Published online Sep 9, 2025. doi: 10.5409/wjcp.v14.i3.102898
Revised: March 12, 2025
Accepted: March 24, 2025
Published online: September 9, 2025
Processing time: 227 Days and 12.3 Hours
Mucopolysaccharidoses (MPS) encompass a spectrum of inherited lysosomal storage disorders caused by deficiencies in enzymes required for glycosami
Core Tip: This study emphasizes the critical role of radiographic imaging in diagnosing and managing mucopolysaccharidoses (MPS), a group of lysosomal storage disorders marked by skeletal abnormalities and multisystem involvement. Key innovations include identifying characteristic radiographic markers—such as the J-shaped sella turcica and odontoid hypoplasia—that are essential for early MPS detection and monitoring. The findings support the value of routine imaging in assessing the progression of skeletal deformities and guiding timely interventions, even as enzyme replacement therapies remain limited in reversing structural changes. This approach enhances both diagnostic precision and therapeutic outcomes, offering a roadmap for optimal care in MPS patients.
- Citation: Teixeira de Castro Gonçalves Ortega AC, Amorim Moreira Alves G, Perera Molligoda Arachchige AS. Radiographic assessment of mucopolysaccharidoses: A pictorial review. World J Clin Pediatr 2025; 14(3): 102898
- URL: https://www.wjgnet.com/2219-2808/full/v14/i3/102898.htm
- DOI: https://dx.doi.org/10.5409/wjcp.v14.i3.102898
Mucopolysaccharidoses (MPS) are a group of inherited lysosomal storage disorders resulting from deficiencies in glycosaminoglycan (GAG)-degrading enzymes. This deficiency leads to the accumulation of unprocessed or partially processed GAGs—such as dermatan sulfate, heparan sulfate, keratan sulfate, and chondroitin sulfate—in lysosomes, causing progressive tissue damage, particularly affecting the heart, respiratory system, bones, joints, and central nervous system. There are seven distinct clinical types of MPS caused by 11 different enzymatic deficiencies, with an overall incidence of about 1 in 25000 live births. All types are inherited in an autosomal recessive manner, except MPS II, which is X-linked. The clinical onset of the disease is variable depending on the type; however, it is important to note that the radiographic onset may be possible even at birth. Early diagnosis is important for prompt, specific treatment with enzyme replacement therapy (ERT), which is currently available for types I, II, IV and VI. Early start of treatment helps reduce the accumulation of intracellular GAGs and the deceleration of progressive multiorgan worsening, improving lung and heart function and final growth rate[1-3]. A combination of clinical picture and analysis of urinary GAGs is usually performed to achieve the diagnosis of MPS, even though this method cannot recognize subtypes; definitive diagnosis is usually possible through measuring enzyme activity in cultured fibroblasts or leukocytes. Treatment consists mainly of symptomatic and supportive care, including decompression of the craniocervical narrowing, tracheostomy insertion and corneal transplantation. In recent years the development of new therapies—represented by enzyme replacement therapies, substrate inhibition therapy and haematopoietic cell transplantation—have changed the treatment of these patients, with a fundamental shift in the approach from symptomatic management to therapeutic intervention[4,5]. In this review we discuss the different types of MPS along with relevant radiographic findings as well as their clinical implications.
MPS type I includes three subtypes: Hurler syndrome (MPS I-H), Hurler-Scheie syndrome (MPS I-H/S), and Scheie syndrome (MPS I-S) inherited in an autosomal recessive manner[6,7]. These subtypes vary in severity, with MPS I-H being the most severe, MPS I-S the attenuated, and MPS I-H/S representing an intermediate form. All subtypes result from a deficiency of the enzyme alpha-L iduronidase, causing the accumulation of GAGs, such as heparan sulfate and dermatan sulfate, in various tissues[8-11].
MPS I-H: This severe form is usually diagnosed between 6 and 24 months of age. Patients often present with skeletal dysplasia (kyphosis, genu valgum, dwarfism), organomegaly, and distinctive facial features such as a broad nose and enlarged tongue. Cognitive decline and developmental delay are common, alongside characteristic radiographic findings like macrocephaly, J-shaped sella turcica, and gibbus deformity. ERT and bone marrow transplantation (BMT) offer some improvement, but progression remains likely without early intervention[11,12].
MPS I-H/S: This intermediate form presents with symptoms that overlap with both Hurler and MPS I-S. Patients typically exhibit corneal clouding, joint stiffness, and mild cognitive impairment. Skeletal deformities such as shortened metacarpals and small carpal bones are common, requiring close monitoring through radiographic imaging. Life expectancy is longer than in MPS I-H, though progressive skeletal and joint changes remain challenging[8,11].
MPS I-S: The mildest form of MPS I, MPS I-S, manifests after 5 years of age with stiff joints, corneal clouding, and milder skeletal deformities. Intelligence is typically normal, and life expectancy can approach normal with appropriate treatment. However, joint stiffness in the hands and feet often necessitates surgical intervention to maintain mobility. Radiographic imaging is essential to monitor the progression of deformities and assess the need for intervention[11,12].
As discussed above radiographic imaging is the primary tool for detecting early skeletal changes such as macrocephaly, J-shaped sella turcica, and gibbus deformity. These abnormalities, especially in MPS I-H, require ongoing monitoring of the spine, pelvis, and hands for signs of progressive deformity[8,11]. Radiologists should also monitor for secondary complications such as carpal tunnel syndrome, which is common in MPS I-H and MPS I-H/S, using both ultrasound and radiographic imaging. Regular imaging is essential in assessing the effectiveness of ERT and BMT, which may slow disease progression but are unlikely to reverse established skeletal changes. These interventions guide critical decisions on surgical planning for joint stiffness, claw hand deformity, and other progressive complications[8,11]
MPS type II (MPS II), or Hunter syndrome, is an X-linked recessive disorder caused by a deficiency of iduronate-2-sulfatase, leading to the accumulation of heparan sulfate and dermatan sulfate. The disease predominantly affects males, with symptom onset typically between 2 and 4 years[13]. Clinically, patients present with dysostosis multiplex, organomegaly, and coarse facial features, while neurological decline is variable—milder forms spare cognitive function, whereas severe forms lead to progressive intellectual disability and developmental delays[11,12]. Neuroimaging findings frequently include hydrocephalus, optic nerve sheath enlargement, and arachnoid cysts, particularly in the anterior temporal lobes, due to GAG accumulation[10]. Skeletal abnormalities mirror those in other MPS types, with joint stiffness and deformities contributing to mobility restrictions. Radiologists are essential for tracking disease progression, particularly through neuroimaging and the evaluation of dysostosis multiplex. ERT with idursulfase is the mainstay of treatment, though it has limited efficacy on central nervous system symptoms. Supportive therapies and regular imaging are critical for managing complications such as cardiovascular involvement and airway obstruction[10,11].
MPS type III (MPS III), also known as Sanfilippo syndrome, is an autosomal recessive disorder characterized by a deficiency in one of four enzymes responsible for the degradation of heparan sulfate, a GAG. The four subtypes—A, B, C, and D—are each linked to different enzyme deficiencies. The disease primarily affects the central nervous system, leading to severe cognitive and behavioral issues. Type A is associated with a lack of heparan N-sulfatase, while Type B stems from a deficiency in alpha-N-acetylglucosaminidase. Types C and D involve defects in acetyl CoA: Alpha-glucosaminide N-acetyltransferase and N-acetylglucosamine-6-sulfatase, respectively[8,12]. The most prominent aspect of MPS III is the progressive neurological deterioration, often seen as worsening intellectual disability combined with behavioral challenges like hyperactivity, aggression, and persistent sleep disturbances[14]. Physical symptoms, though less severe than in other MPS types, include macrocephaly, joint stiffness, and mild dysostosis multiplex. Patients may also exhibit coarse facial features and hepatosplenomegaly[8,9]. Radiologically, patients with MPS III exhibit skeletal abnormalities such as kyphosis and coxa vara, though these are milder compared to other MPS types. Radiologists should remain alert to these findings, which help track disease progression[9]. Currently, there is no cure for MPS III, and management remains supportive. Symptomatic treatments focus on improving quality of life through physical therapy, behavioral management, and speech therapy. Ongoing research, including gene therapy, aims to address the underlying enzyme deficiencies but is still in experimental stages[8,9].
