Published online Nov 18, 2023. doi: 10.5312/wjo.v14.i11.827
Peer-review started: August 14, 2023
First decision: September 28, 2023
Revised: October 9, 2023
Accepted: October 23, 2023
Article in press: October 23, 2023
Published online: November 18, 2023
Processing time: 93 Days and 16.7 Hours
Spondyloepiphyseal dysplasia congenita (SEDC) is a rare autosomal dominant hereditary disease caused by COL2A1 mutations. SEDC primarily involves the skeletal system, with typical clinical manifestations, including short stature, hip dysplasia, and spinal deformity. Due to the low incidence of SEDC, there are only a few case reports regarding the surgical treatment of SEDC complicated with spinal deformities.
We report a case of a 16-year-old male patient with SEDC. He presented with typical short stature, atlantoaxial dysplasia, scoliosis, and hip dysplasia. Cervical magnetic resonance imaging showed spinal canal stenosis at the atlas level and cervical spinal cord compression with myelopathy. The scoliosis was a right thoracic curve with a Cobb angle of 65°. He underwent atlantoaxial reduction, decompression, and internal fixation from C1–C2 to relieve cervical myelopathy. Three months after cervical surgery, posterior correction surgery for scoliosis was performed from T3 to L4. Scoliosis was corrected from 66° to 8° and remained stable at 2-year follow-up.
This is the first case report of a patient with SEDC who successfully underwent surgery for atlantoaxial dysplasia and scoliosis. The study provides an important reference for the surgical treatment of SEDC complicated with spinal deformities.
Core Tip: This study describes the case of a 16-year-old male patient diagnosed with spondyloepiphyseal dysplasia congenita (SEDC) and treated with surgeries for multiple spinal deformities. SEDC is a rare genetic disorder, which mainly affects skeletal development, with an incidence of approximately 3/1000000. Due to the low incidence, there are very few reports on surgical treatment of skeletal deformities in patients with SEDC. We believe that our study makes a significant contribution to the literature because this is the first case report of a patient with SEDC who successfully underwent surgeries for atlantoaxial dysplasia and scoliosis.
- Citation: Jiao Y, Zhao JD, Huang XA, Cai HY, Shen JX. Surgical treatment of atlantoaxial dysplasia and scoliosis in spondyloepiphyseal dysplasia congenita: A case report. World J Orthop 2023; 14(11): 827-835
- URL: https://www.wjgnet.com/2218-5836/full/v14/i11/827.htm
- DOI: https://dx.doi.org/10.5312/wjo.v14.i11.827
Spondyloepiphyseal dysplasia congenita (SEDC) is a rare autosomal dominant genetic disorder with an incidence of approximately 3/1000000[1]. SEDC affects bone development, particularly of the spine and the long bones of the limbs[2]. SEDC is caused by mutations in the COL2A1 gene, which regulates the synthesis of type II collagen, a major com
A 16-year-old male patient presented with a 5-year history of progressive scoliosis.
At the age of 11, the patient was diagnosed with scoliosis and was treated with a brace for more than 12 h daily and had regular check-ups. However, his scoliosis continued to deteriorate.
The patient experienced difficulty walking and had challenges with physical activities since the age of 1 year. His growth and development were delayed compared to those of his peers. Radiography of the hip joints revealed bilateral hip dysplasia.
The patient denied any family history of similar diseases or malignant tumors.
Physical examination revealed that the patient was 128 cm in height, weighed 46 kg, and was unable to ambulate steadily. There was a right-sided thoracic spine curvature, resulting in a "razorback" deformity. The patient exhibited decreased proximal muscle strength in both lower limbs (grade IV), and bilateral Hoffman signs and Babinski signs were positive.
Ventilation function, electrocardiogram, echocardiogram, and other preoperative examination results were normal. Blood calcium, phosphorus, vitamin D, and alkaline phosphatase levels were normal for bone metabolism.
