Published online Jan 18, 2021. doi: 10.5312/wjo.v12.i1.51
Peer-review started: July 27, 2020
First decision: September 21, 2020
Revised: October 5, 2020
Accepted: October 20, 2020
Article in press: October 20, 2020
Published online: January 18, 2021
Processing time: 164 Days and 22.7 Hours
First metatarsophalangeal joint arthritis (FMTPA), also known as hallux rigidus, is the most frequent degenerative disease of the foot. Diagnosis is made through both clinical and radiological evaluation. Regenerative medicine showed promising results in the treatment of early osteoarthritis. The aim of the present study was to report the results of a case of FMTPA treated with the injection of autologous adipose-derived mesenchymal stem cells.
A gentleman of 50 years of age presented with a painful hallux rigidus grade 2 resistant to any previous conservative treatment (including nonsteroidal anti-inflammatory drugs and hyaluronic acid injections). An injection of autologous adipose-derived mesenchymal stem cells into the first metatarsophalangeal joint was performed. No adverse events were reported, and both function and pain scales improved after 9 mo of follow-up.
The FMTP joint injection of mesenchymal stem cells improved symptoms and function in our patient with FMTPA at 9 mo of follow-up.
Core Tip: Recently, the use of intra-articular injections of stem cells has been proposed as a promising treatment in early osteoarthritis. In particular, autologous adipose-derived stem cells (aASCs) have attracted considerable attention, considering the easy access to fat tissue and the absence of adverse events registered. These characteristics make aASCs one of the most promising cell types used in regenerative medicine. Hallux rigidus, is the most frequent degenerative disease of the foot. Patients with hallux rigidus present a history of pain, gait discomfort, articular effusion, and a reduction in range of motion. Different types of treatment are available, both conservative and operative, but both are often ineffective. aASCs might overcome the gap between these two methods of treatment.
- Citation: Braile A, Toro G, De Cicco A, Cecere AB, Zanchini F, Schiavone Panni A. Hallux rigidus treated with adipose-derived mesenchymal stem cells: A case report. World J Orthop 2021; 12(1): 51-55
- URL: https://www.wjgnet.com/2218-5836/full/v12/i1/51.htm
- DOI: https://dx.doi.org/10.5312/wjo.v12.i1.51
First metatarsophalangeal joint arthritis (FMTPA), also known as hallux rigidus, is the most frequent degenerative arthritis disease of the foot[1]. Depending on the severity of the disease, the symptoms in the case of FMTPA are pain, gait discomfort, articular effusion, and a reduction in range of motion. The diagnosis is generally completed by observing the typical findings of osteoarthritis (OA) revealed by X-rays. Coughlin and Shurnas proposed a classification for FMTPA based on both clinical features and imaging[2] (Table 1).
| Grade | Dorsiflexion | Radiographs | Clinical |
| 0 | 40°-60° | Normal | No pain, stiffness with loss of motion |
| 1 | 30°-40° | Dorsal osteophytes; Minimal narrowing; Minimal flattening | Mild pain and stiffness pain with maximum dorsiflexion/plantar flexion |
| 2 | 10°-30° | Global osteophytes, mild/moderate narrowing | Moderate to severe pain and stiffness relatively constant, pain near extreme ROM |
| 3 | < 10° | Cystic changes | Nearly constant pain and stiffness, no midrange pain |
| 4 | < 10° | Same as grade 3 | Grade 3 + midrange pain |
Different types of treatments had been proposed, both conservative [i.e., orthosis, nonsteroidal anti-inflammatory drugs, hyaluronic acid (HA) injections] and operative (i.e., cheilectomy, arthroplasty, arthrodesis)[3].
Recently, emerging evidence has supported the use of autologous adipose-derived mesenchymal stem cells (aAMSCs) for the treatment of early OA[4-7].
The aim of the present study was to report the clinical results of a case of FMTPA treated with the injection of aAMSCs.
A gentleman of 50 years of age presented with a painful FMTP joint in the left foot.
His symptoms were resistant to both nonsteroidal anti-inflammatory drugs and HA injections.
The patient was already scheduled for an aAMSCs injection due to right knee Kellgren-Lawrence grade 2 OA.
Personal and family history were negative for foot pathologies.
The clinical examination showed a positive axial grind test and joint pain, exacerbated by the dorsiflexion that impaired the patient’s ability to walk.
Laboratory tests did not support the final diagnosis.
Antero-posterior and latero-lateral standard X-rays showed FMTPA stage 2.
FMTPA stage 2 was diagnosed.
The patient was already scheduled for an aAMSCs injection due to right knee Kellgren-Lawrence grade 2 OA, and therefore a similar injection was proposed to treat the hallux rigidus.
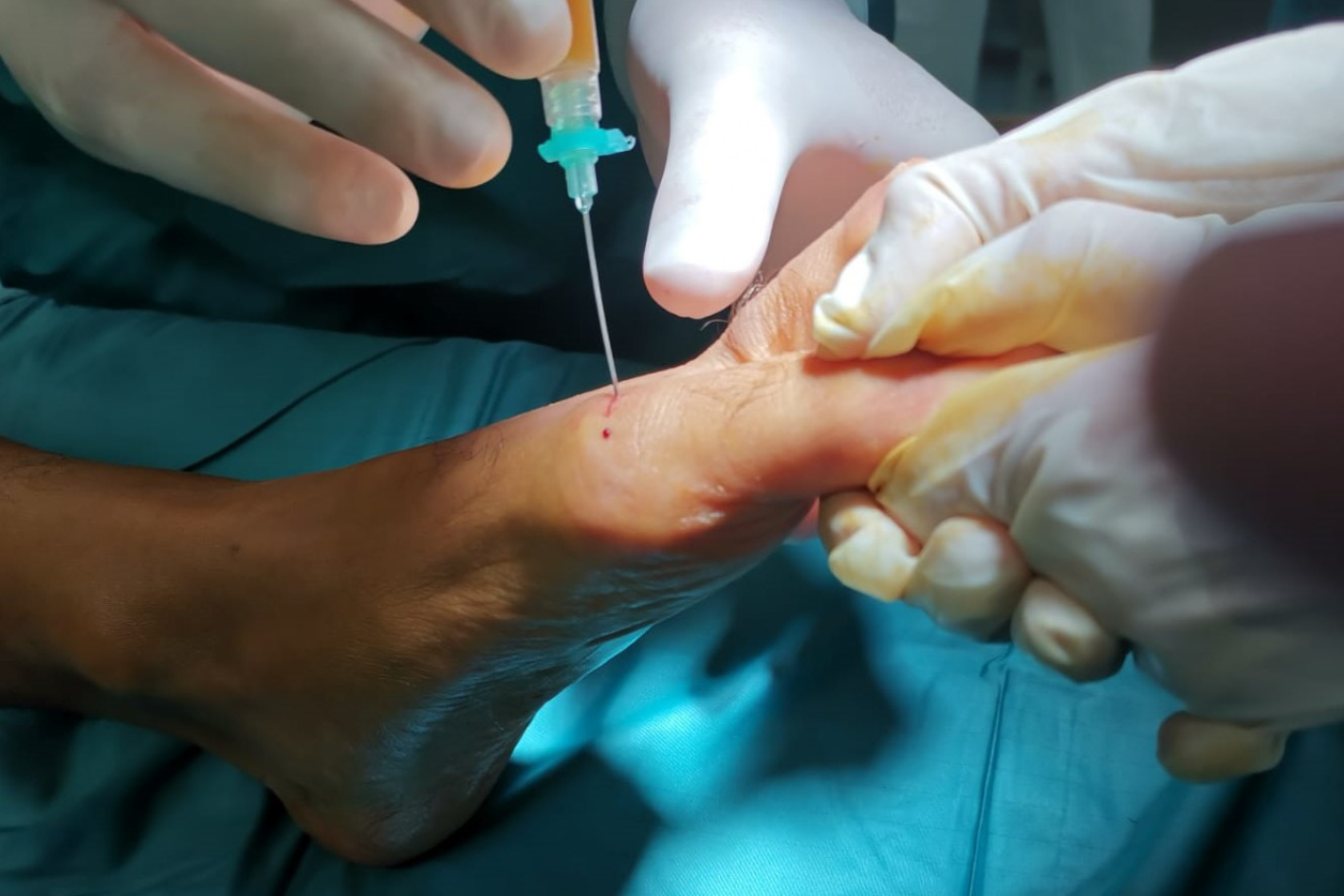
Concentrated aAMSCs were obtained from the abdomen, filtered as previously reported[5], and then injected into the FMTP joint after its distraction (Figure 1).
Protected weight bearing was prescribed during the first week after the procedure.
The patient was followed up at regular intervals. The clinical evaluation was completed using both the American Orthopedic Foot and Ankle Society (AOFAS) for hallux metatarsophalangeal-interphalangeal scale and the Visual Analog Scale (VAS).
No adverse effects were reported, excluding a transient pain in the FMPT joint in the first week after surgery. During the entire follow-up, an improvement in both VAS and AOFAS scales were observed (Table 2), leading to a final VAS scale of 0 and an AOFAS of 78 at 9 mo of follow-up.
| Pre-operatively | 6-mo follow-up | 9-mo follow-up | |
| AOFAS score | |||
| Pain | 30 | 30 | 30 |
| Function | 35 | 40 | 40 |
| Alignment | 8 | 8 | 8 |
| Total score | 73 | 78 | 78 |
| VAS | 7 | 5 | 0 |
Recently, the use of regenerative medicine principles has been proposed for various applications in both trauma and orthopedics, and especially for the treatment of early OA[4-6,8-11]. While conventional therapies for early OA (i.e. physical therapy, glucosamine and chondroitin sulfate supplementation) showed little benefits, regenerative medicine was demonstrated to be a promising option, due to the paracrine, anti-apoptotic, anti-inflammatory, and anti-aging effects of stem cells[12,13].
aAMSCs showed some theoretical advantages over other sources of stem cells. In fact, aAMSCs are easy to harvest, because of the wide availability of adipose tissue, and their sampling is generally associated with minimal discomfort, considering that it can be carried out using local anesthesia[7]. Moreover, aAMSCs demonstrated a high capacity for proliferation and fibroblastic differentiation[14]. Hass et al[15] showed that adipose tissue should be considered a primary source of cells for regenerative medicine as it contains 500 times more MSCs than the same volume of bone marrow.
Emerging literature has underlined the role of aAMSCs in the treatment of early OA. Schiavone Panni et al[5] conducted a study of 52 patients with early knee OA treated with arthroscopic debridement and aAMSCs injection, and showed improvement in both function and pain at an average of 15.3 mo of follow-up. Similarly, Song et al[16] reported the amelioration of pain, function and cartilage volume of the knee after multiple injections of aAMSCs. The efficacy of aAMSCs in OA was recently confirmed in a systematic review conducted by McIntyre et al[17].
FMTPA is a degenerative disease with an incidence of 2.5% in patients over 50 years of age. Its treatment might be frustrating for both the orthopedic and the patient, considering the conflicting outcomes reported after conservative treatment, including HA injections. Petrella et al[18] in their study of 47 patients with FTMPA, described the long-term improvement in both pain and function after multiple HA injections; however, Munteanu et al[19] in their randomized controlled trial did not observe any differences when HA was compared with placebo.
Pons et al[20] in a randomized study compared the use of sodium hyaluronate with triamcinolone acetonide in FMTPA, and reported an improvement in pain relief and function at 3 mo after the injections. However, a high percentage of patients in both groups required subsequent surgery after 1 year of follow-up, due to further progression of the disease with worsening of both pain and function.
To the best of our knowledge, this is the first report on the use of aAMSCs for FMTPA. A single injection was effective in treating FMTPA, improving both the AOFAS and VAS score at 9 mo of follow-up.
The present case report indicates that the injection of aAMSCs might be a promising treatment for FMTPA. Obviously, larger cohorts and longer follow-up studies are needed to confirm these findings.
Manuscript source: Unsolicited manuscript
Specialty type: Orthopedics
Country/Territory of origin: Italy
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Long X S-Editor: Zhang L L-Editor: Webster JR P-Editor: Li JH
| 1. | Ho B, Baumhauer J. Hallux rigidus. EFORT Open Rev. 2017;2:13-20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 83] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 2. | Coughlin MJ, Shurnas PS. Hallux rigidus. Grading and long-term results of operative treatment. J Bone Joint Surg Am. 2003;85:2072-2088. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 394] [Cited by in RCA: 361] [Article Influence: 17.2] [Reference Citation Analysis (1)] |
| 3. | Herrera-Pérez M, Andarcia-Bañuelos C, de Bergua-Domingo J, Paul J, Barg A, Valderrabano V. [Proposed global treatment algorithm for hallux rigidus according to evidence-based medicine]. Rev Esp Cir Ortop Traumatol. 2014;58:377-386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 4. | Damia E, Chicharro D, Lopez S, Cuervo B, Rubio M, Sopena JJ, Vilar JM, Carrillo JM. Adipose-Derived Mesenchymal Stem Cells: Are They a Good Therapeutic Strategy for Osteoarthritis? Int J Mol Sci. 2018;19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 51] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 5. | Schiavone Panni A, Vasso M, Braile A, Toro G, De Cicco A, Viggiano D, Lepore F. Preliminary results of autologous adipose-derived stem cells in early knee osteoarthritis: identification of a subpopulation with greater response. Int Orthop. 2019;43:7-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 59] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 6. | Ha CW, Park YB, Kim SH, Lee HJ. Intra-articular Mesenchymal Stem Cells in Osteoarthritis of the Knee: A Systematic Review of Clinical Outcomes and Evidence of Cartilage Repair. Arthroscopy 2019; 35: 277-288. e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 117] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 7. | Nöth U, Steinert AF, Tuan RS. Technology insight: adult mesenchymal stem cells for osteoarthritis therapy. Nat Clin Pract Rheumatol. 2008;4:371-380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 191] [Cited by in RCA: 195] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 8. | Caggiari G. Efficacy of platelet-rich plasma in experiental instrumented interbody spinal fusion. EuroMediterranean Biomed J. 2016;141–147. [DOI] [Full Text] |
| 9. | Doria C, Mosele GR, Caggiari G, Puddu L, Ciurlia E. Treatment of Early Hip Osteoarthritis: Ultrasound-Guided Platelet Rich Plasma vs Hyaluronic Acid Injections in a Randomized Clinical Trial. Joints. 2017;5:152-155. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 54] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 10. | Toro G, Moretti A, Toro G, Tirelli A, Calabrò G, Toro A, Iolascon G. Surgical treatment of neglected hip fracture in children with cerebral palsy: case report and review of the literature. Clin Cases Miner Bone Metab. 2017;14:317-323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 11. | Toro G, Lepore F, Calabrò G, Toro G, Rossini M, Vasso M, Schiavone Panni A. Humeral shaft non-union in the elderly: Results with cortical graft plus stem cells. Injury. 2019;50 Suppl 2:S75-S79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 12. | Wehling P, Evans C, Wehling J, Maixner W. Effectiveness of intra-articular therapies in osteoarthritis: a literature review. Ther Adv Musculoskelet Dis. 2017;9:183-196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 113] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 13. | Platas J, Guillén MI, Pérez Del Caz MD, Gomar F, Castejón MA, Mirabet V, Alcaraz MJ. Paracrine effects of human adipose-derived mesenchymal stem cells in inflammatory stress-induced senescence features of osteoarthritic chondrocytes. Aging (Albany NY). 2016;8:1703-1717. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 61] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 14. | McIntosh KR, Frazier T, Rowan BG, Gimble JM. Evolution and future prospects of adipose-derived immunomodulatory cell therapeutics. Expert Rev Clin Immunol. 2013;9:175-184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 35] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 15. | Hass R, Kasper C, Böhm S, Jacobs R. Different populations and sources of human mesenchymal stem cells (MSC): A comparison of adult and neonatal tissue-derived MSC. Cell Commun Signal. 2011;9:12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1330] [Cited by in RCA: 1243] [Article Influence: 88.8] [Reference Citation Analysis (0)] |
| 16. | Song Y, Du H, Dai C, Zhang L, Li S, Hunter DJ, Lu L, Bao C. Human adipose-derived mesenchymal stem cells for osteoarthritis: a pilot study with long-term follow-up and repeated injections. Regen Med. 2018;13:295-307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 176] [Article Influence: 25.1] [Reference Citation Analysis (0)] |
| 17. | McIntyre JA, Jones IA, Han B, Vangsness CT Jr. Intra-articular Mesenchymal Stem Cell Therapy for the Human Joint: A Systematic Review. Am J Sports Med. 2018;46:3550-3563. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 104] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 18. | Petrella RJ, Cogliano A. Intra-articular Hyaluronic Acid Treatment for Golfer's Toe: Keeping Older Golfers on Course. Phys Sportsmed. 2004;32:41-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 19. | Munteanu SE, Zammit GV, Menz HB, Landorf KB, Handley CJ, Elzarka A, Deluca J. Effectiveness of intra-articular hyaluronan (Synvisc, hylan G-F 20) for the treatment of first metatarsophalangeal joint osteoarthritis: a randomised placebo-controlled trial. Ann Rheum Dis. 2011;70:1838-1841. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 40] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 20. | Pons M, Alvarez F, Solana J, Viladot R, Varela L. Sodium hyaluronate in the treatment of hallux rigidus. A single-blind, randomized study. Foot Ankle Int. 2007;28:38-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 50] [Article Influence: 2.8] [Reference Citation Analysis (0)] |