MPS type IV (MPS IV), or Morquio syndrome, is an autosomal recessive lysosomal storage disorder that leads to severe skeletal dysplasia due to the accumulation of GAGs. Morquio syndrome is divided into two subtypes: MPS IVA, caused by a deficiency in N-acetylgalactosamine-6-sulfatase, and MPS IVB, associated with a beta-galactosidase deficiency. Both subtypes lead to the buildup of keratan sulfate in tissues[12,15]. The disease predominantly affects the skeletal system, manifesting as short stature, kyphosis, and characteristic dysostosis multiplex. Notably, patients often present with odontoid hypoplasia, a critical skeletal abnormality leading to atlantoaxial instability and a high risk of spinal cord compression, which can result in severe morbidity and mortality if left untreated. Joint stiffness, hypermobile joints, and genu valgum are also common features[8,15]. Radiologists are pivotal in diagnosing Morquio syndrome. Radiographic findings, such as paddle-shaped ribs, odontoid hypoplasia, and vertebral deformities, are crucial for early identification of the disease. A comprehensive skeletal survey is essential to detect craniocervical instability, which poses significant risks, particularly during anesthesia for corrective surgeries. Early and regular imaging allows for prompt intervention, such as dynamic flexion-extension radiographs to assess the cervical spine for instability[15]. Treatment options for Morquio syndrome are limited. For MPS IVA, ERT with elosulfase alfa has shown some benefits in slowing GAG accumulation and improving mobility, but a definitive cure remains elusive[16-18]. Most management revolves around supportive care, including physiotherapy, orthopedic interventions, and surgery to correct skeletal deformities. Gene therapy remains an area of research but is not yet available as a treatment option[8,15].
MPS type VI (MPS VI), or Maroteaux-Lamy syndrome, is a lysosomal storage disorder caused by a deficiency of N-acetylgalactosamine 4-sulfatase (arylsulfatase B), leading to the accumulation of dermatan sulfate within lysosomes[19]. The clinical manifestations typically appear between 6 to 24 months of age and include progressive growth deceleration, skeletal deformities, coarse facial features, upper airway obstruction, corneal clouding, hepatosplenomegaly, and cardiovascular involvement[20]. Unlike some other MPS types, MPS VI is not typically associated with cognitive impairment, though severe physical limitations may impact development[21]. Radiographic findings are consistent with skeletal dysplasia and include platyspondyly, vertebral beaking, kyphosis or scoliosis (Gibbus deformity), macrocephaly, protruding abdomen, widened iliac wings, short and broad metacarpals/metatarsals, genu valgum, acetabular and femoral head dysplasia, and thoracolumbar vertebral malformations[21-23]. ERT with galsulfase has been shown to improve survival, pulmonary function, endurance, and stabilize cardiac involvement, particularly when initiated early[24]. However, the clinical response may vary, particularly in severe phenotypes, where joint contractures, snoring, sleep apnea, and airway obstruction can persist despite therapy[25]. Epidemiological data show variability in MPS VI incidence, ranging from 1: 5000 in Monte Santo, Brazil, to rare occurrences in Northern Ireland, often linked to parental consanguinity[21]. Early diagnosis and intervention are critical for optimizing long-term outcomes.
MPS type VII, or Sly syndrome, is a rare lysosomal storage disorder caused by pathogenic variants in the GUSB gene, leading to β-glucuronidase deficiency and progressive GAG accumulation[26]. Clinically, MPS VII presents with multisystem involvement, including skeletal dysplasia, coarse facial features, hepatosplenomegaly, developmental delay, and progressive neurological decline[27]. Characteristic skeletal abnormalities, observed in 90% of affected individuals, include vertebral beaking, platyspondyly, odontoid hypoplasia with atlantoaxial instability, kyphoscoliosis, pectus carinatum, and hip dysplasia[28]. Additional radiographic findings include widened iliac wings, genu valgum, irregular acetabular roofs, flattened femoral epiphyses, pseudoarthrosis, and early bone maturation in utero[27]. ERT with vestronidase alfa has shown efficacy in reducing urinary GAGs and improving visceral organ involvement, though it does not cross the blood-brain barrier[29]. Hematopoietic stem cell transplantation, when performed early, may mitigate neurological progression by facilitating enzyme secretion from donor-derived microglial cells[30]. Early diagnosis and intervention are crucial to improving patient outcomes.
