Published online Feb 18, 2017. doi: 10.5312/wjo.v8.i2.149
Peer-review started: September 19, 2016
First decision: October 21, 2016
Revised: November 18, 2016
Accepted: December 7, 2016
Article in press: December 9, 2016
Published online: February 18, 2017
To determine the role of cartilage oligomeric matrix protein (COMP), interleukin (IL)-6, IL-10 and ratio of IL-6/IL-10 as risk factors of symptomatic lumbar osteoarthritis (OA) in postmenopausal women with estrogen deficiency.
Case-control study had been conducted in Sanglah General Hospital from October 2015 until March 2016. The blood samples were obtained and analyzed by enzyme-linked immunosorbent assay (ELISA).
From 44 pairs of samples which divided into 44 samples as case group and 44 samples as control group showed that high level of COMP in estrogen deficiency postmenopausal women were not at risk (OR = 0.7; 95%CI: 0.261-1.751; P = 0.393) for symptomatic lumbar OA (cut-off point 0.946). Estrogen deficiency in postmenopausal women with the high level of IL-6 had 2.7 times risk (OR = 2.7; 95%CI: 0.991-8.320; P = 0.033) for symptomatic lumbar OA from the low level of IL-6 (cut-off point 2.264). At lower level of IL-10, there was no risk for symptomatic lumbar OA (OR = 0.6; 95%CI: 0.209-1.798; P = 0.345) than with the higher level of IL-10 (cut-off point 6.049). While the high ratio of IL-6/IL-10 level in estrogen deficiency postmenopausal women gave 3.4 times risk (OR = 3.4; 95%CI: 1.204-11.787; P = 0.011) for symptomatic lumbar OA than the low ratio of IL-6/IL-10 level (cut-off point 0.364).
High ratio of IL-6/IL-10 plasma level was the highest risk factor for causing symptomatic lumbar OA in postmenopausal women with estrogen deficiency.
Core tip: High levels of cartilage oligomeric matrix protein in estrogen deficiency postmenopausal women were not at risk for symptomatic lumbar osteoarthritis (OA). Estrogen-deficient postmenopausal women with the high levels of interleukin (IL)-6 had higher risk for symptomatic lumbar OA from the low level of IL-6. At lower levels of IL-10, there was no risk for symptomatic lumbar OA than with the higher levels of IL-10. High ratio of IL-6/IL-10 levels in estrogen deficiency postmenopausal women produced higher risk for symptomatic lumbar OA.
- Citation: Suyasa IK, Kawiyana IKS, Bakta IM, Widiana IGR. Interleukin-6 and ratio of plasma interleukin-6/interleukin-10 as risk factors of symptomatic lumbar osteoarthritis. World J Orthop 2017; 8(2): 149-155
- URL: https://www.wjgnet.com/2218-5836/full/v8/i2/149.htm
- DOI: https://dx.doi.org/10.5312/wjo.v8.i2.149
Low back pain is a common symptom in elderly due to spine degeneration process which is termed as osteoarthritis (OA) of the lumbar. The prevalence of OA both in men and women at 50 years are alike, while prevalence in over 50 years are increasing in women. However, the etiology remains unknown. Numerous factors are thought to be the cause of low back pain, such as estrogen changes that often occur in older women at post-menopause[1].
Low back pain in post-menopausal women is a clinical manifestation of degeneration process over the most mobile spine main areas/segments. Lumbar OA is the degeneration of cartilage which involves three joints complex that are characterized by narrowing of the lumbar intervertebral disc, vertebral osteophytes formation and occurrence of OA in the facet joints[2,3]. These pathological processes can be resulted from mechanical stress loads due to weight gain and aging that will lead to cartilage thinning, as well as inflammatory process.
Inflammatory process that occurs in the lumbar OA is a chronic inflammatory process which involve the role of cytokines, either proinflammatory cytokines such as interleukin (IL)-6, or antiinflammatory cytokines such as IL-1ra or IL-10. Production of IL-6 by human chondrocytes are also affected by estradiol, suggesting the possibility of a mechanism that affect the metabolism of cartilage[4]. Increased IL-6 will facilitate degeneration process and stimulates the formation of osteoclast precursors of granulocyte macrophage colony-forming units and increase the number of osteoclasts in vivo which leads to increase bone resorption, contributes to the change in spondiloarthrosis[5]. IL-6 are also produced by fat cells. Inhibitors of IL-6 (including estrogen) are used for the treatment of osteoporosis in post-menopausal women[6].
