Published online Mar 18, 2024. doi: 10.5312/wjo.v15.i3.302
Peer-review started: December 3, 2023
First decision: December 28, 2023
Revised: January 6, 2024
Accepted: January 24, 2024
Article in press: January 24, 2024
Published online: March 18, 2024
Processing time: 103 Days and 4.1 Hours
Tumoral calcinosis is a condition characterized by deposits of calcium phosphate crystals in extra-articular soft tissues, occurring in hemodialysis patients. Calcium phosphate crystals are mainly composed of hydroxyapatite, which is highly infiltrative to tissues, thus making complete resection difficult. An adjuvant method to remove or resolve the residual crystals during the operation is necessary.
A bicarbonate Ringer’s solution with bicarbonate ions (28 mEq/L) was used as the adjuvant. After resecting calcium phosphate deposits of tumoral calcinosis as much as possible, while filling with the solution, residual calcium phosphate deposits at the pseudocyst wall can be gently scraped by fingers or gauze in the operative field. A 49-year-old female undergoing hemodialysis for 15 years had swelling with calcium deposition for 2 years in the shoulders, bilateral hip joints, and the right foot. A shoulder lesion was resected, but the calcification remained and early re-deposition was observed. Considering the difficulty of a complete rection, we devised a bicarbonate dissolution method and excised the foot lesion. After resection of the calcified material, the residual calcified material was washed away with bicarbonate Ringer’s solution.
The bicarbonate dissolution method is a new, simple, and effective treatment for tumoral calcinosis in hemodialysis patients.
Core Tip: Tumoral calcinosis, which occurs in 2%-3% of hemodialysis patients, involves calcium phosphate deposits, thus making surgical resection challenging. Hydroxyapatite, the main component of tumoral calcinosis, infiltrates tissues extensively. A bicarbonate Ringer’s solution is used post-resection. A 49-year-old hemodialysis patient with calcified shoulder, hip, and foot lesions underwent the bicarbonate dissolution method. After resection, the operative field was washed with the solution. This simple and effective treatment offers a novel approach for managing tumoral calcinosis in hemodialysis patients.
- Citation: Noguchi T, Sakamoto A, Kakehi K, Matsuda S. New method of local adjuvant therapy with bicarbonate Ringer’s solution for tumoral calcinosis: A case report. World J Orthop 2024; 15(3): 302-309
- URL: https://www.wjgnet.com/2218-5836/full/v15/i3/302.htm
- DOI: https://dx.doi.org/10.5312/wjo.v15.i3.302
Tumoral calcinosis is a condition characterized by solitary or multifocal calcium phosphate deposits in extra-articular soft tissues. The joints of the hips, shoulders, and elbows are most often involved; the hands, feet, and knees are less often involved. Tumoral calcinosis often presents as painful tumor-like masses that limit joint range of motion; cosmetic problems also occur[1-5].
Tumoral calcinosis has a primary type with no associated disease, which is due to a genetic abnormality, and a secondary type that is associated with other disorders, especially chronic renal failure[1,6]. The prevalence of tumoral calcinosis has been reported to range from 0.5%-3% among dialysis patients[7-9]. The exact mechanisms underlying tumoral calcinosis are unclear. The role of repeated joint microtrauma has been suggested to cause tumoral calcinosis[10]. Previous studies have reported that elevated calcium phosphate production is closely associated with soft tissue calcification[11,12]. Regardless of the etiology, hyperphosphatemia is associated with tumoral calcinosis. Medical treatment for tumoral calcinosis in hemodialysis patients includes dietary phosphorus restriction, calcium-free phosphate binders, and frequent dialysis with low-calcium dialysis solutions; however, these treatments are usually ineffective[7,13,14].
