA group, who were enrolled in the study to serve as a control group. These patients were selected from among children with GERD-related symptoms, who had undergone pH-impedance testing for suspected GERD (with indications to confirm the diagnosis of GERD[10-12]) and who had undergone upper gastrointestinal endoscopy. Patients were excluded based on history of any abdominal surgery.
Based on the results of the 24-h MII/pH monitoring and endoscopies, control patients with proven GERD were divided into groups of those with reflux esophagitis (RE) (n = 25, 37.9%) and those with nonerosive reflux disease (NERD) (n = 41, 62.1%). The clinical and demographic features of both control groups are listed in Table 2.
| Characteristic | RE, n = 25 | NERD, n = 41 | P value |
| Age in yr, as median | 8.68 (95%CI: 6.5796 to 10.7804) | 5.74 (95%CI: 4.4583 to 7.0295) | 0.0113 |
| Male/female, n | 19/6 | 26/15 | 0.276 |
| Gastrointestinal symptoms | 10 (40) | 6 (14.6) | 0.017 |
| Respiratory symptoms | 7 (28) | 16 (39) | 0.366 |
| Combined symptoms | 8 (32) | 16 (39) | 0.569 |
| Asymptomatic | - | 3 (7.4) | 0.167 |
We used the Gross classification system to define the type of EA, whereby long-gap EA was defined as any distance (> 2 vertebral bodies) between the esophageal (pouch) ends in a newborn too wide for a primary anastomosis[13-14].
A detailed clinical history and parental reported symptoms in all patients were analyzed. Patient data on GERD-related symptoms were collected via a study-specific questionnaire that queried the frequency, strength/intensity, relationship with mealtimes and body position related to GERD symptoms, the previous treatment(s) (i.e., PPIs, alginates, antacids, histamine 2 receptor antagonists, prokinetics), the history of atopy, the birth history, and any accompanying illnesses. Also evaluated were predominant symptom(s) at presentation, timing of symptom(s) onset after EA repair, and type of EA. Parents were instructed to fill out the questionnaire and then, throughout the study period, to maintain a diary of written descriptions of any GERD-related symptoms, body position (prone and supine), and mealtimes (beginning and end). Patients and their parents were instructed to avoid extremely hot or ice-cold drinks and food, “acid” foods, and carbonated beverages.
The study was performed in all patients while off PPI therapy, using a Digitrapper MII ambulatory system (Medtronic, Dublin, Ireland) and disposable MII/pH catheters adjusted for age and height. The study was performed according to standardized protocol and, therefore, correct catheter position was confirmed by X-ray or under visual endoscopic inspection[15]. A single patient-length appropriate catheter with at least 6 impedance and 1 pH channel was used to perform the MII/pH monitoring. Depending on the age of the patient, the pH channel was placed 2 cm to 5 cm above the lower esophageal sphincter. The MII-pH catheters used were of 2.13 mm (6.4 Fr) diameter. All refluxes were then registered via the Digitrapper pH/ZÔ.
The following pH-impedance parameters were analyzed in the study: acid exposure time (AET), as percentage; longest acid exposure, in min; total number of retrograde bolus movements (RBM); number of proximal events; symptom association probability (SAP); and, distal mean nocturnal baseline impedance (MNBI). The impedance data of all patients with EA and GERD were subject to automatic analysis by the Medtronic software but also reviewed manually.
MNBI is considered an accurate method for characterizing esophageal baseline impedance[16-17]. Its measurement consists of determining the baseline impedance at 3 cm or 5 cm above the lower esophageal sphincter during overnight rest, which represents the mean of values obtained during three 10-min time intervals in a period of no swallowing[16]. Even in EA patients without esophagitis, baseline impedances are known to be 44% lower than in control patients with esophagitis[18]. Low baseline impedances impair bolus detection, resulting in an underestimation of the reflux burden in EA patients. This is a major limitation of MII/pH in EA patients[7,9].
We determined distal MNBI in all patients at the same distance of the esophagus depending on age (1 year to 10 years: 3 cm above the lower esophageal sphincter; older than 10 years: 5 cm above the lower esophageal sphincter) and automatically calculated when neither reflux episodes nor swallowing were present, using a specific software function[16,17].
All of the reflux events were evaluated manually for their proximal extent. Retrograde bolus movements that reached at least channel 2 (the second most proximal channel) in the upper esophagus were considered high refluxes[7].
The index of proximal events (IPE) was calculated as the ratio of the number of proximal refluxes to the total number of refluxes per day.
The endoscopy procedure was performed using the Evis Exera III imaging platform (Olympus Corp., Tokyo, Japan) under pharyngeal anesthesia or deep sedation. Mucosal biopsies were taken from the esophagus (a minimum of at least four samples from various parts of the esophagus), the stomach, and the duodenum. RE was diagnosed based on the Los Angeles classification system[19].
Statistical processing of the results was carried out using MedCalc Statistical Software, version 19.2 (MedCalc Software Ltd., Ostend, Belgium; https://www.medcalc.org; 2020). Descriptive statistics included the arithmetic mean and median [both with 95% confidence interval (CI)], standard deviation, and standard error of the mean. Analysis of consistency of signs’ distribution type to the normal distribution law was carried out using the Shapiro-Wilk test; the sign distribution was considered a departure from normality at P < 0.05. Depending on the consistency/inconsistency of the distribution of the analyzed signs to the normal distribution law, the parametric Student’s t-test and the nonparametric Mann-Whitney U-test were used to evaluate differences between the groups.
For regression analysis, the type of regression equation was chosen according to the highest F-ratio and lowest P value, with maximum of P < 0.01. Measures of central tendency and data dispersion were subjected to one-way analysis of variance (ANOVA) to test the difference between the means of several subgroups of a variable; when the test was positive, post hoc testing (i.e. Student-Newman-Keuls) was performed. The Kruskal-Wallis test was used to analyze the effect of a classification factor on ordinal data; if the test resulted in a P ≤ 0.05, post hoc testing (i.e., Dunn’s test) was performed.
The diagnostic performance of data or the accuracy of a test to discriminate diseased cases from normal cases was evaluated using receiver operating characteristic (ROC) curve analysis. The ROC method used was based on Delong et al[20] with binominal extracted CI for the area under the curve (AUC).
A little over one-half (20/37, 54.1%) of the EA patients were diagnosed with GERD. The EA patients with GERD and those without GERD showed similar clinical characteristics, history of atopy, and upper endoscopy data.