IL-6 also plays an important role in bone metabolism via induction of osteoclastogenesis and stimulates osteoclast activity[7]. IL-6 increases the formation of osteoclasts, especially when estrogen levels decline[8]. IL-6 stimulates formation of osteoclast precursors of granulocyte macrophage colony-forming units and increases the number of osteoclasts in vivo, leading to increased bone resorption, which contributes to spondiloarthrosis and degeneration of intervertebral discs[5]. Increasing amount of IL-6 in patients with aging and menopause are suspected that IL-6 is one of cytokine which plays an important role in the process of bone resorption, by affecting activity of osteoclasts, including the subkondral bone, followed by destruction of cartilage[5].
IL-10 is formerly known as cytokine synthesis inhibitory factor, plays an important role as an anti-inflammatory and immunosuppressive cytokines. IL-10 is produced from regulatory of T cells, also produced by a large number of other cells including macrophages[9]. IL-10 is very effective when suppressing macrophages to release tumor necrosis factor (TNF)-α[5].
Degradation of cartilage resulting in increasing level of cartilage oligomeric matrix protein (COMP) in synovial fluid and serum. The products of cartilage degradation will be fagocyted by the synovium and stimulate the inflammatory process. Synovium cells are activated and produce various catabolic, proinflammatory mediators and proteolytic enzymes which will cause cartilage damage[10]. Increased of COMP level indicates an increase in cartilage damage, as well as the IL-6 increasing the number of osteoclasts which leads to increased bone resorption including subchondral bone[11]. Estrogen deficiency will affect the metabolism of the chondrocytes.
In post-menopausal women with estrogen deficiency prone to have cartilage damage. It is supported by the study of the OA prevalence in post-menopausal women with and without hormone replacement therapy (HRT) showed strong evidence of the benefits of estrogen on OA. Identification over two estrogen receptors: ERα and ERβ proves that the cartilage chondrocytes sensitive to estrogen[1]. Several in vivo and in vitro studies showed that chondrocytes respond to estrogen and its mechanisms that affect the metabolism of the chondrocytes[3,12].
To date, it is still unclear whether high levels of COMP and IL-6 and low level of IL-10 in postmenopausal women with estrogen deficiency could be determined as risk factors for symptomatic lumbar OA. In this study, the authors aimed to prove that the COMP, IL-6 and IL-10 are risk factors for symptomatic lumbar OA in postmenopausal women with estrogen deficiency. By determining the role of COMP, IL-6 and IL-10 as risk factors for the occurrence of symptomatic lumbar OA in postmenopausal women estrogen deficiency, it is expected that early prediction, prevention and management can be recognized in the future.
The study was conducted from October 2015 until March 2016 at Sanglah General Hospital, Denpasar, Bali. The aim of this study was to determine the role of COMP, IL-6, IL-10 and IL-6 to IL-10 ratio as risk factors of lumbar symptomatic OA in postmenopausal women with estrogen deficiency.
This was a case control study with consecutive sampling method. It started with identification for group of cases which was defined as postmenopausal women with estrogen deficiency and symptomatic lumbar OA. The pair of the cases was taken with control group, which was defined as postmenopausal women with estrogen deficiency and asymptomatic lumbar OA. The independent variables were measured retrospectively. The analysis continued with comparison of exposure probability to risk factors. The case group later compared with the control group to describe baseline characteristics and analyzed using two by two table to obtain odds ratio (OR). Odds ratio described as the risk effects, which result from exposure to the risk factors.
Forty four post-menopausal women from the population were identified and defined for both cases and controls and matched by age and body mass index. The blood sampling was performed to measure serum COMP levels and plasma cytokine levels consisting of IL-6 and IL-10 using ELISA. The obtained data were analyzed for normality and the characteristics of cases and controls equality were analyzed by comparing the mean length of menopause, age, BMI and estrogen levels. Analysis of risk factors for symptomatic lumbar OA performed with bivariate analysis (McNemar’s Chi Square). The risk estimation was calculated with OR.
