Copyright
©The Author(s) 2015.
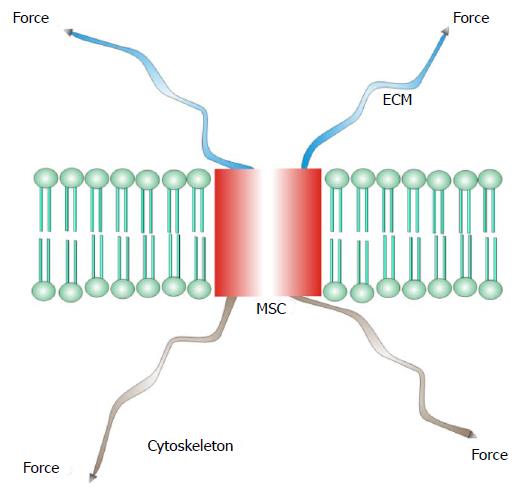
Figure 1 A mechanosensitive channel from a differentiated sensory organ where stress is passed through the channel from the extracellular matrix to the cytoskeleton.
MSC: Mechanosensitive channel; ECM: Extracellular matrix.
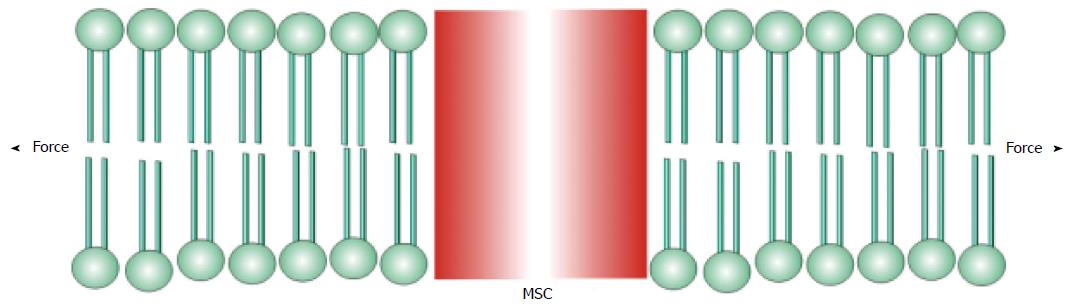
Figure 2 A mechanosensitive channel activated by tension (force) in the bilayer.
MSC: Mechanosensitive channel.
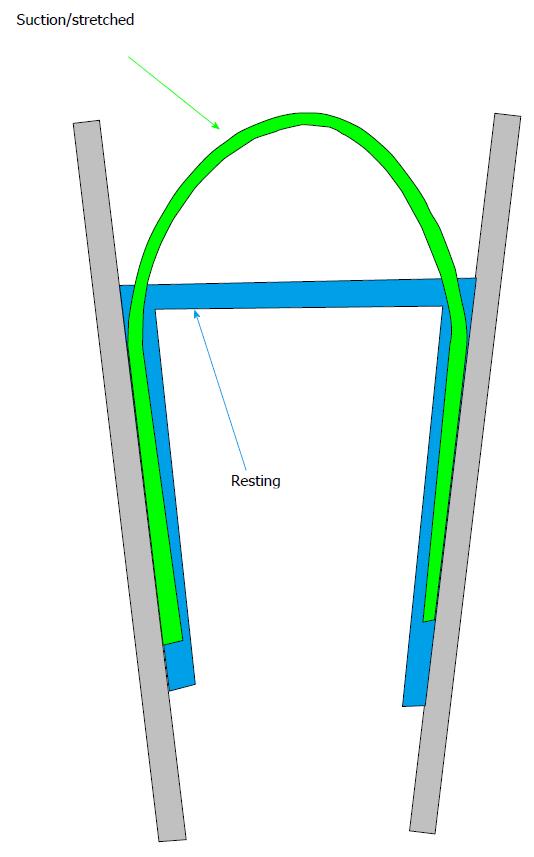
Figure 3 Cartoon of a patch pipette holding a lipid membrane at rest (pulled tight by adhesion to the glass) and under suction.
Notice that suction peels the membrane off the glass a bit and functional channels.
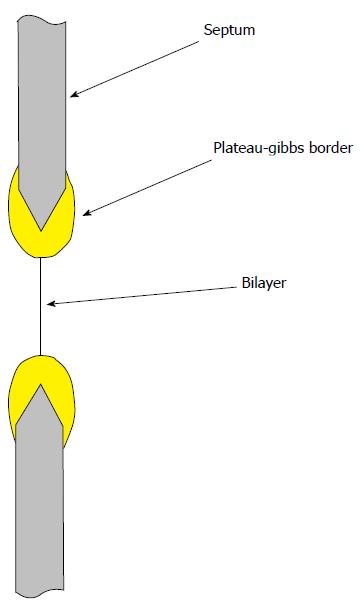
Figure 4 The planar bilayer is under tension, approximately 6 mN/m due to adhesion of the bulk lipid to the septum support.
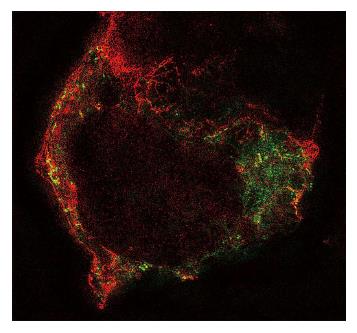
Figure 5 Structured illumination image of an human embryonic kidney cell cotransfected with human PIEZO1 channels labelled green and TREK-1 channels labelled red using green fluorescent protein mutants.
Notice that the channels are in different structural domains and thus feel different forces. Notice also that TREK-1 tends to follow underlying cytoskeletal fibers. (Courtesy Gottlieb P).
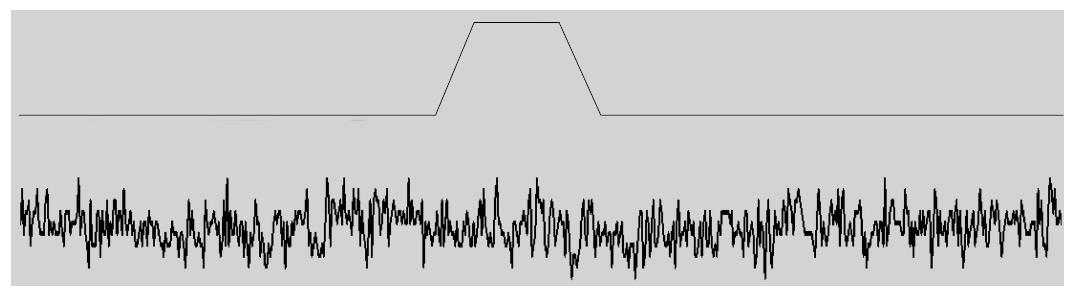
Figure 6 A whole-cell current recording (lower trace) from an human embryonic kidney cell in response to indentation of a few μm with a fire-polished micropipette in a piezoelectric manipulator (upper trace shows the cell indentation).
There is no current associated with the indentation even though human PIEZO1 was cloned from the same cells! RNA expression does not mean the presence of functional channels, and even cells with no obvious endogenous currents may have channels, but they can’t be activated because of mechanoprotection. They may become visible with large and/or repeated stimuli or treatment with agents like cytochalasin. (The trace is 1 s long and the RMS current noise is about 1 pA). RMS: Root-mean-square.
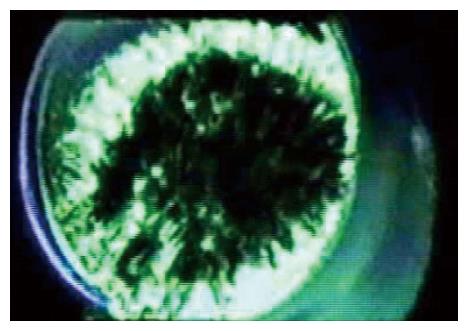
Figure 7 A frame from tomographic reconstruction of a patch of a Xenopus oocyte using high voltage electron microscope tomography[1].
The image shows cytoskeleton spanning the pipette and the bilayer is attached to the upper side but is not visible in this reconstruction due to its low density.
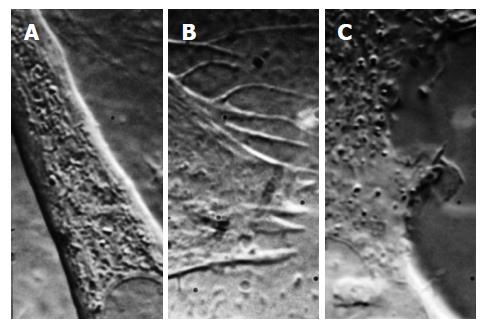
Figure 8 Differential interference contrast images of three different cell types (mouse myotube, A; rat astrocyte, B, human embryonic kidney cell, C) showing the variability of membrane structure and how patch clamp recordings are expected to be variable.
The structures above change rapidly over time (this is a frame from a movie, Courtesy Suchyna T).
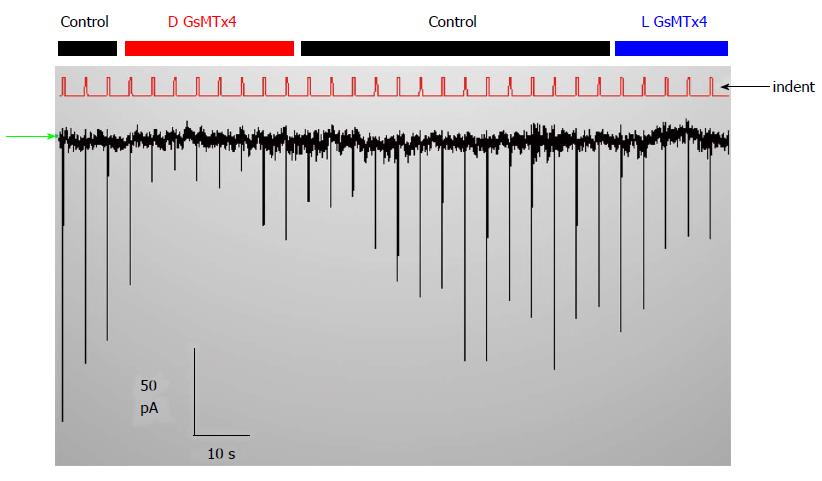
Figure 9 There are no PIEZO1 currents in this resting whole-cell recording unless the cell are indented (the red trace labelled indent shows the stimuli).
The inhibitor GsMtx4 is effective in suppressing the stimulus-induced current but causes no change in the holding current. The stimuli are activated by computer control (http://www.qub.buffalo.edu). The baseline current (green arrow at left) is not affected by adding the D or the L enantiomers of GsMtx4, but the currents that are evoked by indentation are inhibited. Thus, tension in the resting cell is insufficient to activate PIEZO1.
- Citation: Sachs F. Mechanical transduction by ion channels: A cautionary tale. World J Neurol 2015; 5(3): 74-87
- URL: https://www.wjgnet.com/2218-6212/full/v5/i3/74.htm
- DOI: https://dx.doi.org/10.5316/wjn.v5.i3.74