Endoscopic analysis of the upper part of the gastrointestinal tract demonstrated 16 EA patients had esophagitis grade A (according to Los Angeles classification) (43.2%), 1 patient had gastric metaplasia (2.7%), and 6 patients had chronic gastritis (16.2%). The upper endoscopy data revealed no significant differences between the EA patients with GERD and those without GERD (P = 0.819) (Table 1).
There were 8 children in the EA with GERD group (40%) and 9 children in the EA without GERD group (52.9%) that had been previously treated with PPIs (1-3 mo prior). After therapy, clinical improvement was observed in only 47.05% of patients in both EA groups who had received therapy. The PPI therapy was discontinued in all patients for at least 7 d before the impedance-pH testing.
Only 3 out of 37 patients with EA did not experience any symptoms during pH-impedance monitoring. Before pH-impedance testing their parents reported extraesophageal symptoms (cough and recurrent bronchitis) spontaneously. Thirty-four patients reported symptoms during pH-impedance testing. Positive symptom association was defined in children who had a symptom association probability (SAP) over 95%. SAP was positive in 3/20 (15%) in the EA with GERD group and in 8/17 (47.06%) in the EA without GERD group. The most frequently reported symptom for the EA patients was cough, for both groups.
The NERD and RE patients in the control group were subcategorized by their symptoms (Table 2), namely gastrointestinal (heartburn, vomiting and abdominal pain), respiratory (cough and recurrent bronchitis), and combined (gastrointestinal and respiratory symptoms in the same patient). Only the gastrointestinal symptoms showed a significant difference between the two groups, with RE patients having significantly higher prevalence of these symptoms than the NERD patients (P = 0.017).
Only 3 out of the 66 controls did not experience any symptoms during pH-impedance monitoring. Their previously reported symptoms, from before pH-impedance testing, were respiratory (cough and recurrent bronchitis).
The EA without GERD group had significantly more patients with positive SAP (> 95%) compared to the EA with GERD group [3/20 (15%) vs 8/17 (47.06%), P = 0.03] (Table 3).
| pH-impedance parameter | EA without GERD | EA with GERD | P value |
| AET (%) | 2.59, 95%CI: 1.68 to 3.5 | 11.62, 95%CI: 7.54 to 15.7, P = 0.0066 | < 0.001 |
| Number of RBM | 40.3, 95%CI: 34.3 to 46.3 | 67.3, 95%CI: 55.27 to 79.32 | < 0.005 |
| Longest acid exposure in min | 9.37, 95%CI: 5.26 to 13.5, P = 0.0104 | 46.8, 95%CI: 28.39 to 65.26, P = 0.0061 | < 0.001 |
| Proximal events | 6.59, 95%CI: 3.1 to 10.1, P = 0.0249 | 10.95, 95%CI: 6.24 to 15.56, P = 0.0089 | 0.151 |
| Index of proximal events1 | 0.17, 95%CI: 0.09 to 0.27, P = 0.0052 | 0.16, 95%CI: 0.1 to 0.22 | 0.939 |
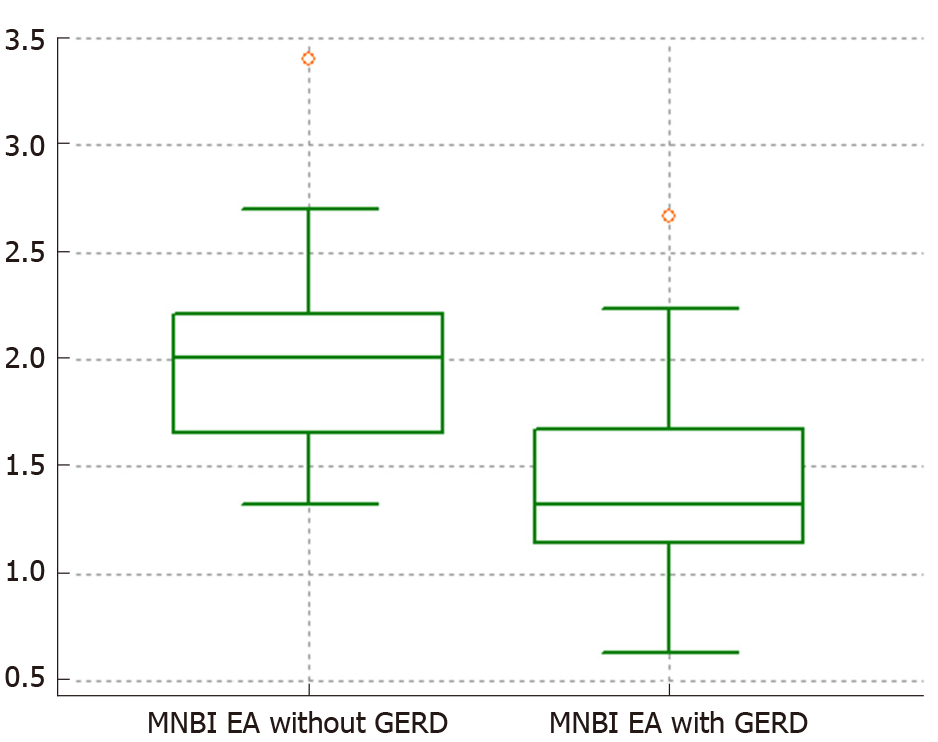
| Distal MNBI in kOhm | 1.99, 95%CI: 1.72 to 2.26 | 1.44, 95%CI: 1.21 to 1.67 | < 0.005 |
| SAP (> 95%/< 95%) | 8/17 | 3/20 | 0.03 |
А comparison of the pH-impedance parameters showed significant differences (P < 0.001) in AET, number of RBM, and duration of the longest reflux event between the EA with GERD group and EA without GERD group. However, Mann-Whitney test (independent samples) indicated no differences in either the number of proximal events (P = 0.151) nor in the IPE (P = 0.939) (Table 3).
Comparison of the MNBI data was carried out using the t-test since the distribution in the groups was normal (Figure 1).
