Published online Aug 6, 2014. doi: 10.5315/wjh.v3.i3.105
Revised: April 20, 2014
Accepted: May 28, 2014
Published online: August 6, 2014
Processing time: 313 Days and 6.9 Hours
AIM: To elucidate risk factors for survival of elderly acute myeloid leukemia (AML) patients in a real-world practice by observational study.
METHODS: We conducted a population-based study in 213 adult and elderly AML patients (127 males and 86 females) in Kagawa Prefecture, Japan. To construct this cohort, we gathered all data for patients diagnosed with AML at 7 hospitals in Kagawa between 2006 and 2010. The primary end point was overall survival (OS) after AML diagnosis. Unadjusted Kaplan-Meier survival plots were used to determine OS in the overall cohort. Multivariate analysis was used to determine the independent adverse prognostic factors for OS, with the covariates of interest including age, gender, race/ethnicity, CCI, education, median income, metropolitan statistical area size and history of myelodysplastic syndrome.
RESULTS: The average population of Kagawa during the study period was 992489, and the incidence of AML was 4.26 per 100000 person-years. A total of 197 patients with non-acute promyelocytic leukemia (non-APL) (119 males and 78 females) were also included. The median age of non-APL patients was 70 years (average 67, range 24-95). The 5-year OS rate was 21.1%. Subsequent analysis by age group showed that the survival rate declined with age; the 5-year OS rates of non-APL patients younger than 64 years, 65-74 years, and older than 75 years were 41.5%, 14.1%, and 8.9%, respectively. Multivariate analysis revealed that unfavorable risk karyotype, older age, poor performance status (PS) (3-4), lack of induction chemotherapy, and antecedent haematological disease were independent prognostic predictors. In the subgroup analysis, we also found that older patients with non-APL had lower complete remission rates and higher early death rates than younger patients, irrespective of PS. However, intensive chemotherapy was a significant predictor for longer survival not only in the patients < 75 years of age, but also in those over 75 with PS 0-2.
CONCLUSION: Age would contribute considerable life expectancy to indicate induction chemotherapy with eligible dose of cytotoxic drugs for a favorable case even in advanced elderly.
Core tip: The prevalence of acute myeloid leukemia (AML) is increasing among elderly patients in Japan. Our population-based observational study revealed that age was an independent prognostic factor in a real-world practice for the treatment of AML patients. Although we found that AML patients older than 75 years had lower complete remission rates and higher early death rates than patients younger than 75 years, an appropriately intensified induction chemotherapy would be helpful to prolong the survival of elderly AML patients with better performance status (PS) (1-2). The intensity of chemotherapy should thus be adjusted according to age and PS.
- Citation: Ohnishi H, Imataki O, Kawachi Y, Ide M, Kawakami K, Waki M, Takimoto H, Hoshijima Y, Fukumoto T, Matsumoto K, Waki F, Matsuoka A, Shintani T, Uemura M, Yokokura S, Taoka T, Matsunaga T. Age is an independent adverse prognostic factor for overall survival in acute myeloid leukemia in Japan. World J Hematol 2014; 3(3): 105-114
- URL: https://www.wjgnet.com/2218-6204/full/v3/i3/105.htm
- DOI: https://dx.doi.org/10.5315/wjh.v3.i3.105
Elderly acute myeloid leukemia (AML) patients often have several comorbidities and poor performance status (PS) at the time of diagnosis, and may be intolerant to intensive chemotherapy, making them poor candidates for intensive induction chemotherapy[1-4]. Compared to young adult AML patients, elderly AML patients also have higher frequencies of adverse prognostic factors such as unfavorable risk karyotype and secondary AML (therapy-related AML)[5], leading to a poorer prognosis[6-8].
Juliusson et al [9] analyzed a population-based cohort of patients aged ≥ 16 years in the Swedish National Acute Leukemia Registry. They found that onset of AML may occur at any age, but is most common in the elderly population, with the highest incidence in individuals aged 80-85 years. The median age of onset was 72 years (range 16-97): 71 years for males and 72 years for females. A number of prospective clinical trials have studied treatments for young adult patients with non-acute promyelocytic leukemia (non-APL)[10-13]. These trials showed that the 5-year overall survival (OS) rate increased to 35%-48% in patients treated with induction chemotherapy using idarubicin and cytosine arabinoside. However, these trials excluded patients with poor PS (3-4) and elderly patients. Considering the distribution of the age onset of AML, these clinical trials included only a small proportion of the total AML population, and the results therefore do not accurately reflect treatment options for the overall AML population.
To accurately evaluate the overall AML population, several retrospective population-based studies have been conducted in Sweden[9,14], the United Kingdom[15,16], and the United States[17]. These studies found relatively low 5-year OS rates of 10%-20%[16,18,19]. Older age and poor PS were reported to be adverse prognostic factors for OS in these population-based studies, but not in prospective clinical trials. However, the retrospective population-based studies did not include multivariate analyses[9,18]. It is therefore still unclear whether older age and poor PS are independent adverse prognostic factors for OS in patients with AML.
In this study, multivariate analysis identified older age, lack of induction chemotherapy, poor PS (3-4), antecedent hematological disease, and unfavorable risk karyotype as independent prognostic factors for poor OS. We also found that older patients had lower complete remission (CR) rates and higher early death rates than younger patients, irrespective of PS. This analysis provides data describing the overall AML population.
