Published online Dec 18, 2019. doi: 10.5312/wjo.v10.i12.424
Peer-review started: August 14, 2019
First decision: August 30, 2019
Revised: September 19, 2019
Accepted: October 18, 2019
Article in press: October 18, 2019
Published online: December 18, 2019
Processing time: 119 Days and 16.2 Hours
Septic arthritis is an orthopedic emergency requiring immediate surgical intervention. Current diagnostic standard of care is an invasive joint aspiration. Aspirations provide information about the inflammatory cells in the sample within a few hours, but there is often ambiguity about whether the source is infectious (e.g. bacterial) or non-infectious (e.g. gout). Cultures can take days to result, so decisions about surgery are often made with incomplete data. Novel diagnostics are thus needed. The “Sepsis MetaScore” (SMS) is an 11-mRNA host immune blood signature that can distinguish between infectious and non-infectious acute inflammation. It has been validated in multiple cohorts across heterogeneous clinical settings.
To study whether the SMS holds diagnostic validity in determining the etiology of acute arthritis.
We conducted a blinded, prospective, non-interventional clinical study of the SMS. All patients undergoing work-up for a septic primary joint were enrolled. Patients proceeded through the normal standard-of-care pathway, including joint aspiration and inflammatory labs [white blood cell (WBC), erythrocyte sedimentation rate (ESR), C-reactive protein (CRP)]. Venous blood was also drawn into PAX gene RNA-stabilizing tubes and mRNAs were measured using Nano String nCounter™. SMS was calculated blinded to clinical results.
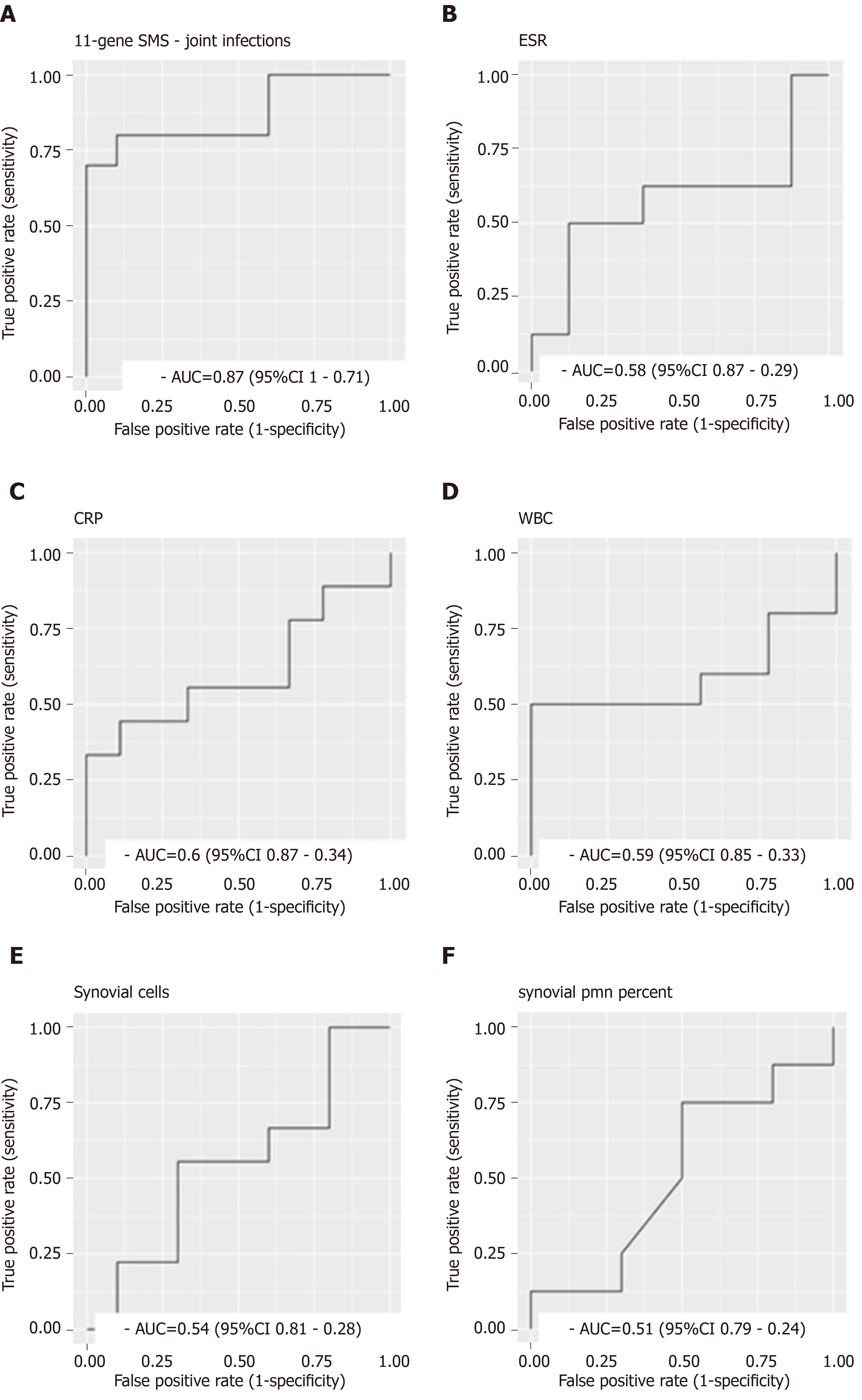
A total of 20 samples were included, of which 11 were infected based on aspiration or intra-operative cultures. The SMS had an area under the ROC curve (AUROC) of 0.87 for separating infectious from non-infectious conditions. For comparison, the AUROCs for ESR = 0.58, CRP = 0.6, and WBC = 0.59. At 100% sensitivity for infection, the specificity of the SMS was 40%, meaning nearly half of non-septic patients could have been ruled out for further intervention.
In this pilot study, SMS showed a high level of diagnostic accuracy in predicting septic joints compared to other diagnostic biomarkers. This quick blood test could be an important tool for early, accurate identification of acute septic joints and need for emergent surgery, improving clinical care and healthcare spending.
Core tip: Acute septic arthritis is an orthopedic emergency. The current gold standard diagnostic tool is synovial fluid culture, but this can take days to results, so decisions about surgery are made with imperfect information. A novel diagnostic “Sepsis MetaScore” (SMS) based on an mRNA signature has been identified that uses a blood sample to rapidly identify differentiate septic vs aseptic inflammation. Our pilot study showed the SMS had higher diagnostic accuracy than current standard of care inflammatory labs, showing potential for use as a rule-out test for septic arthritis, helping to minimize misdiagnosis and avoid unnecessary surgeries.
- Citation: Schultz BJ, Sweeney T, DeBaun MR, Remmel M, Midic U, Khatri P, Gardner MJ. Pilot study of a novel serum mRNA gene panel for diagnosis of acute septic arthritis. World J Orthop 2019; 10(12): 424-433
- URL: https://www.wjgnet.com/2218-5836/full/v10/i12/424.htm
- DOI: https://dx.doi.org/10.5312/wjo.v10.i12.424
Acute arthritis is a common complaint in emergency rooms and orthopedic clinics, with over 13000 hospitalizations per year and over $750 million dollars in healthcare spending in the United States alone[1,2]. The etiology can be septic, commonly from a bacterial infection, or aseptic, such as gout, transient synovitis or other inflammatory, non-infectious etiologies. Acute septic arthritis of native joints is an orthopedic emergency requiring urgent surgical irrigation and debridement (I and D) to prevent irreparable damage to the joint, inpatient hospitalization and an extended course of IV antibiotics. Inflammatory arthritis is typically managed medically on an outpatient basis. The presentation of septic versus aseptic acute arthritis is difficult to distinguish clinically[3]. but making a quick and accurate diagnosis is critical given the drastically different treatments. Currently, clinicians rely heavily on imperfect serum and synovial fluid laboratory values to make acute decisions about emergency surgery[4-7], potentially exposing non-infected patients to unnecessary surgery.
The annual incidence of septic arthritis in native joints is 4-10 patients/100000 patient years, and is continuing to rise with increasing antimicrobial resistance, aging, immunosuppression and the increasing number of invasive or orthopaedic procedures[8-11]. The current diagnostic work-up includes serum inflammatory labs [white blood cell (WBC) counts, erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP)], and an invasive synovial fluid aspiration from the joint. These diagnostics are limited by their turn-around time and specificity. The definitive diagnosis of septic arthritis requires a positive culture from the synovial fluid, which can take multiple days to result. Serum labs result quickly and provide information about general systemic inflammation, but are not specific for infection[4]. Synovial fluid evaluation reveals the inflammatory milieu within the joint, specifically WBC count, percentage of polymorphonuclear cells (PMNs), presence of crystals and a gram stain for bacteria, within a few hours, but again, these are not diagnostic, often leaving ambiguity about whether the source is infectious (e.g. bacterial) or non-infectious (e.g. gout)[4,5]. In addition, the presence of inflammatory cells can be artificially low in patients who are immunocompromised[6,12]. Furthermore, the presence of gouty crystals alone does not rule out a concomitant superimposed bacterial infection, making accurate diagnosis in this setting even more difficult[3]. Procalcitonin has recently been investigated as an inflammatory serum biomarker[13,14]. While it has shown promise in distinguishing septic from aseptic arthritis, it also does not accurately distinguish non-infective inflammation like gout from septic arthritis, and therefore is still a limited diagnostic biomarker[15].
The Sepsis MetaScore (SMS) is a novel diagnostic serum blood test that can efficiently distinguish between infectious and non-infectious acute systemic inflammation[16]. SMS works by interpreting the expression levels of 11 specific mRNAs in peripheral blood (the so-called “host response” to infection). Previous studies have validated its ability to distinguish infection from non-infectious inflammation in a variety of independent clinical settings including medical and surgical patients from ambulatory clinics to the ICU[17-19]. In this study, we hypothesized that the SMS could identify patients presenting acutely with septic arthritis based on positive cultures from those with aseptic arthropathies.
Following Institutional Review Board approval, we enrolled a convenience sample of adult patients presenting to the emergency department at a quaternary referral center with acute, atraumatic onset of a painful, swollen native joint. Non-native joints were excluded due to the different clinical and laboratory diagnostic cut-offs and treatment options for periprosthetic joint infections. Patients were enrolled in the trial at the time of presentation by an orthopaedic surgery resident.
All enrolled patients proceeded through the normal standard-of-care pathway, including inflammatory labs (WBC, ESR, CRP) and a joint aspiration performed by an orthopedic surgery resident. Aspirations were analyzed by the hospital lab for WBC count, percentage of PMNs, culture, gram stain and crystals. If the patient was taken for surgery, an additional intra-operative tissue sample was sent for culture. At the time of the initial lab draw, 2.5 cc of venous blood was also drawn into a PAX gene RNA-stabilizing tube. Blinded, deidentified samples were sent to Inflammatix, where the 11 mRNAs that comprise the SMS were measured using Nano String nCounter™. The SMS was calculated as previously described (difference of geometric means) blinded to clinical results[16]. The SMS score was calculated at the end of study enrollment, so no treating physician was aware of the results during patient care and it was not a factor in any clinical decisions. An independent observer (BS) retrospectively reviewed the charts and patients were diagnosed with septic arthritis if they had a positive culture resulted from the synovial fluid or tissue sample at time of surgery. All other patients were diagnosed with aseptic arthritis.
The primary endpoint of the study was the ROC curve (AUROC) of the SMS to determine clinically adjudicated septic joint status. Secondary endpoints were (1) The specificity of the SMS at the sensitivity > 95%, and (2) The AUROCs of comparator inflammatory biomarkers (serum WBC, CRP, ESR, and synovial WBCs and %PMNs). Student’s t-tests were used to compare continuous variables. Multivariate least-squares logistic regression included only those patients with no missing variables. Significance was set a P < 0.05. Calculations were conducted in R, version 3.5.1.
Our cohort included 20 patients (14 males and 6 females), with an average age of 54.7 years (Table 1). With respect to anatomic location there were fourteen knees, three ankles, two elbows, and one wrist. Ten samples were septic and ten were aseptic based on final culture results. Types of bacterial infections included Staphylococcus aureus, Streptococcus, Pseudomonas aeruginosa, Klebsiella pneumoniae, and Candida[1,6]. There were two cases of a concomitant gout flare with articular bacterial infection and one case of concomitant pseudogout with articular bacterial infection; these three cases were considered septic. There were four cases of gout that were aseptic, one had a surgical I and D due to acute concern for infection, however no aspirate or intra-operative cultures ever grew. In one septic patient the lab was unable to calculate the synovial cell counts because there was not enough fluid. One patient with concomitant gout and articular bacterial infection could not have the synovial PMNs calculated because of the high level of cellular degeneration. One aseptic patient did not have serum inflammatory labs drawn. All other patients had a full set of serum and synovial labs. All patients had an SMS calculated.
| Aseptic | Septic | P value | Number missing date | |
| Number of patients | 10 | 10 | ||
| Age (yr) +/- SD | 54.8 +/- 20.0 | 54.6 +/- 12.1 | 0.98 | 0 |
| Sex (male) | 7 | 7 | 0.99 | 0 |
| Serum WBC (k cells/mm3) | 11.7 +/- 4.0 | 13.4 +/- 8.2 | 0.57 | 1 |
| Serum ESR (mm/hr) | 58.4 +/- 35.2 | 80.4 +/- 50.7 | 0.33 | 4 |
| Serum CRP (mg/dL) | 16.1 +/- 10.1 | 19.6 +/- 12.8 | 0.53 | 2 |
| Synovial WBC (k cells/mm3) | 39.8 +/- 62.8 | 42.8 +/- 46.5 | 0.91 | 1 |
| Synovial % PMNs | 84.8 +/- 13.7 | 80.6 +/- 30.2 | 0.73 | 2 |
| Sepsis MetaScore | -0.33 +/- 0.63 | 1.1 +/- 1.3 | P = 0.008 | 0 |
In the aseptic group (10 patients), average serum WBC = 11.7 cells/mm3, ESR = 58.4 mm/h and CRP = 16.1 mg/dL, and the average synovial WBC = 39881 cells/mm3, PMNs = 84.8% (Table 1). In the septic group (10 patients), the average serum WBC = 13.4 ESR = 80.4 and CRP = 19.6, and the average synovial WBC = 42800, PMNs = 80.6%. No significant statistical difference was found in any inflammatory labs between the septic and aseptic groups. However, there was a significant difference in the Sepsis MetaScore between groups; aseptic = -0.33, septic = 1.1 (P = 0.008).
The SMS had an area under the AUROC of 0.87 (95%CI: 0.71-1) for separating infectious from non-infectious conditions (Figure 1A). Notably, this is very similar to its diagnostic accuracy in multiple other cohorts, lending credence to the stability of the metric[16-18]. For comparison, the AUROCs for serum ESR = 0.58 (95%CI: 0.87-0.29), CRP = 0.6 (95%CI: 0.87-0.34), and WBC = 0.59 (95%CI: 0.85-0.33), and synovial WBC = 0.54 (95%CI: 0.81-0.28) and PMN = 0.51(95%CI: 0.79-0.24) (Figure 1B-F). At 100% sensitivity for infection, the specificity of the SMS was 40%. This suggests that a substantial fraction of non-septic patients could potentially be safely ruled out for further surgical intervention.
In practice, the decision for surgery is not based on one specific inflammatory marker, but rather on the constellation of the clinical and laboratory presentation. To account for this we performed a multivariate logistic regression on all patients with complete laboratory data to measure whether the SMS remained an independent predictor of infection status when accounting for blood and synovial markers of inflammation (Table 2). Note six observations removed due to missingness. SMS was the only significant predictor of infection status when combined with “standard” inflammatory labs, further indicating that it may continue to hold diagnostic utility compared to several standard-of-care labs at once.
| Effect estimate | Std. Error | t value | P value | |
| Intercept | 0.833 | 0.577 | 1.443 | 0.199 |
| CRP | -0.022 | 0.015 | -1.488 | 0.187 |
| ESR | -0.001 | 0.004 | -0.213 | 0.839 |
| WBC | -0.001 | 0.024 | -0.043 | 0.967 |
| synovial WBC | 0.000 | 0.000 | 0.438 | 0.677 |
| synovial % PMN | -0.001 | 0.007 | -0.149 | 0.887 |
| Sepsis metascore | 0.595 | 0.210 | 2.831 | 0.030 |
| Residual standard error: 0.4478 on 6 degrees of freedom | ||||
| Multiple R-squared: 0.6275 | Adjusted R-squared: 0.2551 | |||
| F-statistic: 1.685 | ||||
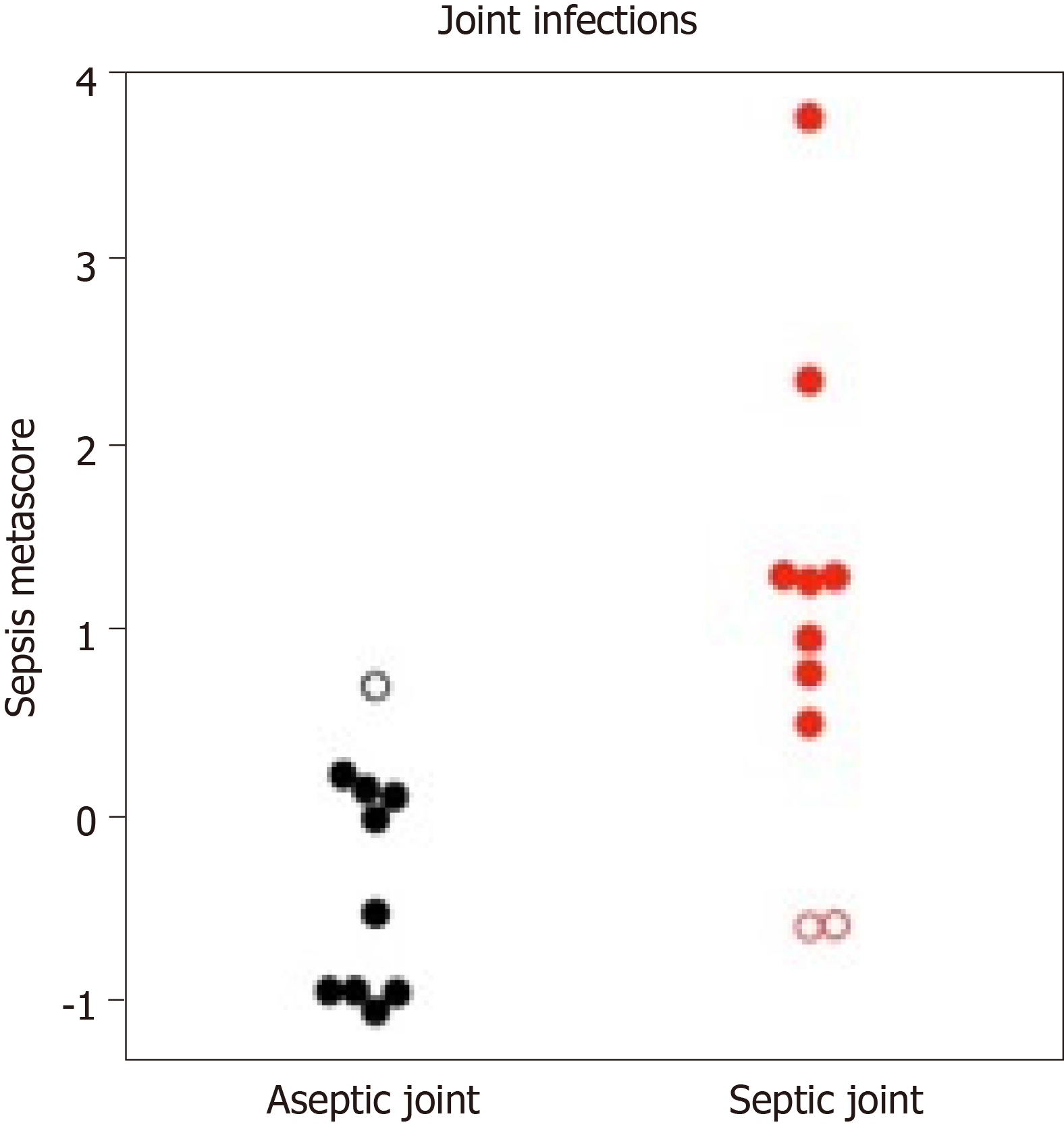
Patients with septic arthritis can also have systemic infections, which can complicate the diagnosis. One patient who was admitted for a bacterial pleural effusion with positive blood cultures also had an acute onset of knee pain (Figure 2). The patient’s knee was aseptic based on a negative aspirate culture and 15111 WBC, but the SMS was elevated. Note, because of the small sample size, a distinct cut-off has not yet been established for the SMS, but as Figure 2 indicates, SMS in the aseptic group tended to be lower (< 0) and SMS is septic group tended to be higher (> 1). This was ruled as a “false positive” since the joint was aseptic, though the SMS did accurately indicate that the patient had a systemic bacterial infection. Notably, if this patient is excluded from the data, the AUROC improves to 0.90 (95%CI: 0.76–1). Additionally, two patients in the septic group received antibiotics prior to SMS draw. Both had at least 12 h of antibiotics, and not surprisingly, their SMS scores were the two lowest of the septic group (Figure 2).
Septic arthritis can be difficult to distinguish from non-infectious arthropathies at the time of presentation. In this pilot study we determined the early diagnostic validity of a novel blood test, the Sepsis Metascore, for septic arthritis. Notably, the SMS had substantially higher AUROCs than standard-of-care inflammatory markers, though this did not reach significance in our small pilot study.
The current laboratory work-up for acute septic arthritis lacks diagnostic accuracy[4,5]. In our cohort, there was a trend towards lower serum WBC, ESR and CRP in the aseptic group compared to the septic group, however, this was not significantly different. The synovial PMN percentages were actually slightly lower in the septic group than the aseptic group, and both groups had synovial WBC averages lower than 50000 cells/mm3 which is the generally accepted cut-off for septic arthritis[5,6,20]. This finding could be from the abnormalities in a few of the septic patients, including immunosuppression and gouty superinfections where the lab noted high levels of cellular degeneration that compromised an accurate cell count. While a larger sample size may decrease the effect of these abnormalities on the lab averages, these cases highlight the overall limited diagnostic potential of the current laboratory work-up. With a reasonable specificity (40%) at 100% sensitivity for infection seen in this study, the SMS offers diagnostic potential as a rule-out test for acute septic arthritis in native joints. Its high sensitivity is ideal for the clinical urgency associated with acute septic arthritis, where a missed diagnosis could lead to devastating, irreversible articular destruction. In such scenarios, the test would have to be available in a rapid timeframe. The SMS has been licensed to Inflammatix for commercial development as part of a point-of-care test with a 30 min turnaround time, which would make it a valuable additional data point for early diagnosis.
The SMS has the potential to be particularly helpful in patients with inflammatory arthropathies and immunocompromise that further complicate septic arthritis diagnosis. Patients with gout can have elevated inflammatory labs and cellular degeneration in the synovial aspirate that make diagnosing a superimposed bacterial infection difficult[3]. In our sample, there was one patient with a history of gout who presented with acute knee pain and a synovial aspirate of 96000 WBC and 86% PMNs with few monosodium urate crystals. Despite no synovial culture results, the high inflammatory markers were concerning for a concomitant bacterial infection and the patient was taken emergently to the OR for a surgical I and D and admitted to the hospital for IV antibiotics. Neither aspirate nor multiple intra-operative cultures grew any bacteria, implying the joint was aseptic. The SMS was -1.05 here. This case was a prime example of a patient who underwent a surgical procedure in the setting of an ambiguous diagnosis that could have been best treated with only medical management.
SMS could be similarly helpful in patients with other inflammatory arthropathies such as rheumatoid arthritis. These patients have an increased risk of developing septic arthritis, especially if they are on immunomodulators, but often experience delay in clinical diagnosis because their inflammatory labs are often elevated at baseline, making it difficult to diagnose acute infection[21,22]. We had an example of this in our study with a patient with seropositive rheumatoid arthritis who presented with acute elbow pain and a synovial aspirate with 189000 WBC and 94% PMNs. Surgical I and D was performed, but neither the aspirate nor intra-operative cultures were positive. The SMS was low at -0.52. They re-presented eight months later with a similar clinical presentation with 176000 WBC with 85% PMNs on aspiration. The patient was taken for a second I and D, again with negative aspirate and intra-operative cultures. Acid fast bacilli, fungal cultures and 16S PCR were also negative. Ultimately our Infectious Disease colleagues diagnosed the patient with recurrent aseptic inflammatory arthritis.
Finally, the SMS could also be useful in patients with immunosuppression who have “falsely” low inflammatory markers[6,12]. There was one patient in the septic group on chemotherapy for leukemia who had suppressed inflammatory markers (WBC = 0.8, ESR = 58, CRP = 27.5, synovial WBC = 139, PMN = 9%) despite a positive aspirate culture that grew Klebsiella. Despite the low inflammatory labs, the SMS was correctly elevated at 1.28, showing its potential as a valuable tool in these special circumstances to prevent missed septic arthritis in patients with a compromised inflammatory response.
Although our pilot study focused on adult patients, the SMS also has potential utility in pediatric and adolescent septic arthritis. The common clinical presentation of transient synovitis of the hip, which is thought to be triggered by a systemic viral infection[23,24], presents similarly to septic arthritis. Additionally, pediatric patients have a high incidence of “culture negative” septic arthritis which makes diagnosis difficult[25]. Given the technical skill and advanced imaging needed to obtain a diagnostic hip aspiration, there would be tremendous benefit if the SMS proved to be an effective rule-out test in this population. Periprosthetic joint infection diagnosis is another area of potential application[26,27]. Although this case does not always require the same urgency that septic native joints require the SMS could potentially add another data point to suggest infection in equivocal cases prior to surgical intervention.
One limitation of this pilot study is its small sample size. A larger sample size, in a rigorously validated, properly statistically powered cohort of patients is necessary to confirm the diagnostic accuracy of the SMS. Another limitation of the study was the timing of SMS lab draw. While our protocol indicated lab draw at the same time as the initial inflammatory lab sample, this was not always possible, and sometimes occurred hours later. Still, we expect the SMS score to decrease with the administration of antibiotics and/or surgical debridement, so the fact that it was still accurate in predicting infection in these patients supports the validity of the test. More generally, a limitation of the SMS is the inability to distinguish systemic vs isolated articular infections. One patient with a bacterial pleural effusion had an aseptic aspirate of their knee. The SMS was elevated, correctly identifying the systemic bacterial infection, but in our data was ruled as a “false positive” since the joint was aseptic (Figure 2). With this in mind, the use of SMS to diagnose septic arthritis in patients with concomitant acute infections may be limited. Finally, a limitation in our data analysis is the reliance on synovial and intra-operative cultures to definitively diagnosing septic arthritis. While this is the current gold-standard diagnostic, it is not 100% sensitive, and can be influenced by administration of antibiotics prior to aspiration[28-30]. Additionally, clinical diagnosis of septic arthritis is not based on one or two lab values, but rather a clinical gestalt factoring in clinical exam, weight bearing status, prior antibiotic use, past medical history and presentation. While the regression model does allow us to compare a combination of lab values to the SMS, further study into the entire patient picture is warranted. Additionally, comparison to newer infection diagnostics such as pro-calcitonin and PCR analysis is warranted[14,31].
The literature is scarce regarding the incidence of patients who undergo emergent I and D for presumed septic arthritis that is ultimately deemed to be non-infected, but anecdotally at our institution this could be as high as 15%-20% of patients who undergo urgent I and D. This highlights the importance of a fast, reliable and less invasive rule-out diagnostic test to give clinicians confidence to choose not to intervene, sparing substantial costs, unnecessary surgery and patient morbidity.
Novel diagnostic tests are needed to quickly and accurately diagnose acute septic arthritis in native joints. In this pilot study, the SMS showed a high level of diagnostic accuracy in predicting septic joints compared to other diagnostic biomarkers. A large, prospective validation study is warranted to better establish the diagnostic accuracy and predictive values of the SMS. When confirmed in larger cohorts and available as a rapid blood test, the SMS could be an important tool for early, accurate diagnosis of acute septic joints and evaluation of need for urgent surgery. Future research should also expand to in investigate infection in non-unions, periprosthetic joints, infected hardware or grafts, transient synovitis, and others.
Septic arthritis in native joints is an orthopedic emergency, requiting urgent surgical intervention. It can present similarly to non-septic arthritis such as grout, transient synovitis or inflammatory arthritis. Non-septic arthritis can be managed medically, so accurate diagnosis is important. Currently, diagnosis is based on a combination of clinic exam and serum and synovial biomarkers which do not reliability differentiate infection from non-infective inflammation. The gold standard of diagnosis is intra-articular aspiration cultures, which can take days to result, so decisions about urgent surgery are often made with incomplete information. Novel diagnostics are needed to improve the speed and accuracy of diagnosis.
Novel diagnostics are needed to improve the speed and accuracy of diagnosis of septic arthritis to prevent the irreversible damage to cartilage seen in septic arthritis of native joints and to avoid unnecessary surgery in patients with aseptic arthritis. The ability to quickly and accurately identify and monitor infection through serum biomarkers, instead of invasive aspirations, has many potential applications across orthopedics, including peri-prosthetic infection, pediatric transient synovitis, hardware infection and in the work-up of fracture non-union.
The main objective was to compare the ability of the Sepsis MetaScore (SMS) to diagnosis acute septic arthritis in native joints compared to current diagnostic serum and synovial biomarkers. The SMS proved more accurate than serum white blood cell (WBC), erythrocyte sedimentation rate, C-reactive protein and synovial WBC and polymorphonuclear cells %. With the ability to result in 30 min without an invasive intra-articular aspiration, there is potential for future research across orthopedics for diagnosis and monitoring of infection.
We conducted a prospective, observational study of adult patients being worked up for acute septic arthritis of native joints in the emergency department. They proceeded through the standard of care work-up including inflammatory labs and aspiration, with an additional venous lab draw into a PAX gene RNA-stabilizing tube that was used to calculate the SMS. Decisions for surgery were made without consideration of SMS which was calculated at the end of the enrollment period, blinded to clinical results. Patients were retrospectively deemed infected or not based on synovial culture results. The SMS and other inflammatory labs were compared to this diagnosis
There was no significant difference in any of the standard serum or synovial labs between the septic and aseptic groups, except for the SMS which was significantly higher in septic patient compared to aseptic patient (P = 0.008). This pilot study data is encouraging, but still needs to be validated in a larger study.
The SMS shows potential as a quicker and more accurate diagnostic tool for acute septic arthritis than current serum and synovial biomarkers. It shows unique potential in complicated patients with histories of gout, inflammatory arthritis or immunocompromise where the current serum biomarkers are known to be less accurate. With development of the 30 min point of care testing, this is a potentially valuable diagnostic aid for decisions about emergency surgery and has potential applications across orthopedics subspecialties for infection diagnosis and monitoring.
Novel serum biomarkers show potential to increase the accuracy and decrease the time to diagnosis of septic arthritis. Future research in a larger study population is needed to validate these findings, which could then be replicated to investigate other topics in orthopedics such as periprosthetic joint infection, septic arthritis in pediatric patients, fracture non-unions and hardware infection.
Manuscript source: Unsolicited manuscript
Specialty type: Orthopedics
Country of origin: United States
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Sitkin S, Ueda H, Yang MS S-Editor: Zhang L L-Editor: A E-Editor: Liu MY
| 1. | Singh JA, Yu S. The burden of septic arthritis on the U.S. inpatient care: A national study. PLoS One. 2017;12:e0182577. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 31] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 2. | Singh JA, Yu S. Septic Arthritis in Emergency Departments in the US: A National Study of Health Care Utilization and Time Trends. Arthritis Care Res (Hoboken). 2018;70:320-326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 27] [Article Influence: 3.9] [Reference Citation Analysis (1)] |
| 3. | Yu KH, Luo SF, Liou LB, Wu YJ, Tsai WP, Chen JY, Ho HH. Concomitant septic and gouty arthritis--an analysis of 30 cases. Rheumatology (Oxford). 2003;42:1062-1066. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 112] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 4. | Li SF, Cassidy C, Chang C, Gharib S, Torres J. Diagnostic utility of laboratory tests in septic arthritis. Emerg Med J. 2007;24:75-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 104] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 5. | McGillicuddy DC, Shah KH, Friedberg RP, Nathanson LA, Edlow JA. How sensitive is the synovial fluid white blood cell count in diagnosing septic arthritis? Am J Emerg Med. 2007;25:749-752. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 75] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 6. | McCutchan HJ, Fisher RC. Synovial leukocytosis in infectious arthritis. Clin Orthop Relat Res. 1990;226-230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 18] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 7. | Ross JJ. Septic Arthritis of Native Joints. Infect Dis Clin North Am. 2017;31:203-218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 163] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 8. | Chander S, Coakley G. What's New in the Management of Bacterial Septic Arthritis? Curr Infect Dis Rep. 2011;13:478-484. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 9. | Kaandorp CJ, Dinant HJ, van de Laar MA, Moens HJ, Prins AP, Dijkmans BA. Incidence and sources of native and prosthetic joint infection: a community based prospective survey. Ann Rheum Dis. 1997;56:470-475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 306] [Cited by in RCA: 245] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 10. | Morgan DS, Fisher D, Merianos A, Currie BJ. An 18 years clinical review of septic arthritis from tropical Australia. Epidemiol Infect. 1996;117:423-428. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 162] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 11. | Weston VC, Jones AC, Bradbury N, Fawthrop F, Doherty M. Clinical features and outcome of septic arthritis in a single UK Health District 1982-1991. Ann Rheum Dis. 1999;58:214-219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 391] [Cited by in RCA: 306] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 12. | Margaretten ME, Kohlwes J, Moore D, Bent S. Does this adult patient have septic arthritis? JAMA. 2007;297:1478-1488. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 378] [Cited by in RCA: 339] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 13. | Zhao J, Zhang S, Zhang L, Dong X, Li J, Wang Y, Yao Y. Serum procalcitonin levels as a diagnostic marker for septic arthritis: A meta-analysis. Am J Emerg Med. 2017;35:1166-1171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 30] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 14. | Maharajan K, Patro DK, Menon J, Hariharan AP, Parija SC, Poduval M, Thimmaiah S. Serum Procalcitonin is a sensitive and specific marker in the diagnosis of septic arthritis and acute osteomyelitis. J Orthop Surg Res. 2013;8:19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 49] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 15. | Chouk M, Verhoeven F, Sondag M, Guillot X, Prati C, Wendling D. Value of serum procalcitonin for the diagnosis of bacterial septic arthritis in daily practice in rheumatology. Clin Rheumatol. 2019;38:2265-2273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 16. | Sweeney TE, Shidham A, Wong HR, Khatri P. A comprehensive time-course-based multicohort analysis of sepsis and sterile inflammation reveals a robust diagnostic gene set. Sci Transl Med. 2015;7:287ra71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 215] [Cited by in RCA: 239] [Article Influence: 26.6] [Reference Citation Analysis (0)] |
| 17. | Sweeney TE, Khatri P. Benchmarking Sepsis Gene Expression Diagnostics Using Public Data. Crit Care Med. 2017;45:1-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 63] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 18. | Sweeney TE, Khatri P. Comprehensive Validation of the FAIM3:PLAC8 Ratio in Time-matched Public Gene Expression Data. Am J Respir Crit Care Med. 2015;192:1260-1261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 19. | Maslove DM, Shapira T, Tyryshkin K, Veldhoen RA, Marshall JC, Muscedere J. Validation of diagnostic gene sets to identify critically ill patients with sepsis. J Crit Care. 2019;49:92-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 33] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 20. | Li SF, Henderson J, Dickman E, Darzynkiewicz R. Laboratory tests in adults with monoarticular arthritis: can they rule out a septic joint? Acad Emerg Med. 2004;11:276-280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 46] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 21. | Al-Ahaideb A. Septic arthritis in patients with rheumatoid arthritis. J Orthop Surg Res. 2008;3:33. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 22. | Galloway JB, Hyrich KL, Mercer LK, Dixon WG, Ustianowski AP, Helbert M, Watson KD, Lunt M, Symmons DP; BSR Biologics Register. Risk of septic arthritis in patients with rheumatoid arthritis and the effect of anti-TNF therapy: results from the British Society for Rheumatology Biologics Register. Ann Rheum Dis. 2011;70:1810-1814. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 155] [Cited by in RCA: 130] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 23. | Nouri A, Walmsley D, Pruszczynski B, Synder M. Transient synovitis of the hip: a comprehensive review. J Pediatr Orthop B. 2014;23:32-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 29] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 24. | Kastrissianakis K, Beattie TF. Transient synovitis of the hip: more evidence for a viral aetiology. Eur J Emerg Med. 2010;17:270-273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 25. | Spyridakis E, Gerber JS, Schriver E, Grundmeier RW, Porsch EA, St Geme JW, Downes KJ. Clinical Features and Outcomes of Children with Culture-Negative Septic Arthritis. J Pediatric Infect Dis Soc. 2018;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 25] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 26. | Ting NT, Della Valle CJ. Diagnosis of Periprosthetic Joint Infection-An Algorithm-Based Approach. J Arthroplasty. 2017;32:2047-2050. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 73] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 27. | Mirza SZ, Richardson SS, Kahlenberg CA, Blevins JL, Lautenbach C, Demetres M, Martin L, Szymonifka J, Sculco PK, Figgie MP, Goodman SM. Diagnosing Prosthetic Joint Infections in Patients With Inflammatory Arthritis: A Systematic Literature Review. J Arthroplasty. 2019;34:1032-1036.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 28. | Carpenter CR, Schuur JD, Everett WW, Pines JM. Evidence-based diagnostics: adult septic arthritis. Acad Emerg Med. 2011;18:781-796. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 224] [Cited by in RCA: 183] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 29. | Hindle P, Davidson E, Biant LC. Septic arthritis of the knee: the use and effect of antibiotics prior to diagnostic aspiration. Ann R Coll Surg Engl. 2012;94:351-355. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 37] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 30. | Shmerling RH, Delbanco TL, Tosteson AN, Trentham DE. Synovial fluid tests. What should be ordered? JAMA. 1990;264:1009-1014. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 31. | Choe H, Deirmengian CA, Hickok NJ, Morrison TN, Tuan RS. Molecular diagnostics. J Am Acad Orthop Surg. 2015;23 Suppl:S26-S31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 1.3] [Reference Citation Analysis (0)] |