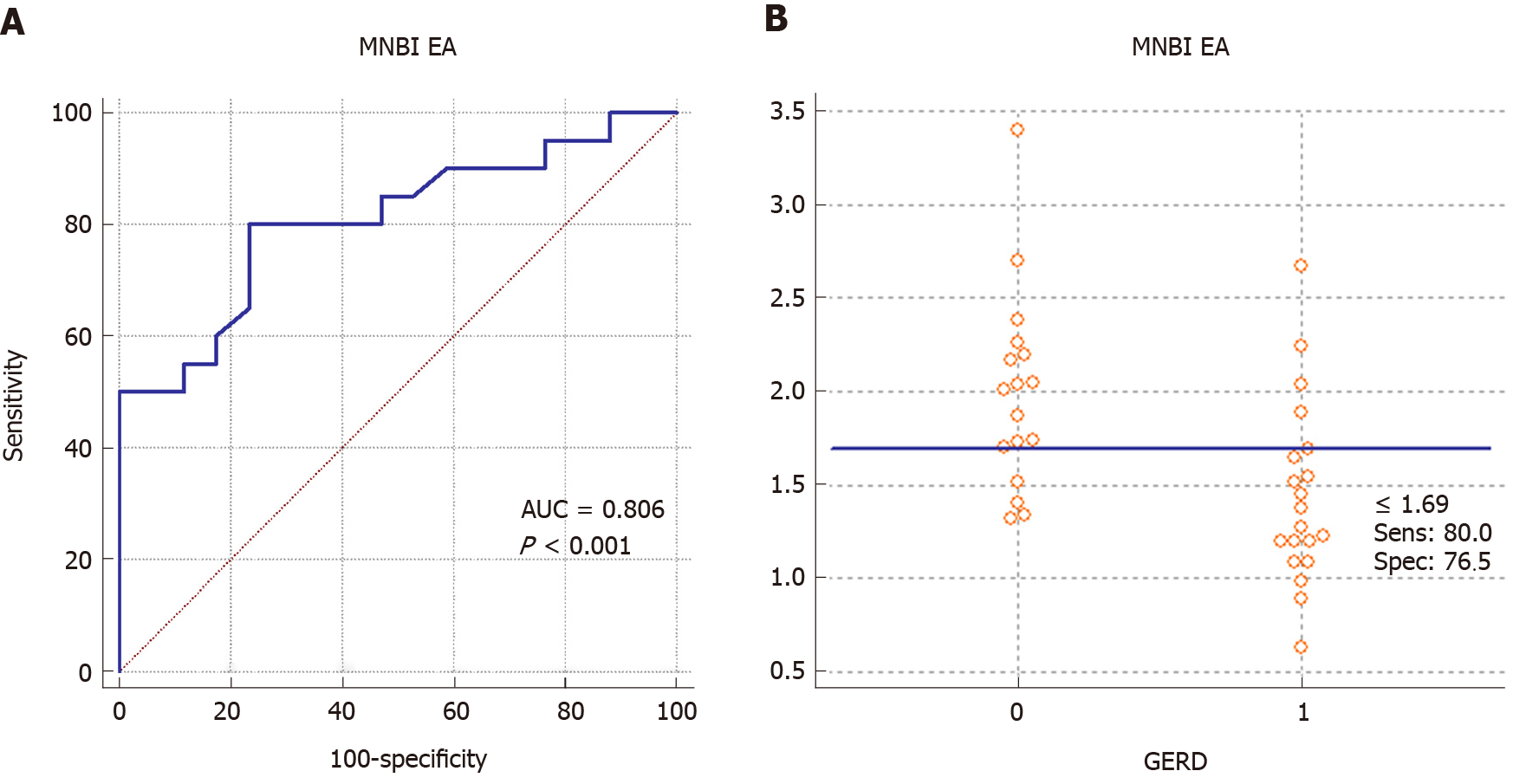
ROC curve analysis used GERD as a classification variable (presence: 1; absence: 0) and MNBI as a variable, and the subsequent results were: AUC = 0.806, P < 0.001 with criterion – 1.69 kOhm, sensitivity 80.0% and specify 76.5% (Figure 2). It should be noted, pairwise comparison of ROC curves for AET (%) and MNBI in the EA group on GERD diagnosis did not show a reliable difference (AUC AET (%) = 0.89). The difference between two areas (calculated as AET~MNBI = 0.0838; 95%CI:
Comparisons between groups (EA with GERD, NERD, and RE) were performed in order to identify specific GERD features. The pH-impedance parameters of the comparison groups are presented in Table 4. The NERD and RE groups were found to share some specific features; in particular, both groups showed a relationship between MNBI and AET, with the NERD group having Spearman's coefficient of rank correlation of -0.46 [P = 0.002; AET(%) = 206364 + -306169 Log(MNBI), P < 0.001] and the RE group having Spearman's coefficient of rank correlation of -0.68 [P < 0.001; AET(%) = 164401 + -243143 Log(MNBI), P = 0.002].
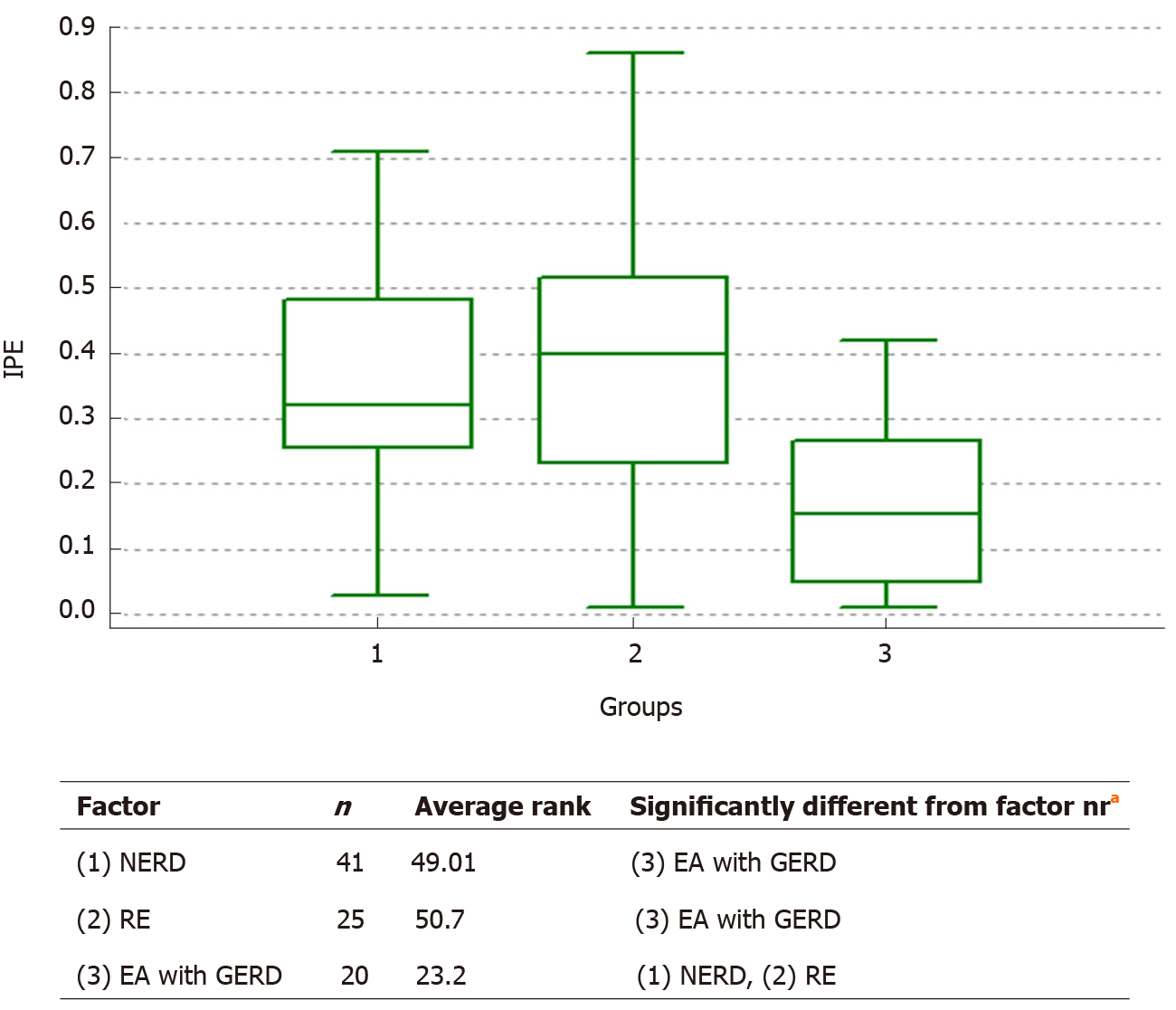
| Parameter | NERD, | RE, | EA with GERD | Kruskal-Wallis test |
| AM (95%CI) | AM (95%CI) | AM (95%CI) | P value | |
| AET (%) | 10.50 (8.05-12.95) | 10.06 (7.33-12.79) | 11.62 (7.53-15.7) | 0.776 |
