Published online Jun 18, 2025. doi: 10.5312/wjo.v16.i6.104853
Revised: February 24, 2025
Accepted: May 21, 2025
Published online: June 18, 2025
Processing time: 165 Days and 18.3 Hours
Congenital scoliosis (CS) is a prevalent spinal deformity with a multifaceted etiology that remains incompletely understood. Recent advances in genetic and epigenetic research have provided novel insights into CS pathogenesis. Herein, we review the current progress in genetics and epigenetics to examine genetic variants, susceptibility factors, and the epigenetic regulatory mechanisms im
Core Tip: Congenital scoliosis (CS) is a common spinal deformity with complex and incompletely understood causes. This review highlights recent advances in genetic and epigenetic research on CS, focusing on the identification of genetic variants, susceptibility factors, and their regulatory mechanisms. By examining genetic markers, chro
- Citation: Zhao R, Zhao JR, Xue X, Ma D. Deciphering the etiology of congenital scoliosis: A genetic and epigenetic perspective. World J Orthop 2025; 16(6): 104853
- URL: https://www.wjgnet.com/2218-5836/full/v16/i6/104853.htm
- DOI: https://dx.doi.org/10.5312/wjo.v16.i6.104853
Congenital scoliosis (CS) is a spinal deformity caused by abnormal vertebral development during early embryogenesis (4-6 weeks of gestation)[1]. It affects 0.5-1 per 1000 individuals, accounting for about 5.19% of spinal deformities[2]. Clinically, CS is classified into three types: Type I (vertebral formation defects, e.g., hemivertebrae), type II (segmentation abnormalities, e.g., block vertebrae), and type III (mixed formation and segmentation defects)[3]. CS is typically characterized by early onset, rapid progression, severe deformity, and frequent complications, including cardiopulmonary dysfunction and neurological impairment in advanced cases. This leads to a significant impact on patient quality of life[4]. The significant physical and mental health burdens of CS highlight the need for research into its etiology and pathogenesis to enable early intervention and potentially mitigate progression.
Genetic factors are considered key drivers of CS etiology, with several genetic defects being implicated in the disorder[5]. Moreover, various environmental influences such as hypoxia[6] and high-altitude exposure[7] have been associated with an increased risk of CS. Epigenetics, the study of heritable changes in gene expression independent of alterations in DNA sequence, offers additional insights into CS pathogenesis. The primary epigenetic mechanisms include DNA methylation, histone modification, non-coding RNAs (ncRNAs), and chromatin remodeling, which collectively regulate gene expression across various biological stages, such as replication, transcription, and translation. Despite extensive research conducted over recent decades, the pathogenic mechanisms underlying CS remain largely elusive. Therefore, this study aims to present new perspectives and potential directions for both research and clinical applications by summarizing the recent advances in the field of epigenetics as they relate to CS.
The genetic susceptibility of CS has been substantiated through multiple studies, largely implicating specific gene mutations. CS patients frequently exhibit familial predisposition, with both autosomal dominant and recessive inheritance patterns observed. The early evidence of the genetic nature of CS can be traced back to 1936, when Haffner[8] documented CS in identical twins, highlighting the hereditary aspect of the disorder. Chromosomal analyses have since revealed that genomic deletions in regions such as 2p13-13, 6q13, and 15q12 are associated with CS[9]. Structural variations in the genome, specifically copy number variations (CNVs), also play a role in various disease states, including scoliosis. Notably, a 16p11.2 microdeletion, which encompasses the T-box transcription factor 6 (TBX6) gene, is related to CS. Compound mutations or specific TBX6 haplotypes involving this microdeletion account for approximately 5%-10% of CS cases across diverse populations[10,11]. When one monozygotic twin is affected, it seems likely that the underlying mechanism is an extrinsic insult rather than genetic. Various environmental factors have also been implicated in CS, including hypoxia and carbon monoxide (CO) exposure. In mice, maternal hypoxia can induce congenital vertebral and rib malformations dependent on the stage of somitogenesis[12]. This is consistent with treatment of pregnant mice with CO producing the highest incidence of spinal malformations at 9.5 days of gestation, which corresponds with tho
Additional CNVs linked to congenital vertebral malformations (CVMs) have been discovered at specific loci, including 10q24.31, 17p11.2, 20p11, and 22q11.2[15,16]. Although the prevalence of CNVs outside of 16p11.2 in CS remains unclear, a study involving 67 patients with congenital hemivertebrae identified 12 scoliosis-associated genes, as well as several rare and recessive CNVs. Chromosomal aneuploidies, including trisomy 22, and specific deletions, such as a 10 Mb deletion of 9q33.1q34.11, have been associated with severe, non-isolated vertebral anomalies.
In one study, the genetic underpinnings of congenital syndromes further found that approximately 3.4% of 237 families had at least two affected members[17]. Moreover, another investigation was conducted to meticulously detail identical twin brothers, one diagnosed with CS and the other exhibiting mild left-sided thoracic scoliosis (10°)[18]. Subsequently, a separate report elucidated three pairs of identical twins manifesting a spectrum of scoliosis presentations[4]. Both twins in the first pair developed scoliosis consequent to hemivertebral malformations. The second pair included one twin with thoracic scoliosis at T10-T11, whereas the other was healthy. The third pair included a twin with scoliosis associated with hemivertebrae at T9 (left) and L4 (right), while the other twin was healthy. These findings further underscore the large genetic components of CS, demonstrated by the heterogeneity in clinical presentation even among genetically identical individuals.
Genetic susceptibility underlying CS is extensive, with numerous studies identifying a range of genetic variants associated with its pathogenesis. These findings have significantly enhanced our knowledge of the genetic basis of CS, providing critical knowledge that may improve early diagnosis and selective therapy. Ongoing research increasingly emphasizes the complex interactions of genetic predisposition and environmental factors in CS, with the aim to refine better prevention and treatment modalities.
Genetic linkage analyses, candidate gene approaches, and genome-wide association studies have been robust methodologies for examining CS under the context of polygenic disease frameworks. Single nucleotide polymorphisms (SNPs), discovered in the human genome at the frequencies exceeding 1%[19], are in both coding and non-coding regions. These variations may affect various processes such as exon splicing and transcription regulation. SNPs have been linked to a wide array of prevalent diseases in the general population, including lumbar disc degeneration[16] and CS[20].
Genes associated with CS development: The fibroblast growth factor receptor 1 (FGFR1) gene encodes a transmembrane receptor belonging to the cytokine receptor family. Structurally, it comprises three extracellular immunoglobulin-like domains (D1, D2, and D3), a transmembrane helix, and an intracellular domain featuring tyrosine kinase activity[21]. This receptor is integral to early human embryonic development, particularly in protoganglionic embryo formation, organ specification, and tissue patterning[22]. Loss-of-function mutations in FGFR1 have been identified in patients with Kallmann syndrome, a condition often linked to distinct skeletal anomalies, including oligodactyly, hemivertebrae, and pterodactyly[23]. Additionally, FGFR1 signaling plays a crucial role throughout osteoblast differentiation, and mouse models with FGFR1 mutations display a range of vertebral anomalies, particularly in the cervical through lumbar regions, thus making FGFR1 a plausible candidate gene for CS[24]. Patients with FGFR1 mutations exhibited skeletal phenotypes[25,26], including oligodactyly on both feet, fusion of metacarpal bones, hemivertebrae, butterfly vertebrae and split hand/foot malformation. Recent studies have discovered four variants of FGFR1 in a cohort of individuals with CS, including one frameshift variant (c.2334dupC) and three missense variants (c.2339T>C, c.1107G>A, c.1261A>G)[22]. Functional assays revealed that three of these variants (c.2334dupC, c.2339T>C, c.1261A>G) attenuated receptor signaling and suppressed protein expression. Despite the comparatively mild impact on protein levels, these variants were associated with mild skeletal phenotypes.
The TBXT gene is crucial for embryonic development, particularly in such processes as mesodermal specification, notochord formation, somitogenesis, and neural tube development[27]. In a recent study, whole-exome sequencing (WES) of a consanguineous family identified a girl with CS. WES analysis revealed a c.512A>G (p.H171R) missense mutation within exon 3 of the TBXT gene, which encodes a transcription factor of the T-box family. Sanger sequencing verified that this mutation was present in a homozygous state in the affected individual and in a heterozygous state in both parents. Notably, this variation was not observed in the control group of 50 healthy individuals from the Tunisian population. The absence further suggests that the c.512A>G mutation in TBXT could be associated with CS in this patient, implying its involvement in CS pathogenesis[28].