MPS type IX (MPS IX) is an extremely rare lysosomal storage disorder caused by a deficiency of hyaluronidase-1, an enzyme responsible for the degradation of hyaluronic acid[31]. Unlike other MPS subtypes, MPS IX does not present with significant neurological or visceral involvement and is primarily characterized by periarticular soft tissue masses, mild short stature, and acetabular erosions[31]. The first reported case, a 14-year-old girl, exhibited multiple non-calcified periarticular masses, beginning at 6 months with the excision of a wrist ganglion and progressing to involve the ankle, knee, and finger joints. Episodes of painful swelling and generalized cutaneous edema, which resolved spontaneously, were also observed[31]. A triad of characteristic radiographic findings includes bilateral soft tissue masses, acetabular erosions, and joint effusions, with preserved joint spaces and no bony erosions on plain radiographs[31]. In later cases, proliferative synovitis affecting multiple joints, including the hands, hips, knees, and ankles, was documented, with synovial biopsies revealing histiocytic infiltrates with vacuolated cytoplasm[32]. In contrast to most MPS types, affected individuals often retain full joint mobility and lack cognitive impairment, hepatosplenomegaly, or significant skeletal deformities[31,32]. Given its rarity and overlapping clinical features with juvenile idiopathic arthritis, MPS IX is likely underdiagnosed, and screening should be considered in patients with poor response to conventional therapies, mono-oligoarticular involvement, and a family history of consanguinity[33]. Due to its autosomal recessive inheritance pattern, further studies are required to better understand its natural history and potential therapeutic approaches.
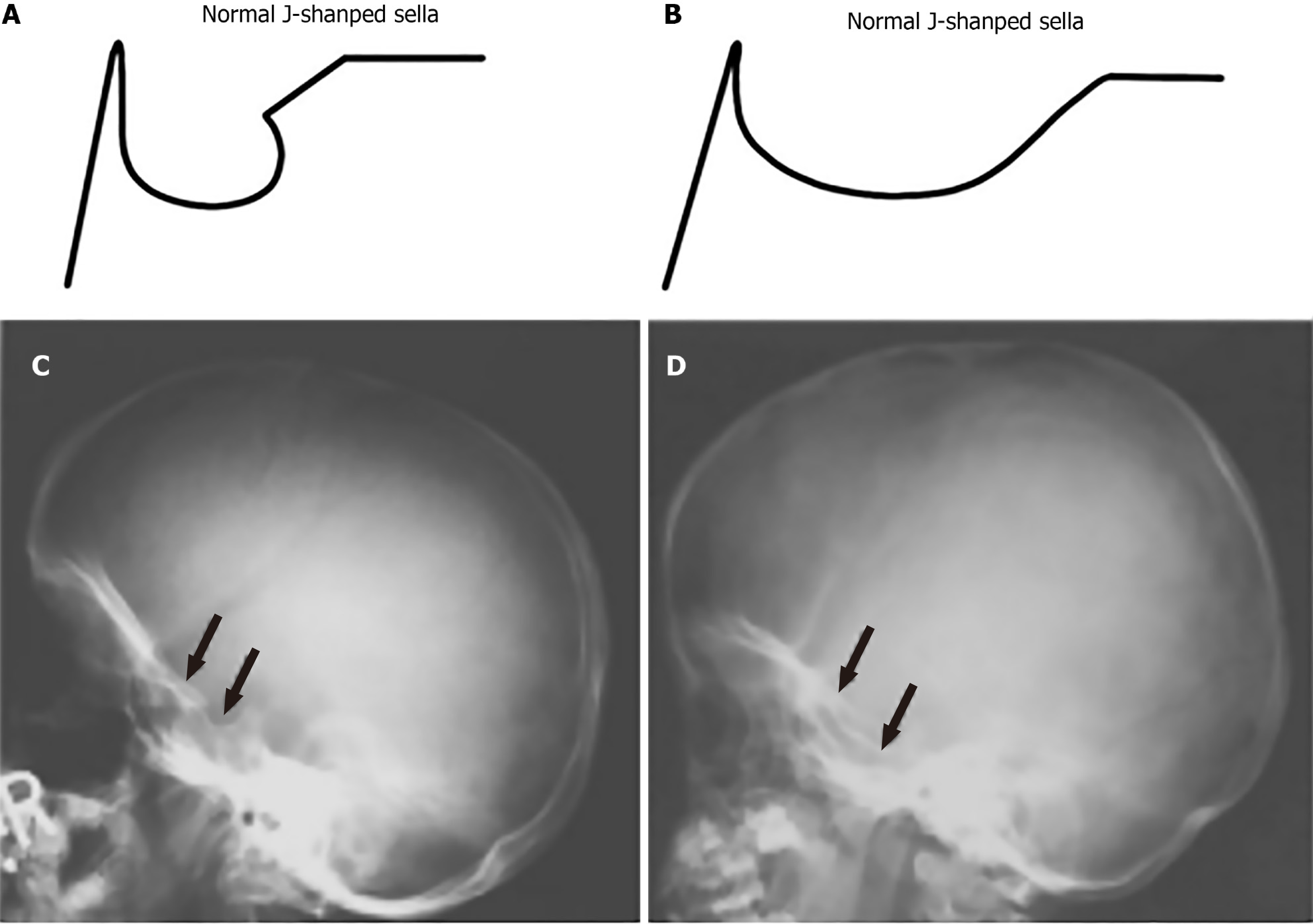
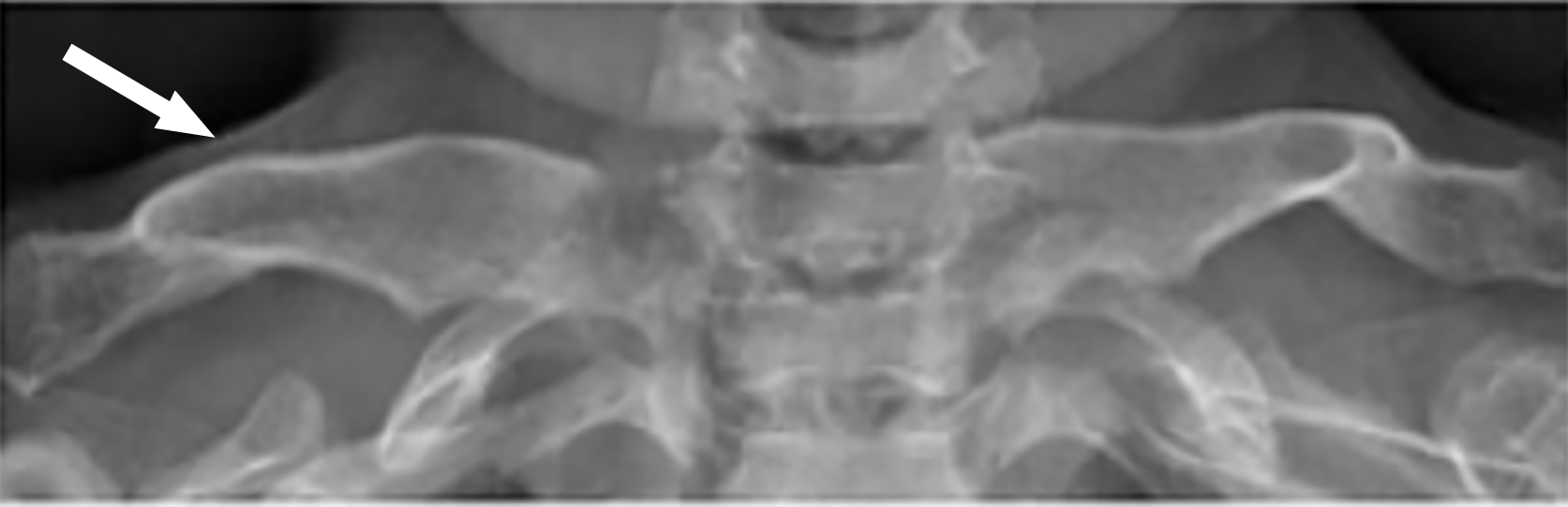
Radiographic imaging plays a crucial role in identifying and managing the characteristic skull abnormalities seen in patients with MPS. These abnormalities often reflect the underlying GAG accumulation and its impact on cranial bone development. One of the most notable findings is the J-shaped sella turcica, a deformity commonly observed across multiple MPS subtypes, particularly in MPS I and MPS II. This abnormality, which features a flattened tuberculum sellae with a normal dorsum sellae, is indicative of the broader skeletal dysplasia associated with MPS, see Figure 1[6,7].
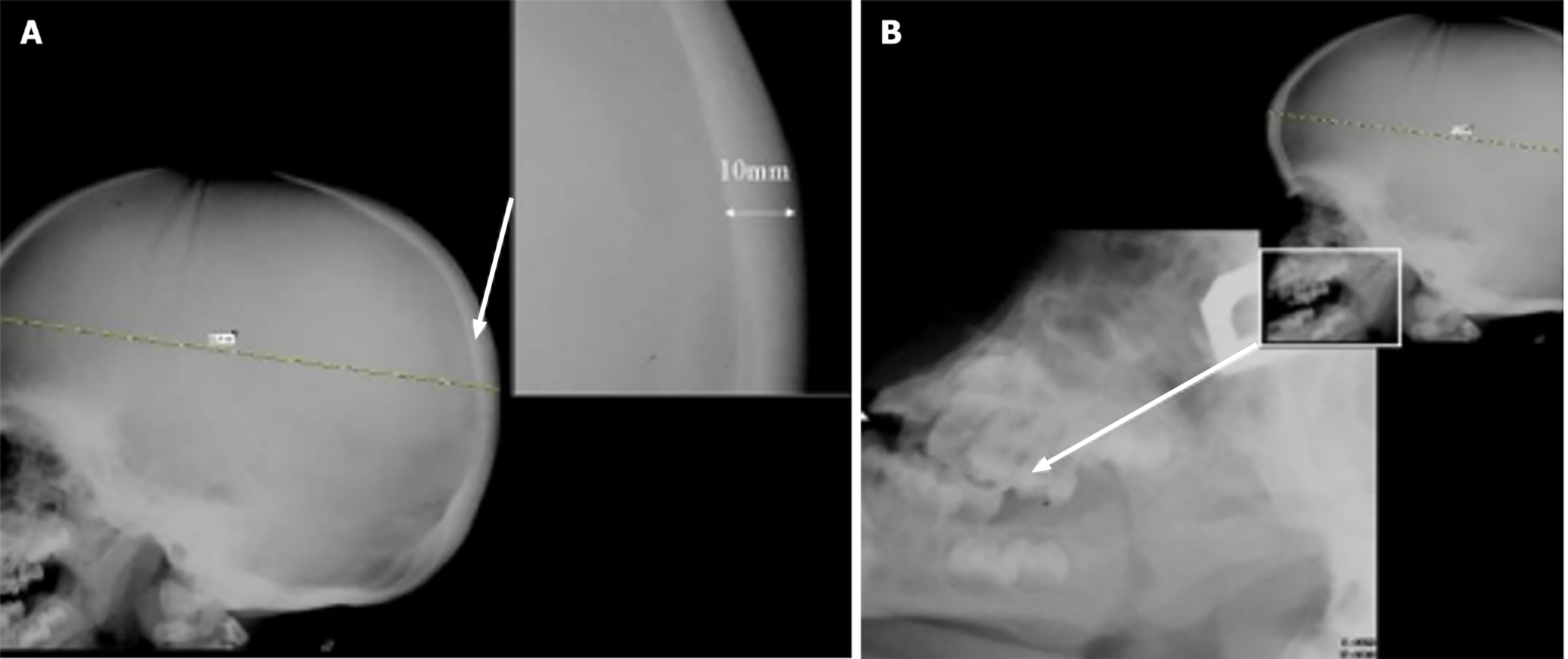
Another frequent finding is thickening of the cortical bone, particularly in the skull, as well as the diploic space, a characteristic feature of cranial involvement that can be clearly identified using X-ray. The progressive thickening of cranial bones, in addition to macrocephaly with dolicocephaly, reflects the disruptive effect of GAG storage on skeletal growth, see Figure 2A[8,9]. Early closure of the sagittal and lambdoid sutures, resulting in an enlarged neurocranium, is a hallmark of this condition[8].
Severe forms of MPS, such as MPS I-H, may also present with gibbus deformities, which, although typically spinal, can affect the overall cranial structure. Additionally, a vertical frontal crest may be seen due to abnormal growth of the frontal bone. Facial anomalies are also prominent, including coarse facial features, prognathism, wide-spaced teeth, and macroglossia. Clinically, these cranial malformations complicate airway management and surgical planning, as seen in patients with progenia and other mandibular abnormalities, such as frontal bossing and square mandibular condyles, see Figure 2B[9]. The structural changes in cranial bones often begin prenatally, making early diagnosis and intervention critical to managing the progression of these deformities[3].
Even with advancements like ERT, long-term studies show that while ERT can help stabilise some symptoms, it does not significantly reverse cranial abnormalities that have already developed. Findings from MPS II patients treated with idursulfase demonstrate that although cranial bone abnormalities may stabilise, the structural changes often persist. This underscores the importance of regular imaging to monitor disease progression and assess therapeutic effectiveness[3,6]. Overall, radiography remains the first-line modality for documenting these skull abnormalities, providing critical information for evaluating the severity of dysostosis multiplex, the broad term encompassing the range of skeletal dysplasia seen in MPS.