We performed a multicenter observational study of adult AML patients aged ≥ 17 years from seven institutions within Kagawa Prefecture (Kagawa Rosai Hospital, Takamatsu Municipal Hospital, Sakaide City Hospital, Kagawa Prefectural Central Hospital, Takamatsu Red Cross Hospital, Mitoyo General Hospital, and Kagawa University Hospital) between January 1, 2006 and December 31, 2010. Data were collected from the medical records. Diagnosis of AML was made according to the criteria of the French-American-British classification. We excluded patients with a previous diagnosis of myelodysplastic syndrome (MDS). Antecedent haematological disease was defined as benign hematological disease other than MDS. The potential effects of consolidation therapy and hematopoietic stem cell transplantation are beyond the scope of this analysis, and patients were not censored at the time of transplantation or any other treatment in the survival analysis. This study was approved by the Institutional Review Boards of Kagawa Rosai Hospital, Takamatsu Municipal Hospital, Sakaide City Hospital, Kagawa Prefectural Central Hospital, Takamatsu Red Cross Hospital, Mitoyo General Hospital, and Kagawa University Hospital.
The karyotypes of non-APL patients were grouped according to the criteria of the National Comprehensive Cancer Network (NCCN) clinical practice guidelines[20], the Southwest Oncology Group (SWOG) classification[21], and the Cancer and Leukemia Group B (CALGB) classification[22].
Induction therapy regimens were chosen by each treating physician based on available clinical data and local standards of care, but not on karyotype. None of the patients were enrolled in clinical trials.
APL patients with a white blood cell (WBC) count of < 3.0 × 109 per liter and a blast plus promyelocyte count of < 1.0 × 109 per liter were started on oral all-trans-retinoic acid (ATRA) (45 mg/m2 per day) alone until the start of consolidation therapy. Patients with a WBC count between 3.0 × 109 per liter and 10.0 × 109 per liter or a blast plus promyelocyte count of ≥ 1.0 × 109 per liter were started on oral ATRA until the start of consolidation therapy plus idarubicin (IDR) (12 mg/m2 per day by 30-min infusion, days 1-2) plus cytarabine (Ara-C) (80 mg/m2 per day by continuous infusion, days 1-5). Patients with a WBC count of ≥ 10.0 × 109 per liter were started on oral ATRA until the start of consolidation therapy plus IDR (days 1-3) plus Ara-C (100 mg/m2 per day by continuous infusion, days 1-5).
Non-APL patients were treated with one of 15 regimens, which we categorized as intensive chemotherapy, less intensive chemotherapy, or best supportive care. The intensive chemotherapy regimens were: (1) full dose IDR (12 mg/m2 per day by 30-min infusion, days 1-3) plus Ara-C (100 mg/m2 per day by continuous infusion, days 1-7); (2) 80% dose IDR (days 1-3) plus Ara-C (days 1-7); (3) full dose daunorubicin (DNR) (50 mg/m2 per day by 30-min infusion, days 1-5) plus Ara-C (days 1-7); (4) 80% dose DNR (days 1-5) plus Ara-C (days 1-7); (5) full dose IDR (days 1-3) plus enocitabine (BHAC) (200 mg/m2 per day by 180-min infusion, days 1-8); (6) 80% dose IDR (days 1-3) plus BHAC (days 1-8); (7) full dose DNR (days 1-5) plus BHAC (days 1-8); (8) 80% dose DNR (days 1-5) plus BHAC (200 mg/m2 per day by 180-min infusion, days 1-8). The less intensive chemotherapy regimens were: (1) less than 80% dose IDR (days 1-3) plus Ara-C (days 1-7); (2) less than 80% dose DNR (days 1-5) plus Ara-C (days 1-7); (3) less than 80% dose IDR (days 1-3) plus BHAC (days 1-8); (4) less than 80% dose DNR (days 1-5) plus BHAC (days 1-8); (5) CAG [Ara-C (10 mg/m2 twice a day by subcutaneous injection, days 1-14) plus aclarubicin (ACR) (14 mg/m2 per day by 30-min infusion, days 1-14) plus granulocyte colony-stimulating factor (G-CSF) (200 g/m2 per day by subcutaneous injection, days 1-14)]; (6) CA [Ara-C (10 mg/m2 twice a day by subcutaneous injection, days 1-14) plus ACR (14 mg/m2 per day by 30-min infusion, days 1-14)]; (7) Low dose Ara-C (10 mg/m2 twice a day by subcutaneous injection, days 1-14).
The χ2 test was used to analyze the significance of differences between two or three groups (Microsoft Excel 2010, version 14.0; Microsoft Corporation Japan, Tokyo). A P value less than 0.05 was considered statistically significant. All other data were analysed using JMP 7.0.1 (SAS Institute Japan, Tokyo, Japan). The Kaplan-Meier method was used to estimate probabilities of OS, and the log-rank test was used to analyse the significance of differences in OS between two or three groups. For the survival analysis, patients were censored at the time of the last follow-up. Multivariate analysis of prognostic factors for OS was performed using the Cox proportional hazards method. All prognostic factors were first analyzed using univariate analysis. Early death was defined as 8-wk mortality after the diagnosis or initiation of chemotherapy. Factors with a P value of less than 0.05 on univariate analysis were included in the multivariate analysis using a stepwise method.
A total of 219 patients were diagnosed with AML between January 1, 2006 and December 31, 2010 at the 7 participating institutions. Considering the average population of Kagawa Prefecture during the study period, the incidence of AML was 4.26 per 100000 person-years.
We focused on analyzing adult patients with AML. Six patients were excluded due to a lack of available clinical data, and the remaining 213 patients were included. These patients were 127 men and 86 women (male to female ratio 1.48) with a median age of 70 years (range 24-95). Thirty-five patients underwent allogeneic hematopoietic stem cell transplantation. The cohort included 16 APL patients and 197 non-APL patients. The estimated 5-year OS rate of the APL patients was 69.2% (95%CI: 55.5-82.9). The median follow-up period for APL survivors was 23.5 mo (range 0-56).