As a member of the T-box gene family, TBX6 functions as a transcription factor essential for proximal mesoderm development and somitogenesis[29]. Pathogenic variants in TBX6 have been consistently linked with CS across various populations. Genotyping analysis of 254 Han Chinese participants, including 127 individuals with CS and 127 controls, identified two SNPs in the TBX6 gene significantly associated with CS. These SNPs - rs2289292 in exon 8 (SNP1) and rs3809624 in the 5’ untranslated region (SNP2) - had P values of 0.017 and 0.033, respectively[30]. Haplotype analysis additionally indicated a significant correlation between the G-A haplotype of SNP1/SNP2 and control status (ratio of 0.71; 95% confidence interval: 0.51-0.99). A further understanding of TBX6’s involvement in CS etiology emerged from an extensive genetic study of 161 Han Chinese patients with sporadic CS and 166 control individuals[31]. TBX6 CNV analysis identified six heterozygous TBX6 null mutations among 17 subjects, including 12 SNPs affecting TBX6 function (one nonsense and four frameshift mutations). Notably, these mutations were not present in the control group. Se
Variations in genes related to the bone morphogenetic protein signaling pathway are associated with a range of skeletal disorders, particularly involving mutations in the growth differentiation factor 3 (GDF3) gene[33]. Among the identified variants, the R266C mutation has pathogenic characteristics. Missense mutations in the GDF3 gene are linked to Klippel-Feil syndrome, a condition marked by cervical spine fusion[34]. In a recent study, five novel mutations were identified through WES in CS patients, including R84C and R84L, both within the first exon of the GDF3 gene[35]. Despite being located at the same locus, these mutations induce distinct amino acid substitutions. The functional predictions from SIFT and Polymorphism Phenotyping v2 classified both variants as pathogenic. Specifically, R84L disrupts GDF3 maturation in the cytoplasm, while R84C appeared to exert minimal impact on GDF3 protein function. However, the substitution of arginine with cysteine in R84C may hinder the pre-peptide cleavage process by altering the folding of adjacent amino acids due to hydrophobic interactions. Therefore, R84L is predicted to play a functional pathogenic role, while R84C is considered a potentially deleterious variant. Moreover, while SIFT and Polymorphism Phenotyping v2 assessed three additional variants - S212L, N215S, and A251T - as benign, molecular analyses still indicated that these loci might exert a significant influence on downstream signaling or protein maturation. As such, these variants can be classified as functional polymorphisms or potentially pathogenic mutations[35].
In a zebrafish model, deletion of protein tyrosine kinase 7 was linked to CS. Four likely deleterious variants in five out of 583 CS patients were identified: one frameshift mutation, c.464_465delAC; and three missense mutations, c.1394A>G, c.1879G>A, c.1955G>T[36]. The c.464_465delAC variant is particularly noteworthy for its role in causing loss-of-function in protein tyrosine kinase 7. Additionally, the SRY-box transcription factor (SOX) gene family member SOX9 has been implicated in CVM[37]. SOX9 mutation analysis in CVM patients may provide an insight into scoliosis pathogenesis and the mechanism behind SOX9-associated skeletal deformities. A previously unreported rare heterozygous missense mutation in the SOX9 gene (NM_000346.3: C.1405A>G, p.M469V) was discovered in three patients[37]. These individuals exhibited clinical manifestations of scoliosis without accompanying systemic malformations. Thus, the mutation in SOX9 at position p.M469V might enhance CVM pathogenesis independently of other systemic anomalies, providing important insight into phenotypic variability within skeletal malformations due to the SOX9 gene. The myosin heavy chain 3
Genes associated with signaling and regulation: In recent years, numerous genetic loci and modifier genes have been increasingly recognized as pivotal contributors to the etiology of CS. Experimental knockout and mutation studies in murine models have implicated a multitude of genes in the development of vertebral malformations. A significant proportion of these genes are implicated in the Notch signaling pathway, where inactivating mutations are associated with the occurrence of hemivertebrae, rib fusion, segmentation anomalies, and thoracic vertebral fusion. The Notch pathway plays a critical role in the establishment of intercellular boundaries. Research in human genetics has uncovered several important genes associated with spinal and rib deformities, including Mesoderm Posterior bHLH transcription factor 2, Hes family bHLH transcription factor 7 (HES7), Lunatic Fringe (LFNG), and Delta like canonical Notch ligand 3 (DLL3)[39-41]. Notably, these cases are associated with SCD, primarily attributed to severe segmentation failures of the spine and ribs. DLL3, a unique member of the DSL family of Notch ligands, which functions not to activate signaling in neighboring cells but rather to inhibit it when expressed in cells possessing the same Notch receptor. Mutations in the DLL3 gene have been linked to SCD, and targeted deletion of DLL3 in murine models results in significant somatic segmentation defects, severely disrupting vertebral body formation[42]. HES7, a bHLH gene whose downstream Notch effector is essential for somitogenesis, displays cyclic mRNA expression in the presomitic mesoderm. As revealed by spatial analyses, HES7 and LFNG transcription occurs in those regions devoid of HES7 protein[43]. HES7 expression within the presomitic mesoderm is dynamic, exhibiting variability, even among embryos at the equivalent stages of development[44]. This expression pattern suggests a cyclic nature, as observed in two bilateral regions (rostral and caudal stripes), potentially contributing to the pronounced deformities of the spine and ribs[39]. Notably, the rs302729 SNP locus is located within the 5’ untranslated region, while the rs1442849 locus is located in the 3’ untranslated region, both of which are involved in mRNA transcription and splicing. Analysis of the influence exerted by HES7 gene on CS patients within the Chinese Han population has revealed correlations between the rs1442849 and rs302729 polymorphisms and the susceptibility to CS. Further analyses have revealed a significant association between the Ha3-GA haplotype and an elevated risk of developing congenital disorders[45].
WES analysis on 16 samples collected from five Japanese patients with congenital disorders and their healthy parents or siblings[46] identified four de novo variants, subsequently validated through Sanger sequencing. These variants included one frameshift mutation in the SHISA3 gene and three missense mutations in ATP/GTP binding protein like 5, histone deacetylation 4, and phosphodiesterase 2A. Furthermore, exome sequencing was performed to reveal a solitary mutation in the molybdenum cofactor sulfurase gene. In a CS patient with both spinal cord atrophy and lower limb dominance, a mutation in DYNC1H1 was identified via WES. WES was applied to detect a congenital disorder patient with two novel missense variants in the LFNG gene. Importantly, only one mutation in LFNG (c.564C>A, p.Phe188 Leu) has been previously reported, which is known to cause scoliosis under the homozygous condition (SCD3, MIM#609813)[47]. In contrast, CS patients harboring LFNG mutations (c.467T>G; p.Leu156Arg and c.856C>T; p.Arg286Trp) exhibited severe scoliosis attributed to the deformities in both the thoracic and lumbar vertebrae, along with multiple rib abnormalities. To elucidate the role of collagen type XI alpha 2 chain (COL11A2) in vertebral development, studies utilized CRISPR/Cas9 technology to generate nonsense mutations (COL11A2L642*) and full locus deletions (COL11A2del) in zebrafish. Vertebral fusion occurred in the caudal vertebrae of both COL11A2L642*/L642* and COL11A2del/del mutant ze
Genes associated with cell function and regulation: The dual serine/threonine and tyrosine protein kinase (DSTYK) is broadly expressed across vertebrate species, and its absence has been found to enhance phosphorylation of transcription factor EB (TFEB), which plays a crucial role in regulating lysosomal biogenesis. This enhancement subsequently inhibits the nuclear translocation of TFEB. Furthermore, an analysis of DSTYK mutants shows a significant decrease in TFEB expression level and various genes targeted by lysosomes. These results suggest that the dysregulation of TFEB function could play a role in the pathogenesis of congenital disorders linked to DSTYK mutations[49]. Several genes, including SHOX, Trk family members, PAX1, DHX40, NBPF20, RASA2, and MYSM1, have already been linked to scoliosis syndromes or are known to play roles in skeletal and spinal development[50]. Additionally, genetic variants within the WNT3A[51] and PAX1[52] genes may not significantly contribute to CS susceptibility or to the varied clinical phenotypes observed within the Chinese Han population.
CS arises from abnormalities in spinal development, and recent investigations have elucidated several genes associated with this condition, providing critical insights into its pathogenesis. In addition to deepening our comprehension of the mechanisms underlying congenital disorders, the discovery of various genes associated with spinal development also opens up new avenues for early diagnosis and tailored treatment strategies. Vital for improving patient prognosis and quality of life, these discoveries integrate genetic insights into CS research and lay a foundation for future therapeutic strategies. With advancements in genomic technologies, additional relevant genes can be identified to further enrich our comprehension of this complex disorder. Table 1 lists the genes with the most compelling findings.
| Gene name | Gene function | Associated research | Mutation type | Associated clinical manifestations | Ref. |
| FGFR1 | Involved in early human embryo development and plays an important role in the formation of protoganglionic embryos, organ specification and tissue patterning | Mutations are associated with CS and skeletal phenotypes | Shift code variants, missense variants | Vertebral malformations, and scoliosis | [22] |
| TBXT | Plays a crucial role in embryonic development, especially in mesoderm formation, notochord development, somite formation, and neural tube closure | Mutations are associated with a wide range of congenital malformations, especially developmental abnormalities of the spine and nervous system | Missense mutations | Scoliosis | [27,28] |
| TBX6 | Transcription factor involved in mesodermal development | Associated with genetic variation in spinal development | Nonsense mutations, code-shifting mutations | Scoliosis and other deformities | [11,29,31] |
| GDF3 | Plays an important role in skeletal development and morphogenesis | Genetic variants are associated with multiple skeletal disorders | Missense mutations | Scoliosis, congenital skeletal malformations | [35] |
| PTK7 | Regulates embryonic and skeletal development | Associated with scoliosis | Shifted code mutation, missense mutation | Scoliosis, skeletal deformities | [36] |
| DLL3 | Plays a role in cell-cell interactions | Mutations associated with spinal dysplasia | Deletion mutations | In spinal dysplasia | [42] |
| HES7 | Involved in vertebral development and differentiation | Mutation is associated with scoliosis and other spinal deformities | Point mutations | Abnormalities in spinal structure | [39,43] |
| LFNG | Involved in the regulation of cell signaling and development, with important roles primarily in the spinal cord and central nervous system | Mutations are strongly associated with specific scoliosis phenotypes | Missense mutations | Scoliosis, vertebral deformities and multiple rib deformities | [47] |
| COL11A2 | Development of the spine and joints | Associated with multiple spine-related disorders such as scoliosis and other skeletal developmental abnormalities | Nonsense mutations | Scoliosis and other skeletal developmental abnormalities | [48] |
| DSTYK | Involved in the regulation of cell signaling and developmental processes, particularly important during embryonic development | Associated with scoliosis | Missing variant | Scoliosis, spinal dysplasia, skeletal deformities | [49] |
| SOX9 | Promotes chondrocyte differentiation during embryonic development | Mutations are associated with the development of several genetic syndromes and spinal deformities | Scoliosis | [37] | |
| DHX40 | Plays an important role in the regulation of gene expression | Mutations associated with scoliosis | Scoliosis, other congenital malformations | [50] | |
| NBPF20 | Involved in the regulation of gene expression, cell differentiation and development | Mutation associated with scoliosis | Scoliosis | [50] |
Epigenetics refers to the heritable changes in gene expression that occur without modifications to the DNA sequence. These changes are observable during both mitotic and meiotic processes. These epigenetic modifications can influence gene expression at various stages, such as replication, transcription, and translation. Alterations to gene expression patterns regulated by epigenetic mechanisms can result in autoimmune diseases, cancer, and numerous other conditions. This study presents a summary of the existing research that clarifies the connection between epigenetics and the development of congenital disorders. Additionally, current studies are classified according to the four main mechanisms of epigenetic variation, emphasizing significant alterations along with the relevant mechanistic theories and regulatory pathways. To conduct a thorough literature review, we employed different keywords, such as “congenital scoliosis”, “epigenetics”, “DNA methylation”, “histone modification”, “chromatin remodeling”, “non-coding RNA”, “miRNA”, “lncRNA”, and “circRNA”, within PubMed, Embase, and Web of Science databases searched up to September 2024. The criteria for inclusion in this study are as follows: (1) Clinical or basic research studies related to the listed keywords; and (2) Articles published in journals without language restrictions. Exclusion criteria include studies that: (1) Are not available in full text; (2) Are of low relevance to the subject; or (3) Contain vague descriptions or insufficient evidence-based evaluations. In total, we identified 68 relevant papers, 13 of which satisfy the inclusion criteria for review. The complete process of literature search is depicted in Figure 1.
DNA methylation represents a crucial epigenetic modification that can modulate gene expression and genomic function through the addition of methyl groups at specific sites within the DNA molecule. This process occurs predominantly at the cytosine residues of CpG dinucleotides, which are commonly situated at transcriptional start sites of genes[53]. As a pivotal epigenetic alteration, DNA methylation regulates the activity of DNA molecules without affecting their structural integrity. Under physiological conditions, DNA methylation maintains a dynamic equilibrium[54], which enables the selective silencing of genes in accordance with cellular requirements, thereby preserving gene expression homeostasis. Conversely, under pathological circumstances, aberrant DNA methylation patterns can lead to dysregulated gene expression. This dysregulation contributes to the improper expression of essential downstream effectors, potentially triggering abnormal cell proliferation or apoptosis. This may ultimately play a role in the development of scoliosis.
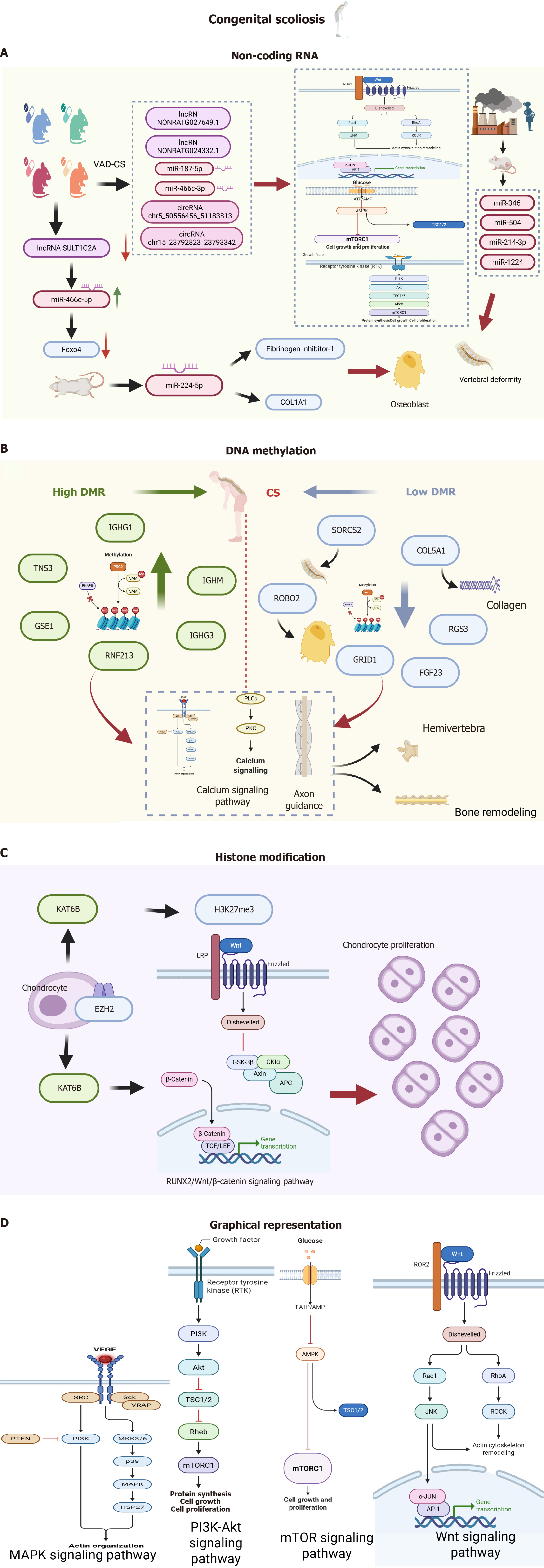
Role of methylation in signaling pathway regulation: A series of differentially methylated regions exist between scoliosis patients and normal controls, with some significant differences. The study of differences in both common and rare SNPs, as well as methylation patterns in twins, uncovered a link between CS and allele-specific methylation in the SVIL gene[55]. Further analysis of functional enrichment found that the genes linked to differentially methylated regions were significantly involved in multiple signaling pathways, including the mitogen-activated protein kinases (MAPK) pathway, calcium signaling pathway, and axon guidance pathway[56].
The MAPK signaling pathway regulates proliferation and differentiation of a variety of cell types, including bone progenitor cells[57], and mutations in MAPK pathway genes have been associated with a variety of disorders that manifest as scoliosis, such as Noonan syndrome[58], Costello syndrome[59], and cardiofaciocutaneous syndrome[60]. Transcriptional regulation, which encompasses microRNA (miRNA) and DNA methylation of the genes linked to the MAPK signaling pathway, has been connected to scoliosis. Consequently, abnormal DNA methylation in genes in this pathway may result in the development of hemi-spine conditions[61]. Calcium metabolism is vitally important for bone formation[62], and abnormal calcium signaling pathways can induce osteoblast dysfunction and scoliosis[63]. Axon guidance proteins are capable in directing axon growth during development[64], and defective axon guidance may lead to several disorders that manifest as scoliosis[65]. Abnormalities in these pathways have the potential to increase the risk of developing hemi-spine. However, further functional investigations are required to elucidate these mechanisms.
Methylation of key genes: The abnormal methylation of key genes during bone formation and development is an important influencing factor in scoliosis development. For clinical analysis, two pairs of identical twins were selected, leveraging Agilent SureSelect XT human methyl sequencing along with thorough validation experiments[66]. A total of 75 promoter regions in the CS group exhibited significant differences in methylation levels compared to the control group. Specifically, 24 patients showed elevated methylation levels while 51 demonstrated reduced levels (P < 0.05). CS patients exhibited increased methylation in the promoter region of tensin-3 (TNS3), thus suppressing TNS3 expression within the cartilage layer of the articular eminence. These findings suggest that TNS3 gene methylation and its corresponding protein could be crucial for CS development. Methylation sequencing of targeted regions was performed using peripheral blood samples from two pairs of identical twins[67]. Patients with CS had lower levels of DNA methylation and higher mRNA levels of the FGF23 gene compared to the control group. Notably, higher FGF23 mRNA levels in CS patient peripheral blood exhibited a negative correlation with computed tomography (CT) values, implying an important association. Receiver operating characteristic curve analysis of FGF23 mRNA levels revealed high sensitivity and specificity for diagnosing CS, suggesting that FGF23 levels in peripheral blood were significantly higher in such patients, causing reduced bone mineral density. Moreover, the heightened levels of peripheral blood FGF23 demonstrated a significant predictive capacity for CS.
These findings suggest significant methylation pattern changes in multiple genes of CS patients compared to a control population, especially in key genes such as TNS3 and FGF23. Observed aberrations in methylation status may affect expression of these genes, thus impacting the associated signaling pathways. These genes may be integral to CS pathogenesis and pathological progression and may emerge as potential biomarkers and therapeutic targets in the future, thereby holding massive potential in clinical applications.
NcRNAs are a crucial class of non-coding regulatory transcripts that encompass three primary subclasses: long ncRNAs (lncRNAs), miRNAs, and circular RNAs (circRNAs). MiRNAs facilitate target mRNA degradation or translation inhibition by directing RNA-induced silencing complexes through base pairing, while lncRNAs and circRNAs exert regulatory influence at multiple levels[68,69]. These include modulation of gene expression through DNA methylation, histone modification, transcription factor recruitment, and miRNA sponging, as well as the lncRNA-mediated regulation of mRNA stability[70]. Furthermore, circRNAs can modulate gene expression by acting as sponges for miRNAs, thereby affecting transcription, selectively altering splicing, and directly interacting with RNA-binding proteins to enhance protein translation through rollover amplification. NcRNAs are essential for regulating and coordinating critical cellular processes, including proliferation, apoptosis, autophagy, differentiation, metabolism, migration, and invasion[71]. Therefore, it is reasonable that ncRNAs are frequently dysregulated in a myriad of diseases, encompassing congenital diseases, endocrine and metabolic disorders. From a clinical perspective, the fluctuating levels of ncRNA in various bodily fluids during disease progression, such as saliva, blood, and urine, have prompted investigations into their potential as valuable biomarkers for early diagnosis and prognosis. Growing evidence has suggested that abnormal expression of ncRNAs is significantly involved in the development of orthopedic conditions, including osteosarcoma, osteoporosis, osteoarthritis, and the degeneration of intervertebral discs[72-75]. Additionally, recent findings suggest that ncRNAs may be dysregulated in scoliosis, suggesting their involvement in disease pathogenesis[75-77]. Although ncRNAs have been implicated in CS pathogenesis, the competitive endogenous RNA (ceRNA) regulatory network in CS remains largely unknown. Chen et al[78] performed sequencing to investigate ncRNA expression in rat embryos (day 9 of gestation) after vitamin A deficiency (VAD) [n = 9 in the VAD-induced CS (VAD-CS) group and n = 4 in the control group]. Expression levels of 749 mRNAs, 56 miRNAs, 685 lncRNAs and 70 circRNAs were significantly different between the two groups. Wnt, phosphatidylinositol 3-kinase (PI3K)-protein kinase B (Akt), forkhead box O (FoxO), epidermal growth factor receptor (EGFR) and mammalian target of rapamycin (mTOR) were the most important pathways involved in VAD-CS pathogenesis. With circRNA/miRNA/mRNA and lncRNA/miRNA/mRNA networks of CS constructed, the ncRNA-mediated gene expression mechanism was revealed through the ceRNA regulatory network. However, future studies should explore these predicted ceRNAs regarding proteomics and associated signaling pathways, which may eventually lead to the full discovery of the underlying CS mechanism.
CircRNA: In recent years, research has highlighted the possibility of using particular circRNAs found in serum as diagnostic biomarkers for treating diseases associated with scoliosis. A comprehensive genome-wide sequencing analysis of circRNAs was performed to identify 22 circRNAs with differential expression in seven patients with CS compared to three healthy controls[79]. As revealed by subsequent quantitative polymerase chain reaction validation of seven circRNAs, only hsa_circ_0006719 was significantly upregulated in the CS cohort compared to the controls (P = 0.036). Receiver operating characteristic curve analysis revealed that hsa_circ_0006719 had a significant diagnostic value for CS, evidenced by an area under the curve of 0.739 (P = 0.001). This finding supports its potential as a new diagnostic biomarker for CS[79]. Population variability, ethnic diversity, and geographic limitations may introduce bias in eva
LncRNA: Animal model studies have provided new insights into CS. In a complementary study, decreased lncRNA SULT1C2A embryonic expression was observed in a VAD-CS rat model, concomitant with miR-466c-5p up-regulation[80]. Various key somatogenesis-related genes, including Foxo4, Sox9, Pax1, and Nkx3-2, were down-regulated on the ninth day of gestation in the context of maternal VAD. Notably, reduced SULT1C2A expression was associated with increased availability of miR-466c-5p, leading to decreased Foxo4 expression. These findings suggest that aberrant down-regulation of lncRNA SULT1C2A in NAD-CS may contribute to reduced Foxo4 expression by enhancing miR-466c-5p abundance[80]. As a transcription factor belonging to the Foxo family, Foxo4 plays a crucial role in the development of cartilage and skeletal muscle during embryogenesis[81]. Therefore, enhancing lncRNA SULT1C2A expression may serve as a viable approach for addressing VAD-CS.
MiRNA: MiRNAs are small RNA molecules that function via signaling pathways to facilitate the progression of scoliosis. In a comprehensive transcriptome sequencing study, the expression profiles of ncRNAs and mRNAs in VAD-CS rat embryos were compared to those in a control group. Specifically, there were 3 down-regulated miRNAs and 53 up-regulated miRNAs in the VAD embryos. Bioinformatics analysis indicated that several critical signaling pathways were involved in the development of VAD-CS, including FoxO, PI3K-Akt, mTOR, EGFR, and Wnt. Selected ncRNAs were confirmed through real-time reverse transcriptase-polymerase chain reaction, which showed dysregulation of miR-187-5p and miR-466c-3p (both miRNAs), NONRATG027649.1 and NONRATG024332.1 (lncRNAs), as well as chr5_50556456_51183813 and chr15_23792823_23793342 (circRNAs). Enrichment analysis indicated that these differentially expressed RNAs were active in different signaling pathways such as FoxO, PI3K-Akt, mTOR, EGFR, and Wnt[78].
Moreover, numerous research efforts have applied animal models to explore the involvement of miRNAs in scoliosis. In a study involving Ishibashi rats, a notable increase in miR-224-5p expression was observed in the lumbar spine of the CS model compared to that of 42-day-old normal rats[82]. The up-regulation of miR-224-5p was associated with enhanced expression of fibrinogen inhibitor-1 and elevated levels of type I collagen in this CS model, indicating enhanced osteoblast differentiation. Although the study failed to further elucidate target genes of miR-224-5p, this finding suggested that miR-224-5p can be considered a contributory factor in CS development via the modulation of target gene expression affecting osteoblast differentiation.
At present, air pollution exposure has markedly escalated, with emerging evidence indicating that such exposure during pregnancy may contribute to congenital defects in progeny. To elucidate the effect of air pollution on miRNA expression, RNA sequencing was performed on rat embryos at day 9 of gestation, with 204 significantly down-regulated miRNAs and 87 up-regulated miRNAs in the embryos of the air pollution exposure group compared to the control group. As indicated by the predicted roles of these miRNAs, they may interfere with a series of crucial biological processes such as mitotic spindle organization, cellular respiration, glycolytic metabolism, and proteasome activity. Additionally, extensive regulatory interactions involving target genes mediated by miR-346, miR-504, miR-214-3p, and miR-1224 were anticipated. Overall, these results imply that miRNAs play a significant role in the development of air pollution-induced congenital spinal defects through the dysregulation of various biological pathways[83].
Histones, integral components of an octameric structure, undergo a myriad of post-translational modifications facilitated by various histone-modifying enzymes[84]. These modifications encompass a diverse array of biochemical alterations, including acetylation, methylation, lactonylation, phosphorylation, dopamylation, and ubiquitination[85]. Such histone modifications serve to modify the binding sites within specific protein complexes and to influence interactions between histones and DNA, as well as among histones themselves, thus regulating gene expression. Notably, the modifications such as methylation and acetylation are epigenetic alterations that critically impact transcriptional activity[86]. The interplay between post-translational histone modifications and their respective modifiers contributes to intricate epigenetic regulatory frameworks. Recent investigations into CS have progressively elucidated the significant role of histone modifications in this pathophysiological context.
The lysine acetyltransferase 6B (KAT6B) gene, located at chromosome 10q22.2, is composed of 18 exons that code for a 2073 amino acid protein. This protein plays an essential role within the histone acetyltransferase complexes as well as the MOZ/MORF complexes[87]. The N-terminal region of KAT6B is associated with transcriptional activation, whereas the C-terminal region is involved in transcriptional repression. Playing a crucial role in the transcriptional activation me
Nucleosomes consist of a histone octamer surrounded by a stretch of DNA. As general gene repressors, nucleosomes can inhibit transcription initiation[91]. Chromatin remodeling complexes are essential for regulating gene expression, with the energy obtained from the hydrolysis of ATP utilized to alter chromatin structural organization. This is achieved through nucleosome movement, sliding, disruption, or reorganization[92]. The remodeling process entails dissociation of genomic DNA at the nucleosome periphery, resulting in the formation of DNA protrusions on the histone octamer’s surface, the wave-like propagation of DNA loops, and the repositioning of DNA without altering the overall number of histone-DNA interactions.
Chromatin remodeling is crucial for several essential biological functions, including DNA replication, gene tran
The intricate correlation between genetic susceptibility and environmental factors remains inadequately understood, despite numerous studies that have elucidated the underlying biological mechanisms. Genetic variants may modulate the capacity of an individual to respond to environmental stressors, resulting in varying susceptibility to morbidity under different environmental conditions. While gene-environment interactions have long been considered as fundamental to the episodic nature of various human diseases, the concrete evidence supporting these mechanisms has largely remained elusive. Notably, environmental factors significantly impact CS etiology[95]. In particular, maternal exposure to various environmental conditions during pregnancy has a considerable effect on CS development[96]. Various maternal ex
Experimental models of intrauterine hypoxia have demonstrated that environmental stressors notably affect the growth rate and severity of CS in mice with genetic predisposition. Furthermore, mouse embryos with null mutations in DLL1 and Notch1 showed a higher occurrence of vertebral defects after short periods of hypoxic exposure, indicating that these genes could be strong candidates for future sequencing studies in CS patients. This model posits that disruptions to Notch signaling, whether via genetic or environmental factors, can induce inappropriate cell fate transitions, with the combined effects of these influences heightening the probability of aberrant outcomes. Additionally, a zebrafish model of somatic cytogenesis suggests that Notch signaling operates not as a binary switch, but rather through a nuanced gradient. According to this model, genetic reduction in Notch signaling is expected to reduce the sensitivity threshold to hypoxia, which would consequently interfere with somitogenesis under higher oxygen conditions.
The importance of vitamin A nutrition and its derivatives throughout the life cycle is well documented[97], with the need for vitamin A beginning with embryonic life, and vitamin A insufficiency in mothers during pregnancy leading to fetal death or congenital abnormalities in the offspring[98]. The retinoic acid receptor and the retinoid X-like receptor are significant transcription factors that function as retinoid receptors, playing a crucial role in genetically regulating vitamin A activity during developmental processes[99]. Vitamin A metabolism maintains retinoic acid homeostasis[81]. Vitamin A undernutrition in early pregnancy is responsible for several pediatric congenital anomalies[100]. VAD is linked to congenital spinal malformations in rats[101]. Research indicates that VAD can suppress RALDH and retinoic acid receptor expression, which are the critical elements of RA signaling. This represents the initial report suggesting a potential link between prenatal VAD and CS. Nonetheless, extensive epidemiological studies are still required to validate this relationship in humans. Furthermore, it is important to investigate how partial, as opposed to total, vitamin A deprivation influences spinal deformities.
Smoking, a significant environmental factor, impacts the onset and progression of CS through multiple mechanisms. Maternal smoking during pregnancy correlates with a range of fetal congenital malformations, including low birth weight, preterm labor, and increased neonatal morbidity and mortality, as well as CS induction[102]. The generation of free radicals and oxidative stress associated with smoking may result in fetal cellular damage and apoptosis, thereby impairing normal spinal cell proliferation and differentiation. Furthermore, by binding to hemoglobin to form carboxyhemoglobin, CO diminishes the oxygen-carrying capacity of blood, potentially leading to localized hypoxia in both the placenta and fetus, which can adversely affect spinal development. In addition, the chemical composition of cigarettes may influence the expression of genes required for spine development through a range of epigenetic mechanisms, including DNA methylation and histone modification[103]. A comprehensive exploration of the interaction between smoking and CS may provide new insights into the prevention and treatment of CS, thereby improving outcomes for CS patients.
Similarly, alcohol, which is harmful to embryonic development, especially the central nervous system and skeletal system, is recognized as a teratogen and has been extensively researched. Fetal alcohol spectrum disorder includes a range of conditions that may manifest in individuals whose mothers drank alcohol during pregnancy[104]. Studies indicate that four out of eight children diagnosed with fetal alcohol syndrome exhibit scoliosis[105]. This suggests that alcohol plays a role in spinal abnormality pathogenesis. Nevertheless, further research is required to elucidate the specific effects of alcohol on CS development.
Various environmental factors play a role in CS pathogenesis. The mother’s nutritional status, medication use, and exposure to harmful substances during pregnancy may affect normal fetal spine development. Collectively, these factors constitute a multidimensional etiological model that emphasizes the interaction between genetics and the environment. By improving maternal nutrition and reducing exposure to harmful substances, the early identification and intervention of these environmental factors may help reduce the incidence of CS.
Epigenetic regulation plays a crucial role in the initiation and progression of CS. In CS individuals, the abnormal methylation patterns in gene promoters lead to the altered expression of key developmental genes. Studies have shown that abnormal methylation of the promoter region of the SOX9 gene disrupts its expression, which impairs normal spinal development and increases the risk of developing CS. Changes in methylation status can be caused by environmental factors or genetic variations, while SNPs can modify methylation sites or affect methyltransferase activity. Genetic variation may have a direct or indirect impact on epigenetic regulation, with SNPs altering methylation sites or regulating epigenetic enzyme function, ultimately affecting gene expression. In addition, genetic variants can alter the sequence or structure of ncRNA, thereby affecting its function and stability. This intricate interaction is crucial in CS pathogenesis. Future studies will delve deeper into this area, thereby enhancing our understanding of the epigenetic mechanisms of CS.
Epigenetic regulation lays a foundation for CS development. This investigation delves into the intricate interactions between CS and epigenetic mechanisms, emphasizing two primary processes: DNA methylation and the regulation of ncRNAs. By scrutinizing the alterations in epigenetic marker expression and their subsequent effects on signaling pathways, researchers have elucidated the pathological mechanisms underlying CS. However, the scarcity of studies available for cross-validation may compromise the robustness and reliability of the conclusions drawn. Furthermore, numerous investigations into the epigenetics of CS have yet to substantiate their proposed theories regarding the modulation of signaling pathways through downstream protein analysis. This leads to a pressing need for more comprehensive studies conducted to rigorously evaluate existing hypotheses and thoroughly assess the specific roles of these epigenetic mechanisms in the pathogenesis of CS. Notably, research on histone modifications and chromatin remodeling remains particularly limited, and the implications for these processes in CS are yet to be fully understood. Given that chromatin remodeling encompasses a diverse array of cellular processes such as replication, transcription, and repair, it is a significant challenge to investigate the reliability of these mechanisms. In conclusion, the field of epigenetic research remains nascent as it pertains to CS, and future investigations should prioritize large-scale epigenomic analyses to identify additional CS-associated epigenetic markers and elucidate their functional mechanisms. Moreover, the enhancement of animal models can provide critical support for the analysis of the epigenetic regulatory network governing CS. The integration of multi-omics data, combining genomic, epigenomic, and transcriptomic analyses, will facilitate a more comprehensive understanding of the pathological processes involved in CS. Examples collected from relevant fields, such as cancer and neurodegenerative diseases, demonstrate the successful application of multi-omics approaches in unraveling complex disease mechanisms. This approach promises not only to yield novel insights into the clinical diagnosis and treatment of the disorder, but also to pave the way for new research avenues and foster the holistic advancement of CS-related epigenetics.
| 1. | Pahys JM, Guille JT. What's New in Congenital Scoliosis? J Pediatr Orthop. 2018;38:e172-e179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 58] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 2. | Giampietro PF, Raggio CL, Blank RD, McCarty C, Broeckel U, Pickart MA. Clinical, genetic and environmental factors associated with congenital vertebral malformations. Mol Syndromol. 2013;4:94-105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 71] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 3. | Weiss HR, Moramarco M. Congenital Scoliosis (Mini-review). Curr Pediatr Rev. 2016;12:43-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 27] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 4. | Cho W, Shepard N, Arlet V. The etiology of congenital scoliosis: genetic vs. environmental-a report of three monozygotic twin cases. Eur Spine J. 2018;27:533-537. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 5. | Lin M, Zhao S, Liu G, Huang Y, Yu C, Zhao Y, Wang L, Zhang Y, Yan Z, Wang S, Liu S, Liu J, Ye Y, Chen Y, Yang X, Tong B, Wang Z, Yang X, Niu Y, Li X, Wang Y, Su J, Yuan J, Zhao H, Zhang S, Qiu G; Deciphering Disorders Involving Scoliosis and COmorbidities (DISCO) study, Ikegawa S, Zhang J, Wu Z, Wu N. Identification of novel FBN1 variations implicated in congenital scoliosis. J Hum Genet. 2020;65:221-230. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 19] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 6. | Sparrow DB, Chapman G, Smith AJ, Mattar MZ, Major JA, O'Reilly VC, Saga Y, Zackai EH, Dormans JP, Alman BA, McGregor L, Kageyama R, Kusumi K, Dunwoodie SL. A mechanism for gene-environment interaction in the etiology of congenital scoliosis. Cell. 2012;149:295-306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 167] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 7. | Hou D, Kang N, Yin P, Hai Y. Abnormalities associated with congenital scoliosis in high-altitude geographic regions. Int Orthop. 2018;42:575-581. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 8. | Haffner J. EINEIIGE ZWILLINGE: mit symmetrischer Wirbelsäulendeformität. Keilwirbel. Acta Radiol. 1936;17:529-541. [RCA] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 9. | Brewer C, Holloway S, Zawalnyski P, Schinzel A, FitzPatrick D. A chromosomal deletion map of human malformations. Am J Hum Genet. 1998;63:1153-1159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 121] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 10. | Feng X, Cheung JPY, Je JSH, Cheung PWH, Chen S, Yue M, Wang N, Choi VNT, Yang X, Song YQ, Luk KDK, Gao B. Genetic variants of TBX6 and TBXT identified in patients with congenital scoliosis in Southern China. J Orthop Res. 2021;39:971-988. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 11. | Lefebvre M, Duffourd Y, Jouan T, Poe C, Jean-Marçais N, Verloes A, St-Onge J, Riviere JB, Petit F, Pierquin G, Demeer B, Callier P, Thauvin-Robinet C, Faivre L, Thevenon J. Autosomal recessive variations of TBX6, from congenital scoliosis to spondylocostal dysostosis. Clin Genet. 2017;91:908-912. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 37] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 12. | Ingalls TH, Curley FJ. Principles governing the genesis of congenital malformations induced in mice by hypoxia. N Engl J Med. 1957;257:1121-1127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 71] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 13. | Loder RT, Hernandez MJ, Lerner AL, Winebrener DJ, Goldstein SA, Hensinger RN, Liu CY, Schork MA. The induction of congenital spinal deformities in mice by maternal carbon monoxide exposure. J Pediatr Orthop. 2000;20:662-666. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 8] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 14. | Farley FA, Loder RT, Nolan BT, Dillon MT, Frankenburg EP, Kaciroti NA, Miller JD, Goldstein SA, Hensinger RN. Mouse model for thoracic congenital scoliosis. J Pediatr Orthop. 2001;21:537-540. [PubMed] |