| Longest acid exposure in min | 21.8425 (17.14 to 26.55) | 25.8560 (18.47 to 33.24) | 46.8 (28.39 to 65.26) | < 0.05 |
| Total number of RBM | 74.61 (63.37 to 85.85) | 82.28 (63.89 to 100.67) | 67.30 (55.28 to 79.32) | 0.697 |
| Total number of proximal reflux | 35.36 (20.76 to 29.97) | 32.91 (21.35 to 44.48) | 10.95 (6.23 to 15.66) | < 0.001 |
| Index of proximal reflux | 0.35 (0.29 to 0.4) | 0.37 (0.29 to 0.46) | 0.16 (0.1 to 0.22) | < 0.001 |
| Distal MNBI in kOhm | 2.25 (2.03 to 2.48) | 1.95 (1.64 to 2.27) | 1.44 (1.21 to 1.67) | < 0.001 |
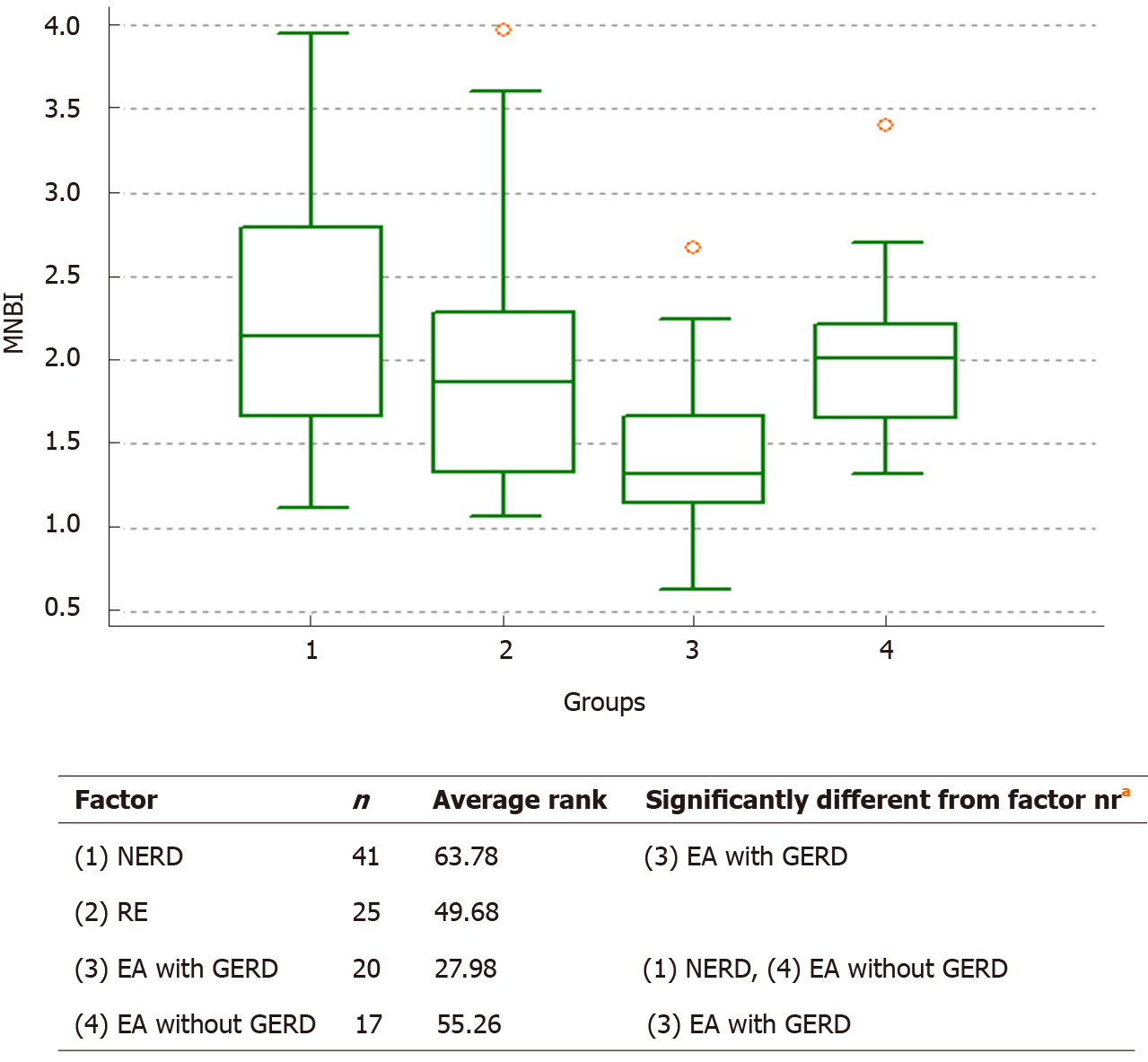
The Kruskal-Wallis test showed absence of difference in AET (%) (P = 0.776) and total number of RBM (P = 0.697) between the groups of EA with GERD, NERD, and RE. However, a significant difference was found in MNBI and the IPE (Figure 3). For the analysis of MNBI, we decided to increase the degree of freedom up to 3-times in the Kruskal-Wallis test, due to the introduction of the EA without GERD group. We based this approach on the fact that the data of MNBI from this group also has great scientific and practical interest when comparing to a group of patients with non-operated esophagus (Figure 4). The ANOVA gave an F-ratio of 6.69 (P < 0.005), and Scheffe test for all pairwise comparison (mean) confirmed the difference between NERD and EA with GERD groups (P < 0.05).