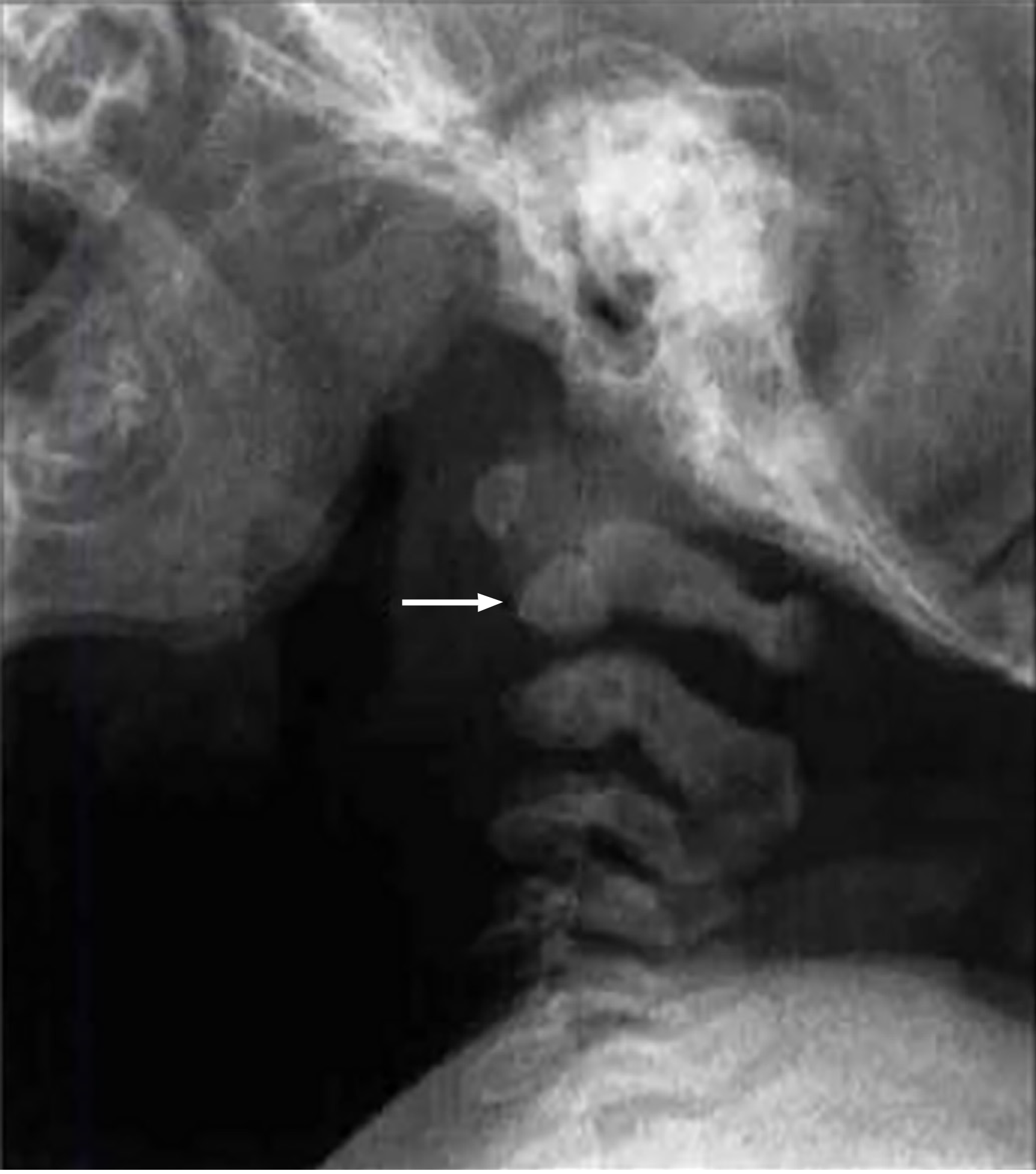
In patients with MPS, the spine is significantly affected by structural abnormalities that result in serious clinical consequences. These abnormalities vary across different regions of the spine, including the cervical, thoracic, and lumbar areas, and can lead to neurological deficits and other complications. One of the most critical issues in MPS is spinal canal stenosis, particularly in the cervical region, where narrowing of the canal can result in compression of the spinal cord. This is especially prevalent at the C1 and C2 vertebrae, leading to severe neurological symptoms such as myelopathy[10]. Odontoid process dysplasia or hypoplasia is a common finding that contributes to cervical instability, increasing the risk of atlantoaxial subluxation and exacerbating spinal cord compression[6,10]. Patients with MPS I and Morquio syndrome (MPS IV) appear to have the highest risk of developing odontoid hypoplasia, with MPS I patients at greater risk than those with MPS IV. Chronic subluxation at the C1-C2 Level may lead to ligamentous hypertrophy and further narrowing in the craniovertebral junction, which results in additional cord compression.
Cervical instability is a major concern in MPS and often requires functional radiographic evaluation in flexion and extension to assess its severity. This instability is frequently observed at the atlantoaxial junction due to odontoid hypoplasia and ligamentous laxity, which predisposes patients to subluxation and further compression of the spinal cord, see Figure 3[6,8]. In addition, periodontoid tissue and ligament thickening further contribute to the stenosis and compression in the craniovertebral junction.
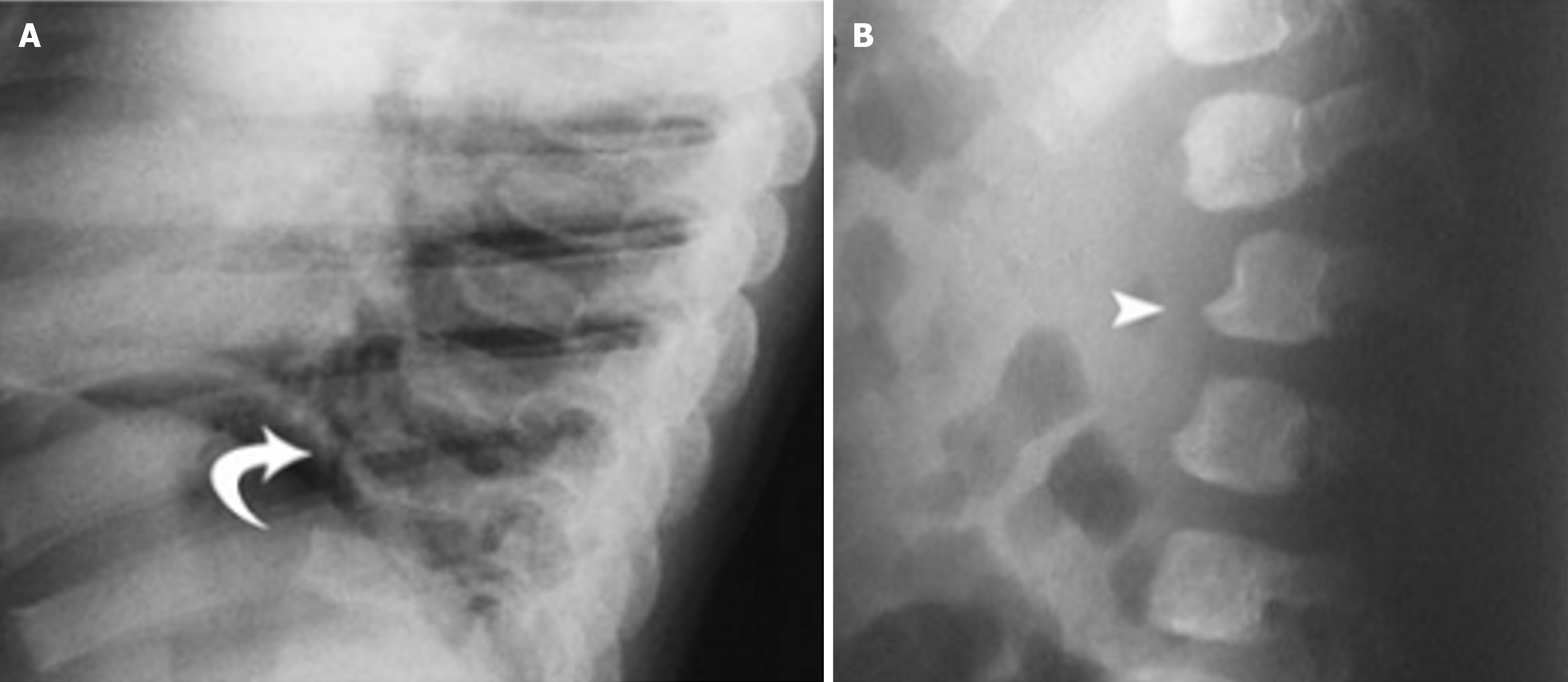
In the thoracic region, kyphosis is frequently observed, particularly dorsal kyphosis, which can contribute to the characteristic spinal profile of MPS. Flattening of the vertebral bodies, known as platyspondyly, is common, and these changes may progress to more severe deformities such as gibbus deformity, a sharp, angular kyphosis that often affects the thoracolumbar junction[8]. Additionally, anterior beaking—the hypoplasia of the anterosuperior corners of the vertebral bodies—is a common finding, further contributing to spinal deformities[11]. Posterior scalloping of the vertebral bodies with widened interpedicular distance is another frequent abnormality seen in MPS, particularly affecting the thoracolumbar spine. In the lumbar spine, gibbus deformity, which often occurs between L1 and L3, can cause significant compression of the cauda equina, leading to neurological deficits such as weakness, numbness, or incontinence[8]. The flattening of vertebral bodies seen in platyspondyly, combined with progressive kyphosis and scoliosis, creates the typical spinal curvature issues associated with MPS, See Figure 4[6].