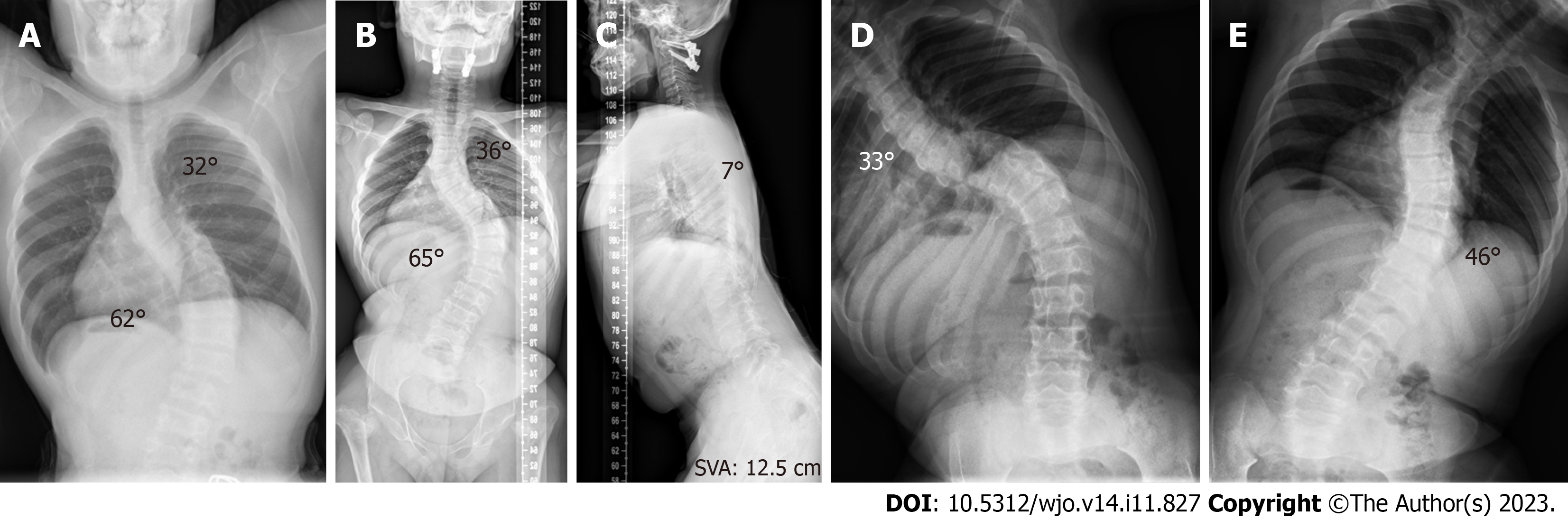
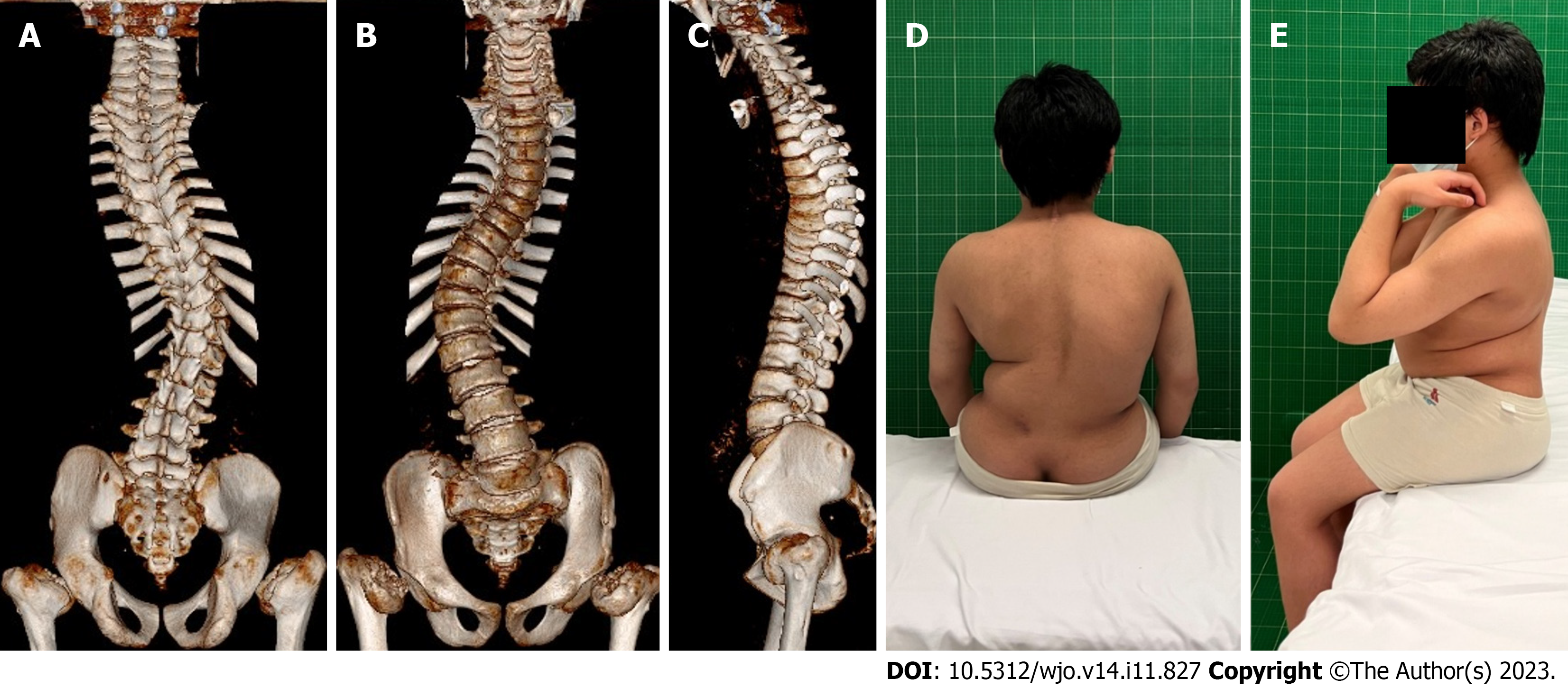
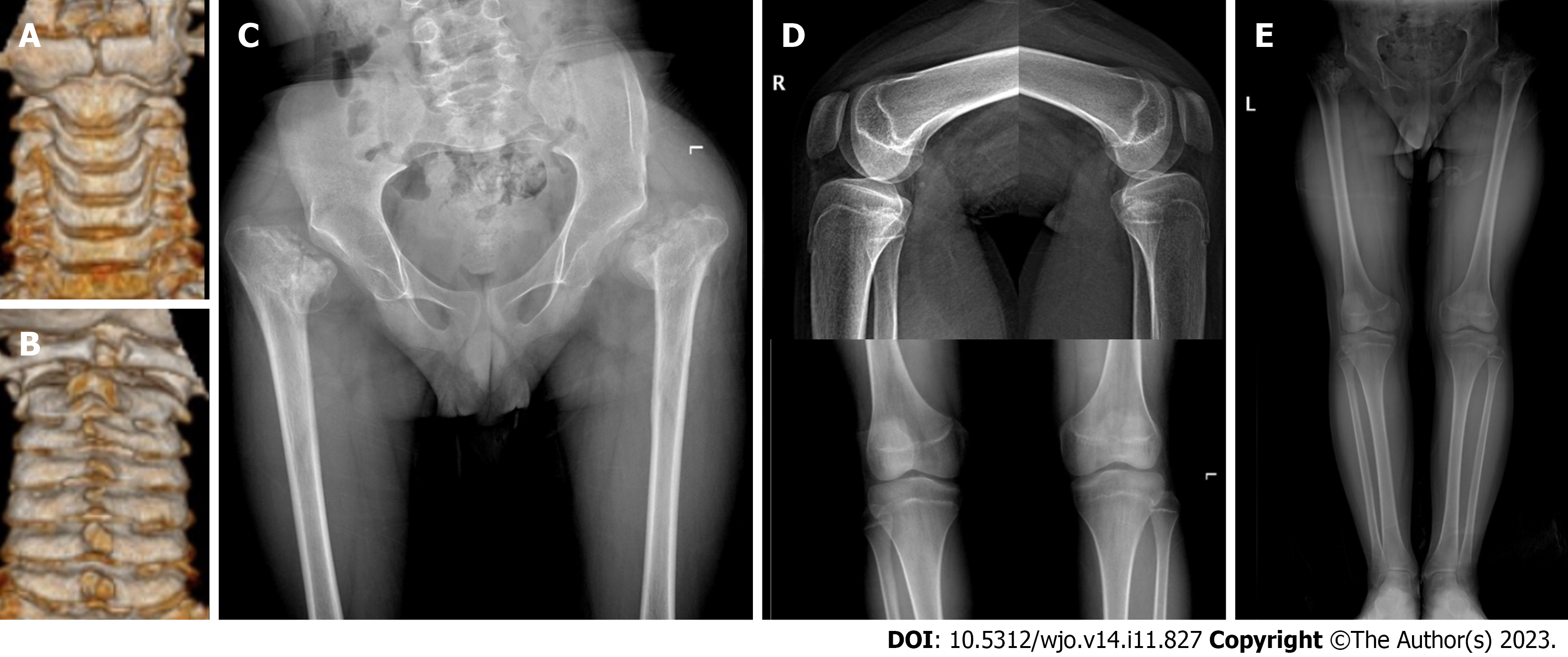
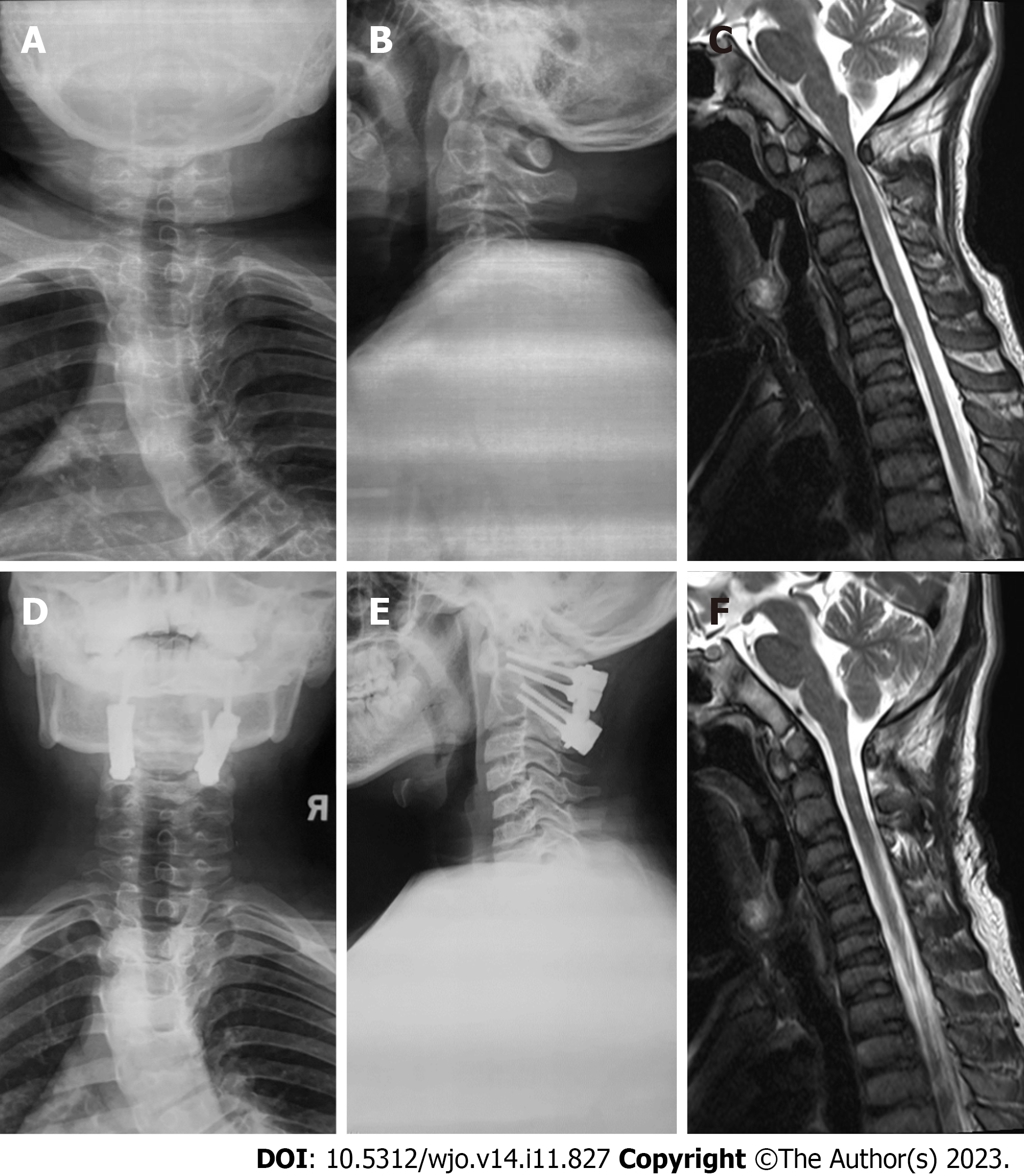
During the initial outpatient examination, the spine X-ray indicated a leftward proximal thoracic curve of 32° and a rightward main thoracic curve of 65° (Figure 1A). Three months following atlantoaxial decompression and fixation surgery, and prior to scoliosis correction, the spine X-rays revealed the proximal thoracic curve was 36°, the main thoracic curve was 65°, the thoracic kyphosis was 7°, and the sagittal vertical axis (SVA) was 12.5 cm (Figure 1B and C). On the bending