The non-APL patients had a median age of 70 years (range 24-95), including 74 patients (37.6%) aged ≤ 64 years, 50 patients (25.6%) aged 65-74 years, and 73 patients (36.8%) aged ≥ 75 years. Table 1 shows the characteristics of the non-APL patients. In our study some data were missing for each parameter. Therefore, the total numbers of patients in each parameter group-that is, the sum of each column-are sometimes different. Some clinical features varied among the different age groups. Overall, 93 patients (47.2%) had one or more features of myelodysplasia (not satisfy the diagnosis criteria for MDS). The frequency of myelodysplastic features was higher in patients aged ≥ 75 years than in younger patients. Approximately half of the patients (48.3%) had Eastern Cooperative Oncology Group PS of 2-4. As age increased, the proportion of patients with good PS (0-1) decreased and that with poor PS (3-4) increased. The chromosomal karyotype is known to be the strongest predictor of prognosis, but the systems used to classify the karyotypes vary among studies. We divided our cohort into three karyotype groups: 5.1%-6.6% of patients were classified as having a favourable risk karyotype, 22.8%-29.9% of patients were classified as having an unfavourable risk karyotype, and category not recognized. A favorable risk karyotype was more frequent in patients aged ≤ 64 years than patients aged ≥ 65 years. The serum lactase dehydrogenase (LDH) level was lower in patients aged 65-74 years than in the other age groups (≤ 64 years and ≥ 75 years); there were significantly more patients with a normal LDH level (< 250 U/L) and fewer patients with an increased LDH level (> 500 U/L) in the group aged 65-74 years than in the other age groups. Twenty-nine patients (14.7%) had renal dysfunction with a serum creatinine level of > 1.3 mg/dL, and 48 patients (24.3%) had an infection at the time of diagnosis. Intensive induction chemotherapy was administered to 102 patients (51.7%) in total, including 71.6% of patients aged ≤ 64 years, 46.0% of patients aged 65-74 years, and 35.6% of patients aged ≥ 75 years.
| Number of patients | ||||||||||
| Parameter | Category | All patients (n = 197) | Age in year | |||||||
| ≤64 (n = 74) | 65-74 (n = 50) | ≥75 (n = 73) | ||||||||
| Gender | Female | 78 | 39.60% | 30 | 40.50% | 19 | 38.00% | 29 | 39.70% | |
| Male | 119 | 60.40% | 44 | 59.50% | 31 | 62.00% | 44 | 60.30% | ||
| FAB classification | M0 | 9 | 4.60% | 3 | 4.10% | 0 | 0.00% | 6 | 8.20% | |
| M1 | 24 | 12.20% | 10 | 13.50% | 4 | 8.00% | 10 | 13.70% | ||
| M2 | 100 | 50.80% | 34 | 45.90% | 27 | 54.00% | 39 | 53.40% | ||
| M4 | 24 | 12.20% | 11 | 14.90% | 7 | 14.00% | 6 | 8.20% | ||
| M5 | 10 | 5.10% | 5 | 6.80% | 3 | 6.00% | 2 | 2.70% | ||
| M6 | 13 | 6.60% | 3 | 4.10% | 4 | 8.00% | 6 | 8.20% | ||
| M7 | 4 | 2.00% | 2 | 2.70% | 1 | 2.00% | 1 | 1.40% | ||
| Myelodysplasia | Yes | 93 | 47.20% | 32 | 43.20% | 20 | 40.00% | 41 | 56.20% | a |
| No | 100 | 50.80% | 40 | 54.10% | 29 | 58.00% | 31 | 42.50% | ||
| Performance status | 0-1 | 98 | 49.70% | 45 | 60.80% | 28 | 56.00% | 25 | 34.20% | |
| 2 | 46 | 23.40% | 13 | 17.60% | 8 | 16.00% | 25 | 34.20% | ||
| 3-4 | 49 | 24.90% | 14 | 18.90% | 13 | 26.00% | 22 | 30.10% | ||
| Karyotype risk category | ||||||||||
| NCCN | F | 13 | 6.60% | 9 | 12.20% | 2 | 4.00% | 2 | 2.70% | a |
| I | 122 | 61.90% | 43 | 58.10% | 27 | 54.00% | 52 | 71.20% | ||
| U | 46 | 23.40% | 16 | 21.60% | 19 | 38.00% | 11 | 15.10% | ||
| SWOG | F | 10 | 5.10% | 7 | 9.50% | 2 | 4.00% | 1 | 1.40% | a |
| I | 97 | 49.20% | 35 | 47.30% | 23 | 46.00% | 39 | 53.40% | ||
| U | 59 | 29.90% | 20 | 27.00% | 21 | 42.00% | 18 | 24.70% | ||
| others | 15 | 7.60% | 6 | 8.10% | 2 | 4.00% | 7 | 9.60% | ||
| CALGB | F | 13 | 6.60% | 9 | 12.20% | 3 | 6.00% | 1 | 1.40% | a |
| I | 100 | 50.80% | 33 | 44.60% | 26 | 52.00% | 41 | 56.20% | ||
| U | 45 | 22.80% | 15 | 20.30% | 16 | 32.00% | 14 | 19.20% | ||
| others | 39 | 19.80% | 17 | 23.10% | 5 | 10.00% | 17 | 23.20% | ||
| Antecedent | No | 158 | 80.20% | 60 | 81.10% | 37 | 74.00% | 61 | 83.60% | |
| hematologic disease | Yes | 36 | 18.30% | 13 | 17.60% | 11 | 22.00% | 12 | 16.40% | |
| Prior chemotherapy | No | 174 | 88.30% | 65 | 87.80% | 41 | 82.00% | 68 | 93.20% | |
| Yes | 19 | 9.60% | 7 | 9.50% | 7 | 14.00% | 5 | 6.80% | ||
| Laboratory findings | ||||||||||
| WBC (× 103/mL) | < 100 | 178 | 90.40% | 65 | 87.80% | 48 | 96.00% | 65 | 89.00% | |
| ≥ 100 | 16 | 8.10% | 7 | 9.50% | 1 | 2.00% | 8 | 11.00% | ||
| Hemoglobin (g/dL) | < 8.0 | 93 | 47.20% | 36 | 48.60% | 23 | 46.00% | 34 | 46.60% | |
| ≥ 8.0 | 101 | 51.30% | 36 | 48.60% | 26 | 52.00% | 39 | 53.40% | ||
| Platelet (× 104/mL) | < 5.0 | 85 | 43.10% | 29 | 39.20% | 23 | 46.00% | 33 | 45.20% | |
| 5.0-10.0 | 64 | 32.50% | 25 | 33.80% | 20 | 40.00% | 19 | 26.00% | ||
| ≥ 10.0 | 45 | 22.80% | 18 | 24.30% | 6 | 12.00% | 21 | 28.80% | ||
| % Blast in blood | < 20 | 89 | 45.20% | 28 | 37.80% | 29 | 58.00% | 32 | 43.80% | |
| 20-50 | 43 | 21.80% | 17 | 23.00% | 11 | 22.00% | 15 | 20.50% | ||
| > 50 | 59 | 29.90% | 27 | 36.50% | 8 | 16.00% | 24 | 32.90% | ||
| % Blast in marrow | < 50 | 98 | 49.70% | 33 | 44.60% | 30 | 60.00% | 35 | 47.90% | |
| ≥ 50 | 84 | 42.60% | 35 | 47.30% | 18 | 36.00% | 31 | 42.50% | ||
| LDH (IU/L) | < 250 | 58 | 29.40% | 15 | 20.30% | 24 | 48.00% | 19 | 26.00% | a |
| 250-500 | 75 | 38.10% | 27 | 36.50% | 14 | 28.00% | 34 | 46.60% | ||
| > 500 | 59 | 29.90% | 29 | 39.20% | 11 | 22.00% | 19 | 26.00% | ||
| Creatinine (mg/dL) | ≤ 1.3 | 162 | 82.20% | 66 | 89.20% | 41 | 82.00% | 55 | 75.30% | |
| > 1.3 | 29 | 14.70% | 5 | 6.80% | 8 | 16.00% | 16 | 21.90% | ||
| Infection at | No | 146 | 74.10% | 52 | 70.20% | 39 | 78.00% | 55 | 75.30% | a |
| induction therapy | Yes | 48 | 24.30% | 20 | 27.00% | 10 | 20.00% | 18 | 24.60% | |
| Intensity of | Intensive | 102 | 51.70% | 53 | 71.60% | 23 | 46.00% | 26 | 35.60% | a |
| induction therapy | Less-intensive | 56 | 28.40% | 13 | 17.50% | 19 | 38.00% | 24 | 32.80% | |
| BSC | 39 | 19.70% | 8 | 10.80% | 8 | 16.00% | 23 | 31.50% | ||
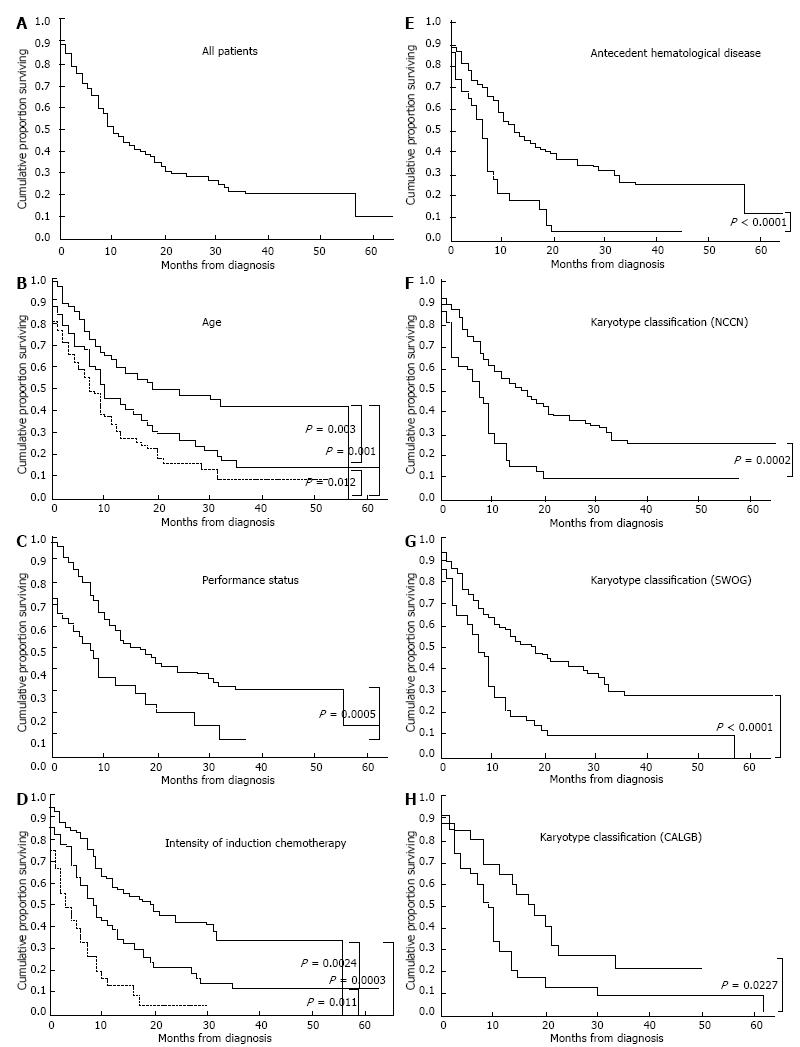
In non-APL patients, the estimated 5-year OS rate was 21.1% (95%CI: 1.7-40.5) (Figure 1A). The median follow-up period among non-APL survivors was 32 mo (range 1.0-59.5). Analysis by age group showed that the 5-year OS rate decreased with increasing age. In non-APL patients aged ≤ 64 years, 65-74 years, and ≥ 75 years, the 5-year OS rates were 41.5% (95%CI: 34.5-48.5), 14.1% (95%CI: 8.8-19.4), and 8.9% (95%CI: 4.1-13.7), respectively; and the median survival times were 19, 10 and 7 mo, respectively (Figure 1B). In addition, poor PS (3-4), lack of induction chemotherapy or less intensive induction chemotherapy, presence of antecedent hematological disease (except for MDS), and unfavourable risk karyotype according to the NCCN, SWOG, or CALGB classifications adversely affected the OS rate (Figure 1C-H). Multivariate analysis revealed that older age, poor PS (3-4), lack of induction chemotherapy, presence of antecedent hematological disease, and unfavourable risk karyotype according to any karyotype classification were adverse prognostic factors (Table 2). Detailed information regarding karyotype categories according to the NCCN, SWOG, and CALGB classifications is shown in Table 3.