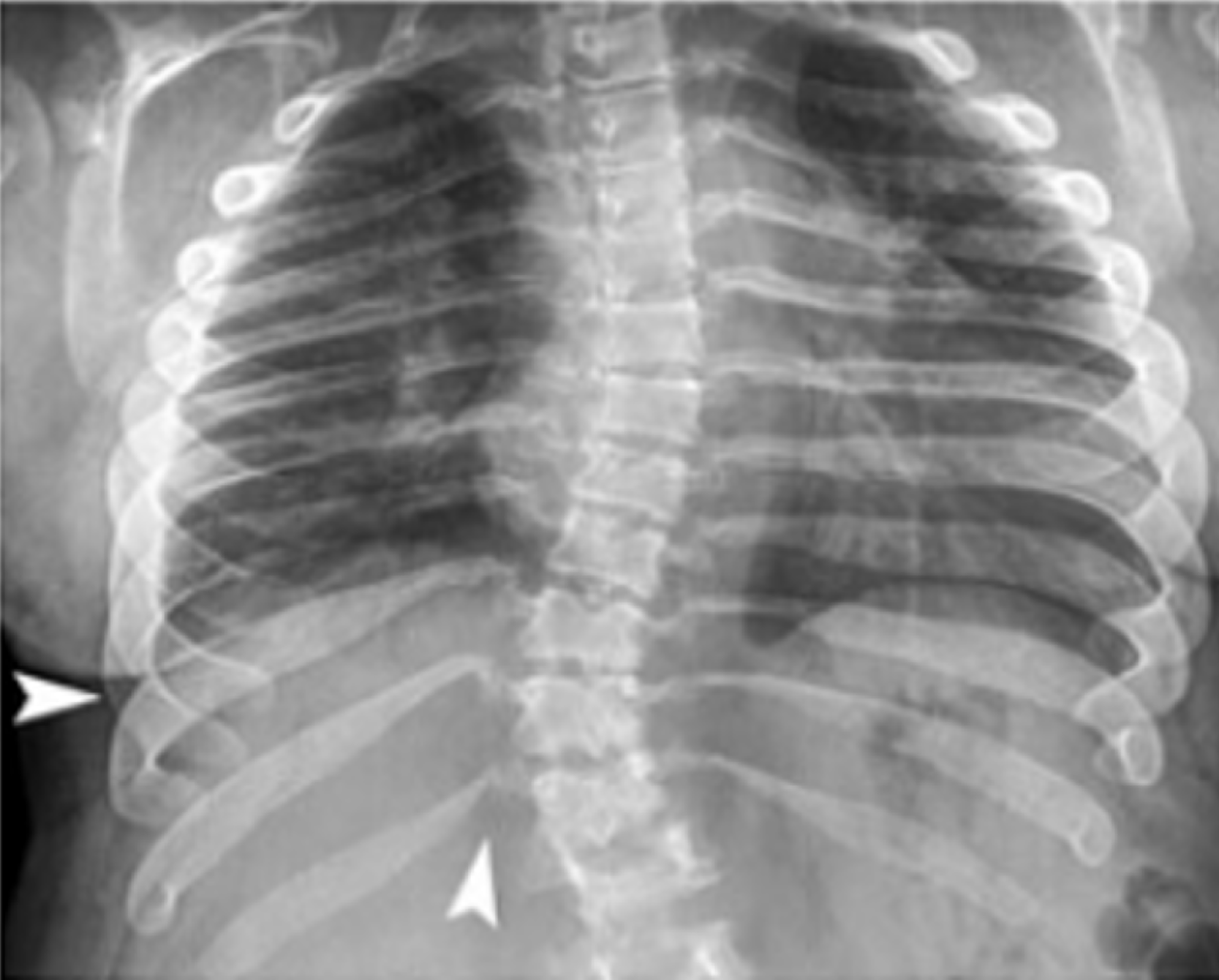
Thoracic abnormalities are a distinctive feature in patients with MPS, significantly contributing to both structural deformities and respiratory complications. Radiographic imaging plays a vital role in identifying these changes, guiding both diagnosis and clinical management. One of the hallmark findings in MPS is paddle-shaped ribs, characterized by anterior widening and posterior tapering. These rib deformities are particularly pronounced in Morquio syndrome (MPS IV) and Maroteaux-Lamy syndrome (MPS VI). The ribs take on an “oar-shaped” appearance, where the anterior arches are broader while the posterior segments taper, see Figure 5[6,11].
These abnormalities can alter the overall thoracic structure, contributing to a shortened, thickened chest wall, which can be visualized on frontal radiographs. Another common thoracic abnormality in MPS patients involves the clavicles, which often appear broad and short, see Figure 6.
This, along with a shortened sternum, further contributes to the distinctive chest wall configuration. The chest can appear compact and thickened, and these changes can be detected early through routine radiographic assessments[11]. The sternum itself may also appear short, further restricting the thoracic cavity and exacerbating respiratory challenges[6]. Additionally, tracheomalacia, the weakening of the tracheal walls, is another concern in MPS patients. This condition is often exacerbated by the deposition of GAGs in the airway, leading to airway obstruction, particularly during expiration. The narrowing of the trachea due to GAG infiltration can cause airway disease, which is resistant to ERT, further complicating respiratory management[3]. Thoracic deformities may also lead to an elevated diaphragm, particularly in patients with hepatosplenomegaly, further reducing respiratory efficiency. This elevation restricts the ability to take deep breaths, contributing to shallow breathing patterns and an overall decline in respiratory function[3]. Radiographic imaging remains a first-line approach for assessing thoracic deformities in MPS patients. The presence of dysostosis multiplex, a term used to describe the constellation of skeletal changes in MPS, is often first detected in the thorax. Radiographs can reveal characteristic malformations such as rib, clavicle, and sternum deformities[6,11]. The thoracic abnormalities in MPS patients not only impact skeletal structure but also have far-reaching effects on respiratory health and quality of life. Proper imaging and early detection of these deformities are critical in managing respiratory complications and planning potential surgical interventions.
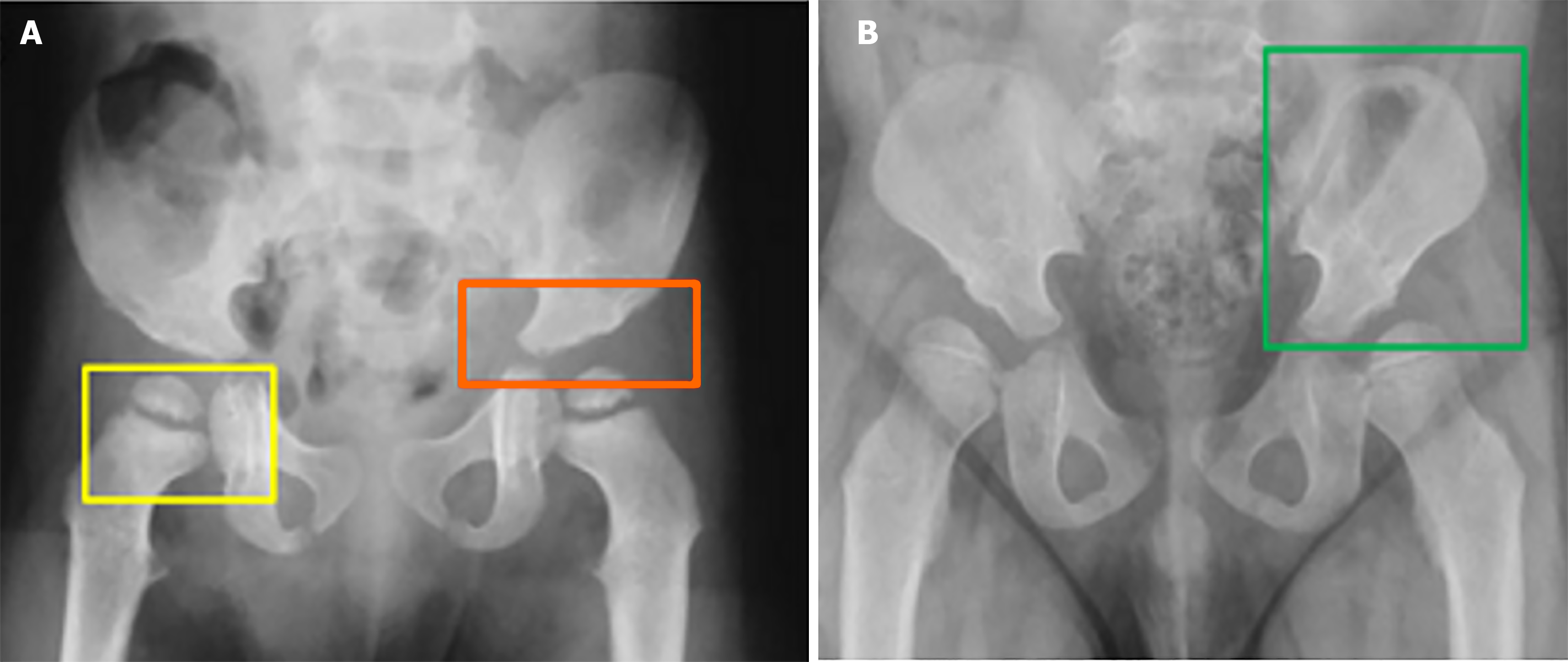
In individuals diagnosed with MPS, the pelvic region is often subject to structural deformities that substantially influence mobility and the functionality of joints. A particularly notable observation is hip dysplasia, a prevalent complication attributed to the inadequate development of the acetabulum and the insufficient growth of the medial aspect of the proximal femoral epiphysis. This condition results in instability of the hip joint, frequently necessitating surgical intervention, especially in patients exhibiting severe manifestations of MPS, such as MPS I, although it may also be observed in less severe forms, such as MPS I-S[6]. Typical radiological manifestations within the pelvis of individuals with MPS encompass rounded iliac wings and inferior tapering of the ileum. These deformities serve as indicators of the extensive skeletal dysplasia that influences various regions of both the axial and appendicular skeleton[11]. The rounded iliac wings can be readily identified through axial imaging, contributing to the distinctive morphology of the pelvis in MPS patients. Concurrently, the inferior tapering of the ileum may modify pelvic morphology, potentially impacting both hip joint stability and gastrointestinal functionality, see Figure 7[11].
