Peer-review started: December 29, 2014
First decision: February 10, 2015
Revised: October 12, 2015
Accepted: November 3, 2015
Article in press: November 4, 2015
Published online: January 18, 2016
Processing time: 140 Days and 5.6 Hours
Cervical myelopathy is a well-described clinical syndrome that may evolve from a combination of etiological mechanisms. It is traditionally classified by cervical spinal cord and/or nerve root compression which varies in severity and number of levels involved. The vast array of clinical manifestations of cervical myelopathy cannot fully be explained by the simple concept that a narrowed spinal canal causes compression of the cord, local tissue ischemia, injury and neurological impairment. Despite advances in surgical technology and treatment innovations, there are limited neuro-protective treatments for cervical myelopathy, which reflects an incomplete understanding of the pathophysiological processes involved in this disease. The aim of this review is to provide a comprehensive overview of the key pathophysiological processes at play in the development of cervical myelopathy.
Core tip: The pathophysiology of cervical myelopathy involves a combination of mechanical static and dynamic factors, triggering a cascade of biomolecular changes to include ischemia, excitotoxicity, neuroinflammation and apoptosis. Development of targeted neuro-protective treatment strategies, specifically modulating these molecular pathways, may optimize neurological recovery following surgical decompression. The aim of this review is to provide an overview of the pathophysiological processes at play in the development of cervical myelopathy.
- Citation: Dolan RT, Butler JS, O’Byrne JM, Poynton AR. Mechanical and cellular processes driving cervical myelopathy. World J Orthop 2016; 7(1): 20-29
- URL: https://www.wjgnet.com/2218-5836/full/v7/i1/20.htm
- DOI: https://dx.doi.org/10.5312/wjo.v7.i1.20
Cervical myelopathy is the most commonly reported spinal cord pathology globally in the > 55 years age cohort[1-3]. Twenty-five percent of spinal cord dysfunction in the Unitd Kingdom is caused by cervical spondylotic myelopathy[4]. The exact biostatistics in relation to the disease remains unknown, however there exists a male preponderance at a ratio of 2.7:1[5]. Originally detailed by Stookey[6] in 1928, compression of the cord by cartilaginous degenerative disc nodules was the primary pathogenic mechanism attributed to this disorder. The key neuropathological characteristics of cervical myelopathy include cystic cavitation, gliosis, Wallerian degeneration of descending and ascending tracts, and loss of anterior horn cells[7,8].
Clinically, cervical myelopathy presents as progressive spinal cord impairment. Symptoms include distorted proprioception, weakness and paresthesia of the hands, spasticity of the lower limbs with resultant gait disturbance, and pyramidal and posterior cord dysfunction[9]. In addition to motor and sensory sequelae, neuropathic pain and functional limitations can be devastating, resulting in significant physical and socioeconomically restrictions for previously healthy individuals. Thus, there exists an urgent requirement to clearly define the pathobiology of the cervical myelopathy to assist discovery of translational interventional strategies.
Researchers have previously postulated that a narrowed spondylotic spinal canal causes compression of the enclosed cord potentially causing local neural ischemia and neurological impairment. The aetiology of cervical cord compression is multifactorial with contributions from disc herniation alone; and osteophytic spur overgrowth in the spinal canal referred to as spondylosis. A decrease in vertebral canal diameter as a consequence of age-related degenerative changes of the joints, intervertebral discs, and ligaments of the cervical vertebrae, significantly contributing to cord compression[1]. Infolding of the ligamentum flavum and facet joint capsule can distort spinal canal anatomy and foraminal dimensions[10]. However, this simple anatomic model has been challenged by falling short of explaining the array of clinical presentations in cervical myelopathy, specifically development of neurological impairment in the absence of static spinal cord compression[11]. Whilst the aetiology of cervical myelopathy is thought to be multifactorial including contributions from age-related degeneration, mechanical stress and biochemical factors, a genetic predisposition has been revisited, due to recent evidence of familial clustering in population studies[12].
Despite advances in the surgical management of cervical myelopathy in addition to earlier diagnosis facilitated by advances in diffusion tensor magnetic resonance imaging (MRI) and kinetic MRI, a significant proportion of patients suffer residual neurological sequele as a consequence of irreversible cord injury[13-17]. Thus, implementation of neuro-protective interventions as an adjunct to surgical decompression may optimise patient outcomes for cervical myelopathy.
In the context of progressive age-related degenerative changes, clinically significant cervical spondylotic myelopathy typically presents in late adulthood. These changes include cervical disc degeneration, osteophytic spur formation and transverse bar formation and osteoarthritic facet hypertrophy. Age-related degenerative changes with respect to supporting ligaments include posterior longitudinal ligament calcification and ligamentum flavum thickening[18-22]. The concept of dynamic stenosis represents progressive impingement on the spinal canal, resulting in transient spinal cord compression during physiological cervical range of motion. However, in some cases, dynamic stenosis may evolve into static compression of the spinal cord, manifesting clinically as classic cervical spondylotic myelopathy.
Although the specific pathophysiological mechanisms contributing to cervical myelopathy remain ambiguous, it is considered a manifestation of long-tract signs resulting from multifactorial compression on the cervical spinal cord[10]. Key factors in the development of cervical myelopathy are categorized as either static or dynamic mechanical factors, resulting in direct injury to neurons and glia, which in turn triggers a cascade of secondary cellular mechanisms (Table 1)[1,23].