| 15. | Gao X, Gotway G, Rathjen K, Johnston C, Sparagana S, Wise CA. Genomic Analyses of Patients With Unexplained Early-Onset Scoliosis. Spine Deform. 2014;2:324-332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 16. | Liu S, Wu N, Liu J, Liu H, Su X, Liu Z, Zuo Y, Chen W, Liu G, Chen Y, Ming Y, Yuan T, Li X, Chen J, Xia Z, Wang S, Chen J, Liu T, Yang X, Ma Y, Zhang J, Shen J, Li S, Wang Y, Zhao H, Yu K, Zhao Y, Huang S, Weng X, Qiu G, Wan C, Zhou G, Wu Z. Association between ADAMTS-4 gene polymorphism and lumbar disc degeneration in Chinese Han population. J Orthop Res. 2016;34:860-864. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 24] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 17. | Purkiss SB, Driscoll B, Cole WG, Alman B. Idiopathic scoliosis in families of children with congenital scoliosis. Clin Orthop Relat Res. 2002;27-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 36] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 18. | Kaspiris A, Grivas TB, Weiss HR. Congenital scoliosis in monozygotic twins: case report and review of possible factors contributing to its development. Scoliosis. 2008;3:17. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 19. | 1000 Genomes Project Consortium; Abecasis GR, Auton A, Brooks LD, DePristo MA, Durbin RM, Handsaker RE, Kang HM, Marth GT, McVean GA. An integrated map of genetic variation from 1,092 human genomes. Nature. 2012;491:56-65. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6041] [Cited by in RCA: 5950] [Article Influence: 457.7] [Reference Citation Analysis (0)] |
| 20. | Wu N, Yuan S, Liu J, Chen J, Fei Q, Liu S, Su X, Wang S, Zhang J, Li S, Wang Y, Qiu G, Wu Z. Association of LMX1A genetic polymorphisms with susceptibility to congenital scoliosis in Chinese Han population. Spine (Phila Pa 1976). 2014;39:1785-1791. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 21. | Trueb B. Biology of FGFRL1, the fifth fibroblast growth factor receptor. Cell Mol Life Sci. 2011;68:951-964. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 111] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 22. | Wang S, Chai X, Yan Z, Zhao S, Yang Y, Li X, Niu Y, Lin G, Su Z, Wu Z, Zhang TJ, Wu N. Novel FGFR1 Variants Are Associated with Congenital Scoliosis. Genes (Basel). 2021;12:1126. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 23. | Jarzabek K, Wolczynski S, Lesniewicz R, Plessis G, Kottler ML. Evidence that FGFR1 loss-of-function mutations may cause variable skeletal malformations in patients with Kallmann syndrome. Adv Med Sci. 2012;57:314-321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 28] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 24. | Bult CJ, Blake JA, Smith CL, Kadin JA, Richardson JE; Mouse Genome Database Group. Mouse Genome Database (MGD) 2019. Nucleic Acids Res. 2019;47:D801-D806. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 440] [Cited by in RCA: 509] [Article Influence: 101.8] [Reference Citation Analysis (0)] |
| 25. | Villanueva C, Jacobson-Dickman E, Xu C, Manouvrier S, Dwyer AA, Sykiotis GP, Beenken A, Liu Y, Tommiska J, Hu Y, Tiosano D, Gerard M, Leger J, Drouin-Garraud V, Lefebvre H, Polak M, Carel JC, Phan-Hug F, Hauschild M, Plummer L, Rey JP, Raivio T, Bouloux P, Sidis Y, Mohammadi M, de Roux N, Pitteloud N. Congenital hypogonadotropic hypogonadism with split hand/foot malformation: a clinical entity with a high frequency of FGFR1 mutations. Genet Med. 2015;17:651-659. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 51] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 26. | Ohtaka K, Fujisawa Y, Takada F, Hasegawa Y, Miyoshi T, Hasegawa T, Miyoshi H, Kameda H, Kurokawa-Seo M, Fukami M, Ogata T. FGFR1 Analyses in Four Patients with Hypogonadotropic Hypogonadism with Split-Hand/Foot Malformation: Implications for the Promoter Region. Hum Mutat. 2017;38:503-506. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 27. | Knezevic V, De Santo R, Mackem S. Two novel chick T-box genes related to mouse Brachyury are expressed in different, non-overlapping mesodermal domains during gastrulation. Development. 1997;124:411-419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 93] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 28. | Alila-Fersi O, Tej A, Maalej M, Kharrat M, Boughamoura L, Chouchen J, Tlili A, Fakhfakh F. Mitochondrial genes modulate the phenotypic expression of congenital scoliosis syndrome caused by mutations in the TBXT gene. Gene. 2024;914:148388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 29. | Campbell GP, Farkas DR, Chapman DL. Ectopic expression of T in the paraxial mesoderm disrupts somite maturation in the mouse. Dev Biol. 2022;485:37-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 30. | Fei Q, Wu Z, Wang H, Zhou X, Wang N, Ding Y, Wang Y, Qiu G. The association analysis of TBX6 polymorphism with susceptibility to congenital scoliosis in a Chinese Han population. Spine (Phila Pa 1976). 2010;35:983-988. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 31] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 31. | Wu N, Ming X, Xiao J, Wu Z, Chen X, Shinawi M, Shen Y, Yu G, Liu J, Xie H, Gucev ZS, Liu S, Yang N, Al-Kateb H, Chen J, Zhang J, Hauser N, Zhang T, Tasic V, Liu P, Su X, Pan X, Liu C, Wang L, Shen J, Shen J, Chen Y, Zhang T, Zhang J, Choy KW, Wang J, Wang Q, Li S, Zhou W, Guo J, Wang Y, Zhang C, Zhao H, An Y, Zhao Y, Wang J, Liu Z, Zuo Y, Tian Y, Weng X, Sutton VR, Wang H, Ming Y, Kulkarni S, Zhong TP, Giampietro PF, Dunwoodie SL, Cheung SW, Zhang X, Jin L, Lupski JR, Qiu G, Zhang F. TBX6 null variants and a common hypomorphic allele in congenital scoliosis. N Engl J Med. 2015;372:341-350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 200] [Cited by in RCA: 236] [Article Influence: 23.6] [Reference Citation Analysis (0)] |
| 32. | Chen W, Lin J, Wang L, Li X, Zhao S, Liu J, Akdemir ZC, Zhao Y, Du R, Ye Y, Song X, Zhang Y, Yan Z, Yang X, Lin M, Shen J, Wang S, Gao N, Yang Y, Liu Y, Li W, Liu J, Zhang N, Yang X, Xu Y, Zhang J, Delgado MR, Posey JE, Qiu G, Rios JJ, Liu P, Wise CA, Zhang F, Wu Z, Lupski JR, Wu N. TBX6 missense variants expand the mutational spectrum in a non-Mendelian inheritance disease. Hum Mutat. 2020;41:182-195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 26] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 33. | Richardson L, Wilcockson SG, Guglielmi L, Hill CS. Context-dependent TGFβ family signalling in cell fate regulation. Nat Rev Mol Cell Biol. 2023;24:876-894. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 24] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 34. | Ye M, Berry-Wynne KM, Asai-Coakwell M, Sundaresan P, Footz T, French CR, Abitbol M, Fleisch VC, Corbett N, Allison WT, Drummond G, Walter MA, Underhill TM, Waskiewicz AJ, Lehmann OJ. Mutation of the bone morphogenetic protein GDF3 causes ocular and skeletal anomalies. Hum Mol Genet. 2010;19:287-298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 115] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 35. | Chen J, Li X, Niu Y, Wu Z, Qiu G. Functional and In Silico Assessment of GDF3 Gene Variants in a Chinese Congenital Scoliosis Population. Med Sci Monit. 2018;24:2992-3001. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 36. | Su Z, Yang Y, Wang S, Zhao S, Zhao H, Li X, Niu Y; Deciphering Disorders Involving Scoliosis And COmorbidities Disco Study Group; Qiu G, Wu Z, Wu N, Zhang TJ. The Mutational Landscape of PTK7 in Congenital Scoliosis and Adolescent Idiopathic Scoliosis. Genes (Basel). 2021;12:1791. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 37. | Wu N, Wang L, Hu J, Zhao S, Liu B, Li Y, Du H, Zhang Y, Li X, Yan Z, Wang S, Wang Y, Zhang J, Wu Z; Disco Deciphering Disorders Involving Scoliosis Comorbidities Study Group; Qiu G. A Recurrent Rare SOX9 Variant (M469V) is Associated with Congenital Vertebral Malformations. Curr Gene Ther. 2019;19:242-247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 38. | Frasuńska J, Pollak A, Turczyn P, Kutkowska-Kaźmierczak A, Pepłowski J, Płoski R, Tarnacka B. A Study of Polish Family with Scoliosis and Limb Contractures Expands the MYH3 Disease Spectrum. Genes (Basel). 2024;15:125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 39. | Bessho Y, Miyoshi G, Sakata R, Kageyama R. Hes7: a bHLH-type repressor gene regulated by Notch and expressed in the presomitic mesoderm. Genes Cells. 2001;6:175-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 147] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 40. | Sparrow DB, Chapman G, Wouters MA, Whittock NV, Ellard S, Fatkin D, Turnpenny PD, Kusumi K, Sillence D, Dunwoodie SL. Mutation of the LUNATIC FRINGE gene in humans causes spondylocostal dysostosis with a severe vertebral phenotype. Am J Hum Genet. 2006;78:28-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 182] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 41. | Turnpenny PD, Whittock N, Duncan J, Dunwoodie S, Kusumi K, Ellard S. Novel mutations in DLL3, a somitogenesis gene encoding a ligand for the Notch signalling pathway, cause a consistent pattern of abnormal vertebral segmentation in spondylocostal dysostosis. J Med Genet. 2003;40:333-339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 101] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 42. | Khan F, Arshad A, Ullah A, Steenackers E, Mortier G, Ahmad W, Arshad M, Khan S, Hayat A, Khan I, Khan MA, Van Hul W. Identification of a Novel Nonsense Variant in the DLL3 Gene Underlying Spondylocostal Dysostosis in a Consanguineous Pakistani Family. Mol Syndromol. 2023;14:191-200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 43. | Bessho Y, Hirata H, Masamizu Y, Kageyama R. Periodic repression by the bHLH factor Hes7 is an essential mechanism for the somite segmentation clock. Genes Dev. 2003;17:1451-1456. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 233] [Cited by in RCA: 240] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 44. | Oda T, Elkahloun AG, Pike BL, Okajima K, Krantz ID, Genin A, Piccoli DA, Meltzer PS, Spinner NB, Collins FS, Chandrasekharappa SC. Mutations in the human Jagged1 gene are responsible for Alagille syndrome. Nat Genet. 1997;16:235-242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 857] [Cited by in RCA: 766] [Article Influence: 27.4] [Reference Citation Analysis (0)] |
| 45. | Gu Z, Qiu G, Zhang Y. Genetic association analysis between polymorphisms of HAIRY-AND-ENHANCER-OF SPLIT-7 and congenital scoliosis. Int J Clin Exp Med. 2015;8:16714-16718. [PubMed] |
| 46. | Murakami K, Kikugawa S, Seki S, Terai H, Suzuki T, Nakano M, Takahashi J, Nakamura Y. Exome Sequencing Reveals De Novo Variants in Congenital Scoliosis. J Pediatr Genet. 2022;11:287-291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 47. | Takeda K, Kou I, Mizumoto S, Yamada S, Kawakami N, Nakajima M, Otomo N, Ogura Y, Miyake N, Matsumoto N, Kotani T, Sudo H, Yonezawa I, Uno K, Taneichi H, Watanabe K, Shigematsu H, Sugawara R, Taniguchi Y, Minami S, Nakamura M, Matsumoto M; Japan Early Onset Scoliosis Research Group, Watanabe K, Ikegawa S. Screening of known disease genes in congenital scoliosis. Mol Genet Genomic Med. 2018;6:966-974. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 20] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 48. | Rebello D, Wohler E, Erfani V, Li G, Aguilera AN, Santiago-Cornier A, Zhao S, Hwang SW, Steiner RD, Zhang TJ, Gurnett CA, Raggio C, Wu N, Sobreira N, Giampietro PF, Ciruna B. COL11A2 as a candidate gene for vertebral malformations and congenital scoliosis. Hum Mol Genet. 2023;32:2913-2928. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 5] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 49. | Sun X, Zhou Y, Zhang R, Wang Z, Xu M, Zhang D, Huang J, Luo F, Li F, Ni Z, Zhou S, Chen H, Chen S, Chen L, Du X, Chen B, Huang H, Liu P, Yin L, Qiu J, Chen D, Deng C, Xie Y, Luo L, Chen L. Dstyk mutation leads to congenital scoliosis-like vertebral malformations in zebrafish via dysregulated mTORC1/TFEB pathway. Nat Commun. 2020;11:479. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 37] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 50. | Lai W, Feng X, Yue M, Cheung PWH, Choi VNT, Song YQ, Luk KDK, Cheung JPY, Gao B. Identification of Copy Number Variants in a Southern Chinese Cohort of Patients with Congenital Scoliosis. Genes (Basel). 2021;12:1213. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 51. | Fei Q, Wu ZH, Wang YP, Zhou X, Wang H, Wang NG, Li X, Qiu GX. [Association study of WNT3A gene polymorphisms with the susceptibility to congenital scoliosis in a Chinese Han population]. Zhonghua Yi Xue Za Zhi. 2011;91:746-751. [PubMed] |
| 52. | Fei Q, Wu ZH, Yuan SM, Wang H, Zhou X, Liu Z, Song HF, Yin RF, Wang YP, Qiu GX. [Association of PAX1 gene polymorphisms with susceptibility to congenital scoliosis in Chinese Han population]. Zhonghua Yi Xue Za Zhi. 2008;88:2597-2602. [PubMed] |
| 53. | Moore LD, Le T, Fan G. DNA methylation and its basic function. Neuropsychopharmacology. 2013;38:23-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2332] [Cited by in RCA: 2963] [Article Influence: 246.9] [Reference Citation Analysis (0)] |
| 54. | Szyf M. The dynamic epigenome and its implications in toxicology. Toxicol Sci. 2007;100:7-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 131] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 55. | Zhang Z, Chen Y, Wu Y, Hao Y, Zhao X, Wang X, Wang Y, Xi Y, Zhang X. A twinpair analysis indicates congenital scoliosis is associated with allelespecific methylation in the SVIL gene. Mol Med Rep. 2020;22:2093-2100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 56. | Liu G, Zhao H, Yan Z, Zhao S, Niu Y, Li X, Wang S, Yang Y, Liu S, Zhang TJ, Wu Z, Wu N. Whole-genome methylation analysis reveals novel epigenetic perturbations of congenital scoliosis. Mol Ther Nucleic Acids. 2021;23:1281-1287. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 57. | Kim JM, Yang YS, Park KH, Oh H, Greenblatt MB, Shim JH. The ERK MAPK Pathway Is Essential for Skeletal Development and Homeostasis. Int J Mol Sci. 2019;20:1803. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 102] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 58. | Kamiya N, Kim HK, King PD. Regulation of bone and skeletal development by the SHP-2 protein tyrosine phosphatase. Bone. 2014;69:55-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 59. | Detweiler S, Thacker MM, Hopkins E, Conway L, Gripp KW. Orthopedic manifestations and implications for individuals with Costello syndrome. Am J Med Genet A. 2013;161A:1940-1949. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 20] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 60. | Stevenson DA, Schwarz EL, Carey JC, Viskochil DH, Hanson H, Bauer S, Weng HY, Greene T, Reinker K, Swensen J, Chan RJ, Yang FC, Senbanjo L, Yang Z, Mao R, Pasquali M. Bone resorption in syndromes of the Ras/MAPK pathway. Clin Genet. 2011;80:566-573. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 84] [Cited by in RCA: 79] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 61. | Hui S, Yang Y, Li J, Li N, Xu P, Li H, Zhang Y, Wang S, Lin G, Li S, Qiu G, Zhao RC, Zhang J, Zhuang Q. Differential miRNAs profile and bioinformatics analyses in bone marrow mesenchymal stem cells from adolescent idiopathic scoliosis patients. Spine J. 2019;19:1584-1596. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 30] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 62. | Hou T, Liu Y, Kolba N, Guo D, He H. Desalted Duck Egg White Peptides Promote Calcium Uptake and Modulate Bone Formation in the Retinoic Acid-Induced Bone Loss Rat and Caco-2 Cell Model. Nutrients. 2017;9:490. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 26] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 63. | Qiu S, Tao ZB, Tao L, Zhu Y. Melatonin induces mitochondrial apoptosis in osteoblasts by regulating the STIM1/cytosolic calcium elevation/ERK pathway. Life Sci. 2020;248:117455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 64. | Van Battum EY, Brignani S, Pasterkamp RJ. Axon guidance proteins in neurological disorders. Lancet Neurol. 2015;14:532-546. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 170] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 65. | Amouri R, Nehdi H, Bouhlal Y, Kefi M, Larnaout A, Hentati F. Allelic ROBO3 heterogeneity in Tunisian patients with horizontal gaze palsy with progressive scoliosis. J Mol Neurosci. 2009;39:337-341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 22] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 66. | Wu Y, Zhang HQ, Tang M, Guo C, Liu S, Li J, Wang Y, Xiao L, Yang G. Abnormal TNS3 gene methylation in patients with congenital scoliosis. BMC Musculoskelet Disord. 2022;23:797. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 67. | Zhang H, Xiang G, Li J, He S, Wang Y, Deng A, Wang Y, Guo C. Promotion effect of FGF23 on osteopenia in congenital scoliosis through FGFr3/TNAP/OPN pathway. Chin Med J (Engl). 2023;136:1468-1477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 68. | Ma B, Wang S, Wu W, Shan P, Chen Y, Meng J, Xing L, Yun J, Hao L, Wang X, Li S, Guo Y. Mechanisms of circRNA/lncRNA-miRNA interactions and applications in disease and drug research. Biomed Pharmacother. 2023;162:114672. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 104] [Reference Citation Analysis (0)] |
| 69. | Shen LP, Zhang WC, Deng JR, Qi ZH, Lin ZW, Wang ZD. Advances in the mechanism of small nucleolar RNA and its role in DNA damage response. Mil Med Res. 2024;11:53. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 70. | Statello L, Guo CJ, Chen LL, Huarte M. Gene regulation by long non-coding RNAs and its biological functions. Nat Rev Mol Cell Biol. 2021;22:96-118. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3257] [Cited by in RCA: 3002] [Article Influence: 750.5] [Reference Citation Analysis (0)] |
| 71. | Heydarnezhad Asl M, Pasban Khelejani F, Bahojb Mahdavi SZ, Emrahi L, Jebelli A, Mokhtarzadeh A. The various regulatory functions of long noncoding RNAs in apoptosis, cell cycle, and cellular senescence. J Cell Biochem. 2022;123:995-1024. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 40] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 72. | Jiang C, Wang P, Tan Z, Zhang Y. Long non-coding RNAs in bone formation: Key regulators and therapeutic prospects. Open Life Sci. 2024;19:20220908. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 73. | Han J, Kong H, Wang X, Zhang XA. Novel insights into the interaction between N6-methyladenosine methylation and noncoding RNAs in musculoskeletal disorders. Cell Prolif. 2022;55:e13294. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 38] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 74. | Zhang Y, Wang Q, Xue H, Guo Y, Wei S, Li F, Gong L, Pan W, Jiang P. Epigenetic Regulation of Autophagy in Bone Metabolism. Function (Oxf). 2024;5:zqae004. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 75. | Yao Q, He T, Liao JY, Liao R, Wu X, Lin L, Xiao G. Noncoding RNAs in skeletal development and disorders. Biol Res. 2024;57:16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 76. | Shi X, Li P, Wu X, Shu J. Whole-transcriptome sequencing identifies key differentially expressed circRNAs/lncRNAs/miRNAs/mRNAs and linked ceRNA networks in adult degenerative scoliosis. Front Mol Neurosci. 2023;16:1038816. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 77. | Raimondi L, De Luca A, Gallo A, Perna F, Cuscino N, Cordaro A, Costa V, Bellavia D, Faldini C, Scilabra SD, Giavaresi G, Toscano A. Investigating the Differential Circulating microRNA Expression in Adolescent Females with Severe Idiopathic Scoliosis: A Proof-of-Concept Observational Clinical Study. Int J Mol Sci. 2024;25:570. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 78. | Chen C, Tan H, Bi J, Li Z, Rong T, Lin Y, Sun L, Li X, Shen J. Identification of Competing Endogenous RNA Regulatory Networks in Vitamin A Deficiency-Induced Congenital Scoliosis by Transcriptome Sequencing Analysis. Cell Physiol Biochem. 2018;48:2134-2146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 24] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 79. | Liu G, Shen J, Chen C, Jiao Y, Li Z, Tan H, Lin Y, Rong T. Genome-Wide Analysis of circular RNAs and validation of hsa_circ_0006719 as a potential novel diagnostic biomarker in congenital scoliosis patients. J Cell Mol Med. 2020;24:7015-7022. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 80. | Chen C, Tan H, Bi J, Li L, Rong T, Lin Y, Sun P, Liang J, Jiao Y, Li Z, Sun L, Shen J. LncRNA-SULT1C2A regulates Foxo4 in congenital scoliosis by targeting rno-miR-466c-5p through PI3K-ATK signalling. J Cell Mol Med. 2019;23:4582-4591. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 81. | Abu-Abed S, Dollé P, Metzger D, Beckett B, Chambon P, Petkovich M. The retinoic acid-metabolizing enzyme, CYP26A1, is essential for normal hindbrain patterning, vertebral identity, and development of posterior structures. Genes Dev. 2001;15:226-240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 448] [Cited by in RCA: 433] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 82. | Ishiwata S, Iizuka H, Sonoda H, Tsunoda D, Tajika Y, Chikuda H, Koibuchi N, Shimokawa N. Upregulated miR-224-5p suppresses osteoblast differentiation by increasing the expression of Pai-1 in the lumbar spine of a rat model of congenital kyphoscoliosis. Mol Cell Biochem. 2020;475:53-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 83. | Li Z, Ma J, Bi J, Guo H, Chan MTV, Wu WKK, Wu Z, Shen J. MicroRNA signature of air pollution exposure-induced congenital defects. J Cell Physiol. 2019;234:17896-17904. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 84. | Park J, Lee K, Kim K, Yi SJ. The role of histone modifications: from neurodevelopment to neurodiseases. Signal Transduct Target Ther. 2022;7:217. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 174] [Cited by in RCA: 142] [Article Influence: 47.3] [Reference Citation Analysis (0)] |
| 85. | Lepack AE, Werner CT, Stewart AF, Fulton SL, Zhong P, Farrelly LA, Smith ACW, Ramakrishnan A, Lyu Y, Bastle RM, Martin JA, Mitra S, O'Connor RM, Wang ZJ, Molina H, Turecki G, Shen L, Yan Z, Calipari ES, Dietz DM, Kenny PJ, Maze I. Dopaminylation of histone H3 in ventral tegmental area regulates cocaine seeking. Science. 2020;368:197-201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 162] [Article Influence: 32.4] [Reference Citation Analysis (0)] |
| 86. | Acharjee S, Chauhan S, Pal R, Tomar RS. Mechanisms of DNA methylation and histone modifications. Prog Mol Biol Transl Sci. 2023;197:51-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 16] [Reference Citation Analysis (0)] |
| 87. | Tajul-Arifin K, Teasdale R, Ravasi T, Hume DA, Mattick JS; RIKEN GER Group; GSL Members. Identification and analysis of chromodomain-containing proteins encoded in the mouse transcriptome. Genome Res. 2003;13:1416-1429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 44] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 88. | Desh H, Gray SL, Horton MJ, Raoul G, Rowlerson AM, Ferri J, Vieira AR, Sciote JJ. Molecular motor MYO1C, acetyltransferase KAT6B and osteogenetic transcription factor RUNX2 expression in human masseter muscle contributes to development of malocclusion. Arch Oral Biol. 2014;59:601-607. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 89. | Wu Y, Zhang H, Tang M, Guo C, Deng A, Li J, Wang Y, Xiao L, Yang G. High methylation of lysine acetyltransferase 6B is associated with the Cobb angle in patients with congenital scoliosis. J Transl Med. 2020;18:210. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 90. | Camilleri ET, Dudakovic A, Riester SM, Galeano-Garces C, Paradise CR, Bradley EW, McGee-Lawrence ME, Im HJ, Karperien M, Krych AJ, Westendorf JJ, Larson AN, van Wijnen AJ. Loss of histone methyltransferase Ezh2 stimulates an osteogenic transcriptional program in chondrocytes but does not affect cartilage development. J Biol Chem. 2018;293:19001-19011. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 45] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 91. | Parmar JJ, Padinhateeri R. Nucleosome positioning and chromatin organization. Curr Opin Struct Biol. 2020;64:111-118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 92. | Luo RX, Dean DC. Chromatin remodeling and transcriptional regulation. J Natl Cancer Inst. 1999;91:1288-1294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 97] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 93. | Ho L, Crabtree GR. Chromatin remodelling during development. Nature. 2010;463:474-484. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 786] [Cited by in RCA: 837] [Article Influence: 55.8] [Reference Citation Analysis (0)] |
| 94. | Mirabella AC, Foster BM, Bartke T. Chromatin deregulation in disease. Chromosoma. 2016;125:75-93. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 94] [Cited by in RCA: 92] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 95. | Li Z, Yu X, Shen J. Environmental aspects of congenital scoliosis. Environ Sci Pollut Res Int. 2015;22:5751-5755. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 21] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 96. | Basu PS, Elsebaie H, Noordeen MH. Congenital spinal deformity: a comprehensive assessment at presentation. Spine (Phila Pa 1976). 2002;27:2255-2259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 135] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 97. | Yang H, Chen K, Zhang X, Wang L, Li C, Tao H, Wang L, Li Q. Vitamin A deficiency results in dysregulation of lipid efflux pathway in rat kidney. Pediatr Nephrol. 2010;25:1435-1444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 98. | Clagett-Dame M, Knutson D. Vitamin A in reproduction and development. Nutrients. 2011;3:385-428. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 277] [Cited by in RCA: 281] [Article Influence: 20.1] [Reference Citation Analysis (0)] |
| 99. | Li N, Sun S, Wang D, Yao P, Yang X, Yan H, Du Y, Ying C, Liu L. Suppression of retinoic acid receptors may contribute to embryonic skeleton hypoplasia in maternal rats with chronic vitamin A deficiency. J Nutr Biochem. 2010;21:710-716. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 100. | Ishaq MU, Kunwar D, Qadeer A, Komel A, Safi A, Malik A, Malik L, Akbar A. Effect of vitamin A on maternal, fetal, and neonatal outcomes: An overview of deficiency, excessive intake, and intake recommendations. Nutr Clin Pract. 2024;39:373-384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 9] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 101. | Li Z, Shen J, Wu WK, Wang X, Liang J, Qiu G, Liu J. Vitamin A deficiency induces congenital spinal deformities in rats. PLoS One. 2012;7:e46565. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 26] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 102. | Ekblad M, Korkeila J, Lehtonen L. Smoking during pregnancy affects foetal brain development. Acta Paediatr. 2015;104:12-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 104] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 103. | Lee KW, Pausova Z. Cigarette smoking and DNA methylation. Front Genet. 2013;4:132. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 225] [Cited by in RCA: 268] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 104. | Lange S, Shield K, Rehm J, Popova S. Prevalence of fetal alcohol spectrum disorders in child care settings: a meta-analysis. Pediatrics. 2013;132:e980-e995. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 92] [Article Influence: 7.7] [Reference Citation Analysis (0)] |