First of all, the main limitation of our study is its high dispersion by age. We included children from 1-year-old to 14-years-old. Second, our institute has no follow-up program for EA open surgery repair patients. We currently examine patients with any GERD-related symptoms, and for this study only 11.7% of the eligible patients with EA open surgical repair were enrolled and included in the analysis. One more limitation of our study is the inability to rule out laryngopharyngeal reflux, because we use probes with one pH-sensor located in the distal part of the probe. This group of patients commonly complain of throat issues, such as chronic cough, throat clearing, or sore throat. Some of our patients had similar complaints. The most common tests in patients suspected of reflux-related laryngeal symptoms or laryngopharyngeal reflux are endoscopy and pH monitoring but these tests have poor sensitivity. The most popular examination of this pathology is proximal or hypo-pharyngeal pH monitoring, but these two probes have sensitivities of only 40%-50% at best, limiting their utility. Thus, there is a need for a better test with increased sensitivity for patients suspected of having laryngopharyngeal reflux[21].
In addition, this was a study, where in not all patients were included but only those who were treated for troublesome symptoms after applying the exclusion criteria and among patients who had contacted our clinic over the past 3 years. Controls were chosen from sex- and gender-matched children with proven GERD in order to find specific features GERD in EA patients. In Belarus, a national follow-up program for EA patients has not yet been developed. So, these patients come to our clinic for examination when they have symptoms. Some of them did not experience any symptoms during pH-impedance monitoring. Before pH-impedance testing their parents reported symptoms spontaneously. The research was carried out at a single institution and as a retrospective study. Further accumulation of study data is needed for a better comparison of data in EA with GERD patients and patients with GERD with nonoperative esophagus. Surely, these data should be evaluated and confirmed with a prospective multicenter study.
Detailed clinical history and parental reported symptoms were analyzed for all patients. Symptoms in study groups were recorded during the study as events and by means of a questionnaire prepared specifically for this study for patients with GERD-related symptoms. We asked parents of children (usually younger than 8 years) to fill out this questionnaire so that we could find out what worries parents of children who cannot explain the symptoms that bother them. Thus, one of the most common symptoms in children younger than 5-6 years are the symptoms noted by their parents, such as coughing, vomiting, feeding difficulties, recurrent bronchitis, and pneumonia. Evaluation of the patient’s and/or parental questionnaires showed that the most frequently observed symptom in EA patients with GERD and without GERD in our groups was cough. We also found that EA patients in our study groups rarely had the typical GERD symptoms of heartburn, regurgitation, and belching. For all symptoms, the comparisons between the EA patients with GERD and those without GERD found no statistical relations.
An intriguing finding in our study was that the number of patients with positive SAP was significantly larger for the EA without GERD group. As such, these children are revealed to have more episodes of symptoms despite the normal data produced upon their pH-impedance testing. This fact can be very important for the accurate evaluation of GERD in symptomatic EA patients before prescribing antireflux medication and especially proceeding fundoplication. On the other hand, we found pathological pH-impedance data in 10% of the asymptomatic patients, meaning that we have to follow-up these patients correctly. Collectively, these results confirm the importance of pH-impedance testing in EA patients in order to evaluate GERD and to individualize the treatment to each patient.
The most frequently observed symptoms in our patients with RE were gastrointestinal (heartburn, vomiting, and abdominal pain). In the group of patients with NERD, respiratory (cough, recurrent bronchitis, and pneumonia) and combined gastrointestinal and respiratory symptoms were more frequently observed. A statistically significant difference was found when the clinical data on gastrointestinal symptoms were compared between the RE patients and NERD patients – showing a significantly higher rate in the former.
The prevalence of GERD in EA patients in our study was high (> 50%). Our results were similar to those from other studies of EA patients with pH-impedance monitoring[7,22-24]. The patients in our study were off PPI therapy for several weeks before examination (minimum being 1 wk).
When we performed investigation into the features of pH-impedance data in our study groups with GERD (EA with GERD, NERD, and RE), we found no significant difference in the pH-impedance parameters (AET and total number of RBM). There were significant differences among all three groups for the longest acid exposure, total number of proximal events, and distal MNBI.
The esophagus is permanently compromised in EA patients, even when successful repair, sometimes under tension, has been achieved. Extrinsic and intrinsic innervations are abnormal and consequently motor function and sphincters are defective. The gastroesophageal reflux event is extremely frequent in patients treated for EA because of serious structural and functional deficiencies[22].
MNBI, a novel pH-impedance metric, may be a surrogate marker of reflux burden. Investigations into the role MNBI in the diagnosis and phenotyping of reflux disease are relatively recent undertakings in the field. They have, however, revealed that MNBI can distinguish different GERD phenotypes from reflux-unrelated symptoms (i.e., functional heartburn) and provides a good predictive value for antireflux therapy[16,17,25-28].
It is known already that EA patients have a significantly lower baseline impendence than normal children with suspected GERD[29,30]. In our study, the EA patients with GERD showed a significantly lower distal MNBI than either the EA patients without GERD or the patients with RE and NERD. Our results show that MNBI can be used as a diagnostic metric for GERD in EA patients after open surgical repair, having sensitivity of 80% and a specificity of 76.5%. We also found that distal MNBI at 1.69 kOhm is the cut-off for diagnosis GERD in EA patients. These results highlight the potential utility/value of distal MNBI for designing a personalized follow-up program for EA patients without high AET or high number of RBM but who have level of distal MNBI < 1.69 kOhm. Such patients require constant monitoring and early treatment of the complications (special follow-up program).