| Static factors |
| Spondylosis |
| Disc degeneration |
| Ossification of the posterior longitudinal ligament |
| Ossification of ligamentum flavum |
| Congenital stenosis |
| Other acquired congenital pathology, e.g., tumors, calcification |
| Dynamic factors |
| Changes in neck flexion/extension - narrow spinal canal |
| Biomolecular factors |
| Ischemic injury - compression of spinal cord vasculature |
| Glutamate - mediated excitotoxicity |
| Oligodendrocyte and neuronal apoptosis |
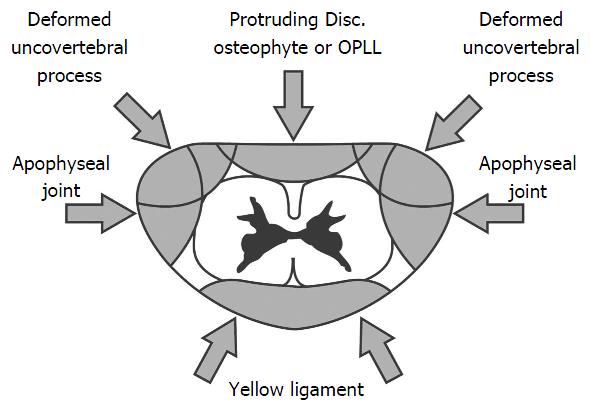
Spondylosis and disc degeneration: Progressive cervical spondylotic changes are a key feature in the pathogenesis of cervical myelopathy due to an increase in the compressive extrinsic canal forces (Figure 1). With advancing age, the intervertebral discs cannot bear load due to a combination of factors to include medial splitting of the disc and gradual loss of the nucleus pulposus. It is this disc degeneration that stimulates a cascade of progressive changes resulting in cervical canal stenosis and the development of cervical spondylotic myelopathy[24]. Anterolateral uncovertebral process flattening increases the load imposed on vertebral articular cartilage. This promotes the development of osteophytic spurs. A combination of osteophytic spur overgrowth ventrally and buckling of the ligamentum flavum posteriorly, can lead to a circumferential mechanical compression of the spinal cord inducing cervical myelopathy[25]. Additionally, the conformational change in bony structures can compromise the integrity of the vertebral artery and spinal nerve leading to demyelination of ascending and descending tracts and chronic pain[1]. Radiological assessment is key, as it assists differentiation of disk-related neck pain, radiculopathy, and myelopathy. Imaging, in the context of pre-operative planning, also aids localization of the site-specific disease[26]. Compared with other radiological studies MRI provides an overview of both bony and soft tissue architecture including intervertebral discs, supporting ligaments, and neural structures. Dynamic weight bearing (kinetic) MRI has been hailed as the gold standard modality for cervical spondylotic myelopathy[15,27]. Myelopathy is represented radiographically with increased cord signal on T2-weighted MRI and a decreased signal on T1-weighted imaging[28]. Diffusion tensor imaging (DTI) improves pathologic specificity by measuring directional diffusivities, which quantify water diffusion parallel and perpendicular to the white matter tracts[16,29]. A recent study of the role of DTI in cervical spondylotic myelopathy suggested that DTI may elucidate pathology of the spinal cord before the development of T2 hyperintensity imaging and thus may be a superior imaging modality in the future[13].
Ectopic ossification of spinal ligaments: Ectopic ossification and calcification of spinal ligaments has also been attributed to the development of spinal canal stenosis and the onset and aggravation of myelopathic symptoms[1,30]. Ossification of the posterior longitudinal ligament (OPLL) is a common pathology[20,31]. Pathological compression by OPLL commonly presents with severe myelopathy and can lead to quadraparesis[32]. The natural course of OPLL suggests a degenerative process as a consequence of environmental factors such as accumulative extrinsic loading on the spine and genetic predisposition. In vivo studies involving the Yoshimura mouse, which develops ossification of the posterior ligaments akin to human OPLL, has identified a mutation within the nucleotide pyrophosphatase (NPPS) gene[31]. Defective NPPS results in reduced production of pyrophosphatase, permiting ectopic ossification of spinal ligaments. Recent genetic studies involving the human NPPS locus have identified female sex, age (< 35 years) and severe ossification (> 10 ossified vertebra) correlated with susceptibility to and severity of OPLL[33]. Further studies involving the Zucker fatty rat have propose a role of a missense mutation in the leptin receptor gene in the promotion of ectopic ossification[34]. Thus, it is credible that NPPS and leptin receptor genes function in synergy in the pathogenesis of ectopic ossification and myelopathy. In light of these novel findings, there is a requirement to investigate this association in human OPLL.
It has been postulated that stenosis and subsequent cord compression may occur as a consequence of ossification of ligamentum flavum (OLF)[21]. The major diagnostic difference is grossly anatomical. In OLF, calcifications allow ligamentum flavum to fuse with adjacent lamina, whereas in OPLL the ligament adheres to the posterior aspects of the vertebral bodies and intervertebral discs. Despite this anatomical disparity, studies report the role of bone morphogenic protein-2 and transforming growth factor-β in matrix hyperplasia and ossification in both OPLL and OLF[35].
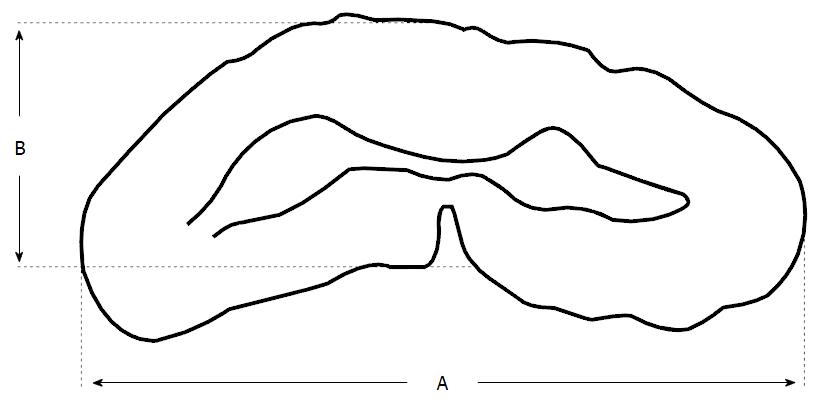
Congenital spinal canal stenosis: It is reported that cervical stenosis has an incidence of approximately 4.9% of the adult population[36]. Cervical stenosis is influenced by two factors: (1) degenerative cervical spondylosis; and (2) a developmentally narrow canal. Recent cadaveric studies have revealed that females patients and individuals over 60 years old, have narrower canals[37]. The presence of congenital or developmental canal stenosis is highly predictive of later myelopathic cord dysfunction[1]. The normal anteroposterior dimenions of the subaxial canal has been reported as 17-18 mm between C3 and C7[38,39]. Numerous MRI studies have concluded that a congenital sagittal spinal canal diameter of < 13 mm is predictive of development of cervical spondylotic myelopathy (CSM)[18,40]. The shape and cross-sectional area of the cord are important independent predictors of the development of CSM and specifically, of neurologic deficit. A transverse area of cord < 60 mm2 and a banana-shaped cord have both been correlated with the clinical presentation of myelopathy[41,42]. The anteroposterior compression ratio (AP) is calculated by dividing the anterior-posterior diameter of the cord by the transverse diameter of the cord (Figure 2)[43]. It has been suggested that an AP ratio < 40% is representative of substantial flattening of the cord and strongly predictive of significant neurologic dysfunction[43]. MRI has been reported as the most accurate method to quantify spinal canal diameter as it assesses both the bony and soft tissue components when estimating spinal canal diameter. This is particularly relevant in the context of CSM whereby central stenosis is often multifactorial to include both bony and soft tissue pathology, e.g., osteophytic spurring, OPLL and posterior disc protrusion[44,45]. Spinal MRI for assessment of CSM should include imaging sets obtained in the axial and saggital planes using a combinations of T1-weighted, and T2-weighted sequences. Fast spin-echo MRI is the best modality to diagnose disc fragments and osteophytes[46].
Dynamic canal space narrowing: It is intuitive that the extent of dynamic mechanical compression of the spinal cord could be significantly manipulated by movement of the cervical spine. During extremes of flexion and extension, a dynamic canal space of < 11 mm has been reported as a critical level for spinal functioning and is strongly predictive of cervical myelopathy[38,47]. Hyperextension of the neck causes canal narrowing by inducing buckling the ligamentum flavum and shingling the laminae. This “pincer effect” induces spinal cord compression between the inferior surface of one vertebra and the lamina or ligamentum flavum of the adjacent vertebra[23,47]. In flexion, the spinal cord lengthens and takes a more anterior position in the canal. If the cord impinges against a disc or vertebral body anteriorly, this will induce a higher intrinsic pressure, resulting in increased axial tension and potential ischemic injury[19].
Effects of stretch and shear: Studies suggest that stretch-associated injury is a major contributor to myelopathy. This claim is supported by evidence in numerous experimental models to include neural injury, tethered cord syndrome, and diffuse axonal injury[11,48,49]. During flexion/extension movements of the spine, elongation of the canal results in longitudinal strain of the spinal cord[50]. This is consistent with Euler’s Theorem, which implicates distraction of the complex portion of a structure that is placed in a flexion mode. The cord can stretch up to a quarter of its length which can correspond to an elongation of 17.6 mm measured at the level of the cervical spine during neck flexion. The increased stretch at this level results in a significant cord compression, translating to increased stress in the white matter and higher stress in gray matter[51]. This is further compounded by craniocervical flexion, which results in longitudinal transmission of stretching force and increases in intramedullary pressure in the lower cervical spine[48]. The rapid occurrence of low-grade mechanical stretching on neural tissues can exceed the biomenchanics of the tissue. This can lead disruption of the tissue and may result in transient or permanent neurological injury. Dynamic forces induced by flexion and extension of the spinal column contribute to axial cord strain with potentially detrimental stretch-induced axonal injury[11]. Cadaveric studies have demonstrated that ongoing longitudinal strain, even within physiological limits, will eventually surpass its material tolerance thereby permitting neurological injury[19]. In canine models, the elastic modulus of the spinal cord tissue decreases with increasing load. The canine spinal cord stripped of pia begins to rupture at a strain close to that developed in the cervicothoracic spinal cord of humans. Deleterious stresses to the spinal cord should be viewed in aggregate and in the context of movement. In the absence of repetitive movement, viscoelastic relaxation of the spinal cord mitigates stress resulting from deformation[52]. In a dynamic formulation, cord deformation form an osteophytic spur or calcified disc herniation adds abnormal plane loading and shear to strain resulting from flexion at the craniocervical and cervicothoracic junctions, significantly increasing overload stress.
The neurobiology of stretch-associated axonal and myelin injury is now understood as a result of work in primates and other animals and from studies of diffuse axonal injury in the human brain. Numerous studies have reproducibly demonstrated that axonal injury frequently occurs at sites of maximal mechanical stress and occurs as a series of recognized events: Myelin stretch injury, altered axolemmal permeability, calcium entry, cytoskeletal collapse, compaction of neurofilaments and microtubules, disruption of anterograde axonal transport, accumulation of organelles, axon retraction, bulb formation, and secondary axotomy[11,53,54].
There is clearly support for the contention that myelopathy increases in the presence of abnormal or excessive motion in the neck[55-57]. It should also be noted that stress is multifactorial and may include elements of gliosis within the spinal cord, acute and chronic changes in cord compliance, and altered gray and white viscoelastic relaxation gradients[51]. Understanding the deleterious effects of abnormal/excessive neck motion highlights the need to eliminate deformation of the spinal cord by minimizing abnormal mobility, and restoring normal sagittal spinal cord contour.
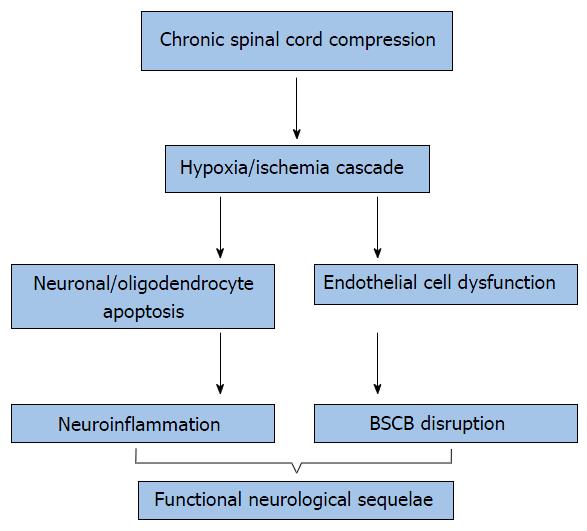
Sufficient pathophysiologic parallels between traumatic spinal cord injury (SCI) and cervical myelopathy have been identified to warrant a brief discussion of these novel mechanisms[58]. It has been proposed that primary mechanical injuries caused by static and dynamic forces to include compression, shear and distraction, induce a secondary injury at the molecular level. Several cellular mechanisms pertaining to these secondary injuries are discussed briefly to include ischemia, glutamatergic toxicity, neuroinflammation and apoptosis (Figure 3)[59-65].
Ischemic injury: Recent studies propose that progressive ischemia of the spinal cord may be central to the origin of cervical myelopathy[36]. The association of ischemia and myelopathy was first hypothesised from the observation of hyalinization and hypertrophy of the walls of the anterior spinal artery upon post-mortem histologic evaluation[66]. Later post-mortem studies of symptomatic cervical myelopathy patients demonstrated further abnormal histologic findings, to include spinal cord ischemic necrosis. Further evidence to implicate spinal cord ischemia in the pathogenesis of cervical myelopathy is that the lower cervical spinal cord, the commonest site of cervical myelopathy, also has the most vulnerable blood supply[67].
Ischemia of the cord secondary to compression was later proposed following experimental observations in canine models. These studies exposed the vulnerability of corticospinal tracts in ischemia conditions and the development of intramedullary cavitation secondary to peripial arterial plexus ischemia[66,68-70]. The use of balloon catheters to assess spinal compression in monkeys revealed hypoperfusion through the transverse arterioles originating from the anterior sulcal arteries and intramedullary branches in the central gray matter, upon anterior and posterior compression[71]. At the cellular level, there is evidence to suggest that oligodendroglia may be hypersensitive to ischemic injury and can apoptose after acute trauma[61,72,73]. These mechanisms may contribute to the demyelinating process observed in chronic cervical myelopathy[74]. The role of ischemic injury has also been proposed with evidence of edema and gliosis represented on MRI. T2-weighted MRI can detect high-intensity signal change, and demyelination and necrosis detected as a hypoenhancement on T1-weighted MRI[75]. MRI changes are an important prognostic indicator. The high intensity signal change seen on T2 images are associated with pathologies that are potentially reversible whereas the low intensity changes seen on T1 images are not. However, it has been proposed that all signal change on MRI is indicative of significant underlying pathology as it is representative of extensive neuronal hypoplasia, glial cell substitution of stroma, and white matter degradation[76].
Disruption of the blood-spinal cord barrier: It has been postulated that the ongoing cord compression observed in cervical myelopathy results in remodelling of the spinal cord[36]. This conformational change may result in endothelial cell loss and dysfunction of the local vasculature[7]. Damage to endothelial cells from resultant ischemia may also impact the integrity of the blood-spinal cord barrier (BSCB). BSCB dysfunction is accompanied by a disruption in vascular permeability leading to cord edema. BSCB permeability in neurotrauma is viewed as a detrimental event to the central nervous system (CNS), secondary to entry of blood-borne inflammatory mediators and increased edema. However, CSM animal models examining mechanisms of BSCB dysfunction and neuroinflammatory responses, remain incomplete.
Glutamatergic toxicity: Glutamate is the major excitatory neurotransmitter in the human CNS. Excitotoxicity is the capacity of excitatory neurotransmitters to control apoptosis of neuronal and oligodendrocyte cells. In the clinical setting these cellular changes have been observed in multiple neuropathologies to include cerebrovascular accidents, traumatic spinal cord injuries and seizural activity[77]. Secondary excitotoxicity refers to neuronal and oligodendrocytic dysfunction mediated by glutamate. It is activated by fluctuations in neuronal metabolism, and has been associated with an array of chronic neurodegenerative diseases[77,78]. Researchers have proposed two structural properties specific to motor neurones that may increase their susceptibility to neurodegeneration. Calcium-mediated toxic events increase their susceptibility to neurodegeneration due to underexpression of both calcium binding proteins and GluR2 AMPA receptors. Based on these findings, future innovations may focus on targeting glutamate receptors in chronic neurodegenerative diseases.
Apoptosis: Apoptosis is defined as programmed cell death, recognised following ischemic and traumatic injury to the CNS[72,79-81]. Decreased perfusion and subsequent ischemia may be important pro-apoptotic events in cervical myelopathy, given the hypersensitivity of neurons and oligodendrocytes to ischaemic injury. In traumatic SCI, there is thought to be a cascade of degenerative changes at the lesion epicentre and demyelination of tracts distant to the injury[61]. The delayed degeneration of anterior horn cells in cervical myelopathy may reflect the effects of apoptosis. It is postulated that axonal degeneration is preceded by oligodendrocyte apoptosis in cervical myelopathy. This series of cellular events has been observed in histological analysis of in vivo models of spinal cord compression, whereby intact demyelinated axons have been observed in the presence of apoptotic oligodendrocytes[58,82]. This is of major therapeutic significance given recent evidence supporting the anti-apoptotic role of novel pharmocolgic inhibitors of the calcium-activated calpain, c-Jun N-terminal kinase and Fas-mediated apoptotic pathways[2,83,84]. Targeting these pro-apoptotic receptors in patients with cervical myelopathy may be employed as neuro-protective treatment strategies in attempt to to diminish the degree of neural degeneration.
Neuroinflammation: Although neuroinflammation is considered integral to wound healing in the setting of neurotrauma, recent evidence suggests that the inflammatory response is a key player in the pro-apoptotic pathway following this event[85]. It is becoming apparent that the innate and adaptive immune responses to acute spinal injuries and chronic cervical myelopathy are dissimilar. Unique to cervical spondylotic myelopathy is a slow inflammatory process, driven by chronic progressive inflammation[86]. Recruitment of neutrophils, monocytes/macrophages, and lymphocytes has been demonstrated in a human model of CSM[2]. However, the identity of the beneficial and detrimental inflammatory mediators in this process remains unknown. Future modulation of this inflammatory cascade has the potential to provide a basis for development of therapeutics for chronic spinal compression disorders.
Effect of neuromodulators on CNS microvasculature: Studies regarding the neurovascular complications of cocaine (benzoylmethylecgonine) report that its use exacerbates and accelerates the natural history of neurological pathology. Cocaine is a serotonin-norepinephrine-dopamine reuptake inhibitor that acts as a powerful CNS stimulant. The compound produces vasoconstriction of the CNS microvasculature, including the anterior spinal artery. Additionally, cocaine primes the responsiveness of platelets to arachidonic acid, leading to an increase in thromboxane release and platelet aggregation. Both these mechanisms independently contribute to infarction of the CNS microvasculature, including the spinal cord.
Recent studies have demonstrated a positive correlation between cocaine abuse and CNS infarction of the middle cerebral artery, vertebrobasilar artery territories, anterior spinal artery, and lateral medulla. However, there is a paucity of data relating to the chronic sequelae of cocaine use on the neurological microenvironment. Studies have demonstrated moderate and persistent alterations in cerebral and spinal blood flow and increased incidence of cerebral vasculitis among cocaine users. A recent study sought to establish the effect of chronic cocaine use on post-operative neurological recovery following surgical decompression of the cervical spine in a cohort of 95 patients diagnosed with symptomatic cervical spondylotic myelopathy[87]. The ability of the spinal cord to heal after surgical decompression is based on the intrinsic ability of the spinal cord to heal itself. Thus, the pre-operative health of the cord is paramount to post-operative improvement. This study revealed that chronic cocaine users had poorer post-operative neurological recovery than non-users and adds further credence to the negative impact of cocaine on spinal cord integrity. These data may influence pre-operative counseling and patient selection in attempt to optimize outcomes following surgical decompression for cervical myelopathy.
Smoking has been identified as a common risk factor for spinal degenerative diseases[88]. A recent in vitro study investigating the effects of nicotine exposure on nucleus pulposus proliferation and extracellular matrix synthesis demonstrated a significant anti-proliferative effect[89]. This suggests that nicotine may contribute to the pathogenesis of vertebral disc degeneration and the development of cervical myelopathy however, further in vivo studies are required to elucidate its role at the molecular level.
The role of heredity in the development of cervical myelopathy was first suggested by Bull et al[90] in 1969. Upon evaluation of several hundred cervical spine radiographs, the authors observed a higher prevalence of simultaneous CSM among twins. MRI of the spines of 172 monozygotic and 154 dizygotic twins revealed that heritability accounted for almost three-quarters of all findings, among a cohort of patients with severe CSM[91].
Flourishing interest in the role of heredity has been supported by evidence of families with a high incidence of CSM[92,93]. The authors’ imply that these cases may represent extreme cases of genetic influence or it may depict the presence of a separate entity classified as “familial cervical spondylosis”.
Previous studies have suggested a correlation between variants of collagen gene expression and degenerative intervertebral disc disorders[94,95]. Collagen IX, a structural component of nucleus pulposus and annulus fibrosis of the intervertebral disc, acts as a bridge between proteins in these tissues. Collagen IX is essential to the proper formation of the collagen II/IX/XI heteropolymer[96]. Recent studies have suggested that collagen tryptophan IX genetic material influence lumbar disc degeneration in patients with herniated nucleus pulposus. Interestingly, it has recently been demonstrated that patients with collagen IX polymorphisms who are smokers have a significantly higher predisposition to developing cervical myelopathy[88]. This landmark study suggests that smoking abstinence is important for reducing cervical myelopathy risk in patients with a genetic predisposition.
Despite the paucity of conclusive evidence to clearly delineate the aetiology of CSM, a multifactorial aetiology to include age-related degeneration, biomechanical factors and heredity is supported.
Whilst acute SCI models have unveiled some cellular mechanisms involved in the pathobiology of CSM, this unique condition and its specific pathomechanisms are poorly defined. A limitation in advancing our knowledge of CSM has been a paucity of reproducible in vivo models to replicate CSM. Several models of acute cord compression have been developed, but relatively little work has focused on the development of an in vivo chronic, graded, cervical cord compression[64,92,97].
However, a recently developed animal model of CSM, employed a chronic compression device on the cervical spine of Sprague-Dawley rats, to achieve chronic and progressive cord compression[4]. This model has the potential to reproduce quantifiable neurobehavioral, neurophysiological, and neuropathological deficits akin to human CSM. Ultimately, this innovation may act as an important catalyst in the translation of targeted therapeutic strategies for cervical myelopathy[4].
Regarding technological advancements, innovations in neuro-imaging will continue to play a key role by facilitating timely diagnosis of soft tissue and osseous pathology in CSM, assist in optimal patient selection for surgical intervention and provide prognostic information in the post-operative period. In addition to advances in kinetic magnetic resonance imaging and DTI, metabolic neuroimaging, has been compared with functional assessments following clinical examination and findings on MRI, of patients selected for surgery for CSM. FDG-PET findings were highly sensitive displayed strong correlations with pre- and postoperative scores, and postoperative rehabilitation[98,99]. The major limitation of this technology is the poor resolution of PET scans. Despite this, future innovations in PET imaging may allow identification of early spinal cord damage and provide indications for surgical intervention.
The cervical spine adapts to the challenges of gravity and the effects of mechanical loading through both structural and biochemical chnages. These adaptations may result in significant physical disability, and in turn stimulate altered biochemical pathways. The pathophysiology of CSM involves a combination of static and dynamic mechanical factors, which induce cellular changes to include neuroischemia, excitotoxicity, neuroinflammation and apoptotic events. There exists an urgent requirement for the development of novel neuro-protective treatment strategies to inhibit neural degeneration and optimize neurological recovery following surgical decompression for CSM.
P- Reviewer: Aota Y, Mori K
S- Editor: Ji FF L- Editor: A E- Editor: Li D
| 1. | Baptiste DC, Fehlings MG. Pathophysiology of cervical myelopathy. Spine J. 2006;6:190S-197S. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 194] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 2. | Yu WR, Liu T, Kiehl TR, Fehlings MG. Human neuropathological and animal model evidence supporting a role for Fas-mediated apoptosis and inflammation in cervical spondylotic myelopathy. Brain. 2011;134:1277-1292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 113] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 3. | Toledano M, Bartleson JD. Cervical spondylotic myelopathy. Neurol Clin. 2013;31:287-305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 116] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 4. | Lee J, Satkunendrarajah K, Fehlings MG. Development and characterization of a novel rat model of cervical spondylotic myelopathy: the impact of chronic cord compression on clinical, neuroanatomical, and neurophysiological outcomes. J Neurotrauma. 2012;29:1012-1027. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 41] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 5. | Broomfield SJ, Da Cruz M, Gibson WP. Cochlear implants and magnetic resonance scans: A case report and review. Cochlear Implants Int. 2013;14:51-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 6. | Stookey B. Differential section of the trigeminal root in the surgical treatment of trigeminal neuralgia. Ann Surg. 1928;87:172-178. [PubMed] |
| 7. | Bohlman HH, Emery SE. The pathophysiology of cervical spondylosis and myelopathy. Spine (Phila Pa 1976). 1988;13:843-846. [PubMed] |
| 8. | Swagerty DL. Cervical spondylotic myelopathy: a cause of gait disturbance and falls in the elderly. Kans Med. 1994;95:226-227, 229. [PubMed] |
| 9. | Nakamura I, Ikegawa S, Okawa A, Okuda S, Koshizuka Y, Kawaguchi H, Nakamura K, Koyama T, Goto S, Toguchida J. Association of the human NPPS gene with ossification of the posterior longitudinal ligament of the spine (OPLL). Hum Genet. 1999;104:492-497. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 136] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 10. | Rao R. Neck pain, cervical radiculopathy, and cervical myelopathy: pathophysiology, natural history, and clinical evaluation. J Bone Joint Surg Am. 2002;84-A:1872-1881. [PubMed] [DOI] [Full Text] |
| 11. | Henderson FC, Geddes JF, Vaccaro AR, Woodard E, Berry KJ, Benzel EC. Stretch-associated injury in cervical spondylotic myelopathy: new concept and review. Neurosurgery. 2005;56:1101-1103; discussion 1101-1103. [PubMed] |
| 12. | Patel AA, Spiker WR, Daubs M, Brodke DS, Cannon-Albright LA. Evidence of an inherited predisposition for cervical spondylotic myelopathy. Spine (Phila Pa 1976). 2012;37:26-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 13. | Kara B, Celik A, Karadereler S, Ulusoy L, Ganiyusufoglu K, Onat L, Mutlu A, Ornek I, Sirvanci M, Hamzaoglu A. The role of DTI in early detection of cervical spondylotic myelopathy: a preliminary study with 3-T MRI. Neuroradiology. 2011;53:609-616. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 85] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 14. | Lao LF, Zhong GB, Li QY, Liu ZD. Kinetic magnetic resonance imaging analysis of spinal degeneration: a systematic review. Orthop Surg. 2014;6:294-299. [PubMed] [DOI] [Full Text] |
| 15. | Harada T, Tsuji Y, Mikami Y, Hatta Y, Sakamoto A, Ikeda T, Tamai K, Hase H, Kubo T. The clinical usefulness of preoperative dynamic MRI to select decompression levels for cervical spondylotic myelopathy. Magn Reson Imaging. 2010;28:820-825. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 22] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 16. | Xu J, Shimony JS, Klawiter EC, Snyder AZ, Trinkaus K, Naismith RT, Benzinger TL, Cross AH, Song SK. Improved in vivo diffusion tensor imaging of human cervical spinal cord. Neuroimage. 2013;67:64-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 66] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 17. | Yone K, Sakou T, Yanase M, Ijiri K. Preoperative and postoperative magnetic resonance image evaluations of the spinal cord in cervical myelopathy. Spine (Phila Pa 1976). 1992;17:S388-S392. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 108] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 18. | Bohlman HH. Cervical spondylosis and myelopathy. Instr Course Lect. 1995;44:81-97. [PubMed] |
| 19. | Breig A, Turnbull I, Hassler O. Effects of mechanical stresses on the spinal cord in cervical spondylosis. A study on fresh cadaver material. J Neurosurg. 1966;25:45-56. [PubMed] |
| 20. | Firooznia H, Benjamin VM, Pinto RS, Golimbu C, Rafii M, Leitman BS, McCauley DI. Calcification and ossification of posterior longitudinal ligament of spine: its role in secondary narrowing of spinal canal and cord compression. N Y State J Med. 1982;82:1193-1198. [PubMed] |
| 21. | Mak KH, Mak KL, Gwi-Mak E. Ossification of the ligamentum flavum in the cervicothoracic junction: case report on ossification found on both sides of the lamina. Spine (Phila Pa 1976). 2002;27:E11-E14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 22. | Payne EE, Spillane JD. The cervical spine; an anatomico-pathological study of 70 specimens (using a special technique) with particular reference to the problem of cervical spondylosis. Brain. 1957;80:571-596. [PubMed] |
| 23. | Fehlings MG, Skaf G. A review of the pathophysiology of cervical spondylotic myelopathy with insights for potential novel mechanisms drawn from traumatic spinal cord injury. Spine (Phila Pa 1976). 1998;23:2730-2737. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 32] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 24. | Parke WW. Correlative anatomy of cervical spondylotic myelopathy. Spine (Phila Pa 1976). 1988;13:831-837. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 84] [Article Influence: 2.3] [Reference Citation Analysis (1)] |
| 25. | Muthukumar N. Ossification of the ligamentum flavum as a result of fluorosis causing myelopathy: report of two cases. Neurosurgery. 2005;56:E622; discussion E622. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 26] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 26. | Green C, Butler J, Eustace S, Poynton A, O’Byrne JM. Imaging modalities for cervical spondylotic stenosis and myelopathy. Adv Orthop. 2012;2012:908324. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 27. | Ferreiro Perez A, Garcia Isidro M, Ayerbe E, Castedo J, Jinkins JR. Evaluation of intervertebral disc herniation and hypermobile intersegmental instability in symptomatic adult patients undergoing recumbent and upright MRI of the cervical or lumbosacral spines. Eur J Radiol. 2007;62:444-448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 31] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 28. | Bednarik J, Kadanka Z, Dusek L, Novotny O, Surelova D, Urbanek I, Prokes B. Presymptomatic spondylotic cervical cord compression. Spine (Phila Pa 1976). 2004;29:2260-2269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 111] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 29. | Basser PJ, Pierpaoli C. Microstructural and physiological features of tissues elucidated by quantitative-diffusion-tensor MRI. 1996. J Magn Reson. 2011;213:560-570. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3041] [Cited by in RCA: 3063] [Article Influence: 105.6] [Reference Citation Analysis (0)] |
| 30. | Morio Y, Nagashima H, Teshima R, Nawata K. Radiological pathogenesis of cervical myelopathy in 60 consecutive patients with cervical ossification of the posterior longitudinal ligament. Spinal Cord. 1999;37:853-857. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 28] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 31. | Okawa A, Nakamura I, Goto S, Moriya H, Nakamura Y, Ikegawa S. Mutation in Npps in a mouse model of ossification of the posterior longitudinal ligament of the spine. Nat Genet. 1998;19:271-273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 329] [Cited by in RCA: 319] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 32. | Hashizume H, Kawakami M, Nishi H, Tamaki T. Histochemical demonstration of nitric oxide in herniated lumbar discs. A clinical and animal model study. Spine (Phila Pa 1976). 1997;22:1080-1084. [PubMed] |
| 33. | Koshizuka Y, Kawaguchi H, Ogata N, Ikeda T, Mabuchi A, Seichi A, Nakamura Y, Nakamura K, Ikegawa S. Nucleotide pyrophosphatase gene polymorphism associated with ossification of the posterior longitudinal ligament of the spine. J Bone Miner Res. 2002;17:138-144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 54] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 34. | Okano T, Ishidou Y, Kato M, Imamura T, Yonemori K, Origuchi N, Matsunaga S, Yoshida H, ten Dijke P, Sakou T. Orthotopic ossification of the spinal ligaments of Zucker fatty rats: a possible animal model for ossification of the human posterior longitudinal ligament. J Orthop Res. 1997;15:820-829. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 16] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 35. | Ono K, Yonenobu K, Miyamoto S, Okada K. Pathology of ossification of the posterior longitudinal ligament and ligamentum flavum. Clin Orthop Relat Res. 1999;18-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 82] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 36. | Kalsi-Ryan S, Karadimas SK, Fehlings MG. Cervical spondylotic myelopathy: the clinical phenomenon and the current pathobiology of an increasingly prevalent and devastating disorder. Neuroscientist. 2013;19:409-421. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 221] [Cited by in RCA: 292] [Article Influence: 22.5] [Reference Citation Analysis (0)] |
| 37. | Lee MJ, Cassinelli EH, Riew KD. Prevalence of cervical spine stenosis. Anatomic study in cadavers. J Bone Joint Surg Am. 2007;89:376-380. [PubMed] |
| 38. | Penning L. Some aspects of plain radiography of the cervical spine in chronic myelopathy. Neurology. 1962;12:513-519. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 121] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 39. | Murone I. The importance of the sagittal diameters of the cervical spinal canal in relation to spondylosis and myelopathy. J Bone Joint Surg Br. 1974;56:30-36. [PubMed] |
| 40. | Arnold JG. The clinical manifestations of spondylochondrosis (spondylosis) of the cervical spine. Ann Surg. 1955;141:872-889. [PubMed] |
| 41. | Penning L, Wilmink JT. Specificity of CT myelographic findings in cervical nerve root symptoms. Neurosurg Rev. 1986;9:99-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 42. | Houser OW, Onofrio BM, Miller GM, Folger WN, Smith PL. Cervical spondylotic stenosis and myelopathy: evaluation with computed tomographic myelography. Mayo Clin Proc. 1994;69:557-563. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 31] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 43. | Ono K, Ota H, Tada K, Yamamoto T. Cervical myelopathy secondary to multiple spondylotic protrusion: a clinico-pathologic study. Spine. 1977;2:109-125. [RCA] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 88] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 44. | Nakamura M, Fujimura Y. Magnetic resonance imaging of the spinal cord in cervical ossification of the posterior longitudinal ligament. Can it predict surgical outcome? Spine (Phila Pa 1976). 1998;23:38-40. [PubMed] |
| 45. | Gundry CR, Fritts HM. Magnetic resonance imaging of the musculoskeletal system. Part 8. The spine, section 2. Clin Orthop Relat Res. 1997;260-271. [PubMed] |
| 46. | Abdulhadi MA, Perno JR, Melhem ER, Nucifora PG. Characteristics of spondylotic myelopathy on 3D driven-equilibrium fast spin echo and 2D fast spin echo magnetic resonance imaging: a retrospective cross-sectional study. PLoS One. 2014;9:e100964. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 47. | White AA, Panjabi MM. Biomechanical considerations in the surgical management of cervical spondylotic myelopathy. Spine (Phila Pa 1976). 1988;13:856-860. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 117] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 48. | Kitahara Y, Iida H, Tachibana S. Effect of spinal cord stretching due to head flexion on intramedullary pressure. Neurol Med Chir (Tokyo). 1995;35:285-288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 29] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 49. | Koçak A, Kiliç A, Nurlu G, Konan A, Kilinç K, Cirak B, Colak A. A new model for tethered cord syndrome: a biochemical, electrophysiological, and electron microscopic study. Pediatr Neurosurg. 1997;26:120-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 17] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 50. | Yuan Q, Dougherty L, Margulies SS. In vivo human cervical spinal cord deformation and displacement in flexion. Spine (Phila Pa 1976). 1998;23:1677-1683. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 52] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 51. | Ichihara K, Taguchi T, Sakuramoto I, Kawano S, Kawai S. Mechanism of the spinal cord injury and the cervical spondylotic myelopathy: new approach based on the mechanical features of the spinal cord white and gray matter. J Neurosurg. 2003;99:278-285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 57] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 52. | Carlson GD, Warden KE, Barbeau JM, Bahniuk E, Kutina-Nelson KL, Biro CL, Bohlman HH, LaManna JC. Viscoelastic relaxation and regional blood flow response to spinal cord compression and decompression. Spine (Phila Pa 1976). 1997;22:1285-1291. [PubMed] |
| 53. | Maxwell WL, Domleo A, McColl G, Jafari SS, Graham DI. Post-acute alterations in the axonal cytoskeleton after traumatic axonal injury. J Neurotrauma. 2003;20:151-168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 62] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 54. | Povlishock JT. Traumatically induced axonal injury: pathogenesis and pathobiological implications. Brain Pathol. 1992;2:1-12. [PubMed] |
| 55. | Iwasaki M, Yamamoto T, Miyauchi A, Amano K, Yonenobu K. Cervical kyphosis: predictive factors for progression of kyphosis and myelopathy. Spine (Phila Pa 1976). 2002;27:1419-1425. [PubMed] |
| 56. | Muhle C, Metzner J, Weinert D, Schön R, Rautenberg E, Falliner A, Brinkmann G, Mehdorn HM, Heller M, Resnick D. Kinematic MR imaging in surgical management of cervical disc disease, spondylosis and spondylotic myelopathy. Acta Radiol. 1999;40:146-153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 35] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 57. | Yonenobu K, Okada K, Fuji T, Fujiwara K, Yamashita K, Ono K. Causes of neurologic deterioration following surgical treatment of cervical myelopathy. Spine (Phila Pa 1976). 1986;11:818-823. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 106] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 58. | Bunge RP, Puckett WR, Becerra JL, Marcillo A, Quencer RM. Observations on the pathology of human spinal cord injury. A review and classification of 22 new cases with details from a case of chronic cord compression with extensive focal demyelination. Adv Neurol. 1993;59:75-89. [PubMed] |
| 59. | Agrawal SK, Fehlings MG. Mechanisms of secondary injury to spinal cord axons in vitro: role of Na+, Na(+)-K(+)-ATPase, the Na(+)-H+ exchanger, and the Na(+)-Ca2+ exchanger. J Neurosci. 1996;16:545-552. [PubMed] |
| 60. | Agrawal SK, Fehlings MG. Role of NMDA and non-NMDA ionotropic glutamate receptors in traumatic spinal cord axonal injury. J Neurosci. 1997;17:1055-1063. [PubMed] |
| 61. | Crowe MJ, Bresnahan JC, Shuman SL, Masters JN, Beattie MS. Apoptosis and delayed degeneration after spinal cord injury in rats and monkeys. Nat Med. 1997;3:73-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 863] [Cited by in RCA: 880] [Article Influence: 31.4] [Reference Citation Analysis (0)] |
| 62. | Delamarter RB, Sherman J, Carr JB. Pathophysiology of spinal cord injury. Recovery after immediate and delayed decompression. J Bone Joint Surg Am. 1995;77:1042-1049. [PubMed] |
| 63. | Fehlings MG, Agrawal S. Role of sodium in the pathophysiology of secondary spinal cord injury. Spine (Phila Pa 1976). 1995;20:2187-2191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 33] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 64. | Fehlings MG, Tator CH. The relationships among the severity of spinal cord injury, residual neurological function, axon counts, and counts of retrogradely labeled neurons after experimental spinal cord injury. Exp Neurol. 1995;132:220-228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 300] [Cited by in RCA: 320] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 65. | Tator CH, Fehlings MG. Review of the secondary injury theory of acute spinal cord trauma with emphasis on vascular mechanisms. J Neurosurg. 1991;75:15-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1106] [Cited by in RCA: 1093] [Article Influence: 32.1] [Reference Citation Analysis (0)] |
| 66. | Gooding MR, Wilson CB, Hoff JT. Experimental cervical myelopathy. Effects of ischemia and compression of the canine cervical spinal cord. J Neurosurg. 1975;43:9-17. [PubMed] |
| 67. | Baron EM, Young WF. Cervical spondylotic myelopathy: a brief review of its pathophysiology, clinical course, and diagnosis. Neurosurgery. 2007;60:S35-S41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 142] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 68. | Ogino H, Tada K, Okada K, Yonenobu K, Yamamoto T, Ono K, Namiki H. Canal diameter, anteroposterior compression ratio, and spondylotic myelopathy of the cervical spine. Spine (Phila Pa 1976). 1983;8:1-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 182] [Cited by in RCA: 165] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 69. | Gooding MR. Pathogenesis of myelopathy in cervical spondylosis. Lancet. 1974;2:1180-1181. [PubMed] |
| 70. | al-Mefty O, Harkey HL, Marawi I, Haines DE, Peeler DF, Wilner HI, Smith RR, Holaday HR, Haining JL, Russell WF. Experimental chronic compressive cervical myelopathy. J Neurosurg. 1993;79:550-561. [PubMed] |
| 71. | Doppman JL. The mechanism of ischemia in anteroposterior compression of the spinal cord 1975. Invest Radiol. 1990;25:444-452. [PubMed] |
| 72. | Shuman SL, Bresnahan JC, Beattie MS. Apoptosis of microglia and oligodendrocytes after spinal cord contusion in rats. J Neurosci Res. 1997;50:798-808. [PubMed] |
| 73. | Li Y, Field PM, Raisman G. Death of oligodendrocytes and microglial phagocytosis of myelin precede immigration of Schwann cells into the spinal cord. J Neurocytol. 1999;28:417-427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 28] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 74. | Gledhill RF, Harrison BM, McDonald WI. Demyelination and remyelination after acute spinal cord compression. Exp Neurol. 1973;38:472-487. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 141] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 75. | Ohshio I, Hatayama A, Kaneda K, Takahara M, Nagashima K. Correlation between histopathologic features and magnetic resonance images of spinal cord lesions. Spine (Phila Pa 1976). 1993;18:1140-1149. [PubMed] |
| 76. | Uchida K, Nakajima H, Sato R, Kokubo Y, Yayama T, Kobayashi S, Baba H. Multivariate analysis of the neurological outcome of surgery for cervical compressive myelopathy. J Orthop Sci. 2005;10:564-573. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 78] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 77. | Choi DW. Excitotoxic cell death. J Neurobiol. 1992;23:1261-1276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1631] [Cited by in RCA: 1685] [Article Influence: 52.7] [Reference Citation Analysis (0)] |
| 78. | Shaw PJ, Ince PG. Glutamate, excitotoxicity and amyotrophic lateral sclerosis. J Neurol. 1997;244 Suppl 2:S3-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 198] [Cited by in RCA: 211] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 79. | Schrank B, Götz R, Gunnersen JM, Ure JM, Toyka KV, Smith AG, Sendtner M. Inactivation of the survival motor neuron gene, a candidate gene for human spinal muscular atrophy, leads to massive cell death in early mouse embryos. Proc Natl Acad Sci USA. 1997;94:9920-9925. [PubMed] |
| 80. | Kato H, Kanellopoulos GK, Matsuo S, Wu YJ, Jacquin MF, Hsu CY, Kouchoukos NT, Choi DW. Neuronal apoptosis and necrosis following spinal cord ischemia in the rat. Exp Neurol. 1997;148:464-474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 73] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 81. | Lipton SA. Molecular mechanisms of trauma-induced neuronal degeneration. Curr Opin Neurol Neurosurg. 1993;6:588-596. [PubMed] |
| 82. | Blight AR. Delayed demyelination and macrophage invasion: a candidate for secondary cell damage in spinal cord injury. Cent Nerv Syst Trauma. 1985;2:299-315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 219] [Cited by in RCA: 225] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 83. | Villa PG, Henzel WJ, Sensenbrenner M, Henderson CE, Pettmann B. Calpain inhibitors, but not caspase inhibitors, prevent actin proteolysis and DNA fragmentation during apoptosis. J Cell Sci. 1998;111:713-722. [PubMed] |
| 84. | Pérez-García MT, Chiamvimonvat N, Ranjan R, Balser JR, Tomaselli GF, Marban E. Mechanisms of sodium/calcium selectivity in sodium channels probed by cysteine mutagenesis and sulfhydryl modification. Biophys J. 1997;72:989-996. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 33] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 85. | Shaked I, Porat Z, Gersner R, Kipnis J, Schwartz M. Early activation of microglia as antigen-presenting cells correlates with T cell-mediated protection and repair of the injured central nervous system. J Neuroimmunol. 2004;146:84-93. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 104] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 86. | Beattie MS, Manley GT. Tight squeeze, slow burn: inflammation and the aetiology of cervical myelopathy. Brain. 2011;134:1259-1261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 37] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 87. | Sadhu A, Ahn NU. Cocaine use and surgical outcomes of cervical spondylotic myelopathy: a retrospective study. Orthopedics. 2012;35:e1640-e1643. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 88. | Wang ZC, Shi JG, Chen XS, Xu GH, Li LJ, Jia LS. The role of smoking status and collagen IX polymorphisms in the susceptibility to cervical spondylotic myelopathy. Genet Mol Res. 2012;11:1238-1244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 26] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 89. | Akmal M, Kesani A, Anand B, Singh A, Wiseman M, Goodship A. Effect of nicotine on spinal disc cells: a cellular mechanism for disc degeneration. Spine (Phila Pa 1976). 2004;29:568-575. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 129] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 90. | Bull J, el Gammal T, Popham M. A possible genetic factor in cervical spondylosis. Br J Radiol. 1969;42:9-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 28] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 91. | Sambrook PN, MacGregor AJ, Spector TD. Genetic influences on cervical and lumbar disc degeneration: a magnetic resonance imaging study in twins. Arthritis Rheum. 1999;42:366-372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 92. | Yoo K, Origitano TC. Familial cervical spondylosis. Case report. J Neurosurg. 1998;89:139-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 27] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 93. | Mukerji N, Todd NV. Cervical myelopathy in rheumatoid arthritis. Neurol Res Int. 2011;2011:153628. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 94. | Falcón-Ramírez E, Casas-Avila L, Miranda A, Diez P, Castro C, Rubio J, Gómez R, Valdés-Flores M. Sp1 polymorphism in collagen I α1 gene is associated with osteoporosis in lumbar spine of Mexican women. Mol Biol Rep. 2011;38:2987-2992. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 95. | Lucas SR, Bass CR, Crandall JR, Kent RW, Shen FH, Salzar RS. Viscoelastic and failure properties of spine ligament collagen fascicles. Biomech Model Mechanobiol. 2009;8:487-498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 22] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 96. | Zhu Y, Wu JJ, Weis MA, Mirza SK, Eyre DR. Type IX collagen neo-deposition in degenerative discs of surgical patients whether genotyped plus or minus for COL9 risk alleles. Spine (Phila Pa 1976). 2011;36:2031-2038. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (1)] |
| 97. | Mukerji SS, Parmar H, Ibrahim M, Bradford C. An unusual cause of recurrent pediatric neck abscess: pyriform sinus fistula. Clin Imaging. 2007;31:349-351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 98. | Uchida K, Nakajima H, Yayama T, Kobayashi S, Shimada S, Tsuchida T, Okazawa H, Mwaka E, Baba H. High-resolution magnetic resonance imaging and 18FDG-PET findings of the cervical spinal cord before and after decompressive surgery in patients with compressive myelopathy. Spine (Phila Pa 1976). 2009;34:1185-1191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 19] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 99. | Uchida K, Kobayashi S, Yayama T, Kokubo Y, Nakajima H, Kakuyama M, Sadato N, Tsuchida T, Yonekura Y, Baba H. Metabolic neuroimaging of the cervical spinal cord in patients with compressive myelopathy: a high-resolution positron emission tomography study. J Neurosurg Spine. 2004;1:72-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 34] [Article Influence: 1.6] [Reference Citation Analysis (0)] |