Surgical resection is only used when these treatments are insufficient[15]. Surgical resection of tumoral calcinosis lesions has been the primary treatment[13] but surgical resection is not curative. In contrast, it has been reported that tumoral calcinosis is reduced or resolved with improved systemic symptoms following parathyroidectomy[16-18]. Parathyroidectomy is effective for tumoral calcinosis patients, especially patients with secondary hyperparathyroidism[5]. Following a parathyroidectomy, calcified tissue is significantly resorbed; however, bone renewal is reduced following parathyroidectomy because of lower parathyroid hormone (PTH) levels, resulting in increased circulating calcium and promotion of vessel and soft tissue calcification[19,20].
Calcium phosphate deposits in tumoral calcinosis have been shown to be comprised of hydroxyapatite [Ca10(PO4)6
A bicarbonate Ringer’s solution was used. The solution had a pH of 7.0 and an osmotic pressure of approximately 1.0. The solution contained HCO3- (28 mEq/L) (Tables 1-3). After resecting calcium phosphate deposits of tumoral calcinosis as much as possible, while filling with bicarbonate Ringer’s solution, residual calcium phosphate deposits at the pseudocyst wall are gently scraped with fingers or gauze in the operative field. Because the concentration of HCO3- (28 mEq/L) is normal in vivo, the amount to be used is not restricted, but we use approximately 1000 mL of the solution for 1 operation. The use of the solution as a washing solution is explained to the patients and consent is obtained.
| Ion | mEq/L |
| Na+ | 130 |
| K+ | 4 |
| Mg2+ | 2 |
| Ca2+ | 3 |
| Cl- | 109 |
| HCO3- | 28 |
| Citrate3- | 4 |
| Bicarbonate solution | |
| Appearance | Colorless and transparent |
| pH | 6.8-7.8 |
| Osmotic pressure | Apraxia 1 |
| Component | 500 mL | 1000 mL | |
| Sodium chloride | NaCl | 2.92 g | 5.84 g |
| Potassium chloride | KCl | 0.15 g | 0.30 g |
| Calcium chloride hydrate | CaCl2·2H2O | 0.11 g | 0.22 g |
| Magnesium chloride | MgCl2 | 0.10 g | 0.20 g |
| Sodium bicarbonate | NaHCO3 | 1.175 g | 2.35 g |
| Sodium citrate hydrate | C6H9Na3O9 | 0.10 g | 0.20 g |
| Citric acid hydrate moderation | HOC(COOH)(CH2COOH)2·H2O | Moderate | Moderate |
Herein we report the successful treatment of a patient with tumoral calcinosis by local adjuvant therapy with bicarbonate Ringer’s solution. The procedure, chemical background, and safety are discussed.
The patient is a 49-year-old woman with a diagnosis of tumoral calcinosis.
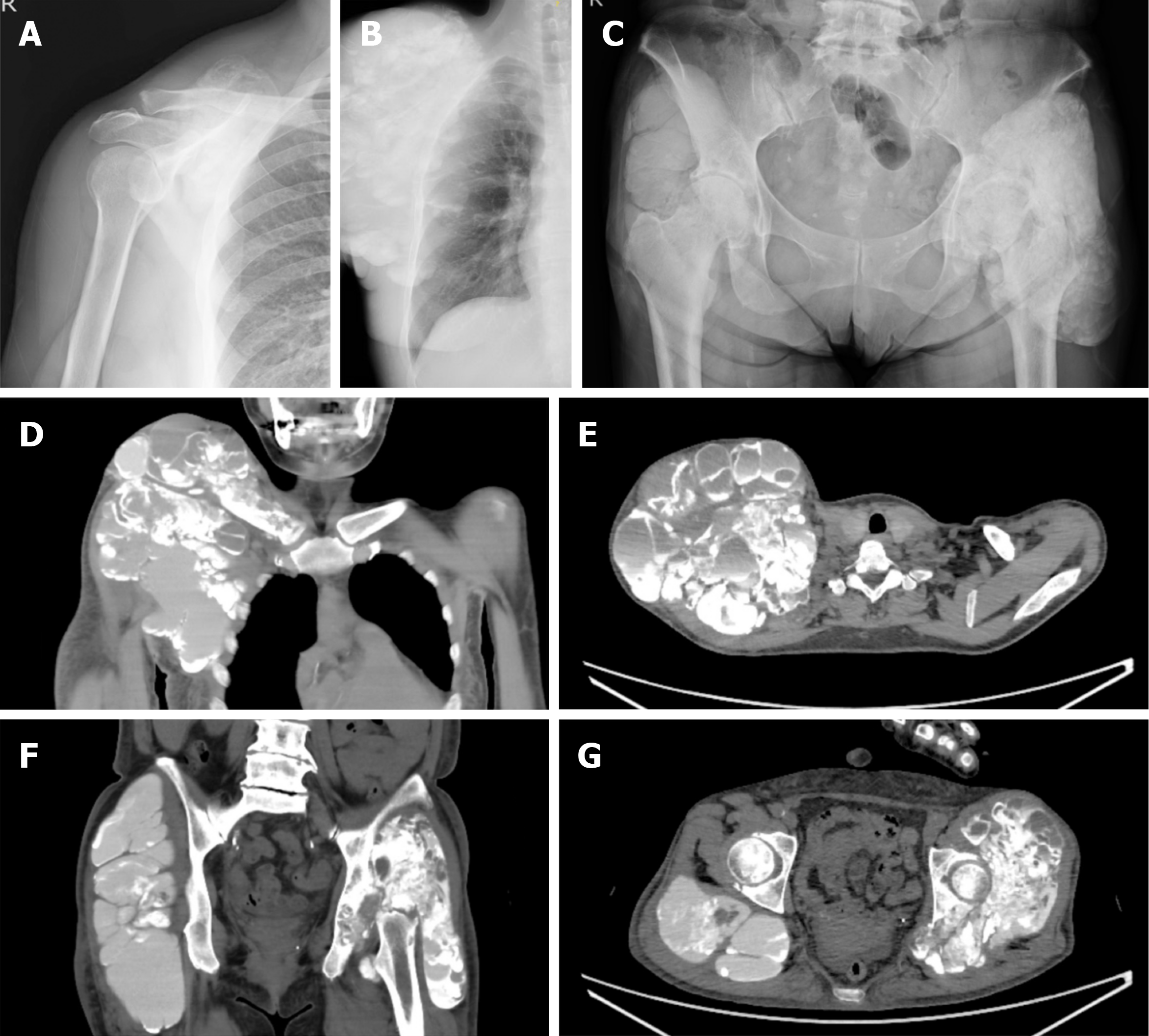
She had noticed swelling in her right shoulder 2 years before the initial assessment. Nine months later, there was bilateral hip and right foot swelling. The size of the right shoulder swelling had increased to be from the supraclavicular fossa to the maxilla, and the size of the swelling was > 20 cm in diameter.
She has a history of hemodialysis for 15 years.
There was no specific personal or family history.
The size of the bilateral hip swelling was > 20 cm in diameter and that of the sole of the foot was 3 cm. Each mass was elastic and soft with fluid palpitation.
The leukocyte count was normal [7140/mm3 (normal range, 3300-8600/mm3)]. The differential was as follows: neutrophils, 68.8% (normal range, 46%-62%); lymphocytes, 22.4% (normal range, 30%-40%); monocytes, 6.7% (normal range, 4%-7%); eosinophils, 1.7% (normal range, 3%-5%); and basophils, 0.4% (normal range, < 1%). Laboratory data showed the following: corrected Ca2+, 9.7 mg/dL (normal range, 8.8-10.1 mg/dL); inorganic phosphorus, 5.5 mg/dL (normal range, 2.7-4.6 mg/dL); creatinine, 5.16 mg/dL (normal range, 0.16-0.79 mg/dL); estimated glomerular filtration rate, 7.8 mL/min/1.73 m2 (normal range, < 90 mL/min/1.73 m2), and PTH-intact, 1110 pg/mL (reference value, 10-65 pg/mL). The serum phosphorus level was elevated, even after medical treatment. The serum PTH level was also elevated, suggesting secondary hyperparathyroidism.
Plain radiographs and computed tomography (CT) showed that the lesions were multilocular, opaque, and homo
The diagnosis was based on the clinical course and the imaging findings were tumoral calcinosis associated with hemo
Because growth of the lesion after medical treatment with low-calcium dialysis failed, surgical intervention was con
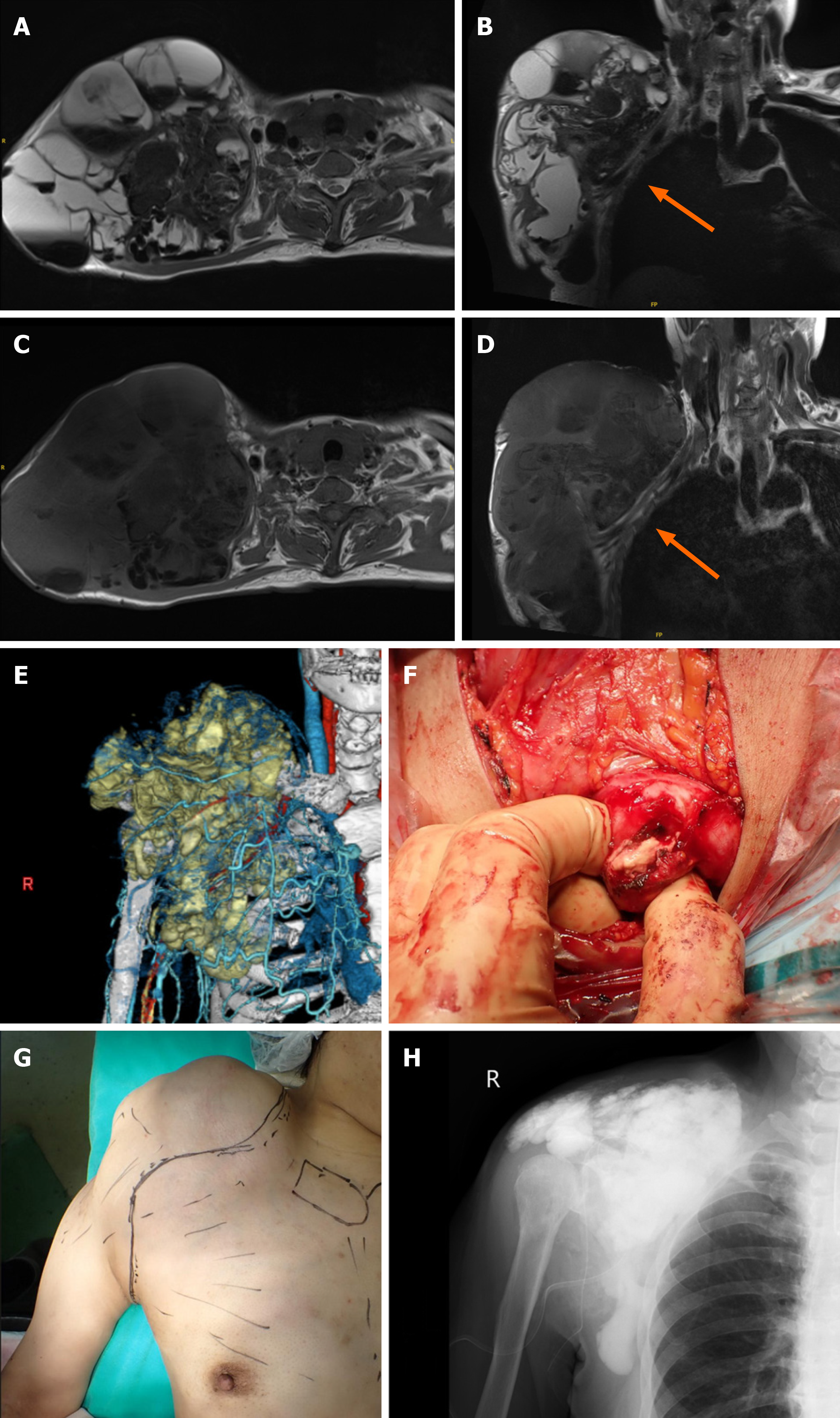
Under general anesthesia, an incision was made from the supraclavicular region to the axilla. A pseudofibrous capsule was noted and white fluid and muddy material extended to the subcutaneous tissue. The fluid material was removed. A solid white material was entrapped in the fibrous wall and the fibrous wall was removed as much as possible. The fibrous wall was continuous with the surrounding tissue without a clear border. Because the brachial plexus and vessels were located between the cyst and chest wall, complete resection was not possible. A curettage was performed for the calcified tissue in the fibrous wall and a massive, calcified lesion was removed. The operative field was routinely washed with normal saline to reduce the possibility of infection. A postoperative plain radiograph showed a diffuse, calcified intensity in the operative field (Figure 2). The fluid collection at the right shoulder gradually increased, and the swelling returned to the preoperative size within 2 months.
