Published online Aug 24, 2025. doi: 10.5306/wjco.v16.i8.109419
Revised: May 25, 2025
Accepted: July 2, 2025
Published online: August 24, 2025
Processing time: 102 Days and 2.7 Hours
Transarterial chemoembolization (TACE) is a main treatment for advanced hepatocellular carcinoma (HCC), but tumors often become resistant. Combining TACE programmed cell death (ligand) 1 [PD-(L)1] inhibitors and molecular targeted therapies (MTT) may improve outcomes, but its role in preventing TACE resistance requires further investigation.
To compare if TACE plus PD-(L)1 inhibitors and MTT reduces TACE resistance and improves survival in advanced HCC compared to TACE alone.
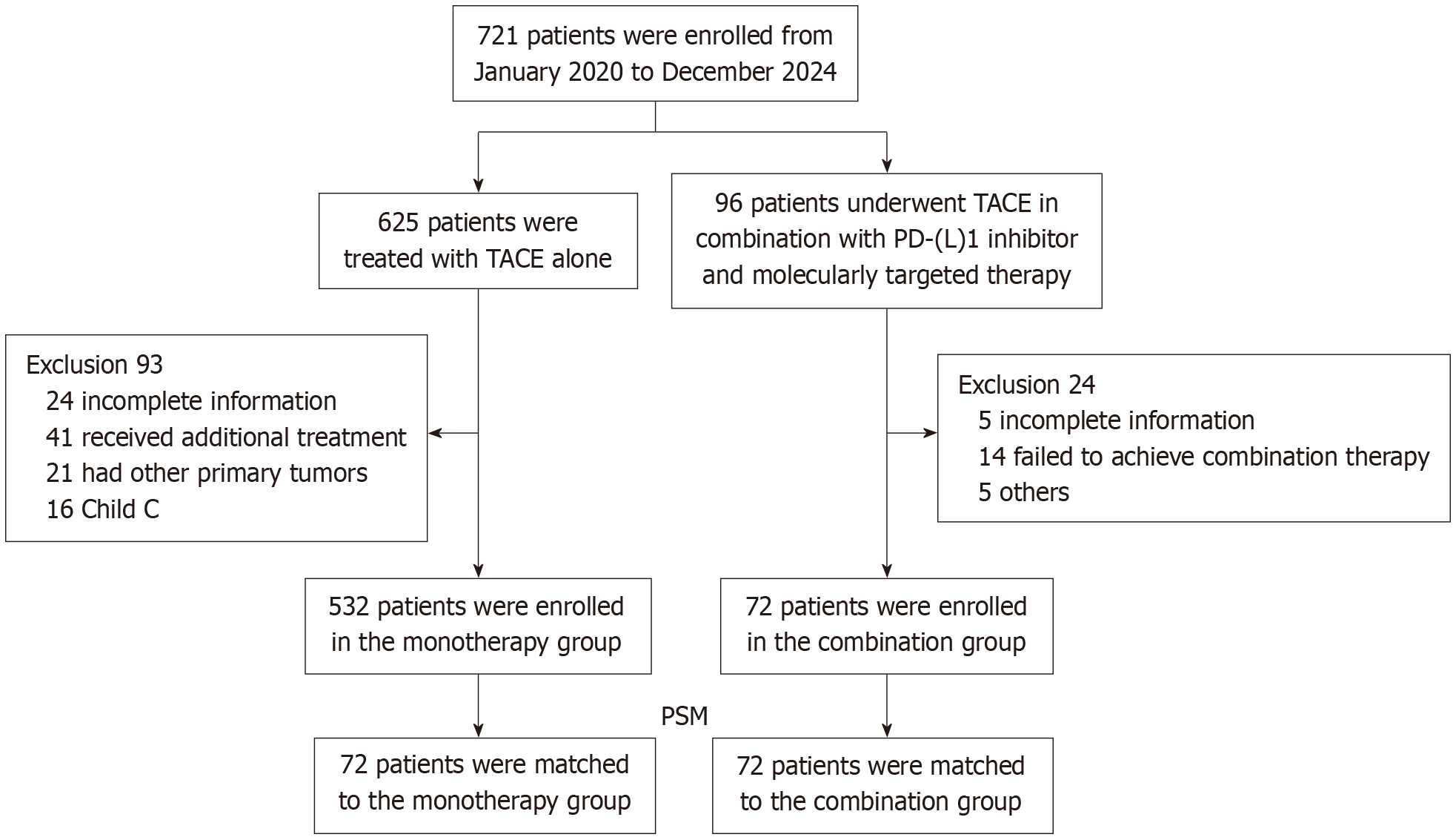
We analyzed 721 patients: 532 received TACE only, and 72 received TACE with PD-(L)1 inhibitors and MTT. After matching patient characteristics, 144 patients (72 pairs) were compared. Tumor progression after 3 treatment cycles was measured.
The combination group exhibited significantly lower TACE resistance rates compared to the monotherapy group (9.7% vs 38.8%, P < 0.001). Moreover, pa
Adding immunotherapy and targeted drugs to TACE significantly reduces treatment resistance and improves survival in advanced liver cancer, suggesting it may become a new standard treatment.
Core Tip: This study demonstrates that combining transarterial chemoembolization (TACE) with programmed cell death (ligand) 1 inhibitors and molecular targeted therapy significantly reduces TACE resistance and improves survival in advanced hepatocellular carcinoma. The combination therapy prolongs progression-free survival and overall survival. Additionally, the study identifies maximum tumor diameter, tumor capsule integrity, and bilobar distribution of tumor as key factors influencing TACE resistance, providing new insights for optimizing treatment strategies.
- Citation: Jiao HY, Yan XM, Li JX, Zhang ZG. Combination therapy reduces transarterial chemoembolization resistance in advanced hepatocellular carcinoma. World J Clin Oncol 2025; 16(8): 109419
- URL: https://www.wjgnet.com/2218-4333/full/v16/i8/109419.htm
- DOI: https://dx.doi.org/10.5306/wjco.v16.i8.109419
Primary liver cancer, a frequently occurring malignancy, currently ranks sixth in global cancer incidence and third in cancer-related mortality. Unfortunately, most patients are diagnosed at advanced stages, missing the optimal window for surgical intervention, leading to poor prognosis[1]. For advanced hepatocellular carcinoma (HCC), TACE remains the primary treatment modality. However, a significant proportion of undifferentiated HCC patients experience disease progression within one year of TACE treatment. This progression is associated with enhanced interactions within the tumor microenvironment post-TACE, potentially contributing to post-treatment deterioration. Clinical studies have shown that immunotherapies, including programmed death 1 (PD-1) and programmed death-ligand 1 (PD-L1) inhibitors, exhibit promising efficacy and safety profiles for advanced HCC[2]. Consequently, combining TACE with immunotherapy may achieve synergistic effects, potentially enhancing therapeutic outcomes. The primary mechanism underlying this combination involves TACE-induced antigen release and upregulation of PD-L1 expression, fostering an im
This study strictly adhered to the guidelines outlined in the Declaration of Helsinki and received formal approval from the Ethics Committee of Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, approval No. TJ-IRB202412142). Given the retrospective nature of the study, written informed consent was waived. The study design was based on the “Chinese Guidelines for the Diagnosis and Treatment of Primary Liver Cancer” and targeted patients clinically or pathologically diagnosed with HCC. Patient data were collected between January 2020 and December 2024, including those who underwent TACE or TACE combined with PD-(L)1 inhibitors and MTT at our institution. Combination therapy was defined as the administration of TACE either concurrently with or up to 30 days prior to PD-(L)1inhibitor therapy, along with the concurrent use of MTT agents in combination with TACE or PD-(L)1 inhibitors. Patients were enrolled based on the following meticulous criteria: (1) Age ≥ 18 years; (2) HCC classified as Barcelona Clinic Liver Cancer stage B or C; (3) Receipt of either TACE combined with PD-L1 inhibitors and MTT, or TACE monotherapy (≥ 3 sessions); (4) Child-Pugh grade A or B liver function; (5) Eastern Cooperative Oncology Group performance status score of 0-1; and (6) Presence of at least one measurable target lesion assessable by Response Evaluation Criteria in Solid Tumors v1.1. Patients were excluded for: (1) Concurrent primary malignancies; (2) Receipt of therapeutic regimens beyond those specified in the study protocol; and (3) Loss to follow-up or incomplete medical records. After rigorous screening, 604 eligible patients were ultimately included, comprising 532 patients receiving TACE alone and 72 patients treated with combination therapy. To minimize confounding biases, the research team employed 1:1 propensity score matching (PSM), resulting in a balanced cohort of 144 patients (Figure 1).
TACE treatment: TACE was typically administered prior to the initiation of PD-(L)1 inhibitors therapy. During the procedure, patients received local anesthesia, followed by catheterization of the celiac trunk or superior mesenteric artery via a 5-French catheter, and subsequent angiography. A 2.7 French microcatheter was then navigated into the specific artery supplying the tumor. A mixture of 20 mg pirarubicin and 10 mL Lipiodol was injected for embolization, followed by additional embolization using gelatin sponge particles until complete cessation of blood flow in the tumor-feeding artery was achieved.
PD-(L)1 inhibitors: For patients receiving combination therapy, various PD-(L)1 inhibitors were selected based on treatment guidelines and drug availability in the Chinese market, including sintilimab, tislelizumab, and pembrolizumab. All selected PD-L1 inhibitors were administered according to their standard dosages and frequencies.
Molecular targeted therapy: For patients undergoing combination therapy, MTT such as sorafenib, lenvatinib, apatinib, and bevacizumab were utilized. All medications were administered in combination with either TACE or PD-(L)1 inhibitors.
All patients underwent computed tomography (CT) and/or magnetic resonance imaging (MRI), liver function tests, complete blood counts, and tumor marker assessments within 1-3 months following each TACE session. TACE efficacy was evaluated using dynamic CT/MRI, and patients were categorized as having complete response, partial response, stable disease, or progressive disease according to modified Response Evaluation Criteria in Solid Tumors criteria. Patients with partial response, stable disease, or progressive disease underwent subsequent TACE or TACE combined with PD-L1 inhibitors and MTT. The occurrence of TACE resistance was determined 1-3 months after three consecutive standardized and refined TACE sessions, based on enhanced CT/MRI findings indicating persistent disease progression of intrahepatic target lesions compared to baseline assessments, as per the Chinese definition of TACE resistance[6].
To control for confounding factors, 1:1 nearest-neighbor PSM was applied to match patients who received TACE alone with those who received TACE combined with PD-(L)1 inhibitors and MTT. We used a 1:1 nearest-neighbor matching algorithm with a caliper width of 0.02 to ensure that matched pairs had similar propensity scores. This process resulted in a balanced cohort of 144 patients (72 pairs) who were comparable in terms of their baseline characteristics, including age, gender, tumor burden, liver function, and performance status. Covariate balance was assessed using standardized mean differences, with a threshold of < 0.1 indicating adequate balance. Continuous variables were compared using in
A total of 604 patients were screened and enrolled, including 532 patients receiving monotherapy and 72 patients undergoing combination therapy. Prior to PSM, the combination group exhibited significantly greater tumor burden (mean maximum tumor diameter: 91.5 mm vs 66.0 mm, P = 0.001) and higher portal vein tumor thrombus incidence (41.7% vs 23.3%, P = 0.001). After 1:1 PSM, the final analysis cohort comprised 144 patients (72 per group). Post-matching cohorts demonstrated balanced baseline characteristics, with comparable demographics: Predominantly male patients (87.5%), majority with viral hepatitis history (79.2%), and similar mean maximum tumor diameter (80 mm in both groups). No statistically significant differences were observed in clinicopathological parameters between groups (Table 1).
| Characteristics | Before PSM | After PSM | ||||||
| Monotherapy group (n = 532) | Combination group (n = 72) | P value | SMD | Monotherapy group (n = 72) | Combination group (n = 72) | P value | SMD | |
| Gender | - | - | 0.882 | - | - | - | 0.325 | - |
| Female | 59 (11.1) | 7 (9.72) | - | -0.023 | 12 (16.7) | 7 (9.72) | - | -0.011 |
| Male | 473 (88.9) | 65 (90.3) | - | 0.023 | 60 (83.3) | 65 (90.3) | - | 0.011 |
| Age (year), mean ± SD | 54.9 ± 10.6 | 56.2 ± 10.5 | 0.33 | - | 58.3 ± 11.0 | 56.2 ± 10.5 | 0.25 | - |
| Comorbid hepatitis B | - | - | 0.184 | - | - | - | 0.669 | - |
| No | 75 (14.1) | 15 (20.8) | - | -0.084 | 12 (16.7) | 15 (20.8) | - | -0.041 |
| Yes | 457 (85.9) | 57 (79.2) | - | 0.084 | 60 (83.3) | 57 (79.2) | - | 0.041 |
| AFP (μg/L) | - | - | 0.826 | - | - | - | 0.17 | - |
| < 400 | 307 (57.7) | 40 (55.6) | - | -0.011 | 49 (68.1) | 40 (55.6) | - | -0.009 |
| ≥ 400 | 225 (42.3) | 32 (44.4) | - | 0.011 | 23 (31.9) | 32 (44.4) | - | 0.009 |
| Primary tumor size (mm) | 66.0 (37.8-100) | 91.5 (57.5-122) | < 0.001 | 0.486 | 85.0 (42.5-120) | 91.5 (57.5-122) | 0.37 | 0.019 |
| Number of tumors | - | - | 0.192 | - | - | - | 0.577 | - |
| Single | 178 (33.5) | 18 (25.0) | - | -0.045 | 22 (30.6) | 18 (25.0) | - | -0.009 |
| Multiple | 354 (66.5) | 54 (75.0) | - | 0.045 | 50 (69.4) | 54 (75.0) | - | 0.009 |
| Tumor capsule | - | - | 0.13 | - | - | - | 0.489 | - |
| No | 163 (30.6) | 29 (40.3) | - | -0.068 | 24 (33.3) | 29 (40.3) | - | -0.018 |
| Yes | 369 (69.4) | 43 (59.7) | - | 0.068 | 48 (66.7) | 43 (59.7) | - | 0.018 |
| Tumor thrombus | - | - | 0.001 | - | - | - | 0.225 | - |
| No | 408 (76.7) | 42 (58.3) | - | -0.422 | 50 (69.4) | 42 (58.3) | - | -0.036 |
| Yes | 124 (23.3) | 30 (41.7) | - | 0.422 | 22 (30.6) | 30 (41.7) | - | 0.036 |
| Bilobar distribution of tumor | - | - | 0.446 | - | - | - | 0.867 | - |
| No | 320 (60.2) | 39 (54.2) | - | -0.076 | 37 (51.4) | 39 (54.2) | - | -0.006 |
| Yes | 212 (39.8) | 33 (45.8) | - | 0.076 | 35 (48.6) | 33 (45.8) | - | 0.006 |
| Child-Pugh grade | - | - | 0.098 | - | - | - | 0.366 | - |
| A | 488 (91.7) | 71 (98.6) | - | -0.072 | 69 (95.8) | 71 (98.6) | - | -0.014 |
| B | 44 (8.3) | 1 (1.4) | - | 0.072 | 3 (4.2) | 1 (1.4) | - | 0.014 |
| ECOG-PS | - | - | 0.719 | - | - | - | 0.918 | - |
| 0 | 456 (85.7) | 60 (83.3) | - | -0.012 | 61 (84.7) | 60 (83.3) | - | -0.003 |
| 1 | 76 (14.3) | 12 (16.7) | - | 0.012 | 11 (15.3) | 12 (16.7) | - | 0.003 |
| BCLC | - | - | 0.042 | - | - | - | 0.58 | - |
| B | 304 (57.2) | 32 (44.4) | - | -0.215 | 34 (47.2) | 32 (44.4) | - | -0.014 |
| C | 228 (42.8) | 40 (53.6) | - | 0.215 | 38 (52.8) | 40 (53.6) | - | 0.014 |
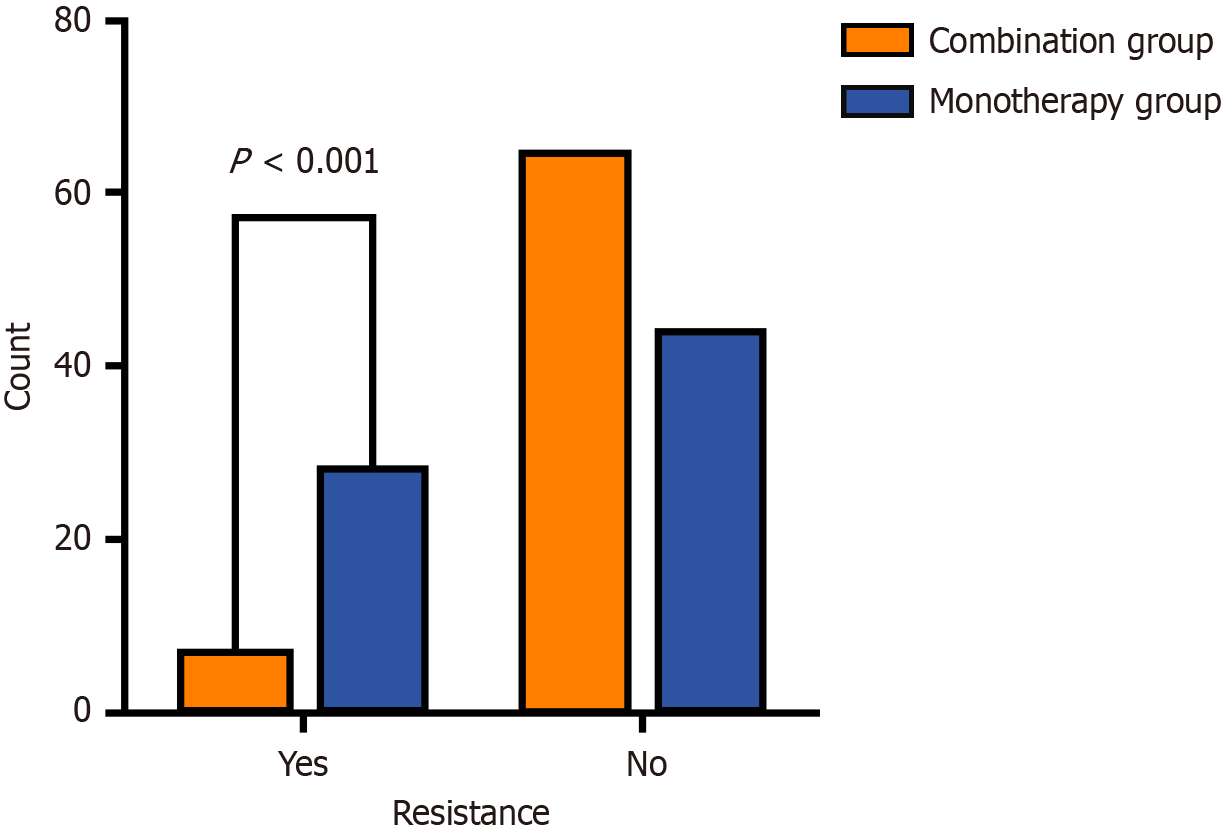
All patients received three consecutive refined treatment cycles. Tumor progression was compared between the combination group and the monotherapy group. TACE resistance occurred in only 7 patients (9.7%) in the combination group, compared to 28 patients (38.8%) in the monotherapy group (Figure 2).
At the time of data cutoff, the median follow-up durations were comparable between the combination and mo
We analyzed risk factors for TACE resistance in 532 patients who developed resistance after TACE monotherapy. Univariate and multivariate logistic regression identified bilobar distribution of tumor, maximum tumor diameter, and tumor capsule absence as independent risk factors for TACE resistance (Table 2).
| Characteristics | Univariate logistic regression analysis | Multivariate logistic regression analysis | ||||
| OR | CI | P value | OR | CI | P value | |
| Age | 0.98 | 0.97-1 | 0.128 | - | - | - |
| Female | 1.15 | 0.59-2.24 | 0.687 | - | - | - |
| Comorbid hepatitis B | 1.06 | 0.56-2.01 | 0.858 | - | - | - |
| AFP ≥ 400 μg/L | 2.07 | 1.34-3.19 | 0.001 | 1.92 | 1.42-2.95 | 0.06 |
| Primary tumor size | 1.01 | 1.01-1.02 | < 0.001 | 1.01 | 1-1.02 | 0.001 |
| Number of multiple tumors | 1.96 | 1.21-3.18 | 0.006 | - | - | - |
| Tumor capsule | 0.18 | 0.11-0.29 | < 0.001 | 0.29 | 0.17-0.48 | 0.002 |
| Tumor thrombus | 4.52 | 2.74-7.47 | 0.03 | - | - | - |
| Bilobar distribution of tumor | 2.46 | 1.59-3.8 | < 0.001 | 2.13 | 1.3-3.48 | 0.003 |
| Child-Pugh grade B | 2.25 | 1.1-4.62 | 0.027 | 2.14 | 1.23-4.21 | 0.07 |
| BCLC-B | 2.56 | 0.92-7.14 | 0.073 | - | - | - |
| BCLC-C | 6.9 | 2.39-19.92 | 0.052 | - | - | - |
The severity of adverse events (AEs) was graded according to the Common Terminology Criteria for AEs, version 5.0. After PSM, 48 of 72 patients (66.6%) in the combination group reported AEs, compared to 35 of 72 patients (48.6%) in the monotherapy group. Grade 3 or higher severe AEs occurred in 19.4% (combination group, 14 cases) and 9.7% (mo
| Variable | Combination group (n = 72) | Monotherapy group (n = 72) |
| Patients with an adverse event from any cause | 48 (66.6) | 35 (48.6) |
| Grade 1 or 2 event | 34 (47.2) | 28 (38.9) |
| Grade 3 event | 12 (16.7) | 7 (9.7) |
| Grade 4 event | 2 (2.7) | 0 |
| Grade 5 event | 0 | 0 |
| Dose interruption of PD-(L)1 inhibitors | 5 (6.9) | 0 |
| Dose interruption of molecular targeted therapies | 7 (9.7) | 0 |
| Dose reduction of PD-(L)1 inhibitors | 8 (11.1) | 0 |
| Dose reduction of molecular targeted therapies | 12 (16.6) | 0 |
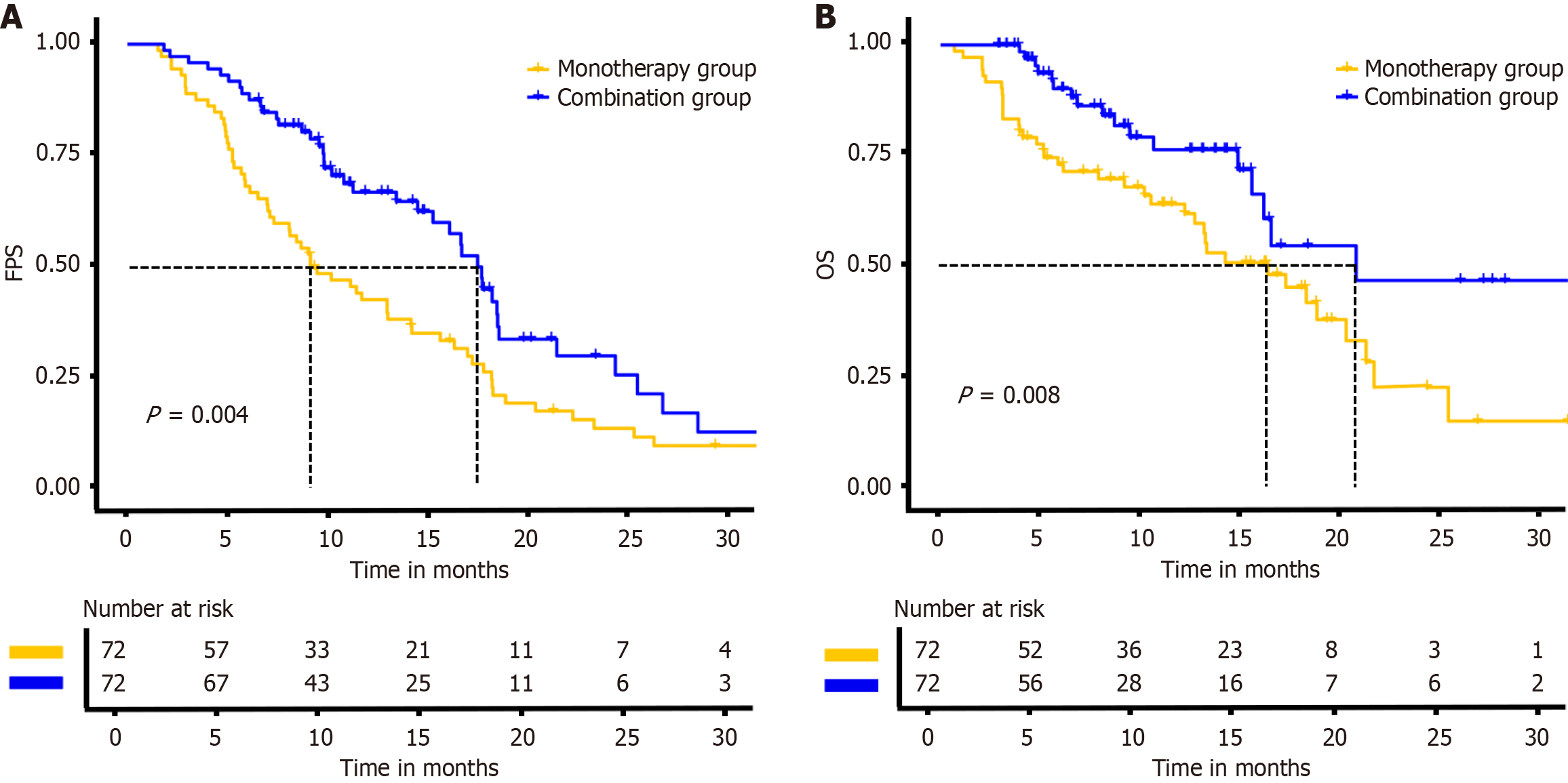
This study innovatively demonstrates that the combination group significantly reverses treatment resistance observed after three TACE cycles. In the core efficacy comparison, only 7 patients (9.7%) in the combination group exhibited target lesion progression, compared to 28 patients (38.8%) in the monotherapy group, with a statistically significant difference (P < 0.001). Survival analysis further revealed a more favorable disease control trend with combination therapy, as evidenced by significantly superior PFS and OS compared to monotherapy. These findings strongly align with the recently published CHANCE001 multicenter retrospective study[2]. This national cohort study (n = 867) demonstrated that combination group provides triple clinical benefits for advanced HCC patients: A 1.5-month prolongation of mPFS (hazard ratio = 0.76, P = 0.015), a 3.5-month prolongation of median OS (hazard ratio = 0.76, P = 0.001), and an increased objective response rate of 28.1%. Notably, this study transcends traditional PFS definitions by innovatively focusing on intrahepatic target lesion progression as the primary endpoint, while considering new lesions, portal vein invasion, and extrahepatic metastases as secondary indicators. This evaluation framework more precisely reflects the biological characteristics of TACE resistance and offers clinically relevant insights for therapeutic assessment.
Furthermore, this study identifies three core biological drivers of TACE resistance in advanced HCC: Maximum tumor diameter, absence of tumor capsule integrity, and bilobar distribution of tumor. These indicators not only serve as independent prognostic markers but also represent critical targets for personalized treatment strategies. From a tumor biological perspective, the absence of tumor capsule integrity constitutes a significant warning signal. The pathological mechanism involves the tumor capsule acting as a natural barrier; its disruption enables tumor cells to breach fibrous matrix constraints and infiltrate along portal vein branches. This infiltrative growth pattern simultaneously compromises the integrity of tumor vascular architecture, promoting the formation of abnormal vascular networks between intra- and extra-tumoral vessels. Clinical research confirms that such vascular remodeling significantly increases the risk of portal vein tumor thrombus formation, which represents an important anatomical basis for TACE treatment failure[7]. The prognostic value of bilobar distribution of tumor is validated in this study, aligning with Chen et al’s meta-analysis[8] of 1238 patients. The mechanism may involve enhanced intrahepatic metastatic potential of tumor cells. This leads to multicentric lesion formation, which significantly increases challenges in treatment coverage[9]. Maximum tumor diameter, established as an independent predictor of early TACE refractoriness, receives robust support in this study. Multiple investigations indicate that tumor size represents a critical prognostic factor in HCC patients[10-12]. Larger tumor diameters (≥ 5 cm) correlate with higher tumor burdens, rendering complete TACE coverage technically challenging[9]. Studies demonstrate that TACE-treated target lesions frequently develop compensatory blood supply through hepatofugal collateral arteries (e.g., inferior phrenic arteries, intercostal arteries)[13]. When the tumor diameter exceeds 5 cm, the incidence of extrahepatic collateral arterial supply increases significantly, which directly compromises therapeutic efficacy and is closely associated with treatment failure and poor prognosis[14,15].These findings carry important clinical implications: For advanced HCC patients exhibiting these high-risk biological characteristics, early combination with systemic therapies (e.g., immuno-oncology combinations) or localized treatment escalation (e.g., embolic agents) should be considered[16]. This precision medicine approach, guided by tumor biological features, has the potential to overcome traditional TACE limitations and substantially improve patient outcomes.
The remarkable efficacy observed in the combination therapy group can be attributed to a synergistic multi-mechanistic interaction. TACE induces ischemic necrosis by embolizing tumor-feeding arteries while simultaneously enabling localized chemotherapy drug release to augment cytotoxic effects. Notably, TACE has been shown to upregulate PD-L1 expression[17], thereby creating a permissive microenvironment for subsequent anti-PD-1/PD-L1 monoclonal antibody therapy. However, persistent hypoxic conditions in residual tumor cells following TACE result in a 2.1 ± 0.8-fold increase in vascular endothelial growth factor (VEGF) expression compared to baseline levels, stimulating tumor angiogenesis and providing a pathophysiological basis for disease progression. Furthermore, studies indicate that embolization-induced reduction in CD8+ T-cell infiltration necessitates molecular targeting agents to promote CD8+ T-cell recruitment[18]. VEGF targeted therapies (e.g., lenvatinib, sorafenib) effectively block tumor vascular regrowth post-TACE by inhibiting VEGF receptor signaling pathways, thereby delaying recurrence kinetics[19,20]. Concurrently, these agents normalize tumor vasculature, significantly optimizing the immune cell infiltration microenvironment and creating favorable conditions for immune checkpoint inhibitor efficacy[3,21]. Clinical evidence suggests that lenvatinib administration before and after TACE enhances drug delivery, maximizing therapeutic outcomes[22]. The PD-1/PD-L1 signaling axis plays a pivotal role in tumor immune evasion by suppressing T-cell activity, enabling tumor cells to escape immune surveillance. PD-(L)1 inhibitors reverse this inhibitory signaling, restoring cytotoxic T-cell function and eliciting robust antitumor immune responses. This tripartite combination establishes a therapeutic closed-loop integrating “vascular disruption-immune activation-immune enhancement”, exerting synergistic antitumor effects through three-dimensional modulation of tumor angiogenesis, immune microenvironment remodeling, and T-cell functional restoration. This strategy significantly elevates disease control rates. This study further confirms that the combination regimen significantly reduces the risk of target lesion progression post-TACE, with mechanisms involving multi-layered interventions including angiogenesis blockade, reversal of immunosuppressive microenvironment, inhibition of metastatic potential, and elimination of drug-resistant clones. By reshaping tumor-immune system interactions, this treatment strategy converts “immunologically cold tumors” into “immunologically hot tumors”, ultimately achieving substantial improvement in disease control rates.
This study has several limitations. The retrospective design carries inherent risks of selection bias, as evidenced by baseline characteristic differences. To mitigate this, we employed PSM and conducted sensitivity analyses commonly used in real-world research. Additionally, the relatively small sample size necessitates larger cohort studies for more in-depth investigations.
This study demonstrates that combination group significantly reduces TACE resistance and improves survival in advanced HCC. Specifically, only 9.7% of patients in the combination group experienced TACE resistance, compared to 38.8% in the monotherapy group. Additionally, the combination group showed significantly prolonged PFS (17.5 months vs 9.1 months) and OS (20.8 months vs 16.4 months). The study also identified maximum tumor diameter, absence of tumor capsule integrity, and bilobar distribution of tumor as independent risk factors for TACE resistance. Additionally, mechanistic studies focusing on tumor hypoxic microenvironment modulation and angiogenesis pathway inhibition may uncover novel targets for reversing therapeutic resistance and improving clinical outcomes.
| 1. | Wang CY, Li S. Clinical characteristics and prognosis of 2887 patients with hepatocellular carcinoma: A single center 14 years experience from China. Medicine (Baltimore). 2019;98:e14070. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 56] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 2. | Zhu HD, Li HL, Huang MS, Yang WZ, Yin GW, Zhong BY, Sun JH, Jin ZC, Chen JJ, Ge NJ, Ding WB, Li WH, Huang JH, Mu W, Gu SZ, Li JP, Zhao H, Wen SW, Lei YM, Song YS, Yuan CW, Wang WD, Huang M, Zhao W, Wu JB, Wang S, Zhu X, Han JJ, Ren WX, Lu ZM, Xing WG, Fan Y, Lin HL, Zhang ZS, Xu GH, Hu WH, Tu Q, Su HY, Zheng CS, Chen Y, Zhao XY, Fang ZT, Wang Q, Zhao JW, Xu AB, Xu J, Wu QH, Niu HZ, Wang J, Dai F, Feng DP, Li QD, Shi RS, Li JR, Yang G, Shi HB, Ji JS, Liu YE, Cai Z, Yang P, Zhao Y, Zhu XL, Lu LG, Teng GJ; CHANCE001 Investigators. Transarterial chemoembolization with PD-(L)1 inhibitors plus molecular targeted therapies for hepatocellular carcinoma (CHANCE001). Signal Transduct Target Ther. 2023;8:58. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 150] [Cited by in RCA: 156] [Article Influence: 78.0] [Reference Citation Analysis (1)] |
| 3. | Llovet JM, De Baere T, Kulik L, Haber PK, Greten TF, Meyer T, Lencioni R. Locoregional therapies in the era of molecular and immune treatments for hepatocellular carcinoma. Nat Rev Gastroenterol Hepatol. 2021;18:293-313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 174] [Cited by in RCA: 593] [Article Influence: 148.3] [Reference Citation Analysis (0)] |
| 4. | Zhang S, Zhu Z, Liu L, Nashan B, Zhang S. Biomarker, efficacy and safety analysis of transcatheter arterial chemoembolization combined with atezolizumab and bevacizumab for unresectable hepatocellular carcinoma. Cancer Immunol Immunother. 2025;74:209. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 5. | Deng W, Xie J, Wang T, Luo L, Zhu G, Xiao Y, Tao J, Lin L, Ge X, Wen W, Wang M, Yu B, Liu Y, Luo R, Wan R, Hu Z, Shan R. The safety and efficacy of tyrosine kinase inhibitors and programmed cell death protein- 1 inhibitors combined with HAIC/TACE in the treatment of recurrent unresectable hepatocellular carcinoma. BMC Cancer. 2025;25:779. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 6. | Zhong B, Zhang S, Zhu H, Wang W, Ni C; Clinical Guidelines Committee of Chinese College of Interventionalists. Transarterial chemoembolization refractoriness in hepatocellular carcinoma: Chinese College of Interventionalists definition and consensus statement. Chin Med J (Engl). 2024;137:2040-2042. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 7. | Choi JY, Lee JM, Sirlin CB. CT and MR imaging diagnosis and staging of hepatocellular carcinoma: part I. Development, growth, and spread: key pathologic and imaging aspects. Radiology. 2014;272:635-654. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 371] [Cited by in RCA: 351] [Article Influence: 31.9] [Reference Citation Analysis (1)] |
| 8. | Chen L, Yu CX, Zhong BY, Zhu HD, Jin ZC, Zhu GY, Zhang Q, Ni CF, Teng GJ. Development of TACE Refractoriness Scores in Hepatocellular Carcinoma. Front Mol Biosci. 2021;8:615133. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 9. | Kudo M, Han KH, Ye SL, Zhou J, Huang YH, Lin SM, Wang CK, Ikeda M, Chan SL, Choo SP, Miyayama S, Cheng AL. A Changing Paradigm for the Treatment of Intermediate-Stage Hepatocellular Carcinoma: Asia-Pacific Primary Liver Cancer Expert Consensus Statements. Liver Cancer. 2020;9:245-260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 212] [Article Influence: 42.4] [Reference Citation Analysis (0)] |
| 10. | Guo X, Song J, Zhu L, Liu S, Huang C, Zhou L, Chen W, Lin G, Zhao Z, Tu J, Chen M, Chen F, Zheng L, Ji J. Multiparametric MRI-based radiomics and clinical nomogram predicts the recurrence of hepatocellular carcinoma after postoperative adjuvant transarterial chemoembolization. BMC Cancer. 2025;25:683. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 11. | Xu B, Dong SY, Bai XL, Song TQ, Zhang BH, Zhou LD, Chen YJ, Zeng ZM, Wang K, Zhao HT, Lu N, Zhang W, Li XB, Zheng SS, Long G, Yang YC, Huang HS, Huang LQ, Wang YC, Liang F, Zhu XD, Huang C, Shen YH, Zhou J, Zeng MS, Fan J, Rao SX, Sun HC. Tumor Radiomic Features on Pretreatment MRI to Predict Response to Lenvatinib plus an Anti-PD-1 Antibody in Advanced Hepatocellular Carcinoma: A Multicenter Study. Liver Cancer. 2023;12:262-276. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 26] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 12. | Mao S, Shan Y, Yu X, Huang J, Fang J, Wang M, Fan R, Wu S, Lu C. A new prognostic model predicting hepatocellular carcinoma early recurrence in patients with microvascular invasion who received postoperative adjuvant transcatheter arterial chemoembolization. Eur J Surg Oncol. 2023;49:129-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 13. | European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J Hepatol. 2018;69:182-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5593] [Cited by in RCA: 6059] [Article Influence: 865.6] [Reference Citation Analysis (3)] |
| 14. | Moustafa AS, Abdel Aal AK, Ertel N, Saad N, DuBay D, Saddekni S. Chemoembolization of Hepatocellular Carcinoma with Extrahepatic Collateral Blood Supply: Anatomic and Technical Considerations. Radiographics. 2017;37:963-977. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 34] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 15. | Shinkawa H, Tanaka S, Kabata D, Takemura S, Amano R, Kimura K, Kinoshita M, Kubo S. The Prognostic Impact of Tumor Differentiation on Recurrence and Survival after Resection of Hepatocellular Carcinoma Is Dependent on Tumor Size. Liver Cancer. 2021;10:461-472. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 47] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 16. | Wu H, Lv S, Zhang R, Gu L, Xu J, Li C, Zhang L, Shen F, Kow AWC, Wang M, Yang T. Next-Generation Flexible Embolic Systems: Targeted Transarterial Chemoembolization Strategies for Hepatocellular Carcinoma. Adv Mater. 2025;e2503971. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 17. | Montasser A, Beaufrère A, Cauchy F, Bouattour M, Soubrane O, Albuquerque M, Paradis V. Transarterial chemoembolisation enhances programmed death-1 and programmed death-ligand 1 expression in hepatocellular carcinoma. Histopathology. 2021;79:36-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 70] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 18. | Zhang S, Xu L, Li JQ, Du MZ, Yin Y, Zhong BY, Liang HS, Li WC, Ni CF, Zhu XL. Transarterial Embolization Enhances Programmed Cell Death Ligand 1 Expression and Influences CD8(+)T Lymphocytes Cytotoxicity in an Orthotopic Hepatocellular Carcinoma Rat Model. Cardiovasc Intervent Radiol. 2024;47:1372-1381. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 19. | Yamauchi M, Ono A, Amioka K, Fujii Y, Nakahara H, Teraoka Y, Uchikawa S, Fujino H, Nakahara T, Murakami E, Okamoto W, Miki D, Kawaoka T, Tsuge M, Imamura M, Hayes CN, Ohishi W, Kishi T, Kimura M, Suzuki N, Arihiro K, Aikata H, Chayama K, Oka S. Lenvatinib activates anti-tumor immunity by suppressing immunoinhibitory infiltrates in the tumor microenvironment of advanced hepatocellular carcinoma. Commun Med (Lond). 2023;3:152. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 19] [Reference Citation Analysis (0)] |
| 20. | Zhao Y, Zhang YN, Wang KT, Chen L. Lenvatinib for hepatocellular carcinoma: From preclinical mechanisms to anti-cancer therapy. Biochim Biophys Acta Rev Cancer. 2020;1874:188391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 142] [Article Influence: 28.4] [Reference Citation Analysis (0)] |
| 21. | Xing R, Gao J, Cui Q, Wang Q. Strategies to Improve the Antitumor Effect of Immunotherapy for Hepatocellular Carcinoma. Front Immunol. 2021;12:783236. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 122] [Article Influence: 30.5] [Reference Citation Analysis (0)] |
| 22. | Peng Z, Fan W, Zhu B, Wang G, Sun J, Xiao C, Huang F, Tang R, Cheng Y, Huang Z, Liang Y, Fan H, Qiao L, Li F, Zhuang W, Peng B, Wang J, Li J, Kuang M. Lenvatinib Combined With Transarterial Chemoembolization as First-Line Treatment for Advanced Hepatocellular Carcinoma: A Phase III, Randomized Clinical Trial (LAUNCH). J Clin Oncol. 2023;41:117-127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 264] [Cited by in RCA: 250] [Article Influence: 125.0] [Reference Citation Analysis (0)] |