Further alterations in the hip joint comprise an increased coxofemoral joint space and coxa valga, both of which exacerbate the compromise of joint function. These observations necessitate meticulous monitoring, as the advancement of dysplasia can result in joint discomfort, mobility challenges, and, in severe instances, the requirement for reconstructive surgical procedures[6]. Radiographic imaging serves an essential function in evaluating the extent of hip dysplasia. An anteroposterior view of the pelvis, particularly when the patient is in an upright position, is paramount for assessing the degree of acetabular underdevelopment. Radiologists employ angular measurements, including the acetabular index, Sharp’s acetabular angle, and the center-edge angle of Wiberg, to evaluate the severity of hip dysplasia. The standard reference values for these angles are Sharp’s angle < 39°, acetabular index < 20°, and center edge angle > 20°[6]. Deviations in these measurements signify a substantial degree of hip instability, which may culminate in joint dysfunction and discomfort over time. The pelvic abnormalities identified in MPS patients not only contribute to hip dysplasia but also possess broader clinical ramifications. Severe hip dysplasia, frequently observed in MPS I, typically exhibits a limited response to medical management, necessitating surgical intervention. Surgical reconstruction of the hip joint may be imperative to rectify the deformities and enhance joint functionality[6]. The radiographic evaluation of the pelvis is indispensable for diagnosing the severity of skeletal dysplasia, formulating surgical strategies, and monitoring the progression of the disease. A comprehensive understanding of the specific pelvic deformities in MPS patients empowers clinicians to effectively manage complications such as joint instability, mobility impairments, and pain, thereby facilitating tailored therapeutic approaches[11].
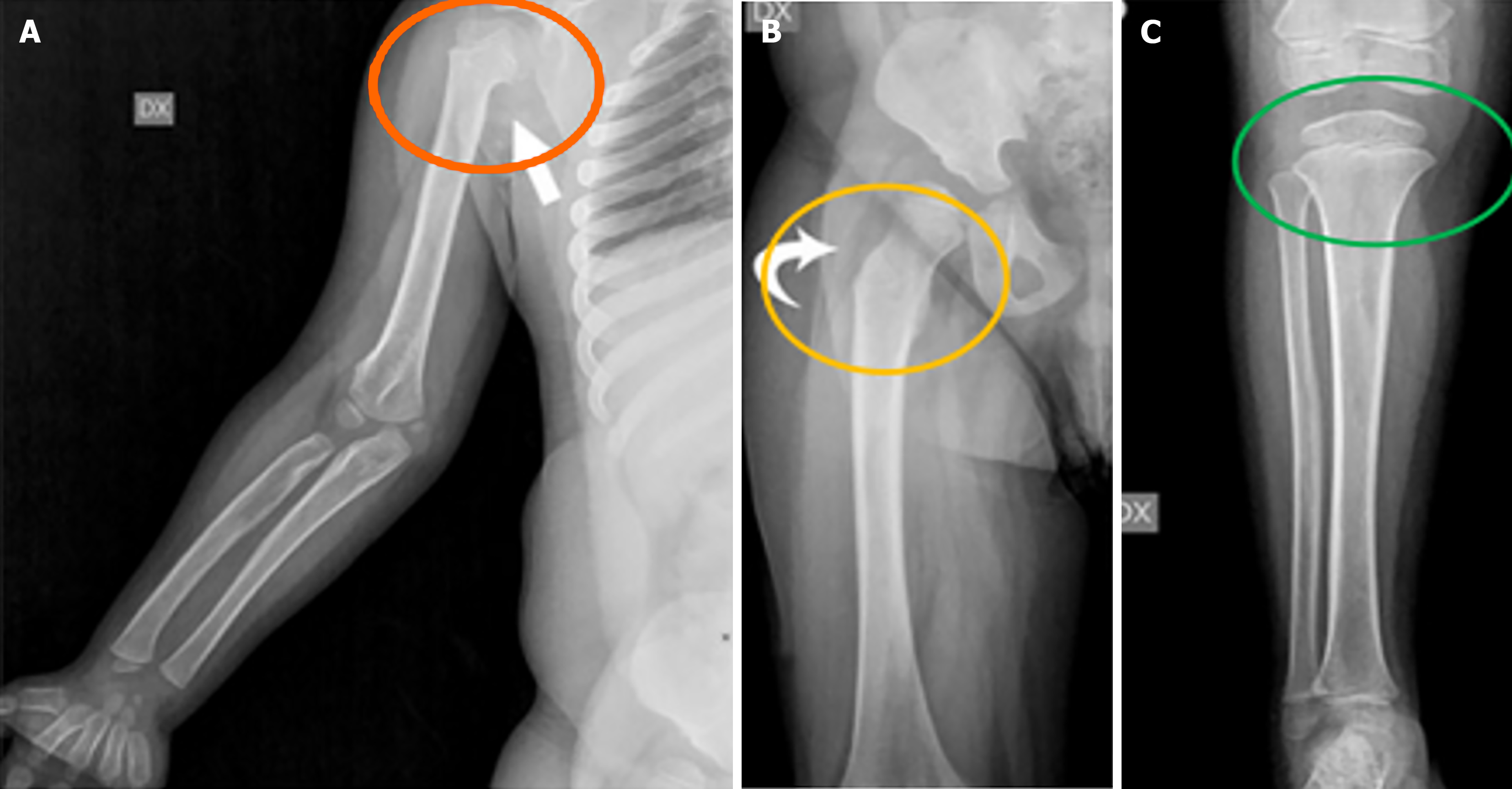
Long bones and knees are profoundly affected by structural abnormalities in patients with MPS, contributing to functional impairments and mobility issues. These deformities are mainly caused by the accumulation of GAGs in the growth plates, which disrupts normal bone development. The diaphyses (shaft) of long bones in MPS patients are often shortened and exhibit a curved appearance, particularly in the distal regions. This curvature can lead to misalignment of the limbs, further affecting limb function and mobility[11]. Additionally, the epiphyses (ends of long bones) tend to be hypoplastic, with thinned cortical bone. This thinning is commonly associated with osteoporosis, increasing the risk of fractures due to the fragility of the bones[11]. Other characteristic features include notching of the proximal humerus, a hatchet-shaped humerus with varus deformity of the humeral neck, and a long, narrow femoral neck, which may lead to functional difficulties and even require surgical intervention in severe cases, see Figure 8[11].
Genu valgum (knock-knees) is one of the most common deformities seen in MPS patients, especially in those with MPS types IV and I. This condition, characterized by inward angling of the knees and outward bowing of the lower legs, often leads to functional difficulties and discomfort during movement[11]. As MPS patients grow, the severity of genu valgum can increase, necessitating surgical correction in some cases to improve mobility and reduce pain. Long-term consequences of knee involvement include advanced arthrosis in adulthood, where significant degeneration of the articular cartilage leads to joint pain and reduced mobility. In severe cases, total knee arthroplasty (knee replacement surgery) may be considered as a treatment option to alleviate symptoms and restore knee function[11]. Although ERT has been shown to alleviate some symptoms of MPS, its impact on long bone deformities and knee function is limited. Research indicates that knee flexion does not significantly improve with ERT, as seen in clinical trials with idursulfase for MPS II. Furthermore, the structural changes in long bones, including shortening and deformity, often remain stable or do not improve substantially, even with treatment[3]. Due to the progressive nature of skeletal changes in MPS, regular monitoring of long bones and knees through imaging is essential. Radiographs and MRIs provide valuable insights into the progression of bone abnormalities and help clinicians plan appropriate interventions. Early detection of issues such as genu valgum or diaphyseal curvature allows for timely surgical or orthopedic management, preventing further functional deterioration[3,11].
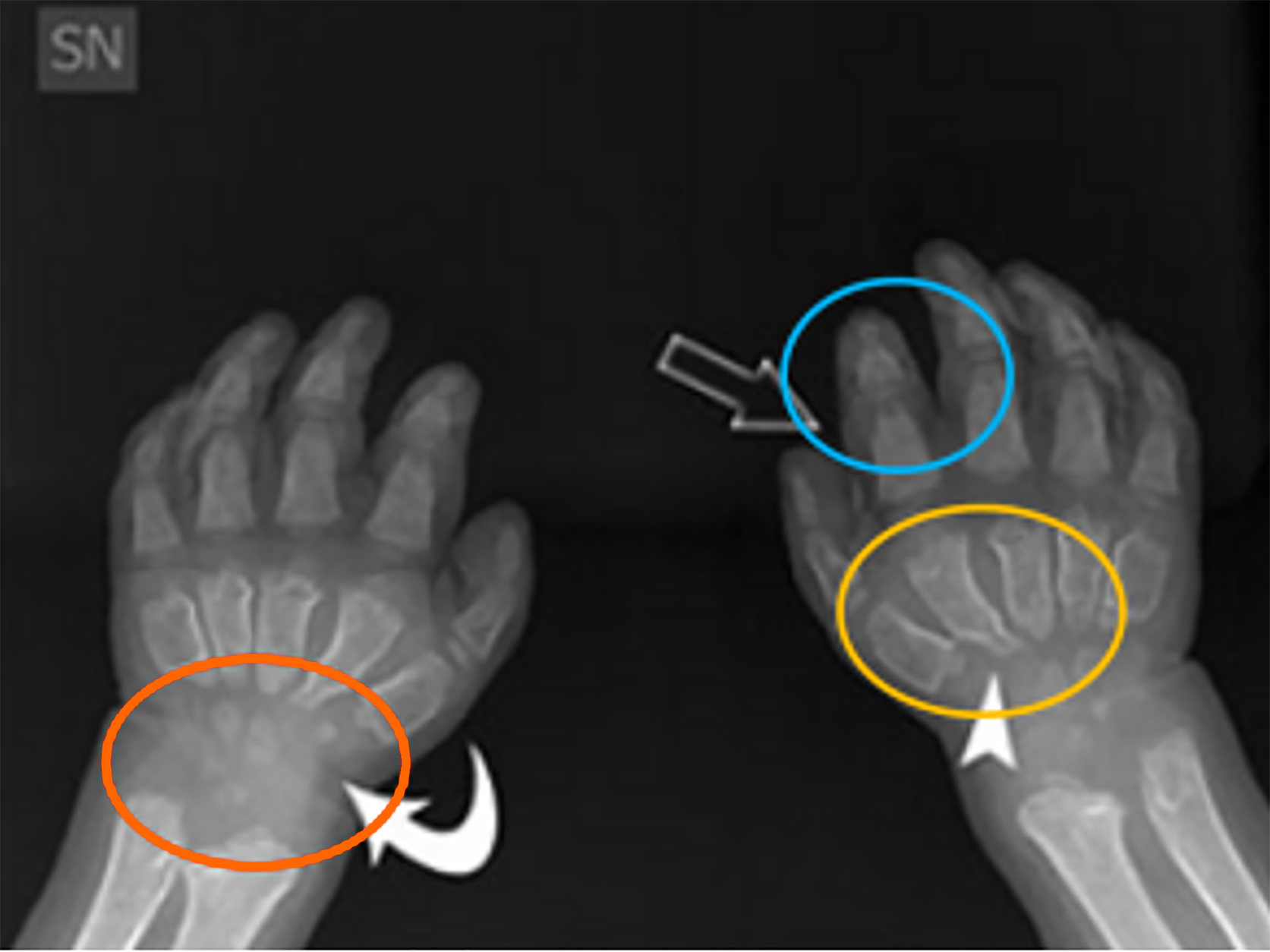
Radiographic imaging is the primary tool for assessing skeletal changes in the hands and feet. Anteroposterior and lateral radiographs are crucial for visualizing key deformities, such as the shortened metacarpals, broad phalanges, and small or absent carpal bones commonly seen in the hands, see Figure 9. These features can lead to functional impairments, including reduced grip strength and dexterity, which are essential for daily activities[6,8]. Additionally, radiologists must pay particular attention to features like Madelung’s deformity, where the distal radius and ulna converge, resulting in wrist pain and limited range of motion[8]. In the feet, deformities such as flat feet (pes planus) and shortened metatarsals can affect mobility and balance, potentially requiring radiographic assessment to evaluate the severity of these abnormalities. Radiographs help identify joint subluxations, dislocations, and hypoplastic bone structures that are characteristic of MPS. Early detection through imaging allows for proactive treatment, including physical therapy or surgical intervention, to address functional impairments[11]. Ultrasound’s ability to visualize soft tissue involvement provides radiologists with additional insights that complement radiographs, particularly in assessing joint stiffness and pain. This tool can differentiate MPS-related joint changes from other rheumatic diseases, helping to tailor treatment strategies[6]. In cases where surgical intervention is considered, radiologists play a pivotal role in pre-surgical planning. Accurate imaging of joint deformities, such as claw hand or dislocations, helps surgeons determine the appropriate course of action, whether it involves corrective osteotomy, joint realignment, or other procedures aimed at improving function[11]. Given the limitations of ERT in reversing skeletal abnormalities, radiological guidance is essential for determining the timing and extent of surgical correction.
Knowledge of the radiographic features of MPS is essential for radiologists (Refer to Table 1 for a summary). Skeletal X-rays may reveal specific changes suggestive of MPS, although they cannot precisely differentiate between types. The radiographic evaluation of MPS plays a crucial role in early diagnosis, monitoring disease progression, and guiding clinical management. Key skeletal abnormalities, such as vertebral beaking, hip dysplasia, and progressive kyphosis, should prompt timely orthopedic assessment to determine the need for surgical intervention. Additionally, radiographs remain essential for assessing the effectiveness of treatments like ERT, with improvements in bone morphology and stabilization of deformities serving as potential indicators of therapeutic response. Future research should focus on standardized radiographic criteria to optimize treatment decision-making and improve patient outcomes.
| Skeletal section | Radiographic findings | Clinical significance |
| Skull | J-shaped sella turcica, cranial thickening, macrocephaly, dolichocephaly, vertical frontal crest, wide-spaced teeth, macroglossia | Suggestive of MPS I (Hurler), II (Hunter); affects airway management and craniofacial development |
| Spine | Odontoid hypoplasia, atlantoaxial instability, gibbus deformity, platyspondyly, anterior beaking, kyphosis, posterior vertebral scalloping | High risk of spinal cord compression (especially in MPS IV Morquio), requires monitoring for surgical stabilization |
| Thorax | Paddle-shaped ribs, short/broad clavicles, short sternum, tracheomalacia, narrowed trachea | Affects respiratory function, increases risk of airway obstruction, complicates anesthesia |
| Pelvis | Hip dysplasia, rounded iliac wings, inferior tapering of the ilium, increased coxofemoral joint space, coxa valga | Leads to joint instability, progressive mobility impairment, often necessitates surgical intervention |
| Long bones and knees | Shortened diaphyses, bowed femur/tibia, proximal humeral notching, genu valgum, flared metaphyses | Functional limitations in mobility, risk of early osteoarthritis, genu valgum common in MPS IV and I |
| Hands and feet | Bullet-shaped phalanges, short metacarpals, small carpal bones, Madelung deformity, claw hand, flat feet (pes planus) | Reduces dexterity, grip strength, and mobility; claw hand may require surgical correction |
| 1. | Machnikowska-Sokołowska M, Myszczuk A, Wieszała E, Wieja-Błach D, Jamroz E, Paprocka J. Mucopolysaccharidosis Type 1 among Children-Neuroradiological Perspective Based on Single Centre Experience and Literature Review. Metabolites. 2023;13:209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 2. | Tylki-Szymańska A, De Meirleir L, Di Rocco M, Fathalla WM, Guffon N, Lampe C, Lund AM, Parini R, Wijburg FA, Zeman J, Scarpa M. Easy-to-use algorithm would provide faster diagnoses for mucopolysaccharidosis type I and enable patients to receive earlier treatment. Acta Paediatr. 2018;107:1402-1408. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 3. | Muenzer J. Early initiation of enzyme replacement therapy for the mucopolysaccharidoses. Mol Genet Metab. 2014;111:63-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 137] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 4. | Ago Y, Rintz E, Musini KS, Ma Z, Tomatsu S. Molecular Mechanisms in Pathophysiology of Mucopolysaccharidosis and Prospects for Innovative Therapy. Int J Mol Sci. 2024;25:1113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 5. | Sawamoto K, Karumuthil-Melethil S, Khan S, Stapleton M, Bruder JT, Danos O, Tomatsu S. Liver-Targeted AAV8 Gene Therapy Ameliorates Skeletal and Cardiovascular Pathology in a Mucopolysaccharidosis IVA Murine Model. Mol Ther Methods Clin Dev. 2020;18:50-61. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 28] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 6. | Spina V, Barbuti D, Gaeta A, Palmucci S, Soscia E, Grimaldi M, Leone A, Manara R, Polonara G. The role of imaging in the skeletal involvement of mucopolysaccharidoses. Ital J Pediatr. 2018;44:118. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 7. | Lachman R, Martin KW, Castro S, Basto MA, Adams A, Teles EL. Radiologic and neuroradiologic findings in the mucopolysaccharidoses. J Pediatr Rehabil Med. 2010;3:109-118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 45] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 8. | Rasalkar DD, Chu WC, Hui J, Chu CM, Paunipagar BK, Li CK. Pictorial review of mucopolysaccharidosis with emphasis on MRI features of brain and spine. Br J Radiol. 2011;84:469-477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 27] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 9. | Eurorad-Brought to You by the ESR. Available from: https://www.eurorad.org/case/5725. |
| 10. | Nicolas-Jilwan M, AlSayed M. Mucopolysaccharidoses: overview of neuroimaging manifestations. Pediatr Radiol. 2018;48:1503-1520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 24] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 11. | Palmucci S, Attinà G, Lanza ML, Belfiore G, Cappello G, Foti PV, Milone P, Di Bella D, Barone R, Fiumara A, Sorge G, Ettorre GC. Imaging findings of mucopolysaccharidoses: a pictorial review. Insights Imaging. 2013;4:443-459. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 48] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 12. | Demczko M. Overview of Lysosomal Storage Disorders. Available from: https://www.msdmanuals.com/professional/pediatrics/inherited-disorders-of-metabolism/overview-of-lysosomal-storage-disorders. |
| 13. | Żuber Z, Kieć-Wilk B, Kałużny Ł, Wierzba J, Tylki-Szymańska A. Diagnosis and Management of Mucopolysaccharidosis Type II (Hunter Syndrome) in Poland. Biomedicines. 2023;11:1668. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 14. | do Valle DA, Santos MLSF, Telles BA, Cordeiro ML. Neurological, neurobehavioral, and radiological alterations in patients with mucopolysaccharidosis III (Sanfilippo's syndrome) in Brazil. Front Neurol. 2022;13:968297. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 15. | Padash S, Obaid H, Henderson RDE, Padash Y, Adams SJ, Miller SF, Babyn P. A pictorial review of the radiographic skeletal findings in Morquio syndrome (mucopolysaccharidosis type IV). Pediatr Radiol. 2023;53:971-983. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (1)] |
| 16. | Tomatsu S, Montaño AM, Oikawa H, Smith M, Barrera L, Chinen Y, Thacker MM, Mackenzie WG, Suzuki Y, Orii T. Mucopolysaccharidosis type IVA (Morquio A disease): clinical review and current treatment. Curr Pharm Biotechnol. 2011;12:931-945. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 165] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 17. | Montaño AM, Tomatsu S, Gottesman GS, Smith M, Orii T. International Morquio A Registry: clinical manifestation and natural course of Morquio A disease. J Inherit Metab Dis. 2007;30:165-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 247] [Cited by in RCA: 248] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 18. | Barak S, Anikster Y, Sarouk I, Stern E, Eisenstein E, Yissar T, Sherr-Lurie N, Raas-Rothschild A, Guttman D. Long-Term Outcomes of Early Enzyme Replacement Therapy for Mucopolysaccharidosis IV: Clinical Case Studies of Two Siblings. Diagnostics (Basel). 2020;10:108. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 19. | Neufeld EF, Muenzer J. The mucopolysaccharidoses. The Online Metabolic and Molecular Bases of Inherited Disease. McGraw-Hill Education, 2019. [DOI] [Full Text] |
| 20. | Harmatz P, Whitley CB, Waber L, Pais R, Steiner R, Plecko B, Kaplan P, Simon J, Butensky E, Hopwood JJ. Enzyme replacement therapy in mucopolysaccharidosis VI (Maroteaux-Lamy syndrome). J Pediatr. 2004;144:574-580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 217] [Cited by in RCA: 194] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 21. | D'Avanzo F, Zanetti A, De Filippis C, Tomanin R. Mucopolysaccharidosis Type VI, an Updated Overview of the Disease. Int J Mol Sci. 2021;22:13456. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 27] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 22. | Mathew J, Jagadeesh SM, Bhat M, Udhaya Kumar S, Thiyagarajan S, Srinivasan S. Mutations in ARSB in MPS VI patients in India. Mol Genet Metab Rep. 2015;4:53-61. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 23. | Azevedo AC, Schwartz IV, Kalakun L, Brustolin S, Burin MG, Beheregaray AP, Leistner S, Giugliani C, Rosa M, Barrios P, Marinho D, Esteves P, Valadares E, Boy R, Horovitz D, Mabe P, da Silva LC, de Souza IC, Ribeiro M, Martins AM, Palhares D, Kim CA, Giugliani R. Clinical and biochemical study of 28 patients with mucopolysaccharidosis type VI. Clin Genet. 2004;66:208-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 83] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 24. | Giugliani R, Lampe C, Guffon N, Ketteridge D, Leão-Teles E, Wraith JE, Jones SA, Piscia-Nichols C, Lin P, Quartel A, Harmatz P. Natural history and galsulfase treatment in mucopolysaccharidosis VI (MPS VI, Maroteaux-Lamy syndrome)--10-year follow-up of patients who previously participated in an MPS VI Survey Study. Am J Med Genet A. 2014;164A:1953-1964. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 65] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 25. | Yoo S, Lee J, Kim M, Yoon JY, Cheon CK. From diagnosis to treatment of mucopolysaccharidosis type VI: A case report with a novel variant, c.1157C>T (p.Ser386Phe), in ARSB gene. J Genet Med. 2022;19:32-37. [DOI] [Full Text] |
| 26. | Sly WS, Quinton BA, McAlister WH, Rimoin DL. Beta glucuronidase deficiency: report of clinical, radiologic, and biochemical features of a new mucopolysaccharidosis. J Pediatr. 1973;82:249-257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 380] [Cited by in RCA: 354] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 27. | Montaño AM, Lock-Hock N, Steiner RD, Graham BH, Szlago M, Greenstein R, Pineda M, Gonzalez-Meneses A, Çoker M, Bartholomew D, Sands MS, Wang R, Giugliani R, Macaya A, Pastores G, Ketko AK, Ezgü F, Tanaka A, Arash L, Beck M, Falk RE, Bhattacharya K, Franco J, White KK, Mitchell GA, Cimbalistiene L, Holtz M, Sly WS. Clinical course of sly syndrome (mucopolysaccharidosis type VII). J Med Genet. 2016;53:403-418. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 103] [Cited by in RCA: 129] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 28. | Lee CL, Chuang CK, Hsu CH, Chiu HC, Tu RY, Lo YT, Chang YH, Lin HY, Lin SP. The first mucopolysaccharidosis type VII in a Taiwanese girl: A case report and review of the literature. J Formos Med Assoc. 2022;121:712-717. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 29. | McCafferty EH, Scott LJ. Vestronidase Alfa: A Review in Mucopolysaccharidosis VII. BioDrugs. 2019;33:233-240. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 35] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 30. | Taylor M, Khan S, Stapleton M, Wang J, Chen J, Wynn R, Yabe H, Chinen Y, Boelens JJ, Mason RW, Kubaski F, Horovitz DDG, Barth AL, Serafini M, Bernardo ME, Kobayashi H, Orii KE, Suzuki Y, Orii T, Tomatsu S. Hematopoietic Stem Cell Transplantation for Mucopolysaccharidoses: Past, Present, and Future. Biol Blood Marrow Transplant. 2019;25:e226-e246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 133] [Article Influence: 22.2] [Reference Citation Analysis (1)] |
| 31. | Natowicz MR, Short MP, Wang Y, Dickersin GR, Gebhardt MC, Rosenthal DI, Sims KB, Rosenberg AE. Clinical and biochemical manifestations of hyaluronidase deficiency. N Engl J Med. 1996;335:1029-1033. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 122] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 32. | Imundo L, Leduc CA, Guha S, Brown M, Perino G, Gushulak L, Triggs-Raine B, Chung WK. A complete deficiency of Hyaluronoglucosaminidase 1 (HYAL1) presenting as familial juvenile idiopathic arthritis. J Inherit Metab Dis. 2011;34:1013-1022. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 58] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 33. | Kiykim E, Barut K, Cansever MS, Zeybek CA, Zubarioglu T, Aydin A, Kasapcopur O. Screening Mucopolysaccharidosis Type IX in Patients with Juvenile Idiopathic Arthritis. JIMD Rep. 2016;25:21-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |