INTRODUCTION AND JUSTIFICATION
Mesenchymal stromal cells or mesenchymal stem cells (MSCs) are a plastic-adherent cell population that expresses specific surface markers and have the capacity to self-renew and differentiate into bone, cartilage, adipose, muscle and neural cells in adequate conditions[1-3]. MSCs are found in a variety of tissues, including the umbilical cord[4-6], which is the focus of this review and provides a non invasive source of abundant stem cells. MSCs are a relatively new source for cell therapy for cartilage and surrounding joint tissues. Regenerative therapies for cartilage defects will profit greatly from the combined applications of available basic science knowledge of matrix biology, hormone and receptor biology, biomechanics, virology, scaffolds and transplantation medicine[7]. Although the therapeutic potential of MSCs is being studied and has clear objectives, the requirements and conditions for their direct chondrogenic differentiation in vitro remains a challenge. This review is intended to summarize our knowledge of umbilical cord MSCs, including their derivation from Wharton’s jelly or umbilical cord blood and their therapeutic application for joint pathologies.
UMBILICAL CORD MSCS
MSCs have been isolated from umbilical cord blood, umbilical vein subendothelium and Wharton’s jelly cells. All these cells are a primitive stromal population that displays the characteristics of MSCs defined by the International Society for Cellular Therapy. Umbilical cord MSCs grow as adherent cells with mesenchymal morphology, are self-renewing, express cell surface markers typical of MSCs (CD14-, CD34- and CD45-negative while expressing high levels of CD73, CD90 and CD105), maintain their differentiation potential and can differentiate in vitro into various cell lineages, including bone, cartilage and adipose tissue[8]. Umbilical cord MSCs also have the capacity to form neurons and glia in vitro[9] and can be successfully trans-differentiated into cardiomyocytes in vitro[10]. Like other stromal cells, MSCs derived from the umbilical cord support the expansion of other stem cells, such as hematopoietic stem cells, and are well-tolerated by the immune system[11]. Human umbilical cord MSCs share similar
in vitro immunosuppressive properties with MSCs obtained from bone marrow and blood. However, the mechanisms and cell interactions used by MSCs to control immune responses are not yet fully elucidated[12]. Functional module analysis has shown different osteogenic potential and differentiation capacity for human MSCs from bone marrow and Wharton’s jelly[13]. There are no known limitations to using umbilical cord–derived MSCs. Wu et al[14] published the first report of umbilical cord MSCs in a human clinical application. They found that umbilical cord MSCs had superior proliferative potential and more suppressive effects on peripheral blood mononuclear cell proliferation than bone marrow MSC. The acute graft-versus-host disease improved dramatically after each of four infusions of umbilical cord MSCs into the two patients. No adverse effects were noted. Both patients are doing well now and this procedure seems both feasible and safe.
Umbilical cord blood
Several studies of MSCs derived from the umbilical cord blood of several species show them to be capable of being isolated and differentiated toward defined mesoderm lineages. Included in these species are equine[15,16], canine[17], porcine[18], ovine[19] and human[20,21]. Studies have also focused on the ability of umbilical cord MSCs to support the ex-vivo expansion of other cell types and their differentiation into other lineages[22,23]. Recent studies by Kim et al[24] have examined the molecular mechanism of differentiation, such as the involvement of NOD1 and NOD2 for differentiation of MSCs derived from human umbilical cord blood, and analyzed the ability of these cells to function as alloantigens in vitro. Current results indicate that human umbilical cord blood-derived MSCs do not provoke allogeneic peripheral blood mononuclear cell proliferation even when their HLA-molecule expression was up-regulated by interferon-(IFN)-γ pre-treatment. This suppressive effect was mediated by soluble factors. Overall it seems that MSCs from umbilical cord blood can suppress the allogeneic response of lymphocytes and may prove useful in allogeneic cell therapies[25]. Umbilical cord blood is a valuable source for hematopoietic progenitor cell therapy. It also contains another non-hematopoietic cell population, mesenchymal progenitor cells (MPCs), which can be expanded ex vivo and differentiated into osteoblasts, chondrocytes and adipocytes. Several studies[4,26] indicate that two sub-populations of MSCs can be isolated from umbilical cord blood by using specific methods. These cells exhibit different morphological phenotypes, the majority of which resemble flattened fibroblasts and the remainder spindle-shaped fibroblasts. Both MSC types share similar cell surface markers, except for CD90, and have similar osteogenic and chondrogenic potential. The spindle-shaped population is positive for CD90 and shows more capability for adipogenesis, while the flattened cell type is CD90 negative and shows less capability for adipogenesis. The larger number of flattened umbilical cord blood MPCs might be linked to their lowered capability for adipogenic differentiation[27].
Wharton’s jelly
The number of studies of MSCs derived from stromal tissues has increased over the last 6 years. However, these cells have not been as widely studied as MSCs derived from umbilical cord blood. Can et al[28] described the noninvasive isolation, culture and basic characterization of human umbilical cord stroma -MSCs. MSCs derived from Wharton’s jelly were isolated using their adherence to plastic and characterized by flow cytometry, looking for cells positive for STRO1, OCT3/4 and SSEA-4, as well as those positive for the classic MSC-markers, CD44, CD73, CD90, Ki67, CD105 and CD106, and negative for CD34 and CD45[29-32] (Figure 1). These MSCs were differentiated towards musculoskeletal tissue[33] and CD10 positive cells that displayed contractile properties[34]. Wharton’s jelly MSCs are also candidates for β cell regeneration, utilizing their immune response inhibiting benefits for treatment of type 1 diabetes[35]. Wharton’s jelly-derived MSCs may prove to be a better clinical alternative to bone marrow-derived MSCs because of their ease of access, higher expansion potential and low immunogenicity. The use of allogenic MSCs would be possible in vivo only if they retain their immune properties in an inflammatory environment. Thus, the focus of a study by Prasanna et al[36] sought to understand and compare the immune properties of MSCs from Wharton’s jelly and from bone marrow primed with the key pro-inflammatory cytokines, IFN-γ and tumor necrosis factor α. Importantly, these authors found that inflammation affects the immune properties of these MSCs differently and that MSCs derived from different tissues may utilize different unique mechanisms of immune-modulation.
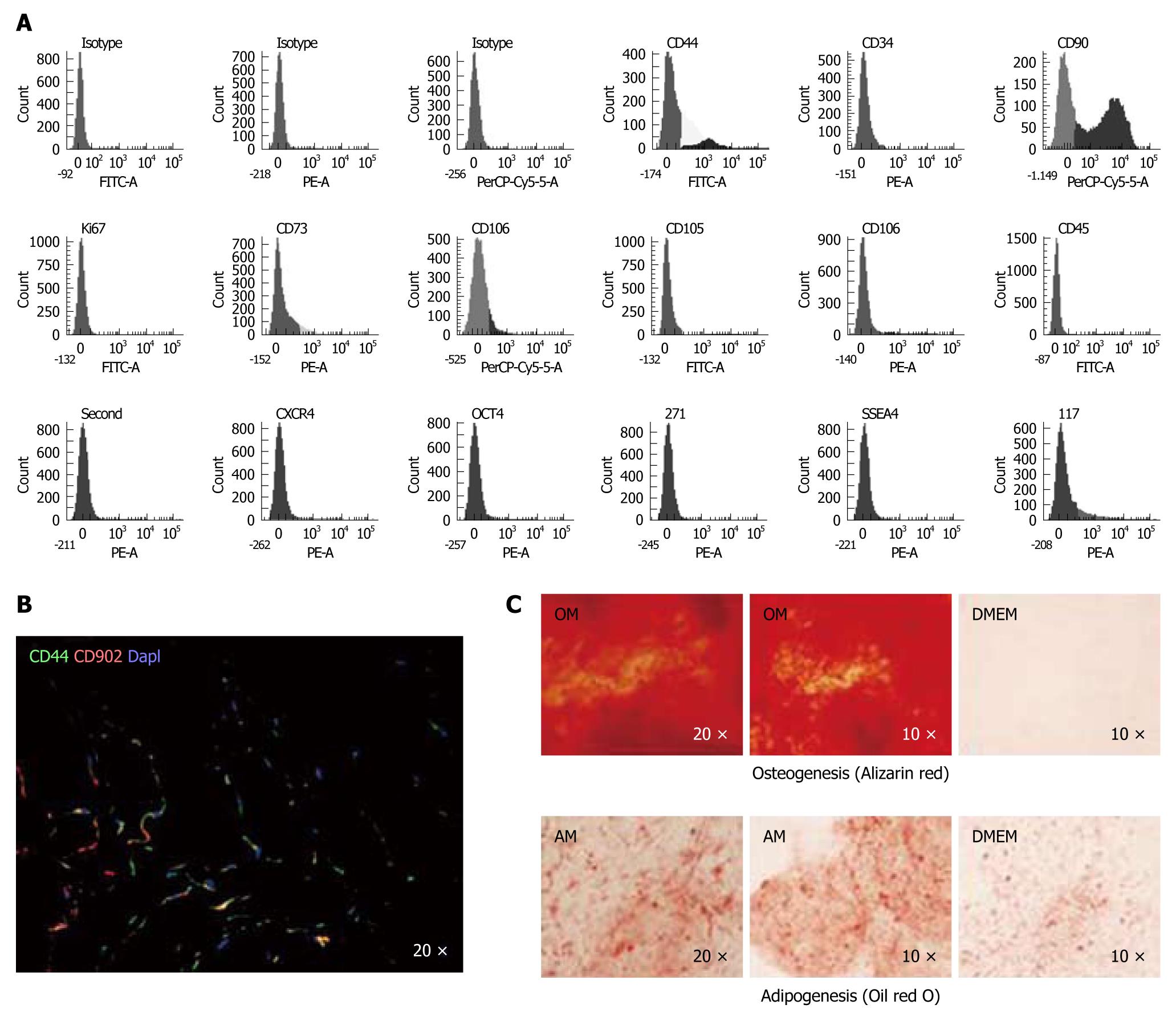
Figure 1 Characterization of the mesenchymal stem cell population from human umbilical cord stroma.
A: Flow cytometry of the principal mesenchymal stem cell (MSC) markers. In each diagram, at the top is the name of the marker, at the bottom the fluorochrome used and at the top right the percentage of positive cells; B: Immunofluorescence analysis of CD44 and CD90 in a human umbilical cord cryosection; DAPI was used to label cell nuclei. (Magnification 20 ×); C: Alizarin red stain (upper) and Oil red O stain (lower) of spheroids engineered from MSCs differentiated in two defined media, proving their pluripotency. OM: Osteogenic medium; AM: Adipogenic medium; DMEM: Control medium with no defined cytokines to promote differentiation.
Recent studies by Huang et al[37] have demonstrated that MSCs can differentiate into germ cells under appropriate conditions. MSCs were induced to differentiate into germ cells in all-trans retinoic acid, testosterone and testicular-cell-conditioned medium prepared from newborn male mouse testes. These MSCs formed “tadpole-like” cells after induction with different reagents and showed both mRNA and protein expression of the germ-cell-specific markers, Oct4 (POUF5), Ckit, CD49 (f) (α6), Stella (DDPA3) and Vasa (DDX4). These results could lead to a new reproductive therapy utilizing human umbilical cord MSCs as well as provide a novel
in vitro model for investigating the molecular mechanisms regulating development of the mammalian germ cell lineage[37]. Proteomic analyses of human MSCs from Wharton’s jelly during in vitro expansion have demonstrated their potential capacity for self-renewal and in vitro expansion[38]. More than 30% of 158 proteins identified by these analyses belong to the cytoskeleton complex. However, several proteins were no longer expressed after the 2nd in vitro passage, suggesting the proliferative potency of these cells was reduced after the initial in vitro stage. This important study provides an essential step for gaining an understanding of the molecular properties of Wharton’s jelly cells[38].
Obstetric parameters, including the gender of the baby, birth weight, the age of the mother at delivery, gestational stage at parturition and mode of delivery were examined by Penolazzi et al[39] who found that osteoblastic potential was not influenced by the baby’s gender and mode of delivery. The highest degree of osteoblastic potential was shown by MSCs from Wharton’s jelly selected from the heaviest full-term babies and with high basal levels RUNX-2. The results of this study are helpful to select optimal umbilical cord donors for efficiently collecting high potential Wharton’s jelly-derived osteoprogenitors. Therefore, the isolation and culture of human umbilical cord-MSCs still need better clarification to ultimately build an optimal standard procedure among laboratories, tissue banks and clinics.
UMBILICAL CORD MSCS AND CHONDROGENESIS
MSCs from equine umbilical cord blood produced a tissue with a cartilage-like morphology that stained positive for proteoglycans and expressed typical cartilage markers[40]. These authors found that MSCs from umbilical cord blood possessed more chondrogenic potential than bone marrow-derived MSCs, based on the cell populations tested and parameters measured[40]. Another study using canine MSCs from umbilical cord blood employed osteogenic and chondrogenic differentiation detected by alizarin red and toluidine blue staining respectively[17]. With osteogenic differentiation, the MSCs were shown to express osteoblastic differentiation genes by reverse transcriptase-polymerase chain reaction (RT-PCR). The results from this study suggest that canine MSCs are capable of multipotential differentiation[17].
MSCs from porcine umbilical cord blood were isolated and their morphology, proliferation, cell cycle status, cell-surface antigen profile and expression of hematopoietic cytokines characterized by Kumar et al[18]. The capacity of porcine MSCs to differentiate in vitro into osteocytes, adipocytes and chondrocytes was also evaluated. These investigators found that the expression of lineage-specific genes was gradually up-regulated during osteogenesis, adipogenesis and chondrogenesis. Porcine umbilical cord blood also contains a population of MPCs capable of self-renewal and of differentiating in vitro into three classical mesenchymal lineages.
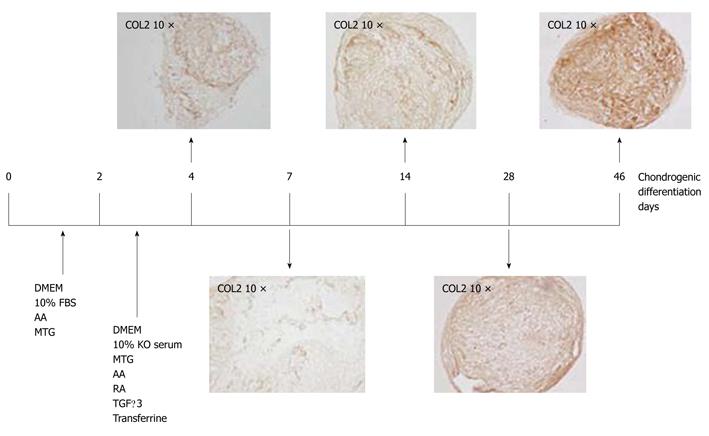
The in vitro growth of human umbilical cord blood MSCs has been achieved; their differentiation into chondrocytes and osteoblasts demonstrates their multipotentiality and predisposition towards a mesodermal fate[41]. Wharton’s jelly contains mucoid connective tissue and fibroblast-like cells. Using flow cytometric analysis, Wang et al[29] found that mesenchymal cells isolated from human Wharton’s jelly express the matrix receptors CD44 and CD105, plus integrin markers CD29 and CD51, but not the hematopoietic lineage markers CD34 and CD45 (Figure 2). Interestingly, these cells also express significant amounts of the MSC markers SH2 and SH3[29]. Isolated and characterized MSCs from human umbilical cord stroma and quantitative RT-PCR analysis of the genes ALP, MEF2C, MyoD, LPL, FAB4 and AMP, characteristic for the differentiated lineages, were used to evaluate early and late differentiation of three germ lines[30]. Direct chondrogenic differentiation was achieved by utilizing spheroid formation by MSCs in a chondrogenic medium; testing for the presence of chondrogenic markers was done at 4, 7, 14, 28 and 46 d of culture. Immunohistochemistry and quantitative RT-PCR analyses were employed to assess the expression of collagen type 1 (COL1), collagen type 2 (COL2) and collagen type X (COLX) at the times studied[30]. These investigators found expression of all chondrogenic markers as early as 4 d of chondrogenic differentiation culture, with their expression increasing with time except for COL1 which decreased in expression in the formed spheroids after 4 d of differentiation. A secretome study to validate this model for in vitro chondrogenic differentiation was performed using the spheroids formed during the chondrogenesis process[30]. The results summarized in Figures 1 and 2 indicate the multipotential capacity of human MSCs from this source; their chondrogenic capacity may prove useful for future cell therapies for articular diseases[30]. It would also be interesting to see if the chondro-type tissues being differentiated from these MSCs can be modified in vitro using biomaterials or other factors to mimic the 5 different layers observed in normal cartilage but it is too early in the research to know this and there is no report of it in the literature.
Figure 2 Representative diagrams of the methodology for spheroid chondrogenesis.
Diagram showing culture times used for a new method for the induction of chondrogenesis. Representative sections of immunohistochemical analyses for collagen type 2 (COL2) expression from spheroids engineered from Wharton´s jelly mesenchymal stem cells at different times of chondrogenesis are shown. (Magnification 10x). AA: Ascorbic acid; MTG: Monothioglycerol; RA: Retinoic acid; KO serum: Knock out serum (GIBCO™); TGFβ-3: Recombinant human transforming growth factor-β3; TRANS: Transferrine; DEX: Dexamethasone.
THERAPEUTIC APPLICATION OF UMBILICAL CORD MSCS FOR JOINT PATHOLOGIES
Human MSCs, with particular reference to Wharton’s jelly MSCs, have an important role as immunomodulators and their multilineage differentiation potential makes their use in tissue regeneration and repair possible[42].
Although bone marrow represents the primary available source of MSCs, the use of bone marrow-derived cells is not always acceptable because of their high degree of viral infection and the significant drop in cell number and both proliferative and differentiation capacity with ageing. Romanov et al[43] isolated MSCs from the subendothelial layer of the umbilical cord and found that cord vasculature contains many MSC-like elements forming colonies of fibroblastoid cells that express several mesenchymal cell markers and can be successfully expanded in culture. They proposed that umbilical cord/placenta stroma may become an alternative source of MSCs for experimental and clinical needs[43]. Scaffolds provide a template for cell distribution, growth, differentiation and extracellular matrix accumulation in three dimensions. Recent studies[44,45] have demonstrated the potential of scaffolding to enhance articular cartilage repair both in vitro and
in vivo. Kao et al[46] demonstrated that chemically synthesized thermoreversible gelation polymer provided a competent three-dimensional culture environment for cord blood MSCs to differentiate into chondrocytes and may prove to be clinically useful to induce chondrogenic differentiation of MSCs from cord blood for cartilage repair. Although bone marrow was the first reported source to contain MSCs, obtaining and utilizing these MSCs is not always practical due to the very invasive technique required for drawing them and the decline in cell number and differentiation capability with increasing age.
Mononuclear cells derived from human umbilical cord blood and obtained by a negative immunoselection technique exhibited either osteoclast-like or mesenchymal-like phenotypes[47]. However, these cells were able to produce homogeneous populations of MSCs displaying a fibroblast-like morphology while expressing mesenchyme-related antigens and showing differentiation capability for osteoblastic and early chondroblastic lineages. This study by Barachini et al[47] is one of the few investigating human umbilical cord blood-derived MSC growth and differentiation on three-dimensional scaffolds. The potential applications in regenerative medicine and tissue engineering of umbilical cord blood-derived MSCs were illustrated by their ability to grow on biodegradable microfiber meshes and their capability to differentiate into mature osteoblasts when cultured inside human plasma clots; this suggests their potential applications in orthopedic surgery. In a 6 wk study by Wang et al[48], a comparison of chondrogenic differentiation of human bone marrow MSCs and human umbilical cord MSCs in a three-dimensional scaffold was conducted for the first time. Cells were seeded on polyglycolic acid scaffolds at 25 M cells/mL and cultured in identical conditions. Cell proliferation, biosynthesis and chondrogenic differentiation were assessed at weeks 0, 3 and 6 after seeding. The authors concluded that human umbilical cord MSCs might provide a desirable option for use as a mesenchymal cell source for fibrocartilage tissue engineering, based on the abundant COL1 and aggrecan production of human umbilical cord MSCs in a three-dimensional matrix. Further investigations of the optimal signals to promote COL2 production from human umbilical cord MSCs are necessary for their use in hyaline cartilage engineering.
MSCs from Wharton’s jelly have been shown to be therapeutic in several pre-clinical animal models for neurodegenerative disease, cancer, heart disease, etc. The preclinical work suggested that MSCs from Wharton’s jelly are therapeutic by mechanisms of trophic rescue and immune modulation. MSCs from human Wharton’s jelly represent a promising source for progenitor cells with the potential to repair and regenerate solid tissues[49]. Collagen-embedded MSCs from Wharton’s jelly have a homogenous growth pattern as well as a constant expression of growth factors and extracellular matrix proteins without any negative effects on the epidermal layer, as shown by histology, electron microscopy, immunohistochemistry and real time-RT-PCR. These results indicate the necessity for an organizing a biomaterial-based scaffold to direct stem cell differentiation, proliferation and paracrine activity, as well as regulation of extracellular matrix deposition[50,51].
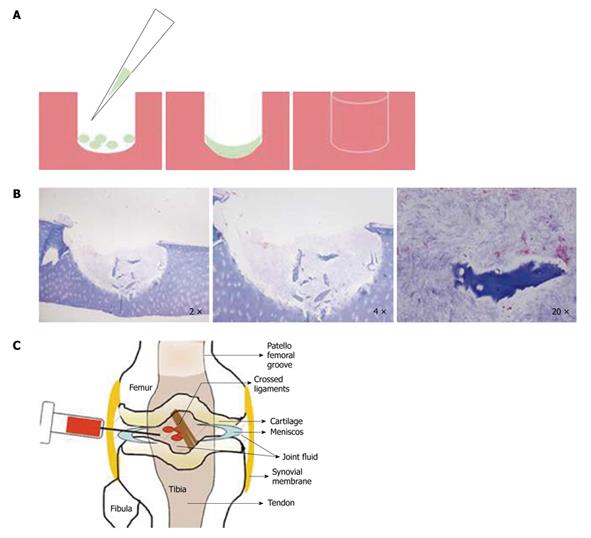
Preliminary in vitro studies done in our group have shown some degree of differentiation and integration of MSCs from Wharton’s jelly into a human osteochondral punch (Figure 3). Further work is needed to determine whether the Wharton’s jelly MSCs can engraft in vivo over the long term and can self-renew cartilage. There are several studies[52] using acellular umbilical veins seeded with MSCs from bone marrow to engineer tendon but umbilical cord-derived MSCs have not yet been tested for this purpose.
Figure 3 Cellular therapy.
A: Graphic illustrating the regeneration of a chondral lesion in vitro; B: Wharton’s jelly mesenchymal stem cells (MSCs) growing in vitro in a human osteochondral punch lesion over 90 d in chondrogenic medium with added transforming growth factor β3. Modified Masson staining shows the MSCs differentiating in the lesion (Magnification 2 ×, 4 × and 20 ×); C: Graphic portraying the technique of intra-articular injection of MSCs into an injured joint to treat lesions in vivo.
New research studies[53,54] involving the use of new scaffold materials made from hyaluronic acid, collagen and other extracellular matrix proteins should be mentioned. These extracellular matrix scaffolds have been used to create three-dimensional structures for supporting in vitro growth and chondrogenesis of MSCs and their possible clinical use for the replacement of damaged joints[51,55-57]. To date, all these studies have utilized only MSCs from bone marrow and none of them have considered the dangerous aspects of such tissue-like inducting malignancies.
In summary, umbilical cord MSCs are multipotent stem cells that may serve many therapeutic and biotechnological roles in cartilage repair opportunities. Considering that acquiring umbilical cord derived-MSCs is a non invasive technique, these cells would appear to be the ideal candidates for clinical cell-based therapies. It would appear to be promising news for use in future in vivo studies and consequently clinical trials for cartilage repair therapies. Successful in vitro and in vivo differentiation to several lineages makes these cells an invaluable stem cell source, deserving further testing as a cellular therapy or other applications in regenerative medicine.
Peer reviewers: Bing Wang, MD, PhD, Assistant Professor, Director, Molecular Therapeutics Laboratory, Department of Orthopaedic Surgery and Neurology University of Pittsburgh School of Medicine, #216 Bridgeside Point II, 450 Technology Drive Pittsburgh, PA 15219, United States; Dr. Monica Mattioli-Belmonte, PhD, Department of Molecular Pathology and Innovative TherapiesUniversità Politecnica delle MarcheVia Tronto 10/A, IT 60126, Ancona, Italy; Dr. Carl Haasper, Department of Trauma, Hannover Medical School (MHH), Carl-Neuberg-Strasse 1, Hannover, 30625, Germany; Dr. Enrique Gomez-Barrena, Department of Orthopaedic Surgery and Traumatology, Hospital La Paz, Universidad Autónoma de Madrid, Madrid, 28034, Spain
S- Editor Sun H L- Editor Roemmele A E- Editor Zheng XM