| Risk factors | |||
| Chromosomal abnormality | NCCN | SWOG | CALGB |
| HR (95%CI) P | HR (95%CI) P | HR (95%CI) P | |
| Age in years | 0.031 | 0.043 | 0.026 |
| ≤ 64 | 1 | 1 | 1 |
| 65-74 | 1.33 (1.06-1.58) | 1.39 (1.13-1.64) | 1.40 (1.15-1.66) |
| ≥ 75 | 1.88 (1.59-2.04) | 1.82 (1.60-2.05) | 1.91 (1.67-2.13) |
| Performance status | 0.012 | 0.028 | 0.019 |
| 0-2 | 1 | 1 | 1 |
| 3-4 | 1.94 (1.15-3.26) | 1.80 (1.06-3.05) | 1.88 (1.11-3.20) |
| Intensity of induction therapy | 0.041 | 0.029 | 0.027 |
| Intensive, less-intensive | 1 | 1 | 1 |
| Best supportive care | 1.74 (2.02-2.96) | 1.80 (1.06-3.07) | 1.83 (1.07-3.14) |
| Antecedent hematological disease | 0.007 | 0.007 | 0.004 |
| No | 1 | 1 | 1 |
| Yes | 1.92 (1.20-3.08) | 1.90 (1.19-3.05) | 2.03 (1.25-3.27) |
| Chromosomal abnormality | 0.001 | < 0.001 | < 0.001 |
| Favorable, intermediate | 1 | 1 | 1 |
| Unfavorable | 1.98 (1.31-2.99) | 1.44 (0.55-3.78) | 1.56 (0.63-3.87) |
| Category | Favorable | Intermediate | Unfavorable | Category not recognized |
| NCCN | t(8;21) | +8, t(9;11), -X, -Y, -6, +1, +4, +7, +11, +13, +21, del(9), del(20), add(12), add(16), add(17), inv(3), t(1;16), t(3;21), t(8;18), t(8;20), t(11;16), t(11;17) | –5, del(5q), -7, non-t(9;11) abn11q23, inv(3), t(9;22), complex karyotype ≥ 3 | - |
| SWOG | t(8;21) | -Y, +8 | abn(3q), -5, -7, t(9;22), abn(9q), abn(11q), abn(17p), abn(20q), abn(21q), complex karyotype ≥ 3 | t(9;11), -X, -6, +1, +4, +7, +11, +13, +21, add(12), add(16), inv(3), t(1;16), t(3;21), t(8;18), t(8;20) |
| CALGB | t(8;21), del(9q) | -Y, del(5q), t(9;11), +11, del(11q), abn(12p), +13, del(20q), +21 | inv(3), -7, +8, complex karyotype ≥ 3 | -X, -6, +1, +4, +7, add(12), add(16), add(17), t(1;16), t(3;21), t(8;18), t(8;20), t(11;16), t(11;17) |
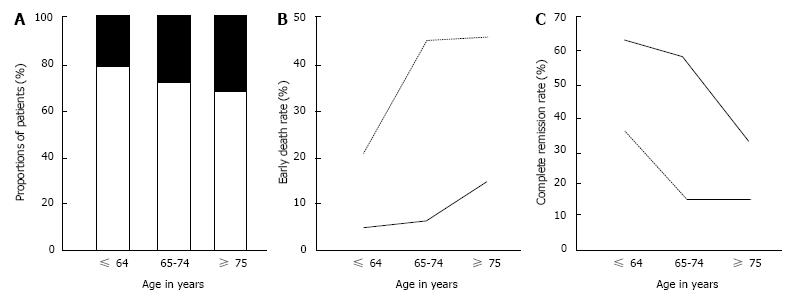
The rates of CR and early death (within 8 wk of diagnosis) according to PS in different age groups are shown in Figure 2. The proportion of patients with poor PS (3-4) increased with age (Figure 2A). Early death rates were related to both age and PS (Figure 2B). Older patients had higher early deaths rates than younger patients, and patients with poor PS had higher early death rates than patients with good PS. CR rates were also related to both age and PS (Figure 2C). Older patients had lower CR rates than younger patients, and patients with poor PS had lower CR rates than patients with good PS.
This study was limited to AML patients in a specific area of Japan, unlike the large scale study conducted by Juliusson et al[9], which included data from the whole of Sweden. However, Kagawa Prefecture is surrounded by ocean and mountain ranges, and residents almost never seek treatment for malignancies elsewhere due to the inconvenience of travelling. This study has almost complete capture of the patient population. We therefore consider that the present results were highly representative of the patient representation. Furthermore, the median and interquartile range of age, and incidence of AML, were similar between this study and the Swedish study by Juliusson et al[9]. This similarity in the distributions of patients between the two reports indicates that the present study was a reliable population-based study. In Japan, all population-based studies of this sort are conducted under a strict registration system which is facilitated by a nation-wide organization. In all of the cases in the present cohort, a primary physician had reached consensus in referring the patient to a general community hospital due to a haematological malignancy.
Dores et al[17] reported that there were no differences in the age of onset or the incidence of AML among non-Hispanic whites, Hispanic whites, Blacks, and Asians/Pacific Islanders in the United States. They also reported that the frequencies of APL, a subtype of AML, differed among these subpopulations, accounting for 6.1%, 14.2%, 9.3%, and 7.0% of the four groups, respectively. Nakase et al[23] reported that although the frequency of t(8;21) AML was higher in the Japanese population than the Australian population (33.1% vs 15.3%, P < 0.05), there was no difference in the frequency of APL between these populations (14.8% vs 11.1%). However, these data may not be generalizable as the patients selected for the study were all diagnosed at a single hospital. The frequency of APL in our cohort was 7.5%, which is similar to the frequencies reported among Australians[23], Asians/Pacific Islanders and non-Hispanic white[17], higher than the frequency reported in the Swedish study[9], and lower than the frequencies reported among both Hispanic whites and Blacks in the United States[17], suggesting that the frequency of APL differs among races.
Several studies[7,8,24,25] have reported on differences between elderly and young adult AML patients in terms of host factors such as physiological functions and biological factors such as characteristics of AML cells. Pollyea et al[8] reported that outcomes in elderly AML patients were affected by host factors such as decreased drug metabolism, compromised immune defence systems, increased frequency of poor PS, increased frequency of hemorrhagic complications, and increased frequency of psychiatric medications. They also demonstrated that AML cells in elderly patients had more immature morphology and expressed higher levels of the multidrug resistance gene MDR1 than AML cells in young adult patients. Dombert et al[7] showed that the frequencies of myelodysplasia and unfavourable risk karyotype were higher in elderly AML patients than young adult AML patients. Our study showed similar results, with elderly patients having higher frequencies of poor PS, myelodysplasia, and unfavourable risk karyotype compared with young adult patients. These data suggest that our study results are a reliable reflection of the overall AML population.
The estimated 5-year OS rate of our non-APL patients was 21.1%, which is similar to the 5-year OS rates reported by retrospective population-based studies conducted in Sweden[9,26,27], the United Kingdom[16], and the United States[17,28,29], but lower than the 5-year OS rates reported by prospective clinical trials for young adult non-APL patients[10-13]. When the data of all AML patients are analysed in a population-based study, patients with poor PS, organ dysfunction, documented infection, and severe comorbidities are included; this lowers the overall long-term OS rates compared with the survival rates in prospective clinical trials of young adult AML patients without these conditions.
Juliusson et al[9] analysed and compared the CR rates of patient cohorts grouped by PS and found that the CR rate decreases as age increases, indicating that PS and age are independent adverse prognostic factors. Our data are consistent with these findings. However, they did not conduct multivariate analysis to determine adverse prognostic factors associated with OS in non-APL patients. Our multivariate analysis identified older age, poor PS (3-4), lack of induction chemotherapy, presence of antecedent haematological disease, and unfavourable risk karyotype as independent adverse prognostic factors for OS. In prospective clinical trials for young adult patients with non-APL, good PS and no organ dysfunction, the presence of antecedent hematological disease and unfavourable risk karyotype were found to be independent adverse prognostic factors for OS[10-12]. Our retrospective population-based study yielded similar results, indicating that the presence of antecedent hematological disease and unfavourable risk karyotype are adverse prognostic factors for OS in all non-APL patients. It has been postulated that the frequencies of myelodysplasia, poor PS, and unfavourable risk karyotype are higher in elderly non-APL patients than young adult non-APL patients, leading to poorer long-term survival in elderly patients[6-8]. As elderly patients and those with poor PS are excluded from prospective clinical trials, population-based studies are necessary to determine whether older age and poor PS are adverse prognostic factors for OS. As described above, a study in Sweden found that older age and poor PS independently affected prognosis in terms of the CR rate and early death rate, but these results were not obtained through statistical analyses[9]. It therefore could not be ruled out that factors such as myelodysplasia and unfavourable risk karyotype were related to older age and influenced the results. Our multivariate analysis shows for the first time that older age is an independent adverse prognostic factor for OS.
This study has several limitations: (1) it is a retrospective study; (2) the number of patients is small; (3) the study includes Japanese patients only; (4) analyses of the data regarding comorbidities and expression of MDR1 on AML cells at the start of treatment could not be performed; and (5) analyses of haemorrhagic and infectious complications and their severity could not be performed.
We propose the following three reasons why older age is an independent adverse prognostic factor for OS in non-APL patients, leading to poor prognosis: (1) epigenetic changes to genes affect the pharmacokinetics of anticancer drugs; (2) preclinical organ dysfunction may not be reflected in the findings of blood tests and functional investigations; and (3) other unknown factors.
It is necessary to perform further large-scale, prospective, population-based observational studies, which take the various parameters that change with age into consideration, in order to definitively determine the adverse prognostic factors associated with older age. Age would contribute a considerable life expectance to indicate induction chemotherapy with eligible dose of cytotoxic drugs for a favorable case even if advanced elderly.
The authors thank Marguerite Elgin of Edanz Group Japan for editorial assistance.
Elderly acute myeloid leukemia (AML) patients have higher frequencies of myelodysplasia, unfavorable risk karyotype, and poor performance status (PS), which are the leading causes of poor long-term prognosis, compared with young adult AML patients. Elderly patients and those with poor PS are often excluded from prospective clinical trials for AML therapies. In such cases, the results of clinical practice may differ from the results reported in a clinical trial. Therefore, it is necessary to include a full cohort of consecutive patients diagnosed with AML in order to elucidate the real-world outcome for patients suffering from AML.