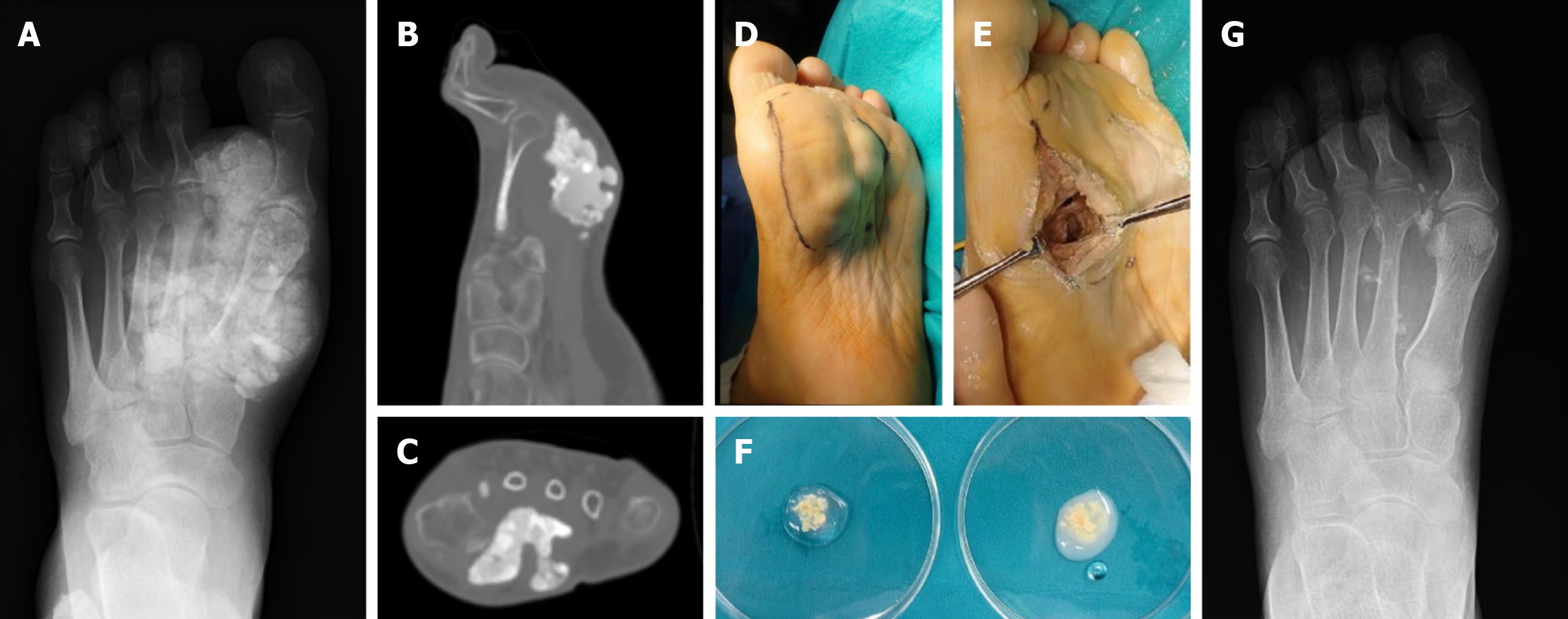
Because of the unsatisfactory results of the resected shoulder lesion, adjuvant therapy was added to the foot lesion resection. Under general anesthesia, a tourniquet was used. The calcified lesion was located on the sole and an incision was made at the lateral side of the sole. Muddy material with the same appearance as the shoulder lesion was removed. A cystic fibrous wall had formed, but was smaller than the shoulder lesion. The fibrous cystic wall was preserved, but calcified materials embedded in the cystic wall were removed as much as possible. Particles of calcified tissue at the fibrous wall were observed, even after resection of the calcified material (Figure 3). After filling with a total of 1000 mL of bicarbonate Ringer’s solution, a significant decrease in calcareous deposits was observed by the naked eye and fluoroscopy during the operation (Figure 3). Intra- and post-operative examinations showed no abnormalities in pH or base excess values. The postoperative course was uneventful. No fluid collection was observed 6 months postoperatively.
The resected materials of tumoral calcinosis were soaked with saline or bicarbonate at the side of the operation theatre. The calcified materials soaked with saline did not change, while the calcified materials soaked with bicarbonate Ringer solution became turbid immediately.
The current case was a typical presentation of tumoral calcinosis associated with hemodialysis. According to the lite
Histologically, tumoral calcinosis has a nodular architecture with fibrous septa coursing between nodules. The fibrous wall shows calcification surrounded by macrophages, osteoclast-like multinucleated giant cells, fibroblasts, and chronic inflammatory cells. Calcified materials are entrapped into the fibrous wall[24,25]. Surgical resection of the lesions is not considered in accordance with the recommendations in the literature. The unsatisfactory results of surgical resection are due to these trapped calcified lesions in the fibrous wall. Calcium phosphate deposits in tumoral calcinosis have been shown to be Ca10(PO4)6(OH)2[21]. We have developed a method to dissolve hydroxyapatite as an adjuvant therapy in surgical resection.
Hydroxyapatite has very low solubility. Hydroxyapatite is easily dissolved by blowing CO2 into the hydroxyapatite suspension. The dissolution amount was 200 times that found without CO2 blowing[22,23]. Ca10(PO4)6(OH)2 reacts with CO2 as soluble Ca(HCO3)2 and liquid phosphoric acid (H3PO4) [Ca10(PO4)6(OH)2 + 20CO2 + 18H2O → 10Ca(HCO3)2 + 6H3PO4][26]. CO2 reacts with H2O to form carbonic acid (H2CO3). Then, H2CO3 further dissociates into HCO3- and a H+. Therefore, CO2 and HCO3- have the same chemical reaction in solution. CaCO3 is a calcium salt with the same insolubility, but dissolves easily in solutions containing CO2. Hydroxyapatite co-existing with CO2 or CaCO3 changes into the highly soluble Ca(HCO3)2[22,23]. The bicarbonate Ringer’s solution had HCO3- 28 mEq/L. The bicarbonate Ringer’s solution was used in emergency cases as well as in liver transplantation for compensation of extracellular fluid and correct metabolic acidosis. A bicarbonate Ringer’s solution is used in the current new method to dissolve hydroxyapatite in tumoral calcinosis.
Use of bicarbonate of Ringer’s solution was shown to be safe. The value of HCO3- (28 mEq/L) was within normal limits in vivo, the solution had a pH of 7.0, and the osmotic pressure was approximately 1.0. In a previous report, critical alkalosis occurred in a patient who was irrigated with 1000 mL of 7% sodium bicarbonate solution intraperitoneally as adjuvant therapy for pseudomyxoma peritonei. The peritoneal capillary vessels easily absorb sodium bicarbonate in the abdominal cavity by diffusion, such as in peritoneal dialysis, and the peritoneal surface area is equal to the body surface area. The predicted amount of absorbed sodium bicarbonate was estimated to be approximately 30% of the entire irrigation volume[27]. The 7% sodium bicarbonate solution was 833 mEq/L, which is much greater than the 28 mEq/L in the solution used in our case. Alkalosis was not reported in a patient who was twice-irrigated with 200 mL of 7% sodium bicarbonate solution for 2 min (total volume = 400 mL; estimated absorbed volume = 85 mL), even for intraperitoneal irrigation for adjuvant therapy for pseudomyxoma peritonei[27].
In conclusion, bicarbonate Ringer’s solution was used as an adjuvant therapy for tumoral calcinosis, which is a common complication in hemodialysis patients. Bicarbonate Ringer’s solution has a role in dissolving Ca10(PO4)6(OH)2. The bicarbonate dissolution method is a new, simple, and effective treatment for tumoral calcinosis in hemodialysis patients. The dissolution mechanism needs to be chemically verified in a corollary study.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Orthopedics
Country/Territory of origin: Japan
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Sanayeh EB, United States S-Editor: Zhang H L-Editor: A P-Editor: Zhao YQ
| 1. | Kadowaki M, Naito K, Tobita M, Kumahashi N, Kono M, Takao M. A case of symptomatic tumoral calcinosis on the great toe and review of the literature. Arch Orthop Trauma Surg. 2008;128:551-554. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 2. | Farzan M, Farhoud AR. Tumoral calcinosis: what is the treatment? Report of two cases of different types and review of the literature. Am J Orthop (Belle Mead NJ). 2011;40:E170-E176. [PubMed] |
| 3. | Chu HY, Chu P, Lin YF, Chou HK, Lin SH. Uremic tumoral calcinosis in patients on peritoneal dialysis: clinical, radiologic, and laboratory features. Perit Dial Int. 2011;31:430-439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 4. | Olsen KM, Chew FS. Tumoral calcinosis: pearls, polemics, and alternative possibilities. Radiographics. 2006;26:871-885. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 162] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 5. | Fathi I, Sakr M. Review of tumoral calcinosis: A rare clinico-pathological entity. World J Clin Cases. 2014;2:409-414. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 69] [Cited by in RCA: 89] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 6. | Ovali GY, Tarhan S, Serter S, Bayindir P, Okcu G, Demireli P, Pabuscu Y. A rare disorder: idiopathic tumoral calcinosis. Clin Rheumatol. 2007;26:1142-1144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 7. | Ke G, Li S, Cui Y, Chen X, Che H, Dou C, Lian Z, Zhang L, Li Z, Ma J, Feng Z, Yang J, Hu Y, Wang Y, Zhang H, Huang H, Su H, Guo J, He C, Liang X, Shi W, Ge P, Liu S. Treatment of Uremic Tumoral Calcinosis in Maintenance Hemodialysis Patients. Blood Purif. 2020;49:658-664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 8. | Hamada J, Tamai K, Ono W, Saotome K. Uremic tumoral calcinosis in hemodialysis patients: clinicopathological findings and identification of calcific deposits. J Rheumatol. 2006;33:119-126. [PubMed] |
| 9. | Cofan F, García S, Combalia A, Campistol JM, Oppenheimer F, Ramón R. Uremic tumoral calcinosis in patients receiving longterm hemodialysis therapy. J Rheumatol. 1999;26:379-385. [PubMed] |
| 10. | Sakhy Y, Taoussi R, Ndayishimiye V, Sabiri M, Labied M, Lembarki G, El Manjra S, Lezar S, Essodegui F. Tumoral calcinosis of unusual location in a chronic hemodialysis patient. BJR Case Rep. 2023;9:20220083. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 11. | Sabeel A, Al-Homrany M. Complete resorption of massive soft tissue calcification in a hemodialysis patient after parathyroidectomy. Am J Nephrol. 2000;20:421-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 12. | Rodríguez-Ortiz ME, Pendón-Ruiz de Mier MV, Rodríguez M. Parathyroidectomy in dialysis patients: Indications, methods, and consequences. Semin Dial. 2019;32:444-451. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 13. | Shen X, Chen W, Wang X, Chen J. Uremic tumoral calcinosis in a patient on hemodialysis. Intern Med. 2012;51:1443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 14. | Kim Y, Hwang E, Park S. Resolution of uremic tumoral calcinosis in a patient on peritoneal dialysis with long-term low-calcium dialysate treatment. Kidney Res Clin Pract. 2014;33:226-228. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 15. | Aristizabal-Alzate A, Nieto-Ríos JF, Trujillo-Agudelo D, Cadavid-Aljure D, Serna-Higuita LM, Zuluaga-Valencia G. Uremic tumoral calcinosis with atypical manifestation. Case report. Nefrologia (Engl Ed). 2020;. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 16. | Niemann KE, Kröpil F, Hoffmann MF, Coulibaly MO, Schildhauer TA. A 23-year-old patient with secondary tumoral calcinosis: Regression after subtotal parathyroidectomy: A case report. Int J Surg Case Rep. 2016;23:56-60. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 17. | Younes M, Belghali S, Zrour-Hassen S, Béjia I, Touzi M, Bergaoui N. Complete reversal of tumoral calcinosis after subtotal parathyroidectomy in a hemodialysis patient. Joint Bone Spine. 2008;75:606-609. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 18. | Sunder S, Verma H, Venkataramanan K. Cervical tumoral calcinosis with secondary hyperparathyroidism in a chronic hemodialysis patient. Hemodial Int. 2013;17:458-462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 19. | Li J, Li X, Dong X, Ma L, Guo Z, Chen X. Different outcomes following parathyroidectomy in patients with uremic tumoral calcinosis: two case reports. BMC Nephrol. 2023;24:55. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 20. | Kakani E, Sloan D, Sawaya BP, El-Husseini A, Malluche HH, Rao M. Long-term outcomes and management considerations after parathyroidectomy in the dialysis patient. Semin Dial. 2019;32:541-552. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 21. | Hatori M, Oomamiuda K, Kokubun S. Hydroxyapatite crystals in tumoral calcinosis: a case report. Tohoku J Exp Med. 1996;180:359-364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 22. | Segnit ER, Holland HD, Biscardi CJ. The solubility of calcite in aqueous solutions—I The solubility of calcite in water between 75° and 200° at CO2 pressures up to 60 atm. Geochim Cosmochim Acta. 1962;26:1301-1331. [DOI] [Full Text] |
| 23. | Langmuir D. Stability of calcite based on aqueous solubility measurements. Geochim Cosmochim Acta. 1968;32:835-851. [DOI] [Full Text] |
| 24. | McKee PH, Liomba NG, Hutt MS. Tumoral calcinosis: a pathological study of fifty-six cases. Br J Dermatol. 1982;107:669-674. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 57] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 25. | Slavin RE, Wen J, Barmada A. Tumoral calcinosis--a pathogenetic overview: a histological and ultrastructural study with a report of two new cases, one in infancy. Int J Surg Pathol. 2012;20:462-473. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 33] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 26. | Toyama T, Nakajima H, Kojima Y, Nishimiya N. Preparation of high-concentration hydroxyapatite solution by carbon dioxide blowing for morphological control of hydroxyapatite. Phosphorus Res Bull. 2012;26:91-94. [DOI] [Full Text] |
| 27. | Shirasawa Y, Orita H, Ishida K, Morimoto Y, Matsumoto M, Sakabe T. Critical alkalosis following intraperitoneal irrigation with sodium bicarbonate in a patient with pseudomyxoma peritonei. J Anesth. 2008;22:278-281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |