Revised: January 12, 2011
Accepted: January 19, 2011
Published online: March 18, 2011
The relationship between the immune system, estrogen deficiency and bone loss is an intriguing and, as yet, unexplained challenge of the past two decades. Here we summarize the evidence that links immune cells, inflammation, cytokine production and osteoclast formation and activity with particular regard to humans.
- Citation: D’Amelio P, Fornelli G, Roato I, Isaia GC. Interactions between the immune system and bone. World J Orthop 2011; 2(3): 25-30
- URL: https://www.wjgnet.com/2218-5836/full/v2/i3/25.htm
- DOI: https://dx.doi.org/10.5312/wjo.v2.i3.25
The bone, hematopoietic and immune systems are in deep physical contact and share several common pathways. Inflammatory disease characterized by systemic and local bone loss is an interesting field with which to explore the relationship between activation of the immune system and bone remodelling. In the last few years, investigators have shed light on this topic and T cells have been recognized as key regulators of osteoclast (OC) and osteoblast (OB)[1] formation and activity in different pathological conditions, such as osteoporosis[2], rheumatoid arthritis (RA)[3], bone metastasis[4,5] and periodontitis[6,7].
Estrogen deprivation induces bone loss. At the same time estrogens are well known regulators of the immune system and T cell functions[8,9]. Thus the immune system can be suggested as a key interface between estrogen deprivation and bone metabolism.
The role of the immune system in bone resorption is still controversial: studies on humans are few and the majority of the data have been derived from animal models and cellular cultures. This review aims to summarize the evidences linking immune activation and bone loss with particular attention to humans.
The Receptor Activator of NFkB Ligand (RANKL) and Macrophage Colony Stimulating Factor (M-CSF) are produced by bone marrow stromal cells[10], OBs[11] and activated T cells[2,12]. The co-stimulation by RANKL and M-CSF is essential for the differentiation of monocytes into OCs[13-15].
M-CSF induces the proliferation of OC precursors, differentiation and fusion of more mature OCs and increases the survival of mature OCs. RANKL promotes the differentiation of OC precursors into fully mature multinucleated OCs and stimulates the capacity of mature OCs to resorb bone.
RANKL is a member of the TNF superfamily, present both as a transmembrane and in secreted form. It binds to its physiological receptor RANK expressed on the surface of OC lineage cells. Its action is opposed by osteoprotegerin (OPG), a neutralizing soluble decoy receptor, produced by marrow stromal cells and OBs[13]. Estrogen deficiency induces the imbalance between RANKL and OPG; this phenomenon is important in the genesis of post-menopausal bone loss[15,16].
Some studies questioned the central role of RANKL and suggested the hypothesis that activated T cells could induce osteoclastogenesis by an independent mechanism since saturating concentrations of OPG failed to neutralize more than 30% of OC formation induced by activated T cells[17]. Rifas and Weitzmann discovered a novel cytokine called Secreted Osteoclastogenic Factor of Activated T cells (SOFAT) in activated T cell medium. This cytokine induces both osteoblastic IL-6 production and functional OC formation in the absence of OBs or RANKL, insensitive to the effects of OPG.
The demonstration that SOFAT is a potent inducer of IL-6 production by OBs suggests that it could play a significant role in the local inflammatory response and also could exacerbate bone destruction in rheumatoid arthritis indirectly through multiple IL-6-mediated events[18].
Estrogen deficiency induces bone loss through a complex modification of cytokine production balance. Primarily, researchers observed increased TNFα production by T cells both in ovariectomized mice[19] and postmenopausal women[2,20]. TNFα enhances OC formation by up-regulating stromal cell production of RANKL and M-CSF and by increasing the responsiveness of OC precursors to RANKL[5,21]. Besides, other studies showed a key role of T cell-produced TNFα in rheumatoid arthritis[3,22], multiple myeloma[23,6] and bone metastasis[4,5]. The effect of TNFα on osteoclastogenesis is up-regulated by IL-1[24]; this cytokine production increases after menopause[20,25], enhancing RANKL expression by bone marrow stromal cells and directly promoting OC differentiation. In fact, treatment with IL-1 receptor antagonist decreases OC formation and bone resorption in ovariectomized mice[26,27], whereas the blockade of both TNFα and IL-1 reduce bone resorption in post-menopausal osteoporosis[28].
IFNγ role in osteoclastogenesis has been hard to define: this cytokine has an anti osteoclastogenic effect in vitro[29] and in vivo in nude mice models[30,31]. Studies in humans indicate an increased level of IFNγ during estrogen deficiency[32,33], in leprosy and rheumatoid arthritis with bone erosions[34,35]. Moreover, data from randomized controlled trials have shown that IFNγ does not prevent bone loss in patients with RA[36,37] nor the bone wasting effect of cyclosporin A[1]. These data are explained by the finding that IFNγ influences OC formation both via direct and indirect effects[32]. It directly blocks OC formation targeting maturing OC[38] and also induces antigen presentation and thus T cell activation. When IFNγ levels are increased in vivo, activated T cells secrete pro-osteoclastogenic factors and this activity offsets the anti-osteoclastogenic effect of IFNγ[39].
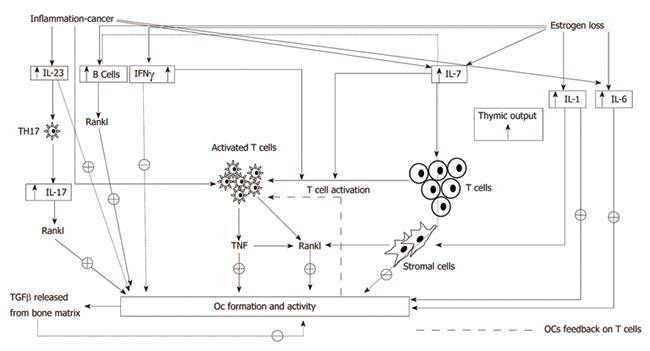
T cells also produce interleukin-7 (IL-7), a cytokine able to enhance B and T cell number and reactivity to antigenic stimulus[40,41]. Some studies have demonstrated that IL-7 promotes osteoclastogenesis by up-regulating T cell-derived osteoclastogenic cytokines including RANKL[42-44] and that the production is up-regulated by estrogen deficiency. In vivo IL-7 blockade is proven to suppress T cell expansion and TNFα and IFNγ production, preventing bone loss due to estrogen deprivation[45,46]. In healthy humans, the expression of IL-7 receptors on T lymphocytes is strictly related to their ability to induce OC formation from peripheral blood mononuclear cells (PBMC)[47]. A comprehensive summary of these pathways can be found in Figure 1.
Both acute and chronic inflammatory diseases in the periosseous tissues are well known to cause bone damage as inflammation increases the OC number and activity. The activation of T cells in autoimmunity or during infection increases RANKL production and promote osteoclastogenesis. The process can be reverted by the administration of OPG in several pathological conditions such as RA and periodontitis[48]. Particularly in autoimmune arthritis, T cell subsets have been examined and Th17 cells, a specialized inflammatory subset, have been identified as responsible for osteoclastogenesis regulation. An inflammatory milieu induces naïve T cells to differentiate into Th17, capable to produce RANKL, TNFα and IL-17, a cytokine that increases RANKL expression by OBs[49].
Researchers recently began to investigate a possible direct role of dendritic cells (DC) in inflammation-related bone damage. DCs are known for their role of antigen presenting cells (APCs) and do not appear to play a role in bone homeostasis in non-pathological conditions, but some data suggest that DC could act as OC precursors in an inflammatory milieu, transforming into DC-derived-OC according to phenotypic and functional characterization studies. Moreover, DCs modulate T cell activity through RANK/RANKL pathway and other cytokines associated with osteoclastogenesis[50-52]. There is a lack of definitive evidence about the physiological relevance of this phenomenon in vivo but DCs could act as an osteo-immune interface, contributing to bone loss in inflammatory diseases.
On the other hand investigators have focused on the role of B-lymphocytes in periodontal inflammation. The host immune response is partly responsible for the bone destruction in cases of periodontitis and the RANK/RANK/OPG signalling axis is important both in bone and immune system communication. Data suggest that B-lymphocyte involvement in the adaptive immune response contributes to bone resorption by up-regulating of RANKL expression through Toll-like receptor pathways. These data align with the known ability of T cells to produce RANKL in the presence of immune stimulus and to increase osteoclastogenesis[53].
Other studies focused on psoriatic arthritis, a chronic inflammatory disease characterized by joint erosions mediated by OCs. These OCs seem to derive from CD14+CD16+ circulating monocytes, present at higher level in patients than in healthy controls when exposed to OC-promoting microenvironment (M-CSF and RANKL). OCs do not derive from this population in healthy controls; thus CD16 can be considered a marker of OC precursors in arthritis[54].
OC precursors circulate within the mononuclear fraction of peripheral blood[2,4,55-57]. This population acts not only as a reservoir for replenishing the pre-OC pool in the bone marrow but also as a potentially abundant source of pre-OCs that can be recruited into bone or joint tissue in response to reparative or pathological signals. On this basis OCs can be considered as immune cells attracted in bone by stimulatory cytokines, expressed on accessory cells and undergoing specific differentiation.
For a long time, osteoimmunology focused on OC regulation by T cells. Recently investigators have paid attention to the feedback action of OCs on T cells. Kiesel et al. observed that OCs are able to present antigenic peptides to T cells and induce FoxP3 expression in CD8+ T cells, thus originating Treg CD8+ cells able to regulate inappropriate activation of the immune response[58].
Senthilkumar et al[59] suggest that the cellular responses in cell-to-cell interactions between T cells and OCs are regulated through reciprocal CD137/CD137L and RANK/RANKL interactions. CD137 is a co-stimulatory member of the TNF receptor induced by T cell receptor activation, characterized by the ability to transduce signals in both directions, through the receptor and into the cell that expresses the ligand. Its ligand CD137L is expressed on APCs and OC precursors; in vitro CD137L ligation suppresses osteoclastogenesis through the inhibition of multi-nucleation. On the other hand, RANKL expressed on T cells bind to RANK on OCs, producing a reverse signal in T cells able to enhance apoptosis.
Periprosthetic osteolysis is another important research field to understand the reciprocal interactions of OC and T cells. Periprosthetic osteolysis patients show T cell-dependent osteoclastogenesis in PBMC cultures; in fact the process was inhibited by RANK-Fc and T cell depletion[60]. In periprosthetic tissues local CD8+ T cells showed a regulatory phenotype, expressing CD25 and FoxP3, while CD4+ T cells did not express activation markers. These data suggest that in an early stage T cells promote osteoclastogenesis, while subsequently OCs activate FoxP3/CD8+ T cells which inhibit CD4+ effector T cells.
The role of estrogen in the regulation of immune function has been demonstrated in animals and humans: immune cells are more responsive to antigenic stimulus in hormone replacement therapy users than non-users[61].
Our group demonstrated that T cells from post-menopausal women show blunt reaction to immune stimulation in respect to pre-menopausal healthy women. At baseline, T cells are more active than in healthy post- and pre-menopausal controls: this implies their greater ability to produce RANKL and TNFα, thus inducing OC formation and activity[2]. We have also demonstrated that OC formation is abolished in T cell-depleted PBMC cultures and this phenomenon is reversed only by the addition of M-CSF and RANKL in cultures.
Grcevic et al[62] suggested a probable role for resting T cells in blunting OC formation; CD4+ and CD8+ T cell-depleted mice have an increased OC formation rate since OPG production is suppressed. T cell-deficient mice show an increased number of OC in basal conditions and a reduced bone density when compared to controls[42].
Estrogen withdrawal up-regulates TNFα production by T cells through a complex pathway involving the thymus and bone marrow. In the bone marrow, ovariectomy promotes T cell activation by increasing antigen presentation by macrophages and DCs[33,63]. Several studies showed the central role of T cells-produced TNF in bone loss induced by estrogen deficiency; in the murine model ovariectomy increases the number of bone marrow T cell-producing TNF[19,64,65]. In fact, ovariectomy stimulates the expression of the gene encoding Class II TransActivator (CIITA), a transcriptional coactivator acting on the MHCII promoter, with the final effect of up-regulation of expression of MHCII on macrophages[33,66,67]. This process proved essential since data show that ovariectomy induces rapid bone loss in wild type (wt) mice but failed to do so in TNFα-deficient [TNFα (-/-)] mice and in T cell-deficient nude mice. Bone loss was restored by adoptive transfer of wt T cells but not by reconstitution with T cells from TNFα (-/-) mice[19], the bone-wasting effect of TNFα is mediated by the p55 TNFα receptor. In fact, ovariectomy caused bone loss in wt mice and in mice lacking p75 TNFα receptor but failed to do so in mice lacking the p55 TNFα receptor[19].
Moreover, RANKL-expression on lymphocytes and marrow stromal cells is significantly elevated during estrogen deficiency in humans and correlates directly with increases in bone resorption markers and inversely with serum estrogen levels[16].
These data demonstrate the causal relationship between estrogen deprivation, T cell activation with increased production of cytokines, and bone resorption.
Estrogen withdrawal has effects on the B cell compartment as well. In ovariectomized mice B220+ IgM-population expands and can differentiate into OC[68-70]. Activated B cells over-express RANKL, contributing to bone resorption[71].
Bone is a dynamic tissue that is constantly formed and resorbed; bone turnover is due to continuous and cyclic bone resorption followed by apposition. These processes are due to the coordinated actions of OC and OB. The action of these two cell types is regulated by paracrine and endocrine factors. In physiological conditions, OB and OC activity is coupled so that the amount of resorption is equal to the amount of formation. However, in pathological conditions or during senescence, resorption is higher than formation, leading to bone loss. OC and OB formation, as well as the coupling between these two cell types, are mediated mainly through cytokines.
Cytokines have pleiotropic functions and regulate several organs and systems. In the last decade several investigators have paid attention to the relationship between estrogen withdrawal, cytokines production, the immune system and skeleton. Today the majority of the data have been obtained in animal models but in recent years, new evidence has been accumulated in humans towards a profound link between estrogen deprivation, immune system deregulation and bone loss. If this relationship is confirmed by future work, postmenopausal osteoporosis should be regarded as an inflammatory disorder sustained by a chronic mild decrease in T cell tolerance.
The relationship between the immune system and bone is complex and depends upon cytokine production and cell to cell contacts. T cells and OCs appear to be the main players in the mechanisms of bone loss. Future studies are needed to fully understand these relationships in humans.
Peer reviewer: Dr. Andrea Giusti, Department of Gerontology and MusculoSkeletal Science, Galliera Hospital, via Sapeto 11 a 18, Genora, 16100, Italy
S- Editor Sun H L- Editor Roemmele A E- Editor Sun H
| 1. | Mann GN, Jacobs TW, Buchinsky FJ, Armstrong EC, Li M, Ke HZ, Ma YF, Jee WS, Epstein S. Interferon-gamma causes loss of bone volume in vivo and fails to ameliorate cyclosporin A-induced osteopenia. Endocrinology. 1994;135:1077-1083. [PubMed] [DOI] [Full Text] |
| 2. | D’Amelio P, Grimaldi A, Di Bella S, Brianza SZ, Cristofaro MA, Tamone C, Giribaldi G, Ulliers D, Pescarmona GP, Isaia G. Estrogen deficiency increases osteoclastogenesis up-regulating T cells activity: a key mechanism in osteoporosis. Bone. 2008;43:92-100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 242] [Cited by in RCA: 243] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 3. | Kotake S, Udagawa N, Hakoda M, Mogi M, Yano K, Tsuda E, Takahashi K, Furuya T, Ishiyama S, Kim KJ. Activated human T cells directly induce osteoclastogenesis from human monocytes: possible role of T cells in bone destruction in rheumatoid arthritis patients. Arthritis Rheum. 2001;44:1003-1012. [DOI] [Full Text] |
| 4. | Roato I, Grano M, Brunetti G, Colucci S, Mussa A, Bertetto O, Ferracini R. Mechanisms of spontaneous osteoclastogenesis in cancer with bone involvement. FASEB J. 2005;19:228-230. [PubMed] |
| 5. | Roato I, Brunetti G, Gorassini E, Grano M, Colucci S, Bonello L, Buffoni L, Manfredi R, Ruffini E, Ottaviani D. IL-7 up-regulates TNF-alpha-dependent osteoclastogenesis in patients affected by solid tumor. PLoS One. 2006;1:e124. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 56] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 6. | Brunetti G, Colucci S, Pignataro P, Coricciati M, Mori G, Cirulli N, Zallone A, Grassi FR, Grano M. T cells support osteoclastogenesis in an in vitro model derived from human periodontitis patients. J Periodontol. 2005;76:1675-1680. [PubMed] |
| 7. | Colucci S, Brunetti G, Cantatore FP, Oranger A, Mori G, Quarta L, Cirulli N, Mancini L, Corrado A, Grassi FR. Lymphocytes and synovial fluid fibroblasts support osteoclastogenesis through RANKL, TNFalpha, and IL-7 in an in vitro model derived from human psoriatic arthritis. J Pathol. 2007;212:47-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 79] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 8. | Weitzmann MN, Pacifici R. T cells: unexpected players in the bone loss induced by estrogen deficiency and in basal bone homeostasis. Ann N Y Acad Sci. 2007;1116:360-375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 62] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 9. | Straub RH. The complex role of estrogens in inflammation. Endocr Rev. 2007;28:521-574. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1188] [Cited by in RCA: 1340] [Article Influence: 74.4] [Reference Citation Analysis (0)] |
| 10. | Khosla S. Minireview: the OPG/RANKL/RANK system. Endocrinology. 2001;142:5050-5055. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 289] [Cited by in RCA: 233] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 11. | Yasuda H, Shima N, Nakagawa N, Yamaguchi K, Kinosaki M, Mochizuki S, Tomoyasu A, Yano K, Goto M, Murakami A. Osteoclast differentiation factor is a ligand for osteoprotegerin/osteoclastogenesis-inhibitory factor and is identical to TRANCE/RANKL. Proc Natl Acad Sci USA. 1998;95:3597-3602. [RCA] [DOI] [Full Text] [Cited by in Crossref: 3136] [Cited by in RCA: 3054] [Article Influence: 113.1] [Reference Citation Analysis (0)] |
| 12. | Horwood NJ, Kartsogiannis V, Quinn JM, Romas E, Martin TJ, Gillespie MT. Activated T lymphocytes support osteoclast formation in vitro. Biochem Biophys Res Commun. 1999;265:144-150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 327] [Cited by in RCA: 306] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 13. | Kong YY, Yoshida H, Sarosi I, Tan HL, Timms E, Capparelli C, Morony S, Oliveira-dos-Santos AJ, Van G, Itie A. OPGL is a key regulator of osteoclastogenesis, lymphocyte development and lymph-node organogenesis. Nature. 1999;397:315-323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2595] [Cited by in RCA: 2512] [Article Influence: 96.6] [Reference Citation Analysis (0)] |
| 14. | Suda T, Takahashi N, Udagawa N, Jimi E, Gillespie MT, Martin TJ. Modulation of osteoclast differentiation and function by the new members of the tumor necrosis factor receptor and ligand families. Endocr Rev. 1999;20:345-357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 535] [Cited by in RCA: 433] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 15. | Hofbauer LC, Khosla S, Dunstan CR, Lacey DL, Boyle WJ, Riggs BL. The roles of osteoprotegerin and osteoprotegerin ligand in the paracrine regulation of bone resorption. J Bone Miner Res. 2000;15:2-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 853] [Cited by in RCA: 794] [Article Influence: 31.8] [Reference Citation Analysis (0)] |
| 16. | Eghbali-Fatourechi G, Khosla S, Sanyal A, Boyle WJ, Lacey DL, Riggs BL. Role of RANK ligand in mediating increased bone resorption in early postmenopausal women. J Clin Invest. 2003;111:1221-1230. [PubMed] |
| 17. | Weitzmann MN, Cenci S, Rifas L, Haug J, Dipersio J, Pacifici R. T cell activation induces human osteoclast formation via receptor activator of nuclear factor kappaB ligand-dependent and -independent mechanisms. J Bone Miner Res. 2001;16:328-337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 114] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 18. | Rifas L, Weitzmann MN. A novel T cell cytokine, secreted osteoclastogenic factor of activated T cells, induces osteoclast formation in a RANKL-independent manner. Arthritis Rheum. 2009;60:3324-3335. [RCA] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 70] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 19. | Roggia C, Gao Y, Cenci S, Weitzmann MN, Toraldo G, Isaia G, Pacifici R. Up-regulation of TNF-producing T cells in the bone marrow: a key mechanism by which estrogen deficiency induces bone loss in vivo. Proc Natl Acad Sci USA. 2001;98:13960-13965. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 425] [Cited by in RCA: 383] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 20. | Pacifici R, Brown C, Puscheck E, Friedrich E, Slatopolsky E, Maggio D, McCracken R, Avioli LV. Effect of surgical menopause and estrogen replacement on cytokine release from human blood mononuclear cells. Proc Natl Acad Sci USA. 1991;88:5134-5138. [RCA] [DOI] [Full Text] [Cited by in Crossref: 404] [Cited by in RCA: 381] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 21. | Hotokezaka H, Sakai E, Ohara N, Hotokezaka Y, Gonzales C, Matsuo K, Fujimura Y, Yoshida N, Nakayama K. Molecular analysis of RANKL-independent cell fusion of osteoclast-like cells induced by TNF-alpha, lipopolysaccharide, or peptidoglycan. J Cell Biochem. 2007;101:122-134. [RCA] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 117] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 22. | Diarra D, Stolina M, Polzer K, Zwerina J, Ominsky MS, Dwyer D, Korb A, Smolen J, Hoffmann M, Scheinecker C. Dickkopf-1 is a master regulator of joint remodeling. Nat Med. 2007;13:156-163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 934] [Cited by in RCA: 977] [Article Influence: 54.3] [Reference Citation Analysis (0)] |
| 23. | Colucci S, Brunetti G, Rizzi R, Zonno A, Mori G, Colaianni G, Del Prete D, Faccio R, Liso A, Capalbo S. T cells support osteoclastogenesis in an in vitro model derived from human multiple myeloma bone disease: the role of the OPG/TRAIL interaction. Blood. 2004;104:3722-3730. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 115] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 24. | Wei S, Kitaura H, Zhou P, Ross FP, Teitelbaum SL. IL-1 mediates TNF-induced osteoclastogenesis. J Clin Invest. 2005;115:282-290. [PubMed] |
| 25. | Zheng SX, Vrindts Y, Lopez M, De Groote D, Zangerle PF, Collette J, Franchimont N, Geenen V, Albert A, Reginster JY. Increase in cytokine production (IL-1 beta, IL-6, TNF-alpha but not IFN-gamma, GM-CSF or LIF) by stimulated whole blood cells in postmenopausal osteoporosis. Maturitas. 1997;26:63-71. [RCA] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 116] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 26. | Kitazawa R, Kimble RB, Vannice JL, Kung VT, Pacifici R. Interleukin-1 receptor antagonist and tumor necrosis factor binding protein decrease osteoclast formation and bone resorption in ovariectomized mice. J Clin Invest. 1994;94:2397-2406. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 251] [Cited by in RCA: 241] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 27. | Kimble RB, Vannice JL, Bloedow DC, Thompson RC, Hopfer W, Kung VT, Brownfield C, Pacifici R. Interleukin-1 receptor antagonist decreases bone loss and bone resorption in ovariectomized rats. J Clin Invest. 1994;93:1959-1967. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 161] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 28. | Charatcharoenwitthaya N, Khosla S, Atkinson EJ, McCready LK, Riggs BL. Effect of blockade of TNF-alpha and interleukin-1 action on bone resorption in early postmenopausal women. J Bone Miner Res. 2007;22:724-729. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 172] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 29. | Takayanagi H, Ogasawara K, Hida S, Chiba T, Murata S, Sato K, Takaoka A, Yokochi T, Oda H, Tanaka K. T-cell-mediated regulation of osteoclastogenesis by signalling cross-talk between RANKL and IFN-gamma. Nature. 2000;408:600-605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1023] [Cited by in RCA: 996] [Article Influence: 39.8] [Reference Citation Analysis (0)] |
| 30. | Sato K, Satoh T, Shizume K, Yamakawa Y, Ono Y, Demura H, Akatsu T, Takahashi N, Suda T. Prolonged decrease of serum calcium concentration by murine gamma-interferon in hypercalcemic, human tumor (EC-GI)-bearing nude mice. Cancer Res. 1992;52:444-449. [PubMed] |
| 31. | Tohkin M, Kakudo S, Kasai H, Arita H. Comparative study of inhibitory effects by murine interferon gamma and a new bisphosphonate (alendronate) in hypercalcemic, nude mice bearing human tumor (LJC-1-JCK). Cancer Immunol Immunother. 1994;39:155-160. [RCA] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 21] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 32. | Gao Y, Grassi F, Ryan MR, Terauchi M, Page K, Yang X, Weitzmann MN, Pacifici R. IFN-gamma stimulates osteoclast formation and bone loss in vivo via antigen-driven T cell activation. J Clin Invest. 2007;117:122-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 326] [Cited by in RCA: 348] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 33. | Cenci S, Toraldo G, Weitzmann MN, Roggia C, Gao Y, Qian WP, Sierra O, Pacifici R. Estrogen deficiency induces bone loss by increasing T cell proliferation and lifespan through IFN-gamma-induced class II transactivator. Proc Natl Acad Sci USA. 2003;100:10405-10410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 246] [Cited by in RCA: 228] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 34. | Arnoldi J, Gerdes J, Flad HD. Immunohistologic assessment of cytokine production of infiltrating cells in various forms of leprosy. Am J Pathol. 1990;137:749-753. [PubMed] |
| 35. | Firestein GS, Alvaro-Gracia JM, Maki R. Quantitative analysis of cytokine gene expression in rheumatoid arthritis. J Immunol. 1990;144:3347-3353. [PubMed] |
| 36. | Cannon GW, Pincus SH, Emkey RD, Denes A, Cohen SA, Wolfe F, Saway PA, Jaffer AM, Weaver AL, Cogen L. Double-blind trial of recombinant gamma-interferon versus placebo in the treatment of rheumatoid arthritis. Arthritis Rheum. 1989;32:964-973. [RCA] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 75] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 37. | Veys EM, Menkes CJ, Emery P. A randomized, double-blind study comparing twenty-four-week treatment with recombinant interferon-gamma versus placebo in the treatment of rheumatoid arthritis. Arthritis Rheum. 1997;40:62-68. [RCA] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 55] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 38. | Takayanagi H, Kim S, Taniguchi T. Signaling crosstalk between RANKL and interferons in osteoclast differentiation. Arthritis Res. 2002;4 Suppl 3:S227-S232. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 117] [Cited by in RCA: 119] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 39. | Pacifici R. Estrogen deficiency, T cells and bone loss. Cell Immunol. 2008;252:68-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 94] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 40. | Komschlies KL, Back TT, Gregorio TA, Gruys ME, Damia G, Wiltrout RH, Faltynek CR. Effects of rhIL-7 on leukocyte subsets in mice: implications for antitumor activity. Immunol Ser. 1994;61:95-104. [PubMed] |
| 41. | Mackall CL, Fry TJ, Bare C, Morgan P, Galbraith A, Gress RE. IL-7 increases both thymic-dependent and thymic-independent T-cell regeneration after bone marrow transplantation. Blood. 2001;97:1491-1497. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 239] [Cited by in RCA: 216] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 42. | Toraldo G, Roggia C, Qian WP, Pacifici R, Weitzmann MN. IL-7 induces bone loss in vivo by induction of receptor activator of nuclear factor kappa B ligand and tumor necrosis factor alpha from T cells. Proc Natl Acad Sci USA. 2003;100:125-130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 219] [Cited by in RCA: 203] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 43. | Giuliani N, Colla S, Sala R, Moroni M, Lazzaretti M, La Monica S, Bonomini S, Hojden M, Sammarelli G, Barill猫 S. Human myeloma cells stimulate the receptor activator of nuclear factor-kappa B ligand (RANKL) in T lymphocytes: a potential role in multiple myeloma bone disease. Blood. 2002;100:4615-4621. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 201] [Cited by in RCA: 187] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 44. | D’Amelio P, Grimaldi A, Bernabei P, Pescarmona GP, Isaia G. Immune system and bone metabolism: Does thymectomy influence postmenopausal bone loss in humans? Bone. 2006;39:658-665. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 17] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 45. | Weitzmann MN, Roggia C, Toraldo G, Weitzmann L, Pacifici R. Increased production of IL-7 uncouples bone formation from bone resorption during estrogen deficiency. J Clin Invest. 2002;110:1643-1650. [PubMed] |
| 46. | Ryan MR, Shepherd R, Leavey JK, Gao Y, Grassi F, Schnell FJ, Qian WP, Kersh GJ, Weitzmann MN, Pacifici R. An IL-7-dependent rebound in thymic T cell output contributes to the bone loss induced by estrogen deficiency. Proc Natl Acad Sci USA. 2005;102:16735-16740. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 98] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 47. | Gendron S, Boisvert M, Chetoui N, Aoudjit F. Alpha1beta1 integrin and interleukin-7 receptor up-regulate the expression of RANKL in human T cells and enhance their osteoclastogenic function. Immunology. 2008;125:359-369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 26] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 48. | Kong YY, Feige U, Sarosi I, Bolon B, Tafuri A, Morony S, Capparelli C, Li J, Elliott R, McCabe S. Activated T cells regulate bone loss and joint destruction in adjuvant arthritis through osteoprotegerin ligand. Nature. 1999;402:304-309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1291] [Cited by in RCA: 1301] [Article Influence: 50.0] [Reference Citation Analysis (0)] |
| 49. | Sato K, Suematsu A, Okamoto K, Yamaguchi A, Morishita Y, Kadono Y, Tanaka S, Kodama T, Akira S, Iwakura Y. Th17 functions as an osteoclastogenic helper T cell subset that links T cell activation and bone destruction. J Exp Med. 2006;203:2673-2682. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1056] [Cited by in RCA: 1176] [Article Influence: 61.9] [Reference Citation Analysis (0)] |
| 50. | Cutler CW, Teng YT. Oral mucosal dendritic cells and periodontitis: many sides of the same coin with new twists. Periodontol 2000. 2007;45:35-50. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 49] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 51. | Alnaeeli M, Park J, Mahamed D, Penninger JM, Teng YT. Dendritic cells at the osteo-immune interface: implications for inflammation-induced bone loss. J Bone Miner Res. 2007;22:775-780. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 63] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 52. | Alnaeeli M, Teng YT. Dendritic cells: a new player in osteoimmunology. Curr Mol Med. 2009;9:893-910. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 21] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 53. | Han X, Lin X, Seliger AR, Eastcott J, Kawai T, Taubman MA. Expression of receptor activator of nuclear factor-kappaB ligand by B cells in response to oral bacteria. Oral Microbiol Immunol. 2009;24:190-196. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 37] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 54. | Chiu YG, Shao T, Feng C, Mensah KA, Thullen M, Schwarz EM, Ritchlin CT. CD16 (FcRgammaIII) as a potential marker of osteoclast precursors in psoriatic arthritis. Arthritis Res Ther. 2010;12:R14. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 73] [Cited by in RCA: 95] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 55. | Ramnaraine M, Pan W, Clohisy DR. Osteoclasts direct bystander killing of cancer cells in vitro. Bone. 2006;38:4-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 56. | Massey HM, Flanagan AM. Human osteoclasts derive from CD14-positive monocytes. Br J Haematol. 1999;106:167-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 175] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 57. | Shalhoub V, Elliott G, Chiu L, Manoukian R, Kelley M, Hawkins N, Davy E, Shimamoto G, Beck J, Kaufman SA. Characterization of osteoclast precursors in human blood. Br J Haematol. 2000;111:501-512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 92] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 58. | Kiesel JR, Buchwald ZS, Aurora R. Cross-presentation by osteoclasts induces FoxP3 in CD8+ T cells. J Immunol. 2009;182:5477-5487. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 83] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 59. | Senthilkumar R, Lee HW. CD137L- and RANKL-mediated reverse signals inhibit osteoclastogenesis and T lymphocyte proliferation. Immunobiology. 2009;214:153-161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 60. | Roato I, Caldo D, D’Amico L, D’Amelio P, Godio L, Patane S, Astore F, Grappiolo G, Boggio M, Scagnelli R. Osteoclastogenesis in peripheral blood mononuclear cell cultures of periprosthetic osteolysis patients and the phenotype of T cells localized in periprosthetic tissues. Biomaterials. 2010;31:7519-7525. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 21] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 61. | Porter VR, Greendale GA, Schocken M, Zhu X, Effros RB. Immune effects of hormone replacement therapy in post-menopausal women. Exp Gerontol. 2001;36:311-326. [RCA] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 83] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 62. | Grcević D, Lee SK, Marusić A, Lorenzo JA. Depletion of CD4 and CD8 T lymphocytes in mice in vivo enhances 1,25-dihydroxyvitamin D3-stimulated osteoclast-like cell formation in vitro by a mechanism that is dependent on prostaglandin synthesis. J Immunol. 2000;165:4231-4238. [PubMed] |
| 63. | Adamski J, Ma Z, Nozell S, Benveniste EN. 17beta-Estradiol inhibits class II major histocompatibility complex (MHC) expression: influence on histone modifications and cbp recruitment to the class II MHC promoter. Mol Endocrinol. 2004;18:1963-1974. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 48] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 64. | Gao Y, Qian WP, Dark K, Toraldo G, Lin AS, Guldberg RE, Flavell RA, Weitzmann MN, Pacifici R. Estrogen prevents bone loss through transforming growth factor beta signaling in T cells. Proc Natl Acad Sci USA. 2004;101:16618-16623. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 126] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 65. | Roggia C, Tamone C, Cenci S, Pacifici R, Isaia GC. Role of TNF-alpha producing T-cells in bone loss induced by estrogen deficiency. Minerva Med. 2004;95:125-132. [PubMed] |
| 66. | Kim JH, Kim K, Youn BU, Jin HM, Kim N. MHC class II transactivator negatively regulates RANKL-mediated osteoclast differentiation by downregulating NFATc1 and OSCAR. Cell Signal. 2010;22:1341-1349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 27] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 67. | Mueller RB, Skapenko A, Grunke M, Wendler J, Stuhlmuller B, Kalden JR, Schulze-Koops H. Regulation of myeloid cell function and major histocompatibility complex class II expression by tumor necrosis factor. Arthritis Rheum. 2005;52:451-460. [RCA] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 68. | Masuzawa T, Miyaura C, Onoe Y, Kusano K, Ohta H, Nozawa S, Suda T. Estrogen deficiency stimulates B lymphopoiesis in mouse bone marrow. J Clin Invest. 1994;94:1090-1097. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 174] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 69. | An J, Ribeiro RC, Webb P, Gustafsson JA, Kushner PJ, Baxter JD, Leitman DC. Estradiol repression of tumor necrosis factor-alpha transcription requires estrogen receptor activation function-2 and is enhanced by coactivators. Proc Natl Acad Sci USA. 1999;96:15161-15166. [RCA] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 153] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 70. | Yang NN, Venugopalan M, Hardikar S, Glasebrook A. Identification of an estrogen response element activated by metabolites of 17beta-estradiol and raloxifene. Science. 1996;273:1222-1225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 323] [Cited by in RCA: 277] [Article Influence: 9.6] [Reference Citation Analysis (0)] |