X-rays, the Cobb angle of the proximal thoracic curve was 33°and the main thoracic curve was 46° (Figure 1D and E). Computed tomography (CT) three-dimensional (3D) reconstruction of the spine showed flattening of the vertebral body and widespread narrowing of the intervertebral space (Figure 2A-C). 3D CT of the cervical spine showed dysplasia of the odontoid process of the axis and the anterior and posterior arches of the atlas (Figure 3A and B). Spinal CT showed shorter pedicle screw lengths in the thoracic and lumbar vertebrae (2.94–5.17 cm), but the cancellous bone of the pedicles was well developed. A hip X-ray revealed dysplasia of bilateral hip joints and severe damage to the femoral heads. No obvious abnormality was found on the X-ray of bilateral knee joints and the full-length of both lower limbs (Figure 3C-E). Magnetic resonance imaging (MRI) of the spine indicated stenosis of the cervical spinal canal in the atlantoaxial region and myelopathy of the cervical spine (Figure 4C). The spinal MRI did not reveal other intraspinal abnormalities. The appearance of the patient before scoliosis surgery is shown in Figure 2D and E.
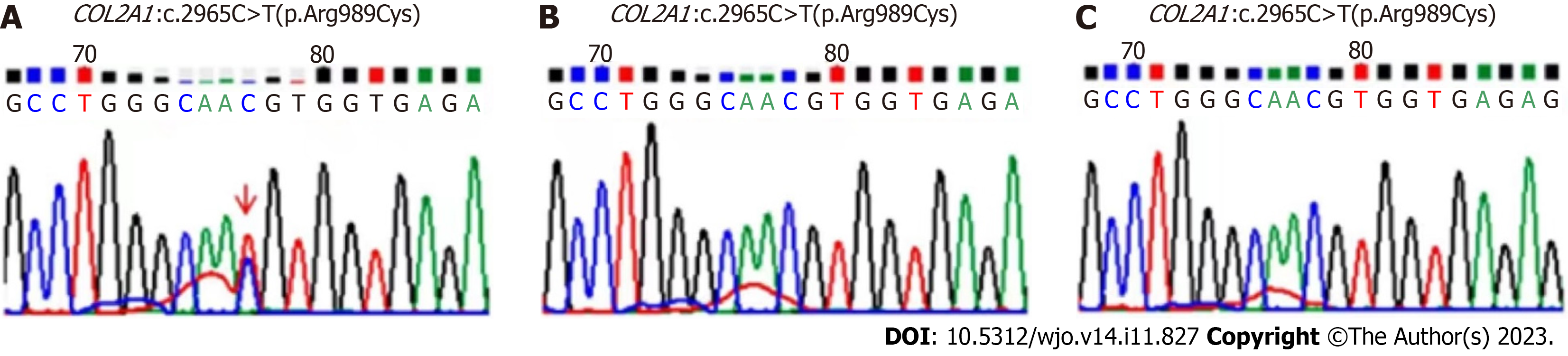
Combined with the patient's medical history and gene detection results (Figure 5), a diagnosis of SEDC was made.