There are many studies in adults and children which have shown a relationship between AET and baseline impedance[31-34]. While this result was found in our group of GERD patients with non-operated esophagus, our study extended the finding to a statistically significant association. However, the statistical analysis indicated the absence of a relationship (significant correlation and logistic regression) between any of the pH-impedance parameters and distal MNBI. This finding is similar to that from a recent study, in which Tong et al[29] proposed that their results could be due to the fact that a significant proportion of their EA cohort (87.9%) and controls (40%) were on PPI therapy during the study, which would have had an effect on the gastroesophageal reflux parameters[29]. In our study, EA patients and patients with proven GERD were off PPI therapy.
The role of reflux height in the clinical picture of GERD in general and extraesophageal symptoms in particular remains unclear. There are studies in EA patients which have shown no relevant correlation of high-reflux events and respiratory symptoms. Statistically, there has been no correlation between the amount of high reflux and symptom scores or reflux index[7]. Yet, as shown in infants by Wenzl[35], there was relevant correlation of high-reflux events with respiratory symptoms.
There was also, in our study, a significant difference between the total number of RBM and the number of proximal events in the same patient. So, one patient may have 100 episodes of RBM and 10 episodes of proximal events, and in another case, the patient may have 20 episodes of RBM and 10 episodes of proximal events; when we compare these cases, the difference will be significant. The first case has 10% proximal events of the total number of RBM, and for the second case it is 50%. We suggest using the IPE for estimation of more adequate assessment of proximal refluxes, as it reflects the share of proximal events in total number of RBM. We calculated this index as the ratio of the number of proximal refluxes to the total number of refluxes per day. Our statistical analysis showed strong correlation with IPE and total proximal events for both EA groups, with and without GERD, and indicated that IPE was significantly lower in both compared to that in GERD patients with non-operated esophagus. Thus, it is obvious that factors other than proximal refluxes are involved in the pathogenesis of respiratory symptoms in EA patients. It is generally known that esophagus after atresia open surgical repair is restored anatomically, but is it restored functionally? An additional question is whether these motility disturbances will disappear with age?
GERD is the most common long-term complication of EA. These patients are predisposed to GERD as a result of the altered anatomy and motility of the esophagus. pH-impedance testing is an effective way to diagnose and monitor for reflux and to individualize the treatment strategy for each patient.
Distal mean nocturnal baseline impedance has good diagnostic value for GERD in children with EA after open surgical repair, with cut-off of < 1.69 kOhm. The difference between two areas (calculated as AUC AET~ AUC MNBI = 0.0838; 95%CI:
The index of proximal events (IPE) is calculated as the ratio of the number of proximal refluxes to the total number of refluxes per day. There was strong correlation with the IPE and total proximal events in each of the EA groups, and our data showed that the IPE in both EA groups was significantly lower than in GERD patients with non-operated esophagus. IPE might be an additional parameter of pH-impedance monitoring.
Esophageal atresia (EA) is the most common congenital anomaly of the gastrointestinal tract. Esophageal dysmotility and gastroesophageal reflux disease (GERD) are frequent and lifelong problems after repair of EA, even after successful surgical repair of the esophagus anatomy. It is important to diagnose and manage GERD to reduce subsequent related respiratory and gastrointestinal problems and their associated short-term and long-term complications. GERD can be asymptomatic and several studies have shown the absence of correlation between symptoms and esophagitis in this population. All EA patients (including asymptomatic patients) should undergo monitoring of GER (impedance/pH-metry and/or endoscopy) at time of discontinuation of anti-acid treatment and during long-term follow-up.
In Belarus, a national follow-up program for EA patients has not yet been developed. So, these patients come to our clinic for examination when they have symptoms. Some of them did not experience any symptoms during pH-impedance monitoring. Before pH-impedance testing their parents reported symptoms spontaneously.
This study was designed to assess clinical symptoms and pH-impedance data in children after EA open surgical repair, and to compare with a control group of children with proven GERD in order to find specific GERD features in these patients and to provide data that will support development of a national program for the follow-up of EA patients. This was accomplished via a retrospective chart review of EA open surgical repair patients with GERD-related symptoms in our clinic from November 2017 to February 2020 using pH-impedance data, upper endoscopy data, medical records and clinic letters.
The main objectives of this study were to assess clinical symptoms and pH-impedance data in children with EA open surgical repair and to compare with a control group of children with proven GERD in order to identify specific features of reflux disease in these groups of patients. According to the results, we hope to develop a national program for the follow-up of EA patients and to personalize their treatment.
Patients with EA who received open surgical repair and combined impedance-pH testing while off proton pump inhibitor therapy and who underwent upper gastrointestinal endoscopy with histological study of mucosa biopsy samples were involved in the study. Data on patient symptoms were collected via a specially-prepared questionnaire for our study patients with GERD-related symptoms. We asked the parents of children (usually younger than 8 years) to fill out this questionnaire so that we could see what worries parents of children who cannot explain the symptoms that bother them. We used the index of proximal events (IPE), calculated as the ratio of the number of proximal refluxes to the total number of refluxes per day. We also determined distal mean nocturnal baseline impedance in all patients at the same distance depending on age (1 year to 10 years: 3 cm above the lower esophageal sphincter; older than 10 years: 5 cm above the lower esophageal sphincter).
We found a strong correlation with IPE and total proximal event in each EA group (EA with GERD: 0.96, P < 0.001; EA without GERD: 0.97, P < 0.001). The level of IPE in both EA groups was significantly lower than in GERD patients without any surgical treatment of esophagus (Kruskal-Wallis test, P < 0.001). Data on distal mean nocturnal baseline impedance in comparison of EA with GERD, EA without GERD, nonerosive reflux disease (commonly referred to as NERD) and reflux esophagitis (commonly referred to as RE) groups showed significant difference between EA with GERD (Kruskal-Wallis test, P < 0.001; one-way analysis of variance: F-ratio 6.69, P < 0.005) and the other two control groups but an absence of difference between EA without GERD, NERD and RE groups. We also found strong correlation with the IPE and total proximal events in each of the EA groups, and our data showed that the IPE in both EA groups was significantly lower than in GERD patients with non-operated esophagus.
Distal mean nocturnal baseline impedance has good diagnostic value for GERD in children with EA after open surgical repair, with cut-off of < 1.69 kOhm, and can be used as an indicator to design a personalized follow-up program for EA patients. The IPE might be an additional parameter of pH-impedance monitoring.