In the field of medical oncology, it remains controversial whether age itself is an independent prognostic factor for prognosis. Generally it is axiomatic that older patients are more likely to have a poorer PS and have more underlying diseases, both of which result in morbid prognosis. Thus, the age and coexistence of related prognostic factors can be confounding. However, biological aging is not always associated with cognitive dysfunctions, and recent studies in geriatric oncology have aggregated sufficient evidence that geriatric assessment for chemotherapy is independent from age.
These multivariate analysis shows for the first time that older age is an independent adverse prognostic factor for overall survival (OS). This was validated in several chromosomal risk categories, i.e., NCCN, SWOG, and CALGB. Though this cohort was limited to a local community in Japan, our results are expected to change the realistic planning of practical treatments for very elderly AML patients, who are not usually assessed in clinical trials.
The clinical outcome of patients over age 65 with AML is poor. However, intensive chemotherapy is a significant predictor for longer survival not only in patients younger than 75, but also in patients over age 75 with PS 0-2. Based on these results, patients with AML over 75 years of age could be candidates for intensive or less-intensive induction chemotherapy to obtain a better remission rate and further survival.
The population-based study is an observational study for longitudinally registered patients without any medical interventions. The population-based cohort is set up to investigate whole populations in order to avoid intentional bias. It is crucial that the cohort be representative of a defined population. The population-based study offers three advantages: (1) it can illustrate the distributions, prevalence, and treatment outcome of the disease; (2) it can assess the risk factors for disease in a realistic manner; and (3) it can carry out unbiased evaluations of relations including confounders. Therefore, the authors believe that the present population-based study has reached a robust conclusion about whether advanced age and poor PS are adverse prognostic factors for OS.
Ohnishi H et al reported for the first time that older age is an independent adverse prognostic factor for overall survival in AML patients through a population-based study cohorting 213 adult AML patients, by using multivariate analysis. Overall, This is a well-written and cafefully discussed paper.
P- Reviewer: Chen SS, Nosaka T, Yokota T S- Editor: Song XX L- Editor: A E- Editor: Lu YJ
| 1. | Yanada M, Naoe T. Acute myeloid leukemia in older adults. Int J Hematol. 2012;96:186-193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 54] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 2. | Appelbaum FR, Gundacker H, Head DR, Slovak ML, Willman CL, Godwin JE, Anderson JE, Petersdorf SH. Age and acute myeloid leukemia. Blood. 2006;107:3481-3485. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 905] [Cited by in RCA: 1032] [Article Influence: 54.3] [Reference Citation Analysis (0)] |
| 3. | Hiddemann W, Kern W, Schoch C, Fonatsch C, Heinecke A, Wörmann B, Büchner T. Management of acute myeloid leukemia in elderly patients. J Clin Oncol. 1999;17:3569-3576. [PubMed] |
| 4. | Löwenberg B, Downing JR, Burnett A. Acute myeloid leukemia. N Engl J Med. 1999;341:1051-1062. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1079] [Cited by in RCA: 1085] [Article Influence: 41.7] [Reference Citation Analysis (0)] |
| 5. | Grimwade D, Walker H, Harrison G, Oliver F, Chatters S, Harrison CJ, Wheatley K, Burnett AK, Goldstone AH; Medical Research Council Adult Leukemia Working Party. The predictive value of hierarchical cytogenetic classification in older adults with acute myeloid leukemia (AML): analysis of 1065 patients entered into the United Kingdom Medical Research Council AML11 trial. Blood. 2001;98:1312-1320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 693] [Cited by in RCA: 699] [Article Influence: 29.1] [Reference Citation Analysis (0)] |
| 6. | Estey E. Acute myeloid leukemia and myelodysplastic syndromes in older patients. J Clin Oncol. 2007;25:1908-1915. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 183] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 7. | Dombret H, Raffoux E, Gardin C. Acute myeloid leukemia in the elderly. Semin Oncol. 2008;35:430-438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 52] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 8. | Pollyea DA, Kohrt HE, Medeiros BC. Acute myeloid leukaemia in the elderly: a review. Br J Haematol. 2011;152:524-542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 124] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 9. | Juliusson G, Antunovic P, Derolf A, Lehmann S, Möllgård L, Stockelberg D, Tidefelt U, Wahlin A, Höglund M. Age and acute myeloid leukemia: real world data on decision to treat and outcomes from the Swedish Acute Leukemia Registry. Blood. 2009;113:4179-4187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 640] [Cited by in RCA: 737] [Article Influence: 43.4] [Reference Citation Analysis (0)] |
| 10. | Ohtake S, Miyawaki S, Fujita H, Kiyoi H, Shinagawa K, Usui N, Okumura H, Miyamura K, Nakaseko C, Miyazaki Y. Randomized study of induction therapy comparing standard-dose idarubicin with high-dose daunorubicin in adult patients with previously untreated acute myeloid leukemia: the JALSG AML201 Study. Blood. 2011;117:2358-2365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 191] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 11. | Miyawaki S, Ohtake S, Fujisawa S, Kiyoi H, Shinagawa K, Usui N, Sakura T, Miyamura K, Nakaseko C, Miyazaki Y. A randomized comparison of 4 courses of standard-dose multiagent chemotherapy versus 3 courses of high-dose cytarabine alone in postremission therapy for acute myeloid leukemia in adults: the JALSG AML201 Study. Blood. 2011;117:2366-2372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 138] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 12. | Berman E, Heller G, Santorsa J, McKenzie S, Gee T, Kempin S, Gulati S, Andreeff M, Kolitz J, Gabrilove J. Results of a randomized trial comparing idarubicin and cytosine arabinoside with daunorubicin and cytosine arabinoside in adult patients with newly diagnosed acute myelogenous leukemia. Blood. 1991;77:1666-1674. [PubMed] |
| 13. | Berman E, Wiernik P, Vogler R, Vélez-Gárcia E, Bartolucci A, Whaley FS. Long-term follow-up of three randomized trials comparing idarubicin and daunorubicin as induction therapies for patients with untreated acute myeloid leukemia. Cancer. 1997;80:2181-5. [DOI] [Full Text] |
| 14. | Mauritzson N, Johansson B, Albin M, Billström R, Ahlgren T, Mikoczy Z, Nilsson PG, Hagmar L, Mitelman F. A single-center population-based consecutive series of 1500 cytogenetically investigated adult hematological malignancies: karyotypic features in relation to morphology, age and gender. Eur J Haematol. 1999;62:95-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 45] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 15. | Taylor PR, Reid MM, Stark AN, Bown N, Hamilton PJ, Proctor SJ. De novo acute myeloid leukaemia in patients over 55-years-old: a population-based study of incidence, treatment and outcome. Northern Region Haematology Group. Leukemia. 1995;9:231-237. [PubMed] |
| 16. | Bhayat F, Das-Gupta E, Smith C, McKeever T, Hubbard R. The incidence of and mortality from leukaemias in the UK: a general population-based study. BMC Cancer. 2009;9:252. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 56] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 17. | Dores GM, Devesa SS, Curtis RE, Linet MS, Morton LM. Acute leukemia incidence and patient survival among children and adults in the United States, 2001-2007. Blood. 2012;119:34-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 379] [Cited by in RCA: 466] [Article Influence: 33.3] [Reference Citation Analysis (0)] |
| 18. | Phekoo KJ, Richards MA, Møller H, Schey SA; South Thames Haematology Specialist Committee. The incidence and outcome of myeloid malignancies in 2,112 adult patients in southeast England. Haematologica. 2006;91:1400-1404. [PubMed] |
| 19. | Pulte D, Gondos A, Brenner H. Improvements in survival of adults diagnosed with acute myeloblastic leukemia in the early 21st century. Haematologica. 2008;93:594-600. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 94] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 20. | Pulte D; NCCN. Guideline. Available from: http: //www.nccn.org/professionals/physician_gls/f_guidelines.asp. |
| 21. | Slovak ML, Kopecky KJ, Cassileth PA, Harrington DH, Theil KS, Mohamed A, Paietta E, Willman CL, Head DR, Rowe JM. Karyotypic analysis predicts outcome of preremission and postremission therapy in adult acute myeloid leukemia: a Southwest Oncology Group/Eastern Cooperative Oncology Group Study. Blood. 2000;96:4075-4083. [PubMed] |
| 22. | Byrd JC, Mrózek K, Dodge RK, Carroll AJ, Edwards CG, Arthur DC, Pettenati MJ, Patil SR, Rao KW, Watson MS. Pretreatment cytogenetic abnormalities are predictive of induction success, cumulative incidence of relapse, and overall survival in adult patients with de novo acute myeloid leukemia: results from Cancer and Leukemia Group B (CALGB 8461). Blood. 2002;100:4325-4336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1199] [Cited by in RCA: 1210] [Article Influence: 52.6] [Reference Citation Analysis (0)] |
| 23. | Nakase K, Bradstock K, Sartor M, Gottlieb D, Byth K, Kita K, Shiku H, Kamada N. Geographic heterogeneity of cellular characteristics of acute myeloid leukemia: a comparative study of Australian and Japanese adult cases. Leukemia. 2000;14:163-168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 44] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 24. | Scholl S, Theuer C, Scheble V, Kunert C, Heller A, Mügge LO, Fricke HJ, Höffken K, Wedding U. Clinical impact of nucleophosmin mutations and Flt3 internal tandem duplications in patients older than 60 yr with acute myeloid leukaemia. Eur J Haematol. 2008;80:208-215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 55] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 25. | Whitman SP, Maharry K, Radmacher MD, Becker H, Mrózek K, Margeson D, Holland KB, Wu YZ, Schwind S, Metzeler KH. FLT3 internal tandem duplication associates with adverse outcome and gene- and microRNA-expression signatures in patients 60 years of age or older with primary cytogenetically normal acute myeloid leukemia: a Cancer and Leukemia Group B study. Blood. 2010;116:3622-3626. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 173] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 26. | Juliusson G, Billström R, Gruber A, Hellström-Lindberg E, Höglunds M, Karlsson K, Stockelberg D, Wahlin A, Aström M, Arnesson C. Attitude towards remission induction for elderly patients with acute myeloid leukemia influences survival. Leukemia. 2006;20:42-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 68] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 27. | Palk K, Luik E, Varik M, Viigimaa I, Vaht K, Everaus H, Wennström L, Stockelberg D, Safai-Kutti S, Holmberg E. The incidence and survival of acute de novo leukemias in Estonia and in a well-defined region of western Sweden during 1997-2001: a survey of patients aged & gt; or=65 years. Cancer Epidemiol. 2010;34:24-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 28. | McClune BL, Weisdorf DJ, Pedersen TL, Tunes da Silva G, Tallman MS, Sierra J, Dipersio J, Keating A, Gale RP, George B. Effect of age on outcome of reduced-intensity hematopoietic cell transplantation for older patients with acute myeloid leukemia in first complete remission or with myelodysplastic syndrome. J Clin Oncol. 2010;28:1878-1887. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 361] [Cited by in RCA: 399] [Article Influence: 26.6] [Reference Citation Analysis (0)] |
| 29. | Oran B, Weisdorf DJ. Survival for older patients with acute myeloid leukemia: a population-based study. Haematologica. 2012;97:1916-1924. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 273] [Cited by in RCA: 332] [Article Influence: 25.5] [Reference Citation Analysis (0)] |