To avoid irreversible damage caused by cervical myelopathy, the patient was recommended to have atlantoaxial decompression and fixation surgery. Skull traction was implemented under anesthesia during the surgical procedure, and the atlantoaxial reduction was observed to be satisfactory. Consequently, one-stage posterior atlantoaxial reduction and fixation procedure was employed. During the procedure, lateral mass screws were placed on both sides of the atlas and pedicle screws were placed on both sides of the axis and fixed with connecting plates, and autogenous iliac cancellous bone were used for bone graft fusion. Three months after the operation, the patient underwent posterior spinal fusion, internal fixation, and bone graft fusion from T3 to L4 to treat his deteriorating scoliosis. During the surgery, Smith–Petersen osteotomies were performed within the fusion range to fully release the spine, and pedicle screws were inserted between T3 and L4. Autogenous combined with allogeneic bone particles were used for Moe bone grafting. Intraoperative blood loss was 450 mL, and the duration of surgery was 3.6 h. No abnormality was identified during intraoperative spinal cord monitoring.
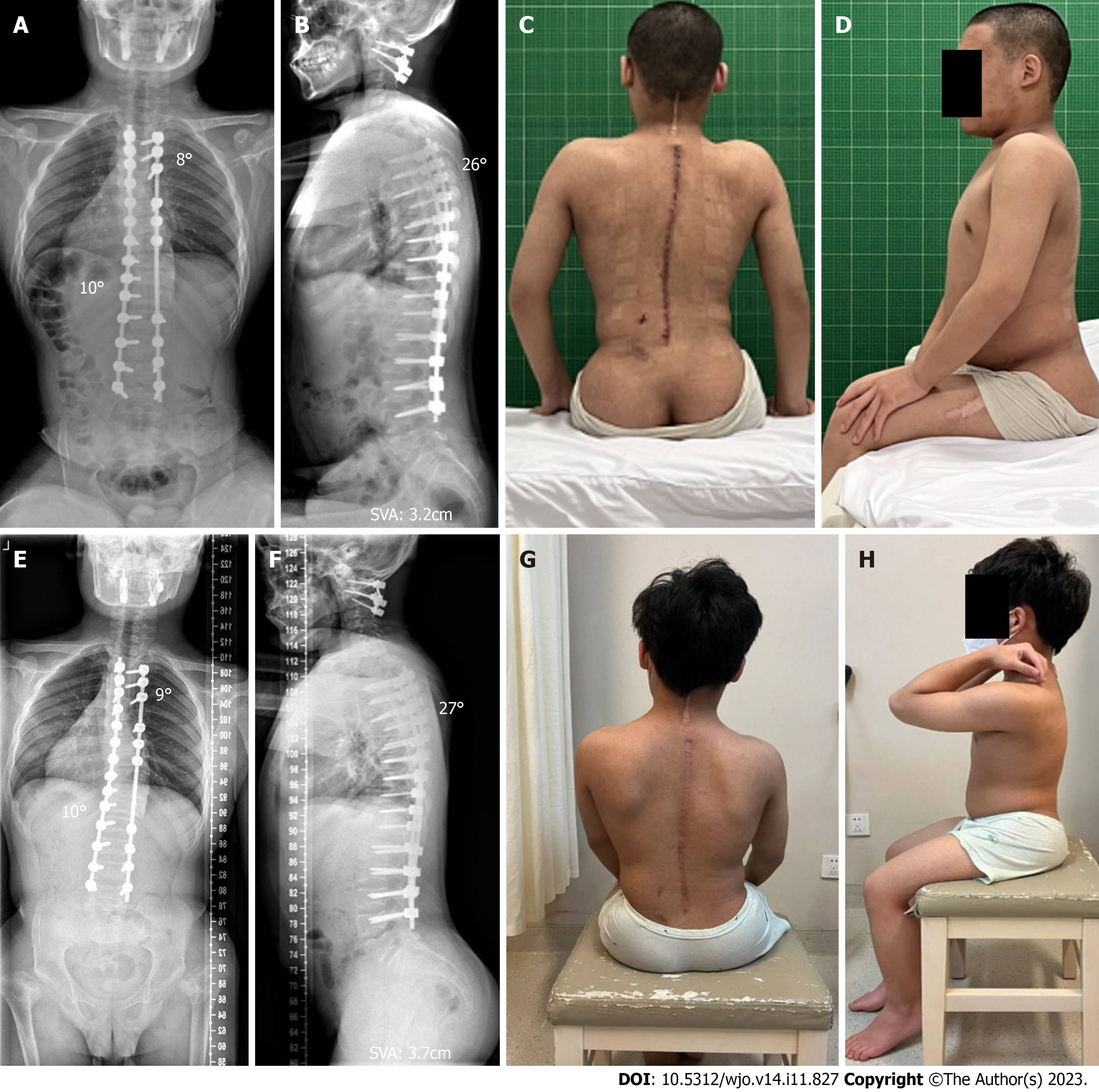
The patient was successfully extubated and recovered smoothly after both surgeries. After the atlantoaxial decompression and fixation surgery, the compression of the cervical spinal cord was significantly relieved, and the instability of the atlantoaxial joint was improved (Figure 4). Before the corrective surgery of scoliosis, the patient’s muscle strength of bilateral proximal lower limbs was significantly improved to grade V. The Hoffman signs and Babinski signs were negative. One week after corrective surgery for scoliosis, the spinal X-ray revealed significant correction of scoliosis with the main thoracic curve corrected from 65° to 10°, thoracic kyphosis corrected from 7° to 26°, SVA corrected from 12.5 cm to 3.2 cm, and considerable improvement in the back’s unevenness (Figure 6A-D). At the postoperative 2-year follow-up, the patient reported no symptoms of discomfort, such as back pain and weakness of the lower extremities, the atlantoaxial joint reduction remained effective and spinal correction retained its stability (Figure 6E-H).
SEDC is a rare genetic disorder that affects skeletal growth and development. Diagnosis of SEDC involves a combination of clinical examinations, radiographic imaging, and genetic testing[1,11]. The radiographic findings typically include flattened vertebrae, shortened long bones, and hip dysplasia. Due to the low incidence of SEDC and its main clinical feature of short stature, it can be easily confused with relatively common skeletal dysplasia that also cause short stature, such as osteogenesis imperfecta, multiple epiphyseal dysplasia, and achondroplasia[12-14]. In addition, mutations in COL2A1 can cause various skeletal dysplasia, such as Kniest and Stickler dysplasia, which can also be easily confused with SEDC[15]. Therefore, it is sometimes difficult to distinguish SEDC from these diseases based only on clinical examination and imaging findings. However, genetic testing can confirm the diagnosis and identify the specific genetic mutation responsible for the disorder. Consequently, genetic testing has become an important tool for the diagnosis of SEDC. Barat-Houari et al[15] analyzed the phenotypes and genetic diagnoses of over 700 patients with type II collagenopathy and identified 415 different COL2A1 mutations. Our patient presented with skeletal dysplasia in infancy; however, owing to the lack of genetic testing at that time, the diagnosis was not confirmed until the patient was 16 years of age. His genetic testing revealed a COL2A1 heterozygous mutation c.2965C>T(p.Arg989Cys), which is the most common mutation associated with SEDC[15]. The spectrum of gene mutations in SEDC will be further expanded and may guide the treatment of SEDC[16]. Genetic testing should be recommended for patients with clinical and radiological evidence suggestive of SEDC. Early diagnosis is crucial for providing appropriate follow-up treatment recommendations and genetic counselling for families affected by SEDC.
Currently, there are no specific therapeutic interventions for COL2A1 mutations, and treatments primarily focus on managing skeletal deformities. Surgical treatment for SEDC aims to correct deformities and prevent complications, such as joint pain, stiffness, and spinal cord compression. Treating hip dysplasia in SEDC can be challenging owing to the severe deformity and small volume of femoral heads, and often concomitant knee and spinal deformities. However, developmental dysplasia of the hip is an important factor that affects the walking ability of patients with SEDC. Shetty et al[17] performed bilateral valgus-extension osteotomy with hybrid external fixation in eight adolescent patients with SEDC who were followed up for 2 years and found significant improvements in the Harris hip score and postoperative mobility. Bayhan et al[10] reported that in children with SEDC, correcting the hip dysplasia by valgus hip osteotomy can maintain satisfactory walking function during 5 years of follow-up and improve hip mobility. Bisht et al[18] used CT to reconstruct the 3D structure of the hip joint and visualize the anatomical structure of the femoral head and neck, which was helpful for selecting implant sizes. Scheduled hip surgery can improve walking ability and delay the progression of hip joint osteoarthritis. However, our patient missed the opportunity for timely hip osteotomy surgery due to a lack of early diagnosis of SEDC. Even though the patient had severe bilateral hip joint damage, the presence of neurological damage due to atlantoaxial dysplasia makes it crucial to prioritize saving neurological function. Additionally, it was observed that the Cobb angle of scoliosis was still increasing three months after atlantoaxial decompression and fixation surgery. To avoid the potential need for more extensive surgery if scoliosis worsens, the decision was made to undergo scoliosis correction surgery before hip replacement surgery.
Among the various skeletal abnormalities associated with SEDC, atlantoaxial dysplasia is common and poses a serious threat to the patient’s health. Liu et al[19] reported a case of SEDC complicated with atlantoaxial instability, spinal cord compression at the cranio-cervical junction, and quadriplegia. The patient underwent decompression of the cranio-cervical region and occipital-C4 fusion surgery, which improved neurological function after the operation. Serhan et al[20] performed upper cervical spine fusion with autogenous iliac crest bone grafting in 20 children clinically diagnosed with SEDC who were followed up for 8 years and reported that the instability of the upper cervical spine was significantly improved, and the bone graft fusion remained stable without any non-union. Miyoshi et al[9] analyzed seven patients with SEDC complicated with atlantoaxial subluxation who underwent reduction and fusion surgery and found that when the diameter of the atlantoaxial spinal canal was less than 10 mm, atlantoaxial plate removal and occipital-cervical fusion were recommended. Al Kaissi et al[21] retrospectively analyzed 10 patients with SEDC and found that orthopedic treatment should begin with cervical spine evaluation to avoid severe neurological dysfunction or death. Multiple skeletal abnormalities were detected during the initial examination of our patient. However, atlantoaxial dysplasia exerted the greatest impact on the patient's quality of life. Therefore, atlantoaxial decompression and fixation surgery were recommended, resulting in improved muscle strength and the prevention of further spinal cord damage. From our perspective, surgery for atlantoaxial deformity should be performed promptly if atlantoaxial instability or cervical spinal cord compression is found.
Reports of successful scoliosis correction surgery in patients with SEDC are rare. Beighton et al[22] reported two cases of SEDC combined with scoliosis, both of which underwent posterior spinal fusion with Harrington instrumentation; however, the surgeries were unsuccessful. Winter et al[23] performed posterior spinal fusion with Harrington instrumentation in two patients with SEDC; during the follow-up period, one patient maintained good correction for 7 years, while the other experienced internal fixation failure due to pseudoarthrosis. Morita et al[7] used Luque rods to treat severe thoracolumbar kyphoscoliosis in a patient with SEDC. Although the correction was stable for 6 years postoperatively, scoliosis and kyphosis correction rates were only 10.5% and 25.9%, respectively. With the advancement of pedicle screws, posterior spinal fusion is believed to achieve a good corrective effect[24]. In this study, a high scoliosis correction rate of 84.6% was achieved through one-stage posterior spinal fusion, and there was no internal fixation failure or loss of correction during the 2-year follow-up. The high correction rate may be due to the use of a pedicle screw. In addition, the appropriate timing of treatment is also an important factor, as our patient's preoperative curve angle was 65°, which was smaller than in the five previously reported cases[7,22,23]. Furthermore, the preoperative spinal CT revealed that although the vertebral body in SEDC patients is small and flat, the cancellous bone of the pedicles developed well, suggesting that pedicle screw technology is suitable for scoliosis correction in SEDC. The selection of the upper and lower instrumented vertebrae (UIV and LIV) was also a crucial aspect of the pre-operative planning. Before surgery, the patient had balanced shoulders. The bending X-ray revealed that the proximal thoracic curve exceeded 25°, indicating the necessity for fusion[25]. Consequently, T3 was chosen as the UIV. As for the LIV, the center sacral vertical line (CSVL) passed between the pedicles of L4 in the bending X-ray. However, CSVL did not touch the pedicle of L3, so we opted to select L4 as the LIV. Our case suggests that a reasonable surgical plan, in combination with early treatment of scoliosis can achieve good results.
This is the first case report of a patient with SEDC who successfully underwent surgeries for atlantoaxial dysplasia and scoliosis. Early diagnosis of SEDC is important for providing appropriate treatment recommendations, and genetic testing is useful in confirming the diagnosis. Orthopedic evaluation of SEDC should begin with the cervical spine, as atlantoaxial dysplasia can be particularly detrimental. Early surgical intervention for hip dysplasia and scoliosis can result in favorable treatment outcomes. The use of pedicle screws may enhance the efficacy of scoliosis correction in patients with SEDC.
Thank the patient and his guardian for their participation.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Orthopedics
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Chhabra HS, India S-Editor: Liu JH L-Editor: A P-Editor: Xu ZH
| 1. | Anderson IJ, Goldberg RB, Marion RW, Upholt WB, Tsipouras P. Spondyloepiphyseal dysplasia congenita: genetic linkage to type II collagen (COL2AI). Am J Hum Genet. 1990;46:896-901. [PubMed] |
| 2. | Oranges CM, Tremp M, Kaempfen A, Schaefer DJ. Spondyloepiphyseal Dysplasia Congenita in a painting of Vicente López y Portaña (1825). J Endocrinol Invest. 2016;39:717. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 3. | Nenna R, Turchetti A, Mastrogiorgio G, Midulla F. COL2A1 Gene Mutations: Mechanisms of Spondyloepiphyseal Dysplasia Congenita. Appl Clin Genet. 2019;12:235-238. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 29] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 4. | Zhou T, Yang X, Chen Z, Zhou Y, Cao X, Zhao C, Zhao J. A novel COL2A1 mutation causing spondyloepiphyseal dysplasia congenita in a Chinese family. J Clin Lab Anal. 2021;35:e23728. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 5. | Terhal PA, Nievelstein RJ, Verver EJ, Topsakal V, van Dommelen P, Hoornaert K, Le Merrer M, Zankl A, Simon ME, Smithson SF, Marcelis C, Kerr B, Clayton-Smith J, Kinning E, Mansour S, Elmslie F, Goodwin L, van der Hout AH, Veenstra-Knol HE, Herkert JC, Lund AM, Hennekam RC, Mégarbané A, Lees MM, Wilson LC, Male A, Hurst J, Alanay Y, Annerén G, Betz RC, Bongers EM, Cormier-Daire V, Dieux A, David A, Elting MW, van den Ende J, Green A, van Hagen JM, Hertel NT, Holder-Espinasse M, den Hollander N, Homfray T, Hove HD, Price S, Raas-Rothschild A, Rohrbach M, Schroeter B, Suri M, Thompson EM, Tobias ES, Toutain A, Vreeburg M, Wakeling E, Knoers NV, Coucke P, Mortier GR. A study of the clinical and radiological features in a cohort of 93 patients with a COL2A1 mutation causing spondyloepiphyseal dysplasia congenita or a related phenotype. Am J Med Genet A. 2015;167A:461-475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 60] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 6. | Turner LM, Steffensen TS, Leroy J, Gilbert-Barness E. Spondyloepiphyseal dysplasia congenita. Fetal Pediatr Pathol. 2010;29:57-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 7. | Morita M, Miyamoto K, Nishimoto H, Hosoe H, Shimizu K. Thoracolumbar kyphosing scoliosis associated with spondyloepiphyseal dysplasia congenita: a case report. Spine J. 2005;5:217-220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 8. | LeDoux MS, Naftalis RC, Aronin PA. Stabilization of the cervical spine in spondyloepiphyseal dysplasia congenita. Neurosurgery. 1991;28:580-583. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 9. | Miyoshi K, Nakamura K, Haga N, Mikami Y. Surgical treatment for atlantoaxial subluxation with myelopathy in spondyloepiphyseal dysplasia congenita. Spine (Phila Pa 1976). 2004;29:E488-E491. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 48] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 10. | Bayhan IA, Abousamra O, Rogers KJ, Bober MB, Miller F, Mackenzie WG. Valgus Hip Osteotomy in Children With Spondyloepiphyseal Dysplasia Congenita: Midterm Results. J Pediatr Orthop. 2019;39:282-288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 11. | Spranger JW, Langer LO Jr. Spondyloepiphyseal dysplasia congenita. Radiology. 1970;94:313-322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 74] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 12. | Rauch F, Glorieux FH. Osteogenesis imperfecta. Lancet. 2004;363:1377-1385. [RCA] [DOI] [Full Text] [Cited by in Crossref: 887] [Cited by in RCA: 818] [Article Influence: 39.0] [Reference Citation Analysis (0)] |
| 13. | Markova T, Kenis V, Melchenko E, Alieva A, Nagornova T, Orlova A, Ogorodova N, Shchagina O, Polyakov A, Dadali E, Kutsev S. Clinical and Genetic Characteristics of Multiple Epiphyseal Dysplasia Type 4. Genes (Basel). 2022;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 14. | Pauli RM. Achondroplasia: a comprehensive clinical review. Orphanet J Rare Dis. 2019;14:1. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 201] [Cited by in RCA: 266] [Article Influence: 44.3] [Reference Citation Analysis (0)] |
| 15. | Barat-Houari M, Sarrabay G, Gatinois V, Fabre A, Dumont B, Genevieve D, Touitou I. Mutation Update for COL2A1 Gene Variants Associated with Type II Collagenopathies. Hum Mutat. 2016;37:7-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 104] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 16. | Silveira KC, Bonadia LC, Superti-Furga A, Bertola DR, Jorge AA, Cavalcanti DP. Six additional cases of SEDC due to the same and recurrent R989C mutation in the COL2A1 gene--the clinical and radiological follow-up. Am J Med Genet A. 2015;167A:894-901. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 17. | Shetty GM, Song HR, Lee SH, Kim TY. Bilateral valgus-extension osteotomy of hip using hybrid external fixator in spondyloepiphyseal dysplasia: early results of a salvage procedure. J Pediatr Orthop B. 2008;17:21-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 18. | Bisht RU, Van Tassel DC, Belthur MV. Spondyloepiphyseal dysplasia congenita: Use of complementary 3D reconstruction imaging for preoperative planning. Clin Imaging. 2022;86:94-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 19. | Liu L, Pang Q, Jiang Y, Li M, Wang O, Xia W. Novel COL2A1 mutations causing spondyloepiphyseal dysplasia congenita in three unrelated Chinese families. Eur Spine J. 2016;25:2967-2974. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 20. | Serhan Er M, Abousamra O, Rogers K, Akyol Y, Palocaren T, Takemitsu M, Campbell JW, Mackenzie WG. Upper Cervical Fusion in Children With Spondyloepiphyseal Dysplasia Congenita. J Pediatr Orthop. 2017;37:466-472. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 21. | Al Kaissi A, Ryabykh S, Pavlova OM, Ochirova P, Kenis V, Chehida FB, Ganger R, Grill F, Kircher SG. The Managment of cervical spine abnormalities in children with spondyloepiphyseal dysplasia congenita: Observational study. Medicine (Baltimore). 2019;98:e13780. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 19] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 22. | Beighton P, Kozlowski K. Spondylo-epi-metaphyseal dysplasia with joint laxity and severe, progressive kyphoscoliosis. Skeletal Radiol. 1980;5:205-212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 43] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 23. | Winter RB, Bloom BA. Spine deformity in spondyloepimetaphyseal dysplasia. J Pediatr Orthop. 1990;10:535-539. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 15] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 24. | Buell TJ, Chen CJ, Nguyen JH, Christiansen PA, Murthy SG, Buchholz AL, Yen CP, Shaffrey ME, Shaffrey CI, Smith JS. Surgical correction of severe adult lumbar scoliosis (major curves ≥ 75°): retrospective analysis with minimum 2-year follow-up. J Neurosurg Spine. 2019;1-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 25. | Lenke LG, Betz RR, Harms J, Bridwell KH, Clements DH, Lowe TG, Blanke K. Adolescent idiopathic scoliosis: a new classification to determine extent of spinal arthrodesis. J Bone Joint Surg Am. 2001;83:1169-1181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1282] [Cited by in RCA: 1264] [Article Influence: 52.7] [Reference Citation Analysis (0)] |