Published online Mar 18, 2023. doi: 10.5312/wjo.v14.i3.155
Peer-review started: November 20, 2022
First decision: January 9, 2023
Revised: January 18, 2023
Accepted: February 17, 2023
Article in press: February 17, 2023
Published online: March 18, 2023
Processing time: 117 Days and 12.2 Hours
Congenital transverse deficiencies are horizontal deficiencies of the long bones that occur with a reported incidence as high 0.38%. They can occur alone or represent a manifestation of a various clinical syndromes. Diagnosis has tradi
To summarize the current state of knowledge on congenital transverse limb deficiencies and to provide an update regarding the radiographic evaluation of congenital transverse limb deficiencies.
This IRB-exempt scoping review followed the PRISMA-ScR checklist for scoping reviews strictly. Five search engines were searched for a total of 265 publications. Four authors reviewed these during the screening process. Of these, 51 studies were included in our article. Prenatal magnetic resonance imaging (MRI), 3D Ultrasound, and multidetector Computed tomography (CT) exist are emerging modalities that have the potential to improve diagnosis.
Use of the appropriate classification system, three-dimensional ultrasonography with a maximum intensity projection, and appropriate use of prenatal MRI and prenatal CT can improve diagnosis and inter-provider communication.
Further scholarly efforts are required to develop improve standardized guidelines regarding the pre-natal radiographic evaluation of congenital limb deficiencies.
Core Tip: Early diagnosis can lead to early, appropriate, family-centered care strategies with the current literature supporting both non-operative and surgical management.
- Citation: Vij N, Goncalves LF, Llanes A, Youn S, Belthur MV. Prenatal radiographic evaluation of congenital transverse limb deficiencies: A scoping review. World J Orthop 2023; 14(3): 155-165
- URL: https://www.wjgnet.com/2218-5836/full/v14/i3/155.htm
- DOI: https://dx.doi.org/10.5312/wjo.v14.i3.155
Congenital transverse deficiencies are horizontal deficiencies involving the long bones. The incidence of congenital limb abnormalities is reported between 0.035%[1] and 0.38%[2], of which transverse limb deficiencies account for approximately half[3]. Congenital transverse limb deficiencies can occur in isolation or as a part of a clinical syndrome. These include femoral and fibular deficiencies[4], Adams-Oliver syndrome[5], Dandy-Walker syndrome[6], and Opitz trigonocephaly[7]. Congenital transverse limb deficiencies can occur in any long bones of the body, though an upper limb and left sided predominance have been demonstrated[8,9]. In the upper extremity, the most common deficiency is the transverse terminal deficiency at the upper third forearm[10].
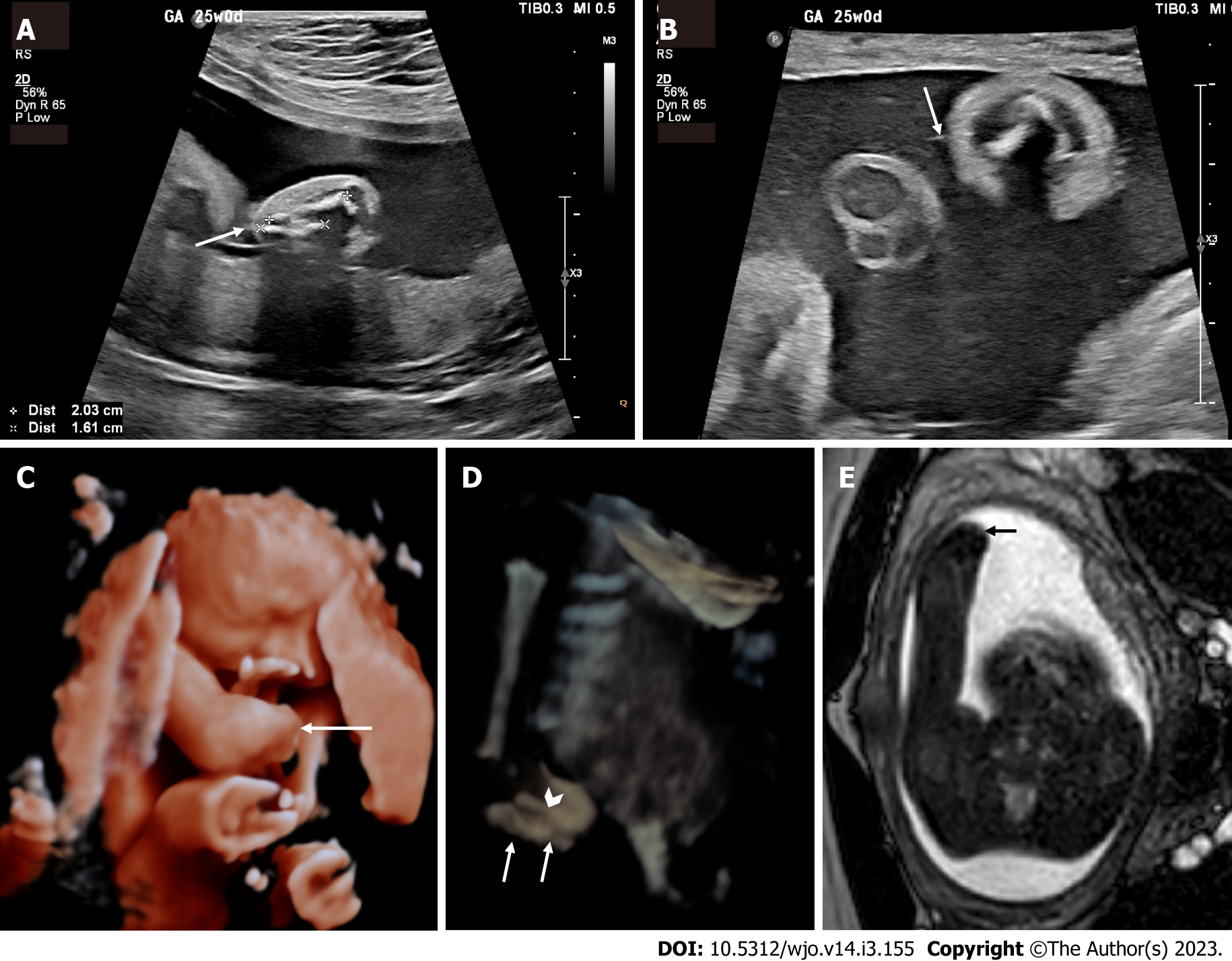
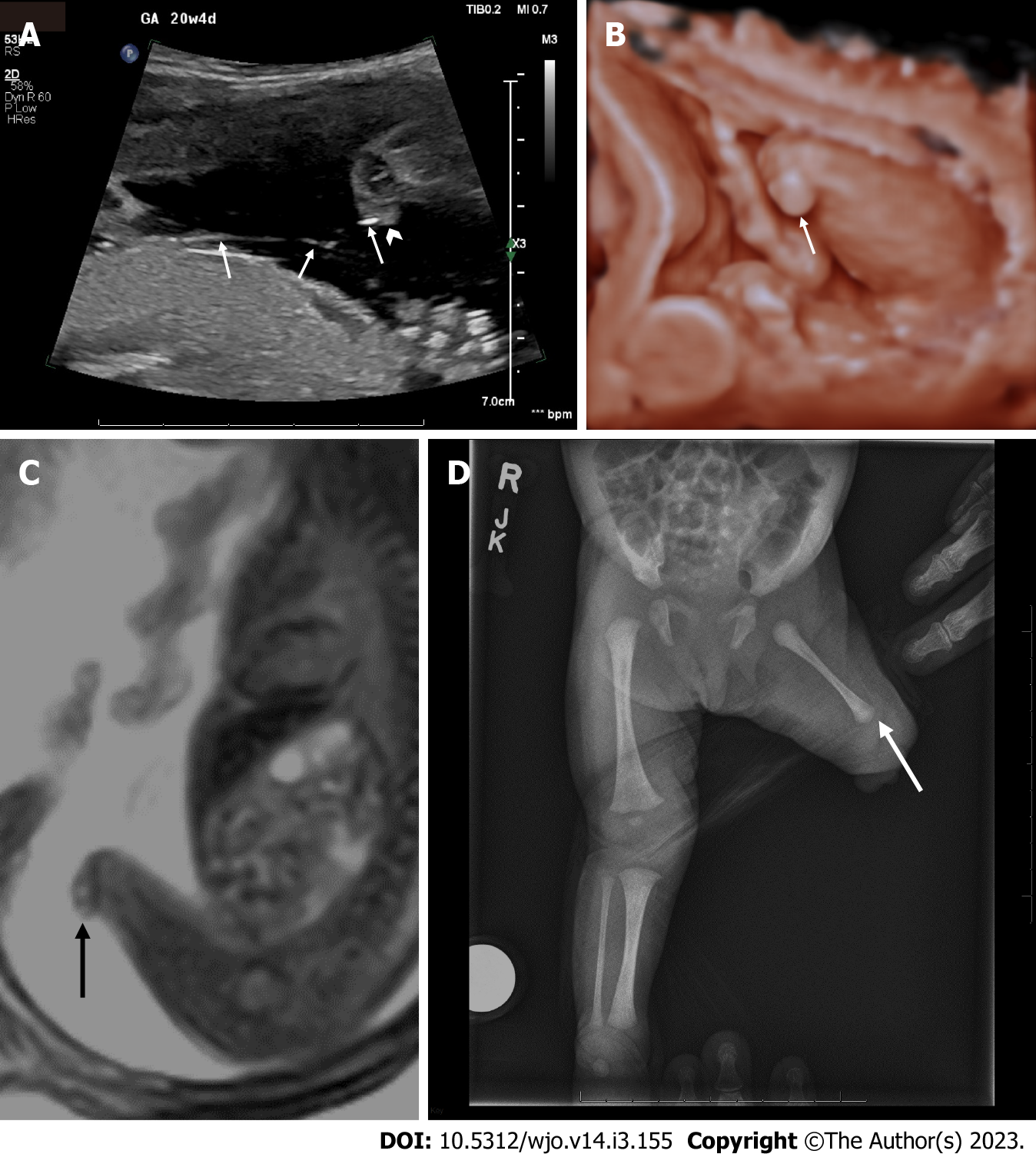
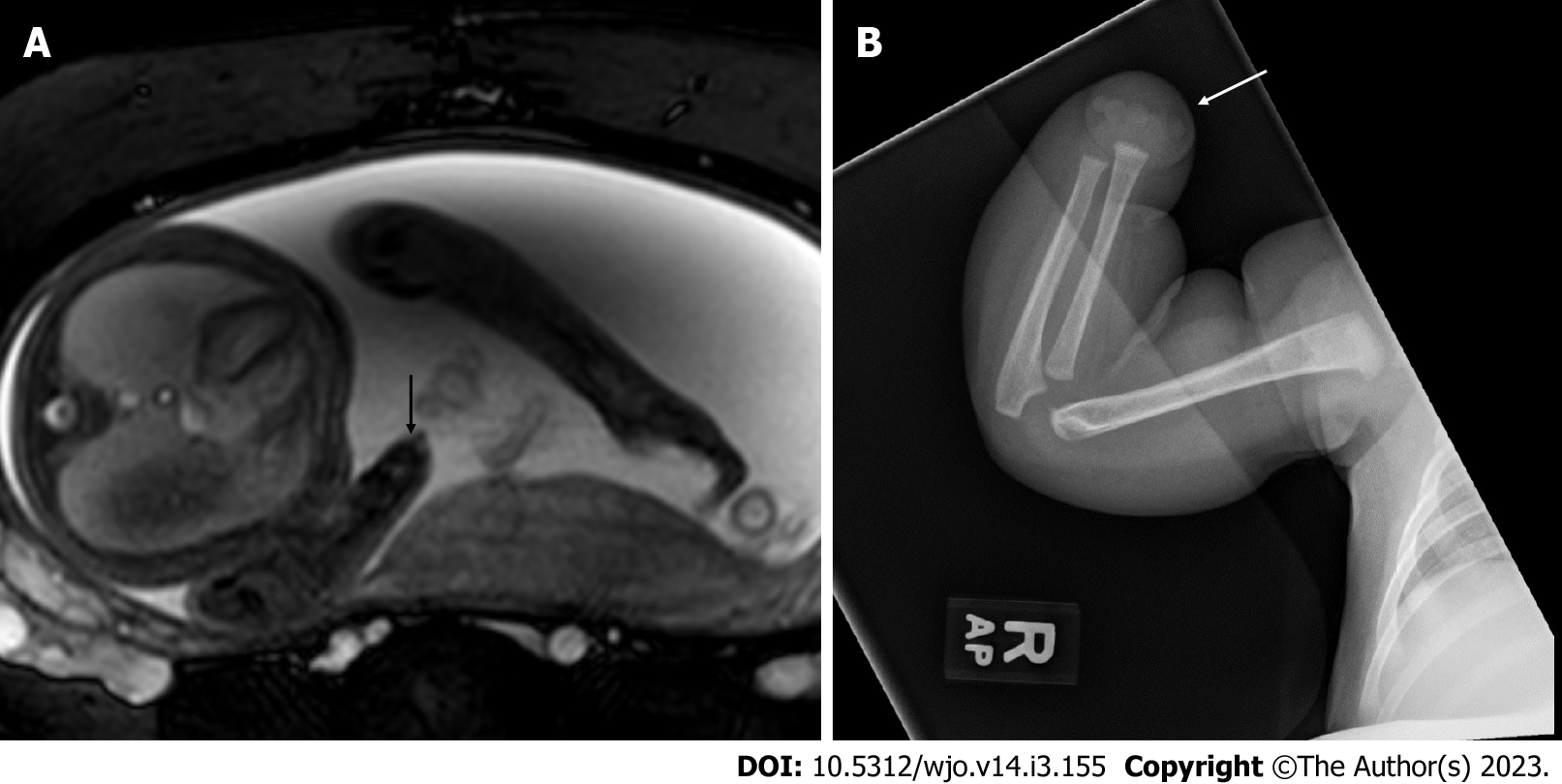
Diagnosis of congenital transverse limb deficiencies is traditionally thought to be largely supported by conventional radiography[11]. In recent years there has been advancement regarding the use of prenatal imaging to assist in early diagnosis. This includes two-dimensional ultrasound (2D US) (Figure 1A and B, Figure 2A), three-dimensional ultrasound (3D US) (Figure 1C and Figure 2B), fetal magnetic resonance imaging (fetal MRI) (Figure 1C and E, Figure 3A) and low-dose multidetector computerized tomography with 3D reconstruction (3D-CT). The purpose of this scoping review article is to summarize the current state of knowledge on congenital transverse limb deficiencies and to provide an update regarding the radiographic evaluation of congenital transverse limb deficiencies.
This was an IRB-exempt scoping review. The scoping review checklist available at the Preferred Reporting Items for Systematic reviews and Meta-Analyses extension for Scoping Reviews Checklist was followed strictly. The International prospective register of systematic reviews (PROPSERO) was contacted regarding our intention of this article and advised that scoping views do not require registration with PROSPERO.
The following search engines were used: Medline, PubMed Advanced Search, Cochrane library, Embase, and Scopus. Cochrane Reviews was also searched per the recommendations of Pautasso et al[12]. The following search items were used: ‘Pediatric Transverse Limb Deficiencies’, ‘Radiography transverse limb deficiencies’, ‘radiographic evaluation’ AND ‘pediatric transverse limb defects’, ‘transverse’ AND ‘limb’ AND ‘defects’, ‘radiographic AND evaluation’ AND ‘transverse’ AND ‘limb’ AND ‘deficiencies’, ‘MRI’ AND ‘transverse limb deficiencies’, and ‘prenatal diagnosis’ AND ‘transverse limb deficiencies.’ This resulted in a total of 265 articles.
Four of the authors (Neeraj Vij, Aaron Llanes, Sean Youn, MV Belthur) screened the article by title and abstract. A preliminary decision to include or exclude an article was made based on relevance of the information within the abstract as determined by our inclusion/exclusion criteria (Table 1) This resulted in a preliminary list of 112 articles. This preliminary list of articles was organized into the following subheadings: Embryology, Etiology, Natural History, Classification Systems, Prenatal Imaging, Non-surgical treatment, and Surgical treatment.