Not all patients were included in this study but only those who were treated for troublesome symptoms (after applying the exclusion criteria) and who had contacted our clinic over the past 3 years. In Belarus, a national follow-up program for EA patients has not yet been developed.
Our results confirm the importance of pH-impedance testing in EA patients in order to evaluate GERD and to individualize the treatment strategy for each patient. This finding has very important implications for the evaluation of GERD in symptomatic EA patients before prescribing antireflux medication and especially in the consideration of proceeding to fundoplication.
Although it is generally known that esophagus after atresia open surgical repair is restored anatomically, whether it is restored functionally remains unknown. Another important unknown for focus of future study is whether these motility disturbances will disappear with age? For such, correct and comprehensive follow-up of surgically-repaired EA patients (such as that designed upon the results of our study presented herein) is needed.
The authors would like to thank the members of the Endoscopic Unit and Department of Surgery of the National Centre of Pediatric Surgery for their technical support.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Belarus
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Borbély Y, Dumitrascu DL S-Editor: Wang DM L-Editor: A P-Editor: Li X
| 1. | Sfeir R, Bonnard A, Khen-Dunlop N, Auber F, Gelas T, Michaud L, Podevin G, Breton A, Fouquet V, Piolat C, Lemelle JL, Petit T, Lavrand F, Becmeur F, Polimerol ML, Michel JL, Elbaz F, Habonimana E, Allal H, Lopez E, Lardy H, Morineau M, Pelatan C, Merrot T, Delagausie P, de Vries P, Levard G, Buisson P, Sapin E, Jaby O, Borderon C, Weil D, Gueiss S, Aubert D, Echaieb A, Fourcade L, Breaud J, Laplace C, Pouzac M, Duhamel A, Gottrand F. Esophageal atresia: data from a national cohort. J Pediatr Surg. 2013;48:1664-1669. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 122] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 2. | Deurloo JA, Klinkenberg EC, Ekkelkamp S, Heij HA, Aronson DC. Adults with corrected oesophageal atresia: is oesophageal function associated with complaints and/or quality of life? Pediatr Surg Int. 2008;24:537-541. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 40] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 3. | Little DC, Rescorla FJ, Grosfeld JL, West KW, Scherer LR, Engum SA. Long-term analysis of children with esophageal atresia and tracheoesophageal fistula. J Pediatr Surg. 2003;38:852-856. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 126] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 4. | Krishnan U, Mousa H, Dall'Oglio L, Homaira N, Rosen R, Faure C, Gottrand F. ESPGHAN-NASPGHAN Guidelines for the Evaluation and Treatment of Gastrointestinal and Nutritional Complications in Children With Esophageal Atresia-Tracheoesophageal Fistula. J Pediatr Gastroenterol Nutr. 2016;63:550-570. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 240] [Article Influence: 26.7] [Reference Citation Analysis (0)] |
| 5. | Vandenplas Y, Rudolph CD, Di Lorenzo C, Hassall E, Liptak G, Mazur L, Sondheimer J, Staiano A, Thomson M, Veereman-Wauters G, Wenzl TG; North American Society for Pediatric Gastroenterology Hepatology and Nutrition; European Society for Pediatric Gastroenterology Hepatology and Nutrition. Pediatric gastroesophageal reflux clinical practice guidelines: joint recommendations of the North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition (NASPGHAN) and the European Society for Pediatric Gastroenterology, Hepatology, and Nutrition (ESPGHAN). J Pediatr Gastroenterol Nutr. 2009;49:498-547. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 431] [Cited by in RCA: 495] [Article Influence: 30.9] [Reference Citation Analysis (0)] |
| 6. | Shay S, Tutuian R, Sifrim D, Vela M, Wise J, Balaji N, Zhang X, Adhami T, Murray J, Peters J, Castell D. Twenty-four hour ambulatory simultaneous impedance and pH monitoring: a multicenter report of normal values from 60 healthy volunteers. Am J Gastroenterol. 2004;99:1037-1043. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 413] [Cited by in RCA: 383] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 7. | Fröhlich T, Otto S, Weber P, Pilic D, Schmidt-Choudhury A, Wenzl TG, Köhler H. Combined esophageal multichannel intraluminal impedance and pH monitoring after repair of esophageal atresia. J Pediatr Gastroenterol Nutr. 2008;47:443-449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 39] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 8. | Hassan M, Mousa H. Impedance Testing in Esophageal Atresia Patients. Front Pediatr. 2017;5:85. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 9. | Vergouwe FWT, van Wijk MP, Spaander MCW, Bruno MJ, Wijnen RMH, Schnater JM, IJsselstijn H. Evaluation of Gastroesophageal Reflux in Children Born With Esophageal Atresia Using pH and Impedance Monitoring. J Pediatr Gastroenterol Nutr. 2019;69:515-522. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 10. | Rosen R, Vandenplas Y, Singendonk M, Cabana M, DiLorenzo C, Gottrand F, Gupta S, Langendam M, Staiano A, Thapar N, Tipnis N, Tabbers M. Pediatric Gastroesophageal Reflux Clinical Practice Guidelines: Joint Recommendations of the North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition and the European Society for Pediatric Gastroenterology, Hepatology, and Nutrition. J Pediatr Gastroenterol Nutr. 2018;66:516-554. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 610] [Cited by in RCA: 530] [Article Influence: 75.7] [Reference Citation Analysis (0)] |
| 11. | Pilic D, Fröhlich T, Nöh F, Pappas A, Schmidt-Choudhury A, Köhler H, Skopnik H, Wenzl TG. Detection of gastroesophageal reflux in children using combined multichannel intraluminal impedance and pH measurement: data from the German Pediatric Impedance Group. J Pediatr. 2011;158:650-654.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 77] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 12. | Wenzl TG, Benninga MA, Loots CM, Salvatore S, Vandenplas Y; ESPGHAN EURO-PIG Working Group. Indications, methodology, and interpretation of combined esophageal impedance-pH monitoring in children: ESPGHAN EURO-PIG standard protocol. J Pediatr Gastroenterol Nutr. 2012;55:230-234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 112] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 13. | Spitz L. Oesophageal atresia. Orphanet J Rare Dis. 2007;2:24. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 272] [Cited by in RCA: 270] [Article Influence: 15.0] [Reference Citation Analysis (2)] |
| 14. | Bagolan P, Valfrè L, Morini F, Conforti A. Long-gap esophageal atresia: traction-growth and anastomosis - before and beyond. Dis Esophagus. 2013;26:372-379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 44] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 15. | Borrelli O, Marabotto C, Mancini V, Aloi M, Macrì F, Falconieri P, Lindley KJ, Cucchiara S. Role of gastroesophageal reflux in children with unexplained chronic cough. J Pediatr Gastroenterol Nutr. 2011;53:287-292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 33] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 16. | Martinucci I, de Bortoli N, Savarino E, Piaggi P, Bellini M, Antonelli A, Savarino V, Frazzoni M, Marchi S. Esophageal baseline impedance levels in patients with pathophysiological characteristics of functional heartburn. Neurogastroenterol Motil. 2014;26:546-555. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 182] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 17. | Frazzoni M, Savarino E, de Bortoli N, Martinucci I, Furnari M, Frazzoni L, Mirante VG, Bertani H, Marchi S, Conigliaro R, Savarino V. Analyses of the Post-reflux Swallow-induced Peristaltic Wave Index and Nocturnal Baseline Impedance Parameters Increase the Diagnostic Yield of Impedance-pH Monitoring of Patients With Reflux Disease. Clin Gastroenterol Hepatol. 2016;14:40-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 233] [Cited by in RCA: 222] [Article Influence: 24.7] [Reference Citation Analysis (0)] |
| 18. | Pedersen RN, Markøw S, Kruse-Andersen S, Qvist N, Hansen TP, Gerke O, Nielsen RG, Rasmussen L, Husby S. Esophageal atresia: gastroesophageal functional follow-up in 5-15 year old children. J Pediatr Surg. 2013;48:2487-2495. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 61] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 19. | Lundell LR, Dent J, Bennett JR, Blum AL, Armstrong D, Galmiche JP, Johnson F, Hongo M, Richter JE, Spechler SJ, Tytgat GN, Wallin L. Endoscopic assessment of oesophagitis: clinical and functional correlates and further validation of the Los Angeles classification. Gut. 1999;45:172-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1518] [Cited by in RCA: 1653] [Article Influence: 63.6] [Reference Citation Analysis (1)] |
| 20. | DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44:837-845. [PubMed] |
| 21. | Vaezi MF. New tests for the evaluation of laryngopharyngeal reflux. Gastroenterol Hepatol (N Y). 2013;9:115-117. [PubMed] |
| 22. | Tovar JA, Fragoso AC. Gastroesophageal reflux after repair of esophageal atresia. Eur J Pediatr Surg. 2013;23:175-181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 43] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 23. | Di Pace MR, Caruso AM, Catalano P, Casuccio A, Cimador M, De Grazia E. Evaluation of esophageal motility and reflux in children treated for esophageal atresia with the use of combined multichannel intraluminal impedance and pH monitoring. J Pediatr Surg. 2011;46:443-451. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 26] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 24. | Iwańczak BM, Kosmowska-Miśków A, Kofla-Dłubacz A, Palczewski M, Grabiński M, Pawłowska K, Matusiewicz K, Patkowski D. Assessment of Clinical Symptoms and Multichannel Intraluminal Impedance and pH Monitoring in Children After Thoracoscopic Repair of Esophageal Atresia and Distal Tracheoesophageal Fistula. Adv Clin Exp Med. 2016;25:917-922. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 10] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 25. | de Bortoli N, Martinucci I, Savarino E, Tutuian R, Frazzoni M, Piaggi P, Bertani L, Furnari M, Franchi R, Russo S, Bellini M, Savarino V, Marchi S. Association between baseline impedance values and response proton pump inhibitors in patients with heartburn. Clin Gastroenterol Hepatol. 2015;13:1082-8.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 107] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 26. | Kandulski A, Weigt J, Caro C, Jechorek D, Wex T, Malfertheiner P. Esophageal intraluminal baseline impedance differentiates gastroesophageal reflux disease from functional heartburn. Clin Gastroenterol Hepatol. 2015;13:1075-1081. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 96] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 27. | Patel A, Wang D, Sainani N, Sayuk GS, Gyawali CP. Distal mean nocturnal baseline impedance on pH-impedance monitoring predicts reflux burden and symptomatic outcome in gastro-oesophageal reflux disease. Aliment Pharmacol Ther. 2016;44:890-898. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 119] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 28. | Frazzoni L, Frazzoni M, de Bortoli N, Tolone S, Furnari M, Martinucci I, Bertani H, Marchi S, Conigliaro R, Fuccio L, Savarino V, Savarino E. Postreflux swallow-induced peristaltic wave index and nocturnal baseline impedance can link PPI-responsive heartburn to reflux better than acid exposure time. Neurogastroenterol Motil. 2017;29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 115] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 29. | Tong S, Mallitt KA, Krishnan U. Evaluation of Gastroesophageal Reflux by Combined Multichannel Intraluminal Impedance and pH Monitoring and Esophageal Motility Patterns in Children with Esophageal Atresia. Eur J Pediatr Surg. 2016;26:322-331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 26] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 30. | Tambucci R, Thapar N, Saliakellis E, Pescarin M, Quitadamo P, Cristofori F, Lindley KJ, Borrelli O. Clinical relevance of esophageal baseline impedance measurement: just an innocent bystander. J Pediatr Gastroenterol Nutr. 2015;60:776-782. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 31. | Kessing BF, Bredenoord AJ, Weijenborg PW, Hemmink GJ, Loots CM, Smout AJ. Esophageal acid exposure decreases intraluminal baseline impedance levels. Am J Gastroenterol. 2011;106:2093-2097. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 186] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 32. | Loots CM, Wijnakker R, van Wijk MP, Davidson G, Benninga MA, Omari TI. Esophageal impedance baselines in infants before and after placebo and proton pump inhibitor therapy. Neurogastroenterol Motil. 2012;24:758-762, e351-e352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 29] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 33. | van der Pol RJ, Loots CM, Peeters L, Vandenplas Y, Hauser B, Devreker T, Omari TI, Benninga MA, van Wijk MP. Outcomes of endoscopy and novel pH-impedance parameters in children: is there a correlation? J Pediatr Gastroenterol Nutr. 2013;56:196-200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 34. | Salvatore S, Salvatoni A, Van Berkel M, Van Steen K, Unmarino D, Ghanma A, Hauser B, Vandenplas Y. Esophageal impedance baseline is age dependent. J Pediatr Gastroenterol Nutr. 2013;57:506-513. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 35. | Wenzl TG. Evaluation of gastroesophageal reflux events in children using multichannel intraluminal electrical impedance. Am J Med. 2003;115 Suppl 3A:161S-165S. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 39] [Article Influence: 1.8] [Reference Citation Analysis (0)] |