Subjects in this study were aged 57 years old in average, with BMI of 25.8 kg/m2 and length of menopause about 6 years with the levels of estrogen approximately 15 pg/mL (Table 1).
| Characteristic | Cases (n = 44) median (interquartile range) | Controls (n = 44) median (interquartile range) |
| Age (yr) | 58 (54-61) | 58 (53-60) |
| Length of menopause (yr) | 7 (4-10) | 8 (3-10) |
| Blood estrogen levels (pg/mL) | 12.7 (9.00-20.87) | 14.16 (9.51-19.23) |
| Body mass indexes (kg/m2) | 25.92 (23.27-28.06) | 25.28 (22.86-27.37) |
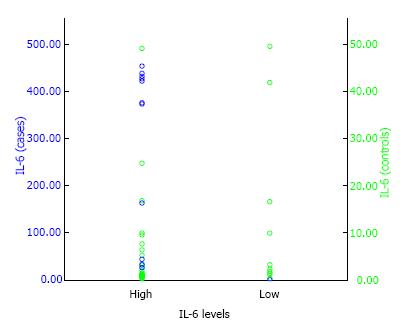
By using the serum COMP levels of 0.946 as the cut-off point, the OR between symptomatic lumbar OA in postmenopausal women with estrogen deficiency and asymptomatic lumbar OA was 0.7 (95%CI: 0.261 to 1.751), and statistically not significant with P value of < 0.05 (P = 0.393) (Table 2). This suggests that postmenopausal women with estrogen deficiency and high levels of serum COMP is not a risk factor for symptomatic lumbar OA. Whereas, by using the plasma IL-6 levels of 2.264 as the cut-off point, the OR between symptomatic lumbar OA and asymptomatic lumbar OA in postmenopausal women with estrogen deficiency was 2.7 (95%CI: 0.991 to 8.320) with P < 0.05 (P = 0.033) (Table 2 and Figure 1). This suggest that postmenopausal women with estrogen deficiency who had high levels of plasma IL-6 were associated with increased risk for symptomatic lumbar OA with calculated risk 2.7 times than those having low levels of plasma IL-6.
| Variables | OR | Pa | 95%CI |
| All subjects (44 pairs) | |||
| COMP | 0.7 | 0.393 | 0.261-1.751 |
| IL-6 | 2.7 | 0.033 | 0.991-8.320 |
| IL-10 | 0.6 | 0.345 | 0.209-1.798 |
| Ratio IL-6/IL-10 | 3.4 | 0.011 | 1.204-11.787 |
The risk difference was statistically significant with P < 0.05. By using the plasma IL-10 levels of 6.049 as the cut-off point, the OR between symptomatic lumbar OA and asymptomatic lumbar OA in postmenopausal women with estrogen deficiency was 0.6 (95%CI: 0.209 to 1.798) with P > 0.05 (P = 0.345). This suggests that low levels of plasma IL-10 in postmenopausal women with estrogen deficiency was not a risk factor for symptomatic lumbar OA (Table 2).
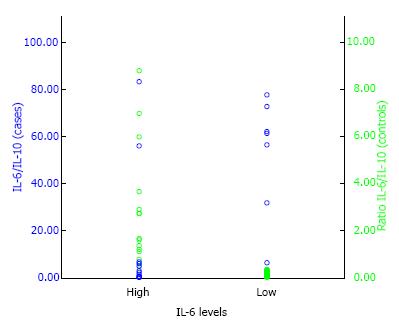
Using the ratio levels of plasma IL-6/IL-10 0.364 as the cut-off point, the OR between symptomatic lumbar OA in postmenopausal women with estrogen deficiency and asymptomatic lumbar OA was 3.4 (95%CI: 1.204 to 11.787) with P value of < 0.05 (P = 0.011) (Table 2 and Figure 2). This suggest that high ratio of plasma IL-6/IL-10 in postmenopausal women with estrogen deficiency had significantly higher risk for symptomatic lumbar OA with calculated risk 23.4 times than those having low ratio of plasma IL-6/IL-10.
Postmenopausal women who enrolled in the study were aged 58 years old in average, most of them had BMI category of overweight, length of menopause about 6 years and estrogen deficiency.
According to Richette et al[1] (2003), there is a relationship between estrogen decline and progression of OA, and it was found that its prevalence increases over age 50 years. Suyasa IK and Setiawan IGNY (2016) also found relationship of aging, BMI and estrogen deficiency with symptomatic lumbar OA[13]. The lumbar OA defined as a degeneration of cartilage which involves three joint complexes which characterized by narrowing of lumbar intervertebral disc, vertebral osteophytes formation and occurrence of OA in facet joints. These pathological conditions potentially caused by mechanical stress load due to aging, weight gain and hormonal changes that occur in joint cartilage thinning process[3].
Two estrogen receptors: ERα and ERβ, identified in chondrocytes and has proven sensitive to estrogen. In vivo animal studies showed that intra-articular injection of estrogen have a dose dependent; supraphysiological dose of 17β estradiol histologically induces OA histologically, on the other hand, it had no effect when given in low dose. In postmenopausal women, estrogen may decrease the speed of subchondral bone remodelling, which is a key factor in the pathophysiology of OA. Furthermore, estrogen receptor expression was shown in sinoviocytes, which are targets effect of estrogen on the joints[1,3,5].
In this study, the levels of serum COMP was not significantly with different risk for symptomatic lumbar OA with OR of 0.7 (95%CI: 0.261 to 1.751; P = 0.393). These results are in contrast to recent research by Goode et al[11] (2012) regarding the relationship between narrowing of the intervertebral discs, COMP and low back pain. Among patients with low back pain, strong correlations between COMP and narrowing of the intervertebral discs was found with OR of 1.82 (95%CI: 1:02 to 3:27). These finding reflects the degeneration of the intervertebral disc which characterized by narrowing of the intervertebral discs and any other symptoms associated with degeneration process[11].
COMP is a non-collagen protein which originated from cartilage extracellular matrix, act as a prognostic marker for OA. The levels of COMP can be used to predict the severity of damage in large joints, in addition, it can also predict the narrowing gap of joints[14]. As a biomarker, the concentration of COMP in synovial fluid or serum can be used as an indicator of early abnormalities[15,16], thus, COMP is very sensitive to detect early occurrence of premature OA in patients whom genetically suffering from OA[17].
OA is characterized by the destruction of cartilage and subchondral bone. Similarly, in lumbar OA which involved three joint complex, those pathological changes will occur in the cartilage of facet joint. Cartilage damage can be induced by mechanical factors, in which antigen release by the joint cartilage occurred. This situation will stimulate immune system, causing immunological reaction such as release of inflammatory mediators and proteases that are destructive and can aggravate the cartilage damage[11]. Cartilage damage characterized by the increase levels of COMP. As a diagnosis indicator, COMP correlated with disease severity. It is proved by detection of COMP levels 10 times higher in the synovial fluid of patients with OA.
Degradation of cartilage resulted in increased levels of COMP in synovial fluid and serum. These cartilage degradation products will be fagocyted by the synovium and stimulate inflammatory process. Synovium cells are activated and produce various catabolic and proinflammatory mediators and proteolytic enzymes which will lead to cartilage damage[10].
However, serum COMP levels can not be stated as a specific indicator that reflects the facet joint cartilage damage because serum COMP levels generally increased as a result of damage to the cartilage in the various joints of the body. This is in correspond with research of Söderlin et al[18] (2004), which stated that increase in serum COMP levels is a common finding in OA that indicate cartilage involvement. These are due to the narrow facet cartilage surface area and the serum COMP does not fully describe the extent of cartilage damage.
According to Neidhart et al[19] (1997), serum COMP levels constantly lower in the synovial fluid than cartilage matrix, so that taking samples from cartilage matrix might provide more accurate results. In addition, the concept of three joint complex in the lumbar OA involves only a small portion of cartilage in the facet joints, because 2/3 of load movement in one single functional unit is in the anterior unit[19].
The levels of serum COMP in this study gathered due to differences in OA stages of the subjects as well as differences in affinity or specificity of primary antibody used in the detection of COMP. Western blot analysis with the mAbs 12C4 showed higher affinity towards COMP fragments which had low molecular weight compared to mAbs 14G4[20]. According to Tseng et al[21] (2009), specificity of COMP against cartilage and specific reagent for degradation of COMP is still lacking. These conditions would limit its usefulness in determining the presence of OA as well as for the examination of dichotomous outcome in the population (normal vs abnormal)[21]. Another study by Lohmander et al[22] (1994) also stated that high concentration of COMP can be found only at the early stages of OA development and not at the advanced stage.
According to Mobasheri and Henrotin[23] (2011), COMP has the ability to mediate interaction between chondrocytes and cartilage extracellular matrix. COMP suppresses apoptosis in primary chondrocytes through activation of caspase-3 and induction of apoptosis inhibitor protein family (IAP) on survival proteins such as Baculoviral IAP Repeat Containing 3 and 5, X-linked IAP (BIRC3, BIRC5, XIAP). This is certainly in accordance with the study where investigated that COMP may be a protective factor against Symptomatic lumbar OA although not proven to be statistically significant.
In this study, IL-6 plasma levels were high (above median) in postmenopausal women with estrogen deficiency. This finding suggested that high levels of IL-6 plasma significantly act as a risk factor for symptomatic lumbar OA with OR of 2.7 (95%CI: 0.991 to 8.320; P = 0.033). This finding was in line with the study of Weber et al[24] (2016) and Valdes[25] (2010), that IL-6 was significantly higher in patients with low back pain (LBP), moreover, IL-6, BMI, duration of symptoms and age were significantly correlated with low back pain. IL-1, IL-6 and IL-10 that involved in the inflammatory process were also correlated with the risk of OA.
IL-6 is a cytokine that acts as an innate and acquired immunity, formed by many cells and affect multiple targets. The main sources of IL-6 are macrophages and lymphocytes in inflammatory area. IL-6 also plays an important role in enhancing the formation of osteoclasts, especially when estrogen levels are declining[8]. IL-6 stimulates formation of osteoclast precursors of granulocyte macrophage colony-forming units and increase the number of osteoclasts in vivo, leading to increased bone resorption, which contributed to spondiloarthrosis and degeneration of intervertebral discs[5]. While Ershler, Harman and Keller[7] (2002) found an increase in IL-6 on aging and menopausal women. The levels of IL-6 increased in older age[26] and significantly increased at the age of 70[27].
In OA, the production of IL-6 is mainly stimulated by increased catabolic cytokines that are IL-1 and TNF-α, and then IL-6 will potentiate the effect of IL-1. In the pathogenesis of OA, IL-6 has dual functions that are as a trigger of the inflammatory process and has the ability to down-regulate the factors involved catabolic role in the degeneration of cartilage[28]. In addition, IL-6 is also plays an important role in bone resorption including subchondral bone through the activity of osteoclasts.
The role of IL-6 plasma against symptomatic lumbar OA is through the activation of transducer glycoprotein 130 (gp130) on neurons due to the formation of complex IL-6/soluble IL-6R. Transducer glycoprotein 130 (gp130) is associated with sensitization taste buds of pain through activation of phosphoinositide 3-kinase, protein kinase C-delta and Janus kinase as well as the regulation of ion channel transient receptor potential cation channels vanilloid 1 (TRPV1)[29].
IL-6 is regarded as a key cytokine, which cause changes in the subchondral bone layer. The effect is largely based on the formation of osteoclasts resulting in bone resorption and also showed synergism with IL-1β and TNF[30].
In this study, the plasma levels of IL-10 were not proven to be significant as a risk factor for symptomatic lumbar OA with OR of 0.6 (95%CI: 0.209 to 1.798; P = 0.345). This is in contrast with the study by John et al[31] (2007) and Wang et al[32] (2001) which found that IL-10 not only inhibits the synthesis of inflammatory cytokines, but also protect the chondrocytes directly to antagonize the role of IL-1. Immunoregulatory cytokine IL-10 modulates a series of apoptosis in TNF-α such as caspase activity in human articular chondrocytes.
IL-10, known as cytokine synthesis inhibitory factor, is an anti-inflammatory and immunosuppressive cytokine, produced by T-regulatory cells and macrophages. IL-10 is a cytokine that is highly effective on suppressing the release of TNF-α by macrophages. There are two main functions of IL-10 such as inhibit the production of several cytokines (TNF, IL-1, and the chemokine IL-12) and inhibit the function of macrophages and dendritic cells in the activation of T-cell, generally it has an immunosuppressive effect. Supression on macrophage function occurs because IL-10 suppressing the expression of MHC class II molecules on macrophages, and reduce the expression of co-stimulatory (a.l. B7-1 and B7-2). The final impact of IL-10 activities is specific and non-specific supression of inflammatory reactions which mediated by T-cells. According to those, IL-10 known as cytokine synthesis inhibitory factor and anti-inflammatory cytokines[9].
IL-10 also able to show chondroprotective effect in the course of OA. Chondrocytes express the cytokines IL-10 and IL-10R receptor. It has been proven that IL-10 is involved in stimulating the synthesis of collagen type II and aggrecan. After administration of IL-10 in vitro, the healthy articular cartilage over the course of OA showed increased in proteoglycan synthesis and its percentage in the extracellular matrix. IL-10 inhibits apoptosis of chondrocytes, which might be a result of stimulation on IL-1β antagonist synthesis, IL-1Ra, tissue inhibitor of metalloproteinase-1 (TIMP-1) and growth factors. IL-10 reduces the effect of TNF-α in synovial fibroblasts in patients with OA[16].
In lumbar OA, IL-10 alone can not be used as a risk factor, because the inflammatory process is chronic involving via complex interaction by various cytokines, both pro-inflammatory cytokines such as TNF-α and IL-6, as well as cytokines anti-inflammatory such as IL-1ra or IL-10. Increased levels of TNF-α and IL-6 will be responded by anti-inflammatory cytokines. IL-10 is very powerful suppressor for TNF-αreleased by macrophages. The low levels of IL-10 can be used as an indicator of failure in the process of TNF-α and IL-6 suppression.
The role of IL-10 plasma in symptomatic lumbar OA is a protective factor although not clearly proved. To determine the role of IL-10 levels as a risk factor, it can be evaluated on the expression of IL-10 ratio against other potential cytokines such as ratio of IL-6/IL-10[33].
In this study, the ratio levels of plasma IL-6/IL-10 were high (above median) in postmenopausal women estrogen deficiency. This finding showed that high ratio levels of plasma IL-6/IL-10 significantly acts as a risk factor for symptomatic lumbar OA with OR of 3.4 (95%CI: 1.204 to 11.787; P = 0.011). The results of this study showed the concept of balance between proinflammatory cytokines and anti-inflammatory cytokines. Until now, data and research on the ratio of IL-6/IL-10 in symptomatic lumbar OA in postmenopausal women with estrogen deficiency does not exist. And the role of inflammatory and anti-inflammatory cytokines in the pathogenesis of OA on inter and intracellular signaling pathways are still under study[30].
Based on the explanation, the levels of IL-6 or IL-10 alone did not reflect the existence of symptomatic lumbar OA in postmenopausal women with estrogen deficiency. The ratio of both IL-6 and IL-10 is required to obtain more accurate result and reflect incidence of symptomatic lumbar OA in postmenopausal women with estrogen deficiency.
The role of the IL-6 to IL-10 ratio levels against symptomatic lumbar OA is through the interaction of pro-inflammatory cytokines with anti-inflammatory cytokines. Both of these cytokines potentially provide accurate information on the risk of symptomatic lumbar OA. IL-6 plasma has a function as a trigger of the inflammatory process and has the ability to down-regulation the catabolic factors that involved in cartilage degeneration, while IL-10 inhibits specific and non-specific inflammatory reactions as a response to the increase of cytokines TNF-α and IL-6.
From the data analysis that had been done in this study, it is concluded that the high ratio of IL-6/IL-10 plasma levels is the strongest risk factor of symptomatic lumbar OA in postmenopausal women with estrogen deficiency.
Plasma COMP can not be considered as a biomarker of symptomatic lumbar OA in postmenopausal women with estrogen deficiency.
Lumbar osteoarthritis (OA) is the degeneration of cartilage which involves three joint complex. Indeed, the inflammatory process, which is a chronic inflammatory process, influence this pathological process especially if alteration of estrogen levels occurs. Furthermore, degradation of cartilage lead to increased cartilage oligomeric matrix protein (COMP) levels. Either proinflammatory cytokines [interleukin (IL)-6] or anti-inflammatory cytokines (IL-1ra or IL-10) and COMP are thought to be relevant as risk factor for symptomatic lumbar OA in postmenopausal women with estrogen deficiency.
IL-6, IL-10, and COMP had been extensively studied in relation to inflammatory process and cartilage damage. Chronic inflammatory process occurs in individuals with lumbar OA and this inflammatory process related to estrogen levels. Thus, it is of interest whether IL-6, IL-10, and COMP are risk factors for symptomatic lumbar OA in postmenopausal women with estrogen deficiency.
The authors confirm that high ratio of IL-6/IL-10 plasma level was the highest risk factor for causing symptomatic lumbar OA in postmenopausal women with estrogen deficiency.
Determining ratio of IL-6/IL-10 could be used as risk factors for causing symptomatic lumbar OA in postmenopausal women with estrogen deficiency. It is expected that early prediction, prevention and management can be recognized.
COMP: Cartilage oligomeric matrix protein; IL-6: Interleukin-6; IL-10: Interleukin-10; OA: Osteoarthritis.
This is an interesting paper.
Manuscript source: Invited manuscript
Specialty type: Orthopedics
Country of origin: Indonesia
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Adams JD, Korovessis P S- Editor: Ji FF L- Editor: A E- Editor: Lu YJ
| 1. | Richette P, Corvol M, Bardin T. Estrogens, cartilage, and osteoarthritis. Joint Bone Spine. 2003;70:257-262. [PubMed] [Cited in This Article: ] |
| 2. | Fujiwara A, Lim TH, An HS, Tanaka N, Jeon CH, Andersson GB, Haughton VM. The effect of disc degeneration and facet joint osteoarthritis on the segmental flexibility of the lumbar spine. Spine (Phila Pa 1976). 2000;25:3036-3044. [PubMed] [Cited in This Article: ] |
| 3. | Sniekers YH, Weinans H, van Osch GJ, van Leeuwen JP. Oestrogen is important for maintenance of cartilage and subchondral bone in a murine model of knee osteoarthritis. Arthritis Res Ther. 2010;12:R182. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 61] [Cited by in F6Publishing: 65] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 4. | Wluka AE, Cicuttini FM, Spector TD. Menopause, oestrogens and arthritis. Maturitas. 2000;35:183-199. [PubMed] [Cited in This Article: ] |
| 5. | Holm S, Mackiewicz Z, Holm AK, Konttinen YT, Kouri VP, Indahl A, Salo J. Pro-inflammatory, pleiotropic, and anti-inflammatory TNF-alpha, IL-6, and IL-10 in experimental porcine intervertebral disk degeneration. Vet Pathol. 2009;46:1292-1300. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 29] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 6. | Bastard JP, Jardel C, Delattre J, Hainque B, Bruckert E, Oberlin F. Evidence for a link between adipose tissue interleukin-6 content and serum C-reactive protein concentrations in obese subjects. Circulation. 1999;99:2221-2222. [PubMed] [DOI] [Cited in This Article: ] |
| 7. | Keller ET, Wanagat J, Ershler WB. Molecular and cellular biology of interleukin-6 and its receptor. Front Biosci. 1996;1:d340-d357. [PubMed] [Cited in This Article: ] |
| 8. | Roitt I, Brostoff J, Male D. Cell-mediated immune reactions. 5th edition. London: Mosby 1998; 121-123. [Cited in This Article: ] |
| 9. | Kresno SB. Imunologi: diagnosis dan prosedur laboratorium. 4th edition. Jakarta: Balai Penerbit Fakultas Kedokteran Universitas Indonesia 2001; 63-68. [Cited in This Article: ] |
| 10. | Yuan GH, Masuko-Hongo K, Kato T, Nishioka K. Immunologic intervention in the pathogenesis of osteoarthritis. Arthritis Rheum. 2003;48:602-611. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 52] [Cited by in F6Publishing: 48] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 11. | Goode AP, Carey TS, Jordan JM. Low back pain and lumbar spine osteoarthritis: how are they related? Curr Rheumatol Rep. 2013;15:305. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 105] [Cited by in F6Publishing: 87] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 12. | Ushiyama T, Ueyama H, Inoue K, Ohkubo I, Hukuda S. Expression of genes for estrogen receptors alpha and beta in human articular chondrocytes. Osteoarthritis Cartilage. 1999;7:560-566. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 13. | Suyasa IK, Setiawan IGNY. The role of aging, body mass index and estrogen on symptomatic lumbar osteoarthritis in post-menopausal women. Int J Res Med Sci. 2016;4:1-4. [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 14. | Conrozier T, Saxne T, Fan CS, Mathieu P, Tron AM, Heinegård D, Vignon E. Serum concentrations of cartilage oligomeric matrix protein and bone sialoprotein in hip osteoarthritis: a one year prospective study. Ann Rheum Dis. 1998;57:527-532. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 69] [Cited by in F6Publishing: 77] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 15. | Sharif M, Kirwan JR, Elson CJ, Granell R, Clarke S. Suggestion of nonlinear or phasic progression of knee osteoarthritis based on measurements of serum cartilage oligomeric matrix protein levels over five years. Arthritis Rheum. 2004;50:2479-2488. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 101] [Cited by in F6Publishing: 113] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 16. | Dragomir AD, Kraus VB, Renner JB, Luta G, Clark A, Vilim V, Hochberg MC, Helmick CG, Jordan JM. Serum cartilage oligomeric matrix protein and clinical signs and symptoms of potential pre-radiographic hip and knee pathology. Osteoarthritis Cartilage. 2002;10:687-691. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 47] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 17. | Bleasel JF, Poole AR, Heinegård D, Saxne T, Holderbaum D, Ionescu M, Jones P, Moskowitz RW. Changes in serum cartilage marker levels indicate altered cartilage metabolism in families with the osteoarthritis-related type II collagen gene COL2A1 mutation. Arthritis Rheum. 1999;42:39-45. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 18. | Söderlin MK, Kastbom A, Kautiainen H, Leirisalo-Repo M, Strandberg G, Skogh T. Antibodies against cyclic citrullinated peptide (CCP) and levels of cartilage oligomeric matrix protein (COMP) in very early arthritis: relation to diagnosis and disease activity. Scand J Rheumatol. 2004;33:185-188. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 32] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 19. | Neidhart M, Hauser N, Paulsson M, DiCesare PE, Michel BA, Häuselmann HJ. Small fragments of cartilage oligomeric matrix protein in synovial fluid and serum as markers for cartilage degradation. Br J Rheumatol. 1997;36:1151-1160. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 181] [Cited by in F6Publishing: 195] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 20. | Arai K, Misumi K, Carter SD, Shinbara S, Fujiki M, Sakamoto H. Analysis of cartilage oligomeric matrix protein (COMP) degradation and synthesis in equine joint disease. Equine Vet J. 2005;37:31-36. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 41] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 21. | Tseng S, Reddi AH, Di Cesare PE. Cartilage Oligomeric Matrix Protein (COMP): A Biomarker of Arthritis. Biomark Insights. 2009;4:33-44. [PubMed] [Cited in This Article: ] |
| 22. | Lohmander LS, Saxne T, Heinegård DK. Release of cartilage oligomeric matrix protein (COMP) into joint fluid after knee injury and in osteoarthritis. Ann Rheum Dis. 1994;53:8-13. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 167] [Cited by in F6Publishing: 177] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 23. | Mobasheri A, Henrotin Y. Biomarkers of osteoarthritis: a review of recent research progresson soluble biochemical markers, published patents and areas for future development. Recent Patents on Biomarkers. 2011;1:25-43. [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 24. | Weber KT, Alipui DO, Sison CP, Bloom O, Quraishi S, Overby MC, Levine M, Chahine NO. Serum levels of the proinflammatory cytokine interleukin-6 vary based on diagnoses in individuals with lumbar intervertebral disc diseases. Arthritis Res Ther. 2016;18:3. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 63] [Cited by in F6Publishing: 74] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 25. | Valdes AM. Molecular pathogenesis and genetics of osteoarthritis: implications for personalized medicine. Personalized Medicine. 2010;7:49-63. [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 26. | Maggio M, Guralnik JM, Longo DL, Ferrucci L. Interleukin-6 in aging and chronic disease: a magnificent pathway. J Gerontol A Biol Sci Med Sci. 2006;61:575-584. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 484] [Cited by in F6Publishing: 461] [Article Influence: 25.6] [Reference Citation Analysis (0)] |
| 27. | Giuliani N, Sansoni P, Girasole G, Vescovini R, Passeri G, Passeri M, Pedrazzoni M. Serum interleukin-6, soluble interleukin-6 receptor and soluble gp130 exhibit different patterns of age- and menopause-related changes. Exp Gerontol. 2001;36:547-557. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 108] [Cited by in F6Publishing: 112] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 28. | Lotz M, Guerne PA. Interleukin-6 induces the synthesis of tissue inhibitor of metalloproteinases-1/erythroid potentiating activity (TIMP-1/EPA). J Biol Chem. 1991;266:2017-2020. [PubMed] [Cited in This Article: ] |
| 29. | Svensson CI. Interleukin-6: a local pain trigger? Arthritis Res Ther. 2010;12:145. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 23] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 30. | Wojdasiewicz P, Poniatowski LA, Szukiewicz D. The role of inflammatory and anti-inflammatory cytokines in the pathogenesis of osteoarthritis: review article. Mediators of Inflammation. USA: Hindawi Publishing Corporation 2014; 1-20. [DOI] [Cited in This Article: ] [Cited by in Crossref: 798] [Cited by in F6Publishing: 972] [Article Influence: 97.2] [Reference Citation Analysis (0)] |
| 31. | John T, Müller RD, Oberholzer A, Zreiqat H, Kohl B, Ertel W, Hostmann A, Tschoeke SK, Schulze-Tanzil G. Interleukin-10 modulates pro-apoptotic effects of TNF-alpha in human articular chondrocytes in vitro. Cytokine. 2007;40:226-234. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 67] [Cited by in F6Publishing: 64] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 32. | Wang Y, Lou S. Direct protective effect of interleukin-10 on articular chondrocytes in vitro. Chin Med J (Engl). 2001;114:723-725. [PubMed] [Cited in This Article: ] |
| 33. | Kaneda H, Waddell TK, de Perrot M, Bai XH, Gutierrez C, Arenovich T, Chaparro C, Liu M, Keshavjee S. Pre-implantation multiple cytokine mRNA expression analysis of donor lung grafts predicts survival after lung transplantation in humans. Am J Transplant. 2006;6:544-551. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 92] [Cited by in F6Publishing: 99] [Article Influence: 5.5] [Reference Citation Analysis (0)] |