| Inclusion criteria | Exclusion criteria |
| Pediatric population (ages 0 – 18) | Absence of etiologic, classification system, radiographic, or outcome data in the abstract |
| Date of publication 1950 – 20221 | |
| Mention of the diagnosis of terminal transverse or intercalary transverse limb deficiencies | |
| Insufficient description of patient characteristics to reasonably draw inferences |
These articles then underwent a full-text screening process by the same four authors (Neeraj Vij, Aaron Llanes, Sean Youn, MV Belthur). The primary purpose of the full-text screening was final inclusion based on the inclusion/exclusion criteria, and placement of the article in a given section. This resulted in a total of 51 included articles. The references of the selected articles were also hand-searched to identify any missing articles. This did not reveal any additional articles. The include articles were then imported and stored into the most up-to-date stable release Mendeley (v2.57.0)[12].
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Limb development begins through paired primordial limb buds at 4 wk of intrauterine development. This is enabled by the undifferentiated mesenchymal cells that underlay the ectoderm on both the forelimb and hindlimb[13]. This structure is referred to as the apical ectodermal ridge and permits both proximodistal development through fibroblastic growth factor, anteroposterior development through the sonic hedgehog protein, and dorsoventral development through the Engrailed-1 protein[14]. The main mechanism of the etiology and embryological level is the disruption of growth of the Apical Epidermal Ridge (AER) along the proximodistal plane[14]. The subsequent result is that the interaxial signaling that is responsible for normal limb development is disrupted and causes a deficiency in the plane orthogonal to the developing limb bud, that is, transversely.
There are several proposed causes for this disruption of the AER. The leading theory includes hypoperfusion, which leads to apoptosis of the AER. This is generally thought to be due to a vasoocclusive event[15] including and other vascular etiologies including thrombosis, vasospasm, and embolism[5,16-18]. Decongestants, non-aspirin non-steroidal anti-inflammatory drugs, and smoking are significantly associated with terminal transverse limb deficiencies[16]. Other etiologies include maternal thrombophilias[9], alpha-thalassemias[19], and amniotic band sequence[16] (Figure 1).
Congenital transverse limb deficiencies are non-progressive disorders. These disorders result from apoptosis failure (usually secondary to a vascular insult). Thus, the condition is of maximum severity at its presentation and does not progress. Though congenital transverse limb deficiencies are characterized by decreased mobility and function, the symptoms do not worsen as the child grows older.
While the deformity itself cannot worsen, there are malformations associated with limb defects that need to be monitored. Preaxial limb defects were shown to occur more frequently with esophageal atresia, heart defects, and unilateral kidney dysgenesis; postaxial defects with hypospadias; transverse defects with craniofacial defects and ring constrictions; and amelia with anorectal atresia, omphalocele, gastroschises, and ring constriction[20]. Therefore, close attention to associated congenital anomalies is necessary to ensure the health and proper growth of the child.
In a significant proportion of congenital transverse limb deficiency cases, surgery and prosthetics are unnecessary and sometimes contraindicated due to the possibility of complications and the lack of functional gain. Therefore, supportive treatment along with aid from occupational therapists can be a viable option[21].
The first classification of congenital limb deficiencies was described in 1961 by Frantz et al[22] The International Society for Prosthetics and Orthotics anatomical classification for congenital limb deficiencies represented a significant expansion of the originally published classification[23]. In this system, congenital limb deficiencies are first described as either longitudinal or transverse. Longitudinal limb deficiencies refer to the complete absence or reduction of the long axis of a limb, whereas transverse limb deficiencies refer to the abrupt absence of an otherwise normally developing limb. Limb deficiencies can be described to specify the affected limb and the extent to which it is affected. By convention, longitudinal limb deficiencies name the bone that is affected such as the ulna or tibia whereas transverse limb deficiencies name the anatomical location that is affected such as the shoulder or leg. The degree of deficiency for both longitudinal and transverse deficiencies can be described as total or partial.
More recent classification systems have expounded upon the anatomy and etiology of congenital limb deficiencies. For instance, McGuirk et al[24] considered an additional category of limb deficiency termed intercalary defects, which refers specifically to the absence or hypoplasia of the middle portion of a long bone with intact distal features.
The most recent classification includes both an anatomic and etiologic component[25]. This greatly expands on that of Day et al[23] and McGuirk et al[24] to include an etiologic component that includes chromosomal abnormalities, Mendelian inheritance, syndromes, teratogenic exposures, vascular disruption defects, or unknown causes[25]. This classification also includes other congenital limb deficiencies, can be used to succinctly describe the location, and allows modification for the presence of nubbins. By allowing subclassification by syndromic, teratogenic, vascular, or other etiologies and by distinguishing between phenotypes within the family of transverse limb deficiencies, the classification proposed by Gold et al[25] can provide improved communication between providers and guide genetic testing[26].
Prenatal 2D US has become the mainstay of the diagnosis of transverse limb deficiencies (Figures 1A and B, 2A) In much of the developed world, prenatal evaluation involves the mid-trimester scan[1,27]. However, a comprehensive cross-sectional study in 12 European countries for a total of 7758 cases found a prenatal detection rate of 22.7% for isolated terminal transverse deficiencies[1]. Another large study from England, demonstrates a second trimester detection rate of 25%[27]. These large studies in the developed world demonstrate a significant barrier to early intervention and counseling for patients’ families.
Of note, is the potential utility of the first-trimester ultrasound scan that has shown to be useful in the detection of transverse limb deficiencies. Liao et al[2] demonstrated a detection rate of 77.8% for all fetal limb abnormalities through the first trimester scan. The first trimester scan may have greater role in diagnosis of these limb deficiencies than previously thought.
Fetal MRI is also used as a secondary diagnostic modality (Figures 1C and E, 3A) It is particularly useful in cases that do not image well by ultrasound including cases of maternal obesity, advanced gestational age, oligohydramnios, and unfavorable fetal position[11]. MRI may uncover other associated soft tissue anomalies that may not otherwise be diagnosed prenatally. 3D US (Figures 1C and D, 2B) and multidetector computed tomography are also emerging as modalities to improve diagnosis and lead to earlier treatment[28]; however, low-dose 3D CT is, in general, more useful in cases of suspected skeletal dysplasias. A set of radiographic pearls have been developed by our institutional experts for the reader (Table 2). However, no standardized algorithm incorporating their use is available.
| Pearl or pitfall | Reasoning and evidence |
| Use the appropriate classification system | Many classification systems exist[25,26]. However, the most recent classification system by Gold et al[27] provides improved communication. When these fail, description of the location and use of modifiers can be helpful to radiologists and non-radiologists, alike |
| Prenatal ultrasound pearl | Three-dimensional ultrasonography using both maximum intensity projection (MIP), thick slabs with MIP, or surface rendering greatly complements the examination using two-dimensional ultrasound. Scanning with new generation high-frequency broad band probes (up to 22 MHz in some cases) allows greater confidence in the identification of amniotic bands compared to standard obstetrical probes[11] |
| Prenatal fetal MRI pearl | MRI is not superior to ultrasound for imaging fetal bones. However, it may prove useful in cases where ultrasonography windows are limited, such as maternal obesity, advanced gestational age, oligohydramnios, and unfavorable fetal position. It also provides an additional opportunity to diagnose associated congenital anomalies for which MRI is more sensitive than ultrasound (i.e., CNS anomalies) |
| Prenatal CT | Current literature supports the use of low-dose CT with 3D reconstruction for the evaluation of skeletal dysplasias[30]. The use of CT for the evaluation of transverse limb deficiencies has not been fully evaluated. The use of CT may be considered when both ultrasound and MRI failed to characterize the phenotype and only after thorough evaluation of benefits versus risks in individual cases |
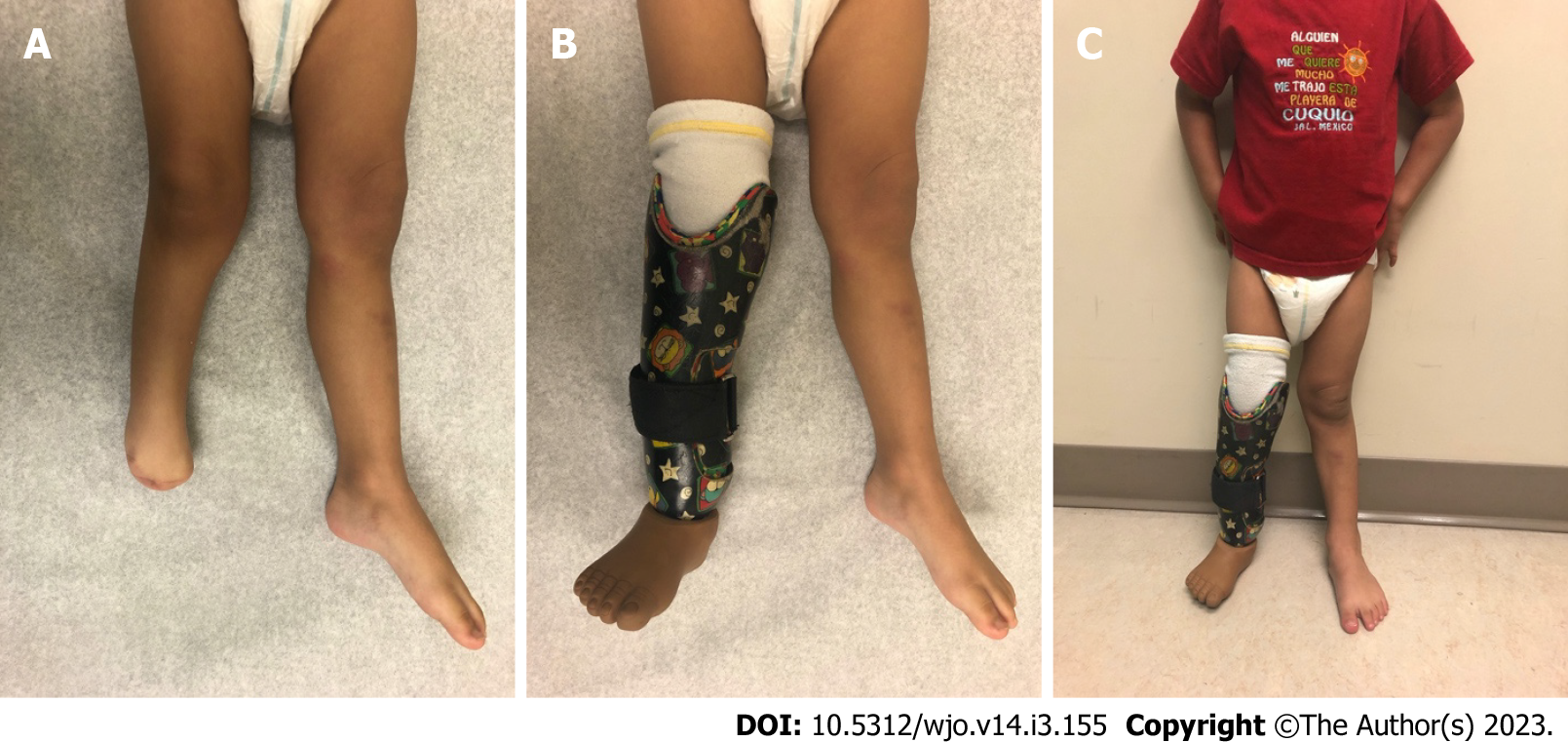
Non-surgical therapies: Non-surgical management of congenital limb deficiencies is comprised of physical therapy, orthoses, and prostheses (Figure 4). Quality of life, functionality, and the degree of limb deficit should all be considered in deciding an appropriate non-surgical strategy[29-31]. Timely implementation of a multidisciplinary strategy is required for reducing pain, function loss, and improving quality of life[32-36].
While there are no clinical guidelines regarding the timeline of treatment, most studies recommend prosthetic fitting before the age of 2 years as the prosthetic rejection rate increases drastically after the age of two[37,38]. It is important to note that children who have treatment can achieve satisfactory degrees of movement and function.
An important facet to treatment is appropriate counseling of the family. This begins with a thorough conversation about the diagnosis, prognosis, natural history, treatment options, and alternatives. Providing an opportunity for the family to meet the family of another affected child has been shown to be very helpful. Doing so can reduce parental anxiety and improve compliance with treatment recommendations[39,40].
Location of the defect is a very important determinant in the treatment strategy (Figure 3B). While lower extremity prosthetic intervention is widely used, the treatment strategy around upper extremity defects is more nuanced[21]. Upper extremity defects are more common and the choice and fit of prosthesis is rapidly evolving[41].
The ideal time range for prosthesis fitting for trans-forearm and trans-humeral deficiencies is between six and twenty-four months of age[42]. This improves the performance of activities of daily living (ADL's) and minimizes prosthetic rejection. Generally, the outcome of prosthesis use in patients with proximal upper limb deficiencies is good[43]. There is evidence to suggest that early myoelectric prosthesis devices between 2.5 and 4 years of age may have potential therapeutic benefit[42]. However, it is important to consider that prosthesis choice and fit needs to be individualized based on the level of amputation and stump choice to prevent the nerve entrapment syndromes associated with prosthesis overuse[44].
There is a reported underuse of upper limb prosthesis in children with congenital transverse reductions[45]. Children tend to use their prostheses for specific activities and not others[45]. A recent study has shown that there is a direct correlation between the number of activities that children perform and daily use of the upper extremity prosthesis[46]. Addressing the issue of sporadic use may lead to better performance on activities of daily living and increased independence[38].
Though the current literature supports the use of prostheses, there are no long-term reports that compare the outcomes of prosthetic treatment in terms of patient-reported or functional outcomes. Further studies are needed to determine the functional outcome for upper extremity prosthesis users and non-users and thus allow clinicians to make evidence-based recommendations.
An important topic of discussion is rehabilitation options for young adults. These management resources are often underutilized. A low rate of referral to the affected child and an interdisciplinary management team has been noted from the Regional Disablement Service in Northern Ireland[47].
Ultimately, this has led to progressive social strains that further worsen the quality of life of individuals with transverse limb deficiencies. Many young adults with transverse upper limb deficiencies have difficulty in finding suitable housing and employment[48]. Further, most young adults with upper limb transversal deficiencies are unaware of available resources and have perceived limited benefit of said resources[49]. Increased focus on age-relevant information as well as dedicated training programs could increase benefit to young adults with upper limb transverse limb defects[49]. These findings suggest that an emphasis on the availability and use of these resources could have a great impact on the quality of life of individuals with transverse limb deficiencies. It remains unclear as to what the ideal age, modality of education, and the role of the physician would be to better utilize these options. More research is required to identify what measures can be taken to increase both awareness and use of available resources.
Surgical treatment is reserved for a minority of patients and ranges from 16.9%[50] to 25%[3] of patients. Generally, surgical treatment involves intermediary correction for prosthesis placement[42]. However, there is a role in corrective surgeries[3] and lengthening procedures[42,51-52] for some patients.
Surgical treatment pursued varies based on the location of the deficiency[3]. An epidemiological study in Japan noted that in both the upper and lower extremity, surgical treatment was more commonly pursued than prosthesis and orthosis combined in the setting of transverse deficiencies at or distal to the level of distal to the carpals. However, in proximal upper and lower extremity defects, surgery was rarely pursued.
When considering surgical options, they can be broadly classified as one of the following: amputation/constriction ring procedures[3], lengthening procedures[42,51], joint reconstructions[52], and digit transfer surgeries[53]. The literature on outcomes in lower extremity transverse limb surgery is sparse; however, the outcomes for upper limb defects in the hand and wrist are summarized below.
To treat upper limb deficiencies at the level of the metacarpal or distal to the metacarpal, finger resurfacing procedures are performed. Amputation and constriction ring release procedures are also performed in preparation for the complete articulation of the limb and prosthetic[3]. However, there are a few surgical options that have been described around the topic of improving orthotic use and increasing functional ability without the need for prostheses.
Reconstruction of the wrist articulation with an iliac crest autograft has been described in the setting of terminal deficiencies. In two patients, these were shown to have cartilage formation on MRI and improved functional abilities[52].
Microsurgical toe-to-hand transfers have also been described with good radiographic outcomes at a mean follow-up of 21 months; however, no patient-reported outcomes have been noted with this surgical option. In the setting of cleft hand, transverse limb deficiencies respond well to cleft hand reconstruction with both radiographic and functional outcomes[53]. Importantly, 4 out of the 11 cleft hands due to underlying transverse limb deficiencies did require additional surgery[54]. Lengthening with either an on-top resurfacing procedure or distraction lengthening has been described with good radiographic outcomes and improved inch function[51].
Though there is some good data regarding radiographic outcomes, the literature is sparse for success rates and re-operation rates when considering these surgical options. Overall, when considering upper extremity transverse deficiencies, a re-operation rate of 5%-20% has been reported. More studies with long-term clinical and functional outcomes of these surgical options and studies investigating causes for re-operation are needed to assist in surgical decision-making.
As a scoping review, this manuscript is limited by the quality of the evidence of the studies included. The lowest quality of evidence included was level IV. Further as a scoping review, this article uses summary date to draw conclusions about the current state of a topic. As such, it is vulnerable to selection bias. This may also result in the inability of our search findings to be completely generalizable.
Transverse limb deficiencies are rare and display an upper limb predominance. Conventionally, the diagnosis was made through radiography; however, 2D US emerged as a prevalent technology with the potential to improve our diagnostic capabilities. Prenatal 3D US, fetal MRI, and low-dose 3D CT are newly emerging modalities that could also potentially improve diagnosis; however, are not routinely employed in diagnosis. To date, there are no standardized international guidelines regarding the pre-natal radiographic evaluation of congenital limb deficiencies. A systematic method would allow for enhanced interdisciplinary communication, early diagnosis, and integration of family values into treatment plans. Management with prosthesis is commonly pursued, especially in the upper extremity, with highly variable use rates. Surgical treatment is less commonly sought out. Though some studies demonstrate good radiographic and clinical outcomes for hand/wrist surgical options, these are limited by the number of patients and quality of evidence. Further long-term studies are needed for both the conservative and operative treatment options to assist in clinical decision-making.
Congenital transverse deficiencies are horizontal deficiencies of the long bones that occur with a reported incidence as high 0.38%.
There has been much advancement regarding prenatal imaging modalities to allow for early diagnosis and appropriate treatment.
The purpose of this scoping review article is to summarize the current state of knowledge on congenital transverse limb deficiencies and to provide an update regarding the radiographic evaluation of congenital transverse limb deficiencies.
This IRB-exempt scoping review followed the PRISMA-ScR checklist for scoping reviews strictly. Five search engines were searched for a total of 265 publications.
Of these, 51 studies were included in our article.
Prenatal magnetic resonance imaging (MRI), 3D Ultrasound, and multidetector computed tomography (CT) exist are emerging modalities that have the potential to improve diagnosis. Use of the appropriate classification system, three-dimensional ultrasonography with a maximum intensity projection, and appropriate use of prenatal MRI and prenatal CT can improve diagnosis and inter-provider communication.
Further scholarly efforts are required to develop improve standardized guidelines regarding the pre-natal radiographic evaluation of congenital limb deficiencies.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Orthopedics
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Liu P, China; Wang J, China S-Editor: Ma YJ L-Editor: A P-Editor: Ma YJ
| 1. | Stoll C, Wiesel A, Queisser-Luft A, Froster U, Bianca S, Clementi M. Evaluation of the prenatal diagnosis of limb reduction deficiencies. EUROSCAN Study Group. Prenat Diagn. 2000;20:811-818. [PubMed] [DOI] [Full Text] |
| 2. | Liao YM, Li SL, Luo GY, Wen HX, Ouyang SY, Chen CY, Yao Y, Bi JR, Tian XX. Routine screening for fetal limb abnormalities in the first trimester. Prenat Diagn. 2016;36:117-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 24] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 3. | Mano H, Fujiwara S, Takamura K, Kitoh H, Takayama S, Ogata T, Haga N. Treatment approaches for congenital transverse limb deficiency: Data analysis from an epidemiological national survey in Japan. J Orthop Sci. 2021;26:650-654. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 4. | Walker JL, White HD, Jacobs CA, Riley SA. Upper extremity anomalies in children with femoral and fibular deficiency. J Pediatr Orthop B. 2020;29:399-402. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 5. | Snape KM, Ruddy D, Zenker M, Wuyts W, Whiteford M, Johnson D, Lam W, Trembath RC. The spectra of clinical phenotypes in aplasia cutis congenita and terminal transverse limb defects. Am J Med Genet A. 2009;149A:1860-1881. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 75] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 6. | Chen CP, Chien SC, Chern SR, Tzen CY, Wang W. Prenatal diagnosis of Dandy-Walker malformation associated with distal limb deficiencies. Genet Couns. 2007;18:343-347. [PubMed] |
| 7. | Fryns JP, Snoeck L, Kleczkowska A, Van den Berghe H. Opitz trigonocephaly syndrome and terminal transverse limb reduction defects. Helv Paediatr Acta. 1985;40:485-488. [PubMed] |
| 8. | Saeed F, Paramasivam G, Wiechec M, Kumar S. Fetal transverse limb defects: case series and literature review. J Clin Ultrasound. 2011;39:454-457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 9. | Ordal L, Keunen J, Martin N, Shehata N, Borschel GH, Clarke HM, Toi A, Shuman C, Chitayat D. Congenital limb deficiencies with vascular etiology: Possible association with maternal thrombophilia. Am J Med Genet A. 2016;170:3083-3089. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 10. | Koskimies E, Lindfors N, Gissler M, Peltonen J, Nietosvaara Y. Congenital upper limb deficiencies and associated malformations in Finland: a population-based study. J Hand Surg Am. 2011;36:1058-1065. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 82] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 11. | Aucourt J, Budzik JF, Manouvrier-Hanu S, Mézel A, Cotten A, Boutry N. Congenital malformations of the hand and forearm in children: what radiologists should know. Semin Musculoskelet Radiol. 2012;16:146-158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 12. | Pautasso M. Ten simple rules for writing a literature review. PLoS Comput Biol. 2013;9:e1003149. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 136] [Cited by in RCA: 114] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 13. | Lin GH, Zhang L. Apical ectodermal ridge regulates three principal axes of the developing limb. J Zhejiang Univ Sci B. 2020;21:757-766. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 14. | Christiaens AB, Deprez PML, Amyere M, Mendola A, Bernard P, Gillerot Y, Clapuyt P, Godfraind C, Lengelé BG, Vikkula M, Nyssen-Behets C. Isolated bilateral transverse agenesis of the distal segments of the lower limbs at the level of the knee joint in a human fetus. Am J Med Genet A. 2016;170A:523-530. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 15. | Hoyme HE, Jones KL, Van Allen MI, Saunders BS, Benirschke K. Vascular pathogenesis of transverse limb reduction defects. J Pediatr. 1982;101:839-843. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 149] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 16. | Adrien N, Petersen JM, Parker SE, Werler MM. Vasoactive exposures and risk of amniotic band syndrome and terminal transverse limb deficiencies. Birth Defects Res. 2020;112:1074-1084. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 17. | Holmes LB, Nasri HZ. Terminal transverse limb defects with "nubbins". Birth Defects Res. 2021;113:1007-1014. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 18. | Gardiner DM, Holmes LB. Hypothesis: terminal transverse limb defects with "nubbins" represent a regenerative process during limb development in human fetuses. Birth Defects Res A Clin Mol Teratol. 2012;94:129-133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 19. | Adam MP, Chueh J, El-Sayed YY, Stenzel A, Vogel H, Weaver DD, Hoyme HE. Vascular-type disruptive defects in fetuses with homozygous alpha-thalassemia: report of two cases and review of the literature. Prenat Diagn. 2005;25:1088-1096. [PubMed] |
| 20. | Rosano A, Botto LD, Olney RS, Khoury MJ, Ritvanen A, Goujard J, Stoll C, Cocchi G, Merlob P, Mutchinick O, Cornel MC, Castilla EE, Martínez-Frías ML, Zampino G, Erickson JD, Mastroiacovo P. Limb defects associated with major congenital anomalies: clinical and epidemiological study from the International Clearinghouse for Birth Defects Monitoring Systems. Am J Med Genet. 2000;93:110-116. [PubMed] [DOI] [Full Text] |
| 21. | Kuyper MA, Breedijk M, Mulders AH, Post MW, Prevo AJ. Prosthetic management of children in The Netherlands with upper limb deficiencies. Prosthet Orthot Int. 2001;25:228-234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 34] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 22. | Frantz CH, OʼRahilly R. Congenital Skeletal Limb Deficiencies. J Bone Jt Surg. 1961;43(8). [RCA] [DOI] [Full Text] [Cited by in Crossref: 212] [Cited by in RCA: 134] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 23. | Day HJ. The ISO/ISPO classification of congenital limb deficiency. Prosthet Orthot Int. 1991;15:67-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 38] [Article Influence: 1.1] [Reference Citation Analysis (1)] |
| 24. | McGuirk CK, Westgate MN, Holmes LB. Limb deficiencies in newborn infants. Pediatrics. 2001;108:E64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 79] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 25. | Gold NB, Westgate MN, Holmes LB. Anatomic and etiological classification of congenital limb deficiencies. Am J Med Genet A. 2011;155A:1225-1235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 59] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 26. | Bergman JEH, Löhner K, van der Sluis CK, Rump P, de Walle HEK. Etiological diagnosis in limb reduction defects and the number of affected limbs: A population-based study in the Northern Netherlands. Am J Med Genet A. 2020;182:2909-2918. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 27. | Kevern L, Warwick D, Wellesley D, Senbaga R, Clarke NM. Prenatal ultrasound: detection and diagnosis of limb abnormalities. J Pediatr Orthop. 2003;23:251-253. [PubMed] [DOI] [Full Text] |
| 28. | Potier A. Prenatal diagnosis of upper limb reduction deficiencies. Chir Main. 2008;27 Suppl 1:S21-S26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 29. | Nelson VS, Flood KM, Bryant PR, Huang ME, Pasquina PF, Roberts TL. Limb deficiency and prosthetic management. 1. Decision making in prosthetic prescription and management. Arch Phys Med Rehabil. 2006;87:S3-S9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 21] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 30. | Johansen H, Østlie K, Andersen LØ, Rand-Hendriksen S. Health-related quality of life in adults with congenital unilateral upper limb deficiency in Norway. A cross-sectional study. Disabil Rehabil. 2016;38:2305-2314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 12] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 31. | Postema SG, Bongers RM, Brouwers MA, Burger H, Norling-Hermansson LM, Reneman MF, Dijkstra PU, van der Sluis CK. Upper Limb Absence: Predictors of Work Participation and Work Productivity. Arch Phys Med Rehabil. 2016;97:892-899. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 32. | Pruitt SD, Varni JW, Setoguchi Y. Functional status in children with limb deficiency: development and initial validation of an outcome measure. Arch Phys Med Rehabil. 1996;77:1233-1238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 35] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 33. | Yigiter K, Ulger O, Sener G, Akdogan S, Erbahçeci F, Bayar K. Demography and function of children with limb loss. Prosthet Orthot Int. 2005;29:131-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 23] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 34. | Chhina H, Klassen AF, Kopec JA, Oliffe J, Iobst C, Dahan-Oliel N, Aggarwal A, Nunn T, Cooper AP. What matters to children with lower limb deformities: an international qualitative study guiding the development of a new patient-reported outcome measure. J Patient Rep Outcomes. 2021;5:30. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 35. | Michielsen A, Van Wijk I, Ketelaar M. Participation and quality of life in children and adolescents with congenital limb deficiencies: A narrative review. Prosthet Orthot Int. 2010;34:351-361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 32] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 36. | Pruitt SD, Seid M, Varni JW, Setoguchi Y. Toddlers with limb deficiency: conceptual basis and initial application of a functional status outcome measure. Arch Phys Med Rehabil. 1999;80:819-824. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 19] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 37. | Toda M, Chin T, Shibata Y, Mizobe F. Use of Powered Prosthesis for Children with Upper Limb Deficiency at Hyogo Rehabilitation Center. PLoS One. 2015;10:e0131746. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 9] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 38. | Scotland TR, Galway HR. A long-term review of children with congenital and acquired upper limb deficiency. J Bone Joint Surg Br. 1983;65:346-349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 46] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 39. | Kerr SM, McIntosh JB. Coping when a child has a disability: exploring the impact of parent-to-parent support. Child Care Health Dev. 2000;26:309-322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 86] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 40. | Watts HG, Clark MW. Who Is Amelia? Pediatr Phys Ther. 1999;11(4). [DOI] [Full Text] |
| 41. | Mano H, Fujiwara S, Takamura K, Kitoh H, Takayama S, Ogata T, Hashimoto S, Haga N. Congenital limb deficiency in Japan: a cross-sectional nationwide survey on its epidemiology. BMC Musculoskelet Disord. 2018;19:262. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 16] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 42. | Farr S, Catena N, Martinez-Alvarez S, Soldado F; EPOS Upper Limb Study Group. Peromelia - congenital transverse deficiency of the upper limb: a literature review and current prosthetic treatment. J Child Orthop. 2018;12:558-565. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 43. | Datta D, Selvarajah K, Davey N. Functional outcome of patients with proximal upper limb deficiency--acquired and congenital. Clin Rehabil. 2004;18:172-177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 122] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 44. | Burger H, Vidmar G. A survey of overuse problems in patients with acquired or congenital upper limb deficiency. Prosthet Orthot Int. 2016;40:497-502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 36] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 45. | Buffart LM, Roebroeck ME, van Heijningen VG, Pesch-Batenburg JM, Stam HJ. Evaluation of arm and prosthetic functioning in children with a congenital transverse reduction deficiency of the upper limb. J Rehabil Med. 2007;39:379-386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 20] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 46. | Mano H, Noguchi S, Fujiwara S, Haga N. Relationship between degree of disability, usefulness of assistive devices, and daily use duration: an investigation in children with congenital upper limb deficiencies who use upper limb prostheses. Assist Technol. 2021;1-6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 47. | Hillan J, Graham LE. Compliance to service standards for congenital upper limb deficiency: the Northern Ireland experience. Prosthet Orthot Int. 2012;36:39-44. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 48. | Lankhorst IMF, Baars ECT, Wijk IV, Janssen WGM, Poelma MJ, van der Sluis CK. Living with transversal upper limb reduction deficiency: limitations experienced by young adults during their transition to adulthood. Disabil Rehabil. 2017;39:1623-1630. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 49. | Huurneman KAM, Lankhorst IMF, Baars ECT, van Wijk I, van der Sluis CK. Opinions on rehabilitation care of young adults with transversal upper limb reduction deficiency in their transition to adulthood. J Pediatr Rehabil Med. 2021;14:103-112. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 50. | Jain SK. A study of 200 cases of congenital limb deficiencies. Prosthet Orthot Int. 1994;18:174-179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 8] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 51. | Ogino T, Kato H, Ishii S, Usui M. Digital lengthening in congenital hand deformities. J Hand Surg Br. 1994;19:120-129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 32] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 52. | Deroussen F, Gouron R, Juvet-Segarra M, Maes-Clavier C, Plancq MC, Collet LM. Use of an iliac crest growth plate for the development of a neo-articulation for congenital transverse deficiencies at the wrist. J Hand Surg Am. 2012;37:2061-2067. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 53. | Chang J, Jones NF. Radiographic analysis of growth in pediatric microsurgical toe-to-hand transfers. Plast Reconstr Surg. 2002;109:576-582. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 17] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 54. | Aleem AW, Wall LB, Manske MC, Calhoun V, Goldfarb CA. The transverse bone in cleft hand: a case cohort analysis of outcome after surgical reconstruction. J Hand Surg Am. 2014;39:226-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |