Published online May 24, 2025. doi: 10.5306/wjco.v16.i5.106292
Revised: March 26, 2025
Accepted: April 8, 2025
Published online: May 24, 2025
Processing time: 88 Days and 6 Hours
The predominance of pituitary adenoma in the etiology of sellar masses often leads to the diagnostic fallacy of “availability bias” so that pituitary adenoma is almost always considered the most likely diagnosis of all sellar masses, even when clinical evidence suggests otherwise. Primary sellar atypical teratoid/rhabdoid tumor (AT/RT) is the most aggressive sellar tumor. Most patients with sellar AT/RT are initially misdiagnosed with pituitary macroadenoma. Early diagnosis of sellar AT/RT is of paramount importance to counsel patients and family on the grave prognosis and to avoid futile surgical procedures. Since there are no discerning imaging features to differentiate AT/RT from other sellar tumors, the acuity of sellar compression symptoms characteristic of AT/RT is the only evidence indicative of the AT/RT diagnosis. Based on the biological and anatomical properties of the sella turcica and its surrounding structures, the nature, order of manifestation, and acuity of the sellar compression symptoms in response to sellar content expansion are mostly predictable. It is concluded that rapidly progressive headache and subsequent similarly rapidly progressive visual symptoms in a female with a large sellar mass are pathognomonic of sellar AT/RT (the “Yu rule”).
Core Tip: The biological and anatomical characteristics of the sella turcica and its surrounding structures determine the nature, order of manifestation, and acuity of the sellar compression symptoms. The acuity of sellar compression symptoms is critically important to make a correct diagnosis of a non-adenoma sellar mass. In particular, rapidly progressive headache and subsequent similarly rapidly progressive visual symptoms in a female with a large sellar mass are pathognomonic of sellar atypical teratoid/rhabdoid tumor.
- Citation: Yu R. Importance of symptoms acuity for clinical diagnosis of primary sellar atypical teratoid/rhabdoid tumor. World J Clin Oncol 2025; 16(5): 106292
- URL: https://www.wjgnet.com/2218-4333/full/v16/i5/106292.htm
- DOI: https://dx.doi.org/10.5306/wjco.v16.i5.106292
Sellar mass is encountered frequently in clinical practice[1]. Although a sellar mass can be neoplastic, hyperplastic, inflammatory, infiltrative, vascular, or hemorrhagic in nature, the vast majority of sellar masses are pituitary adenomas (also known as pituitary neuroendocrine tumors) with a prevalence close to 1 in 1000 in the general population[1,2]. The predominance of pituitary adenoma in the etiology of sellar masses often leads to the diagnostic fallacy of “availability bias” (limiting differential diagnoses to what readily come to mind)[3]. In my experience, pituitary adenoma is almost always considered by radiologists and clinicians as the most likely diagnosis of all sellar masses, and clinicians often rely too heavily on the radiological diagnosis of a sellar mass, not realizing that although a single pituitary magnetic resonance imaging can describe a sellar mass in detail, it cannot convincingly differentiate a pituitary adenoma from non-adenoma lesions in most cases[4,5]. The notion that a sellar mass is pituitary adenoma until proven otherwise can cause harm. For example, a postpartum female with hypophysitis can be misdiagnosed as having a pituitary macroadenoma and inappropriately receive transsphenoidal hypophysectomy, resulting in hypopituitarism[6]. In this article, I will summarize the biological and anatomical properties of the sella turcica and its surrounding structures and their responses to sellar masses and conclude that clinical symptoms are critically important to make a correct diagnosis of a non-adenoma sellar mass, particularly primary sellar atypical teratoid/rhabdoid tumor (AT/RT), which is very rare with only about 60 definitively diagnosed cases reported so far (to be discussed below).
AT/RT is a very aggressive malignancy of the central nervous system[7,8]. AT/RT is derived from primitive cells in the central nervous system, affects both children and adults, and can arise in any region of brain and spinal cord, including the sellar area. Histological diagnosis of AT/RT can be challenging due to poorly differentiated cellular morphology[9]. At the molecular level, AT/RT exhibits loss of the integrase interactor 1 [encoded by switch defective/sucrose non-fermentable (SWI/SNF) related BAF chromatin remodeling complex subunit B1] protein expression in most cases or the Brahma-related gene 1 (encoded by SWI/SNF related BAF chromatin remodeling complex subunit ATPase 4) protein expression in some, which is used to establish the molecular diagnosis by either immunostaining of integrase interactor 1 or sequencing of the SWI/SNF related BAF chromatin remodeling complex subunit B1 gene)[7]. Primary sellar AT/RT belongs to the MYC sub-group, World Health Organization grade 4, by DNA methylation classification of AT/RT, and is mostly found in adult females[10].
Primary sellar AT/RT is the most aggressive sellar tumor[11]. No other sellar tumors, including metastatic small cell lung cancer to the sella, are as aggressive as AT/RT[12]. Most patients inflicted with sellar AT/RT are otherwise healthy but unfortunately follow a typical and tragic clinical course[11,13,14]. They would present with acute headache for weeks and visual changes for days, get a pituitary magnetic resonance imaging, be clinically diagnosed with a pituitary macroadenoma (with the implication that the sellar tumor is benign and manageable), receive transsphenoidal resection, and unexpectedly be diagnosed with AT/RT, a devastating malignancy, based on histology and molecular studies. Sellar AT/RT would grow back quickly and the patients almost invariably succumb to the tumor after re-operation, chemotherapy, and radiotherapy, not to mention the lack of a clear chemotherapy regimen anyway. I have personally seen 3 patients with sellar AT/RT[13,14]. What struck me most was the confusion and frustration of the patients and their family when they had to face the sudden and precipitous deterioration of the patients’ heath after initially being told that the tumor was benign. Early diagnosis of sellar AT/RT is thus of paramount importance to counsel patients and family on the grave prognosis and to avoid futile surgical procedures, and perhaps to guide treatment. Admittedly, no definitive treatment is available; based on retrospective case reviews, early initiation of chemotherapy and radiation seems to be beneficial[11].
In 2015, in a case report on the first sellar AT/RT patient I saw, we proposed that “short duration of headache and visual changes associated with a large sellar mass are diagnostic clues for sellar AT/RT or other rapidly expanding sellar masses[13]”. Based on that observation, I was able to raise the concern of sellar AT/RT in the subsequent 2 cases I saw as they both presented with rapidly progressive sellar compression symptoms[14]. Further clinical experience and literature review now convince me that the acuity (and implicitly severity) of sellar compression symptoms is critically important to make a correct diagnosis of a non-adenoma sellar mass. In particular, rapidly progressive headache and subsequent similarly rapidly progressive visual symptoms are pathognomonic of sellar AT/RT.
Sellar compression symptoms, including headache and visual symptoms, are results of the increase of the volume of sellar content. To best understand the nature, order of manifestation, and acuity of the sellar compression symptoms, two main characteristics of the sella and the surrounding structures need to be emphasized. First, biologically the sella and its surrounding structures such as cavernous sinus and optic chiasm are plastic and can adapt to the expansion of sellar content so long as the expansion is slow[15,16]. Second, anatomically the dura of the sella and the diaphragma sella are the first structure to be affected by the expansion of sellar content (causing headache) while optic chiasm and cranial nerves III, IV, VI, and V (in order of the distance from sella, closest one first) are more distal to the sellar content thus visual symptoms usually occur after headache. When the expansion of sellar content is hyperacute with inflammatory reactions such as in pituitary apoplexy, the sella and its surrounding structures do not have time to adapt so that the sellar compression symptoms are also hyperacute with thunderclap headache and sudden-onset visual impairments[17]. Conversely when the expansion is gradual and slow as in pituitary adenoma, the sella would also gradually expand by tissue remodeling and the optic chiasm and the cranial nerves III, IV, VI, and V are gradually displaced so that sellar compression symptoms are subtle and progress slowly over many months[18,19]. Sellar lymphoma, sellar metastatic malignancies, and hypophysitis have variable, but comparable speed of expansion to that of pituitary adenoma, resulting in slow or subacute symptoms of sellar compression symptoms[20-22].
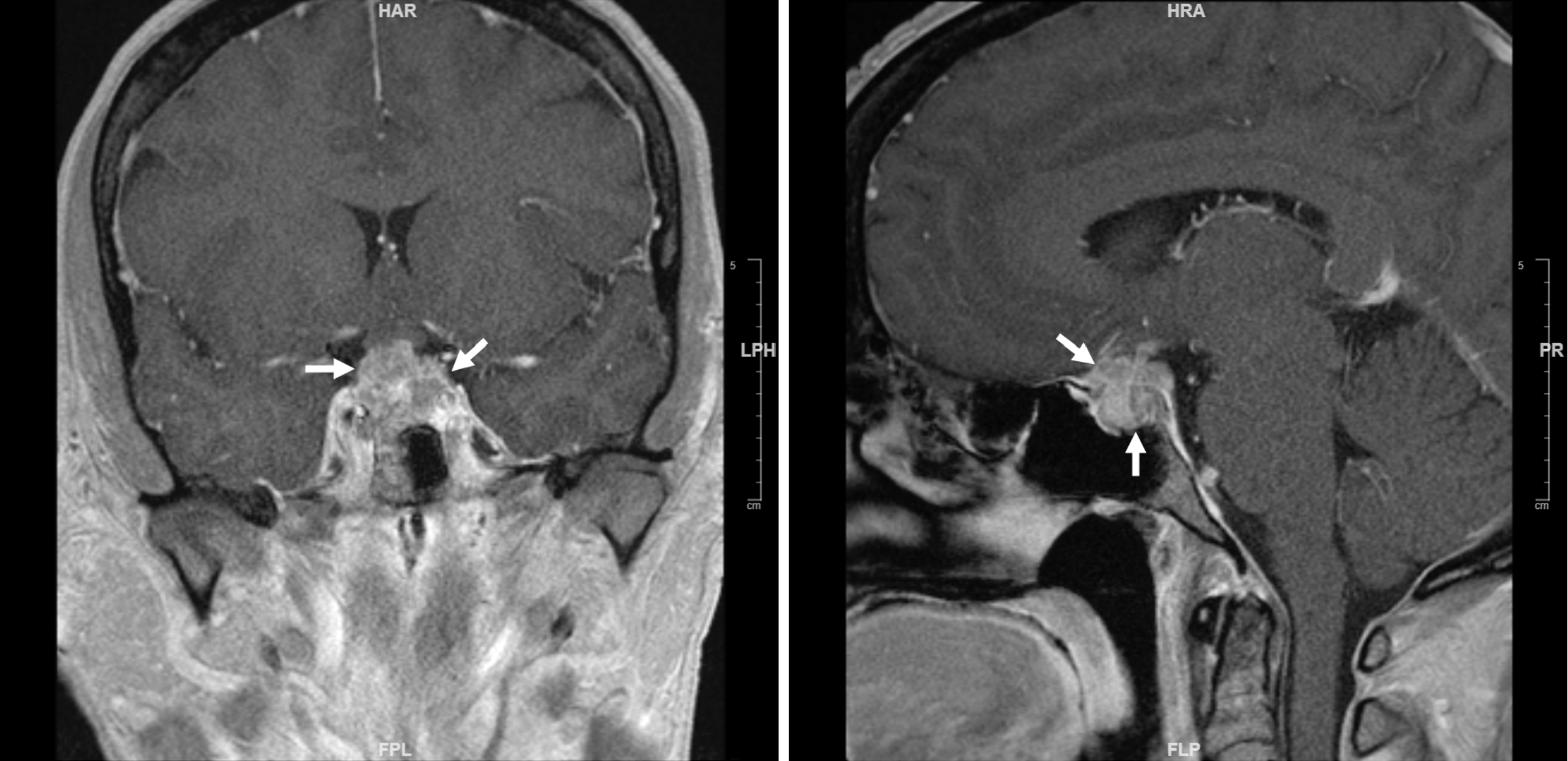
The only known sellar tumor that expands acutely and invades into the surrounding structures aggressively is sellar AT/RT. Sellar AT/RT exhibits extremely high proliferation rates without significant local inflammatory reaction, regardless of the specific histological features of the tumor[7-9]. As sellar AT/RT rapidly grows and invades into the sellar and cavernous sinus, the sella cannot remodel itself timely to accommodate so that the nerves in sellar dura and diaphragma sella are irritated progressively, causing headache initially. When the growth and invasion of AT/RT reach the optic chiasm and cranial nerves III, IV, VI, and V, visual symptoms such as double vision, blurry vision, and eye pain begin to manifest (Figure 1). In all the 3 cases I have seen, headache lasted for about 1 month and visual symptoms about 1 week until they were sufficiently severe and patients felt they needed to be urgently evaluated by medical professionals[13,14].
To my knowledge, there have been 62 cases of primary sellar AT/RT with molecular diagnosis so far reported in the literature, including the 3 cases I have seen (Table 1)[11,13,14,23-55]. The duration of sellar compression symptoms before the patients present to their physicians is unfortunately only described in 1/3 of them. Even among those cases, the description of the duration is incomplete in some. In all the cases where the duration of sellar compression symptoms is described, the headache and visual symptoms manifest acutely and progressively, similar to the clinical course of the 3 cases I have seen. Since duration of any symptoms is essential in the differential diagnosis of the symptoms, I assume that duration of headache and visual symptoms are omitted or neglected in the reports of sellar AT/RT in most cases due to the authors’ unawareness of the critical importance of this information in diagnosing a sellar mass (Table 2).
| Author and year of publication | Age | Sex | Molecular diagnosis | Headache duration | Visual symptoms duration | Pituitary hormones | Imaging features | Surgery | Chemotherapy | Radiation | Outcome |
| Zamudio-Coronado et al[23], 2023 | 60 | F | Loss of INI1 SMARCB1 mutations | No headache | 2 months | Normal | 1.3-cm with cavernous sinus invasion | Total resection, rapid recurrence | Yes | Yes | Did not specify |
| Aldhafeeri et al[24], 2023 | 32 | M | Loss of INI1 | 4 days | ND | Hypopituitarism | Large sellar mass with suprasellar distension | Debulking | No | No | Died 3 months after presentation |
| Yu[14], 2023 | 45 | F | Loss of INI1 (Met) | 3 weeks | 1 week | Hypopituitarism | 1.9 cm × 2.2 cm × 1.8 cm | Debulking | No | No | Alive 1 month after presentation |
| Yu[14], 2023 | 32 | F | Loss of INI1 (Met) | 1 month | 1 week | Normal | 2.1 cm | Debulking | No | Yes | Died 4 months after presentation |
| Baiano et al[25], 2022 | 32 | F | Loss of INI1 (Met) | 2 months | 10 days | Elevated prolactin | Large | Debulking | Yes | No | Died 2 months after presentation |
| Baiano et al[25], 2022 | 42 | F | Loss of INI1 (Met) | Intense | ND | ND | Large | Debulking | Yes | No | Died 2 months after presentation |
| Baiano et al[25], 2022 | 41 | F | Loss of INI1 (Met) | ND | ND | Hypopituitarism | Large | Debulking | No | No | Died 1 month after presentation |
| Baiano et al[25], 2022 | 50 | F | Loss of INI1 | Intense | ND | Hypopituitarism | Large | Debulking | Yes | No | Alive 18 months after presentation |
| Major et al[11], 2022 | 70 | F | Loss of INI1 | 3 months | 4 months | ND | 1.8 cm × 2.2 cm × 1.7 cm | Debulking | Yes | Yes | Died 8.5 months after presentation |
| Duan et al[26], 2022 | 47 | F | Loss of INI1 (Met) | ND | ND | 6/7 have hypopituitarism | Large | Debulking | No | No | Died 6 months after presentation |
| Duan et al[26], 2022 | 52 | F | Loss of INI1 (Met) | ND | ND | 6/7 have hypopituitarism | Large | Debulking | No | No | Died 2.5 months after presentation |
| Duan et al[26], 2022 | 47 | M | Loss of INI1 (Met) | ND | ND | 6/7 have hypopituitarism | Large | Debulking | No | Yes | Died 1 month after presentation |
| Duan et al[26], 2022 | 46 | F | Loss of INI1 (Met) | ND | ND | 6/7 have hypopituitarism | Large | Debulking | No | No | Died 6 months after presentation |
| Duan et al[26], 2022 | 45 | F | Loss of INI1 (Met) | ND | ND | 6/7 have hypopituitarism | Large | Debulking | No | No | Died 1 month after presentation |
| Duan et al[26], 2022 | 73 | F | Loss of INI1 (Met) | ND | ND | 6/7 have hypopituitarism | Large | Debulking | No | No | Died 3 months after presentation |
| Duan et al[26], 2022 | 41 | F | Loss of INI1 (Met) | ND | ND | 6/7 have hypopituitarism | Large | Debulking | No | No | Alive 11 months after presentation |
| Fukuda et al[27], 2021 | 45 | F | Loss of INI1 | ND | A few weeks | Hypopituitarism | Large | Debulking | Yes | Yes | Died 5 months after presentation |
| Liu et al[28], 2020 | 43 | F | Loss of INI1 | 1 month | 1 month | Normal | 4.5 cm | Debulking | No | Yes | Died 4 months after presentation |
| Liu et al[28], 2020 | 52 | F | Loss of INI1 | 2 weeks | ND | Hypopituitarism | 2.8 cm | Debulking | No | No | Died 2 months after presentation |
| Liu et al[28], 2020 | 50 | F | Loss of INI1 | 2 months | 2 months | Hypopituitarism | 2.7 cm | Debulking | No | No | Died 1 month after presentation |
| Liu et al[28], 2020 | 29 | F | Loss of INI1 | 4 months | ND | Hypopituitarism | 1.9 cm | Debulking | Yes | Yes | Died 8 months after presentation |
| Liu et al[28], 2020 | 80 | F | Loss of INI1 | 2 weeks | 2 weeks | Hypopituitarism | 2.2 cm | Debulking | No | No | Died 1 month after presentation |
| Bokhari et al[29], 2020 | 40 | F | Loss of INI1 | Severe | ND | ND | 2.9 cm × 1.7 cm × 2.3 cm | Debulking | None | None | ND |
| Madhavan et al[30], 2020 | 58 | F | Loss of INI1 | ND | ND | Hypopituitarism | 2.1 cm sellar mass with 3.1 cm suprasellar extension | Debulking | No | No | ND |
| Siddiqui et al[31], 2019 | 55 | F | Loss of INI1 | 1 week | 1 week | ND | 3.5 cm × 1.7 cm | Debulking | No | No | Died 1 .5 months after presentation |
| Asmaro et al[32], 2019 | 62 | F | Loss of INI1 | Several months | ND | Hypopituitarism | Large | Debulking | No | No | Died 2 months after presentation |
| Lawler and Robertson[33], 2019 | 27 | F | Loss of INI1 | ND | ND | ND | Large | Debulking | ND | ND | ND |
| Su and Su[34], 2018 | 37 | F | Loss of INI1 | ND | 2 months | ND | 2.57 cm × 1.96 cm × 3.63 cm | Debulking | No | No | Died 3 months after presentation |
| Nishikawa et al[35], 2018 | 42 | F | Loss of INI1 | Rapid | Rapid | ND | 1.9 cm × 2.0 cm | Debulking | Yes | Yes | Died 11 months after presentation |
| Barresi et al[36], 2018 | 59 | F | Loss of INI1 | Days | Days | 2.3 cm × 1.2 cm | Debulking | No | No | Died 2 months after presentation | |
| Paolini et al[37], 2018 | 31 | F | Loss of INI1 | ND | ND | ND | ND | Debulking | No | No | Died 2 months after presentation |
| Paolini et al[37], 2018 | 36 | F | Loss of INI1 | ND | ND | ND | ND | Debulking | Yes | Yes | Alive 22 months after presentation |
| Paolini et al[37], 2018 | 46 | F | Loss of INI1 | ND | ND | ND | ND | Debulking | No | No | Died after 2nd resection |
| Paolini et al[37], 2018 | 47 | F | Loss of INI1 | ND | ND | ND | ND | Debulking | Yes | Yes | Alive 62 months after presentation |
| Paolini et al[37], 2018 | 65 | f | Loss of INI1 | ND | ND | ND | ND | Debulking | Yes | Yes | Died 23 months after presentation |
| Johann et al[38], 2018 | 20 | F | Loss of INI1 (met) | ND | ND | ND | ND | Debulking | Yes | No | Died 10 years after presentation |
| Johann et al[38], 2018 | 48 | F | Loss of INI1 (Met) | ND | ND | ND | ND | Debulking | No | No | Alive 4 months after presentation |
| Huq et al[39], 2018 | 62 | F | Loss of INI1 | 2 months | Finally | Hypopituitarism | Large | Debulking | No | No | Died 2 months after presentation |
| Pratt et al[40], 2017 | 47 | F | Loss of INI1 | Severe | ND | ND | 2.6 cm × 3.9 cm × 3.2 cm | Debulking | No | Yes | ND |
| Nakata et al[41], 2017 | 31 | F | Loss of INI1 | ND | ND | ND | ND | Debulking | Yes | Yes | Died 28 months after presentation |
| Nakata et al[41], 2017 | 56 | F | Loss of INI1 | ND | ND | ND | ND | Debulking | No | Yes | Died 23 months after presentation |
| Nakata et al[41], 2017 | 44 | F | Loss of INI1 | ND | ND | ND | ND | Debulking | Yes | Yes | Died 17 months after presentation |
| Nakata et al[41], 2017 | 26 | F | Loss of INI1 | ND | ND | ND | ND | Debulking | Yes | Yes | Died 33 months after presentation |
| Nakata et al[41], 2017 | 21 | F | Loss of INI1 | ND | ND | ND | ND | Debulking | Yes | Yes | Died 35 months after presentation |
| Nakata et al[41], 2017 | 69 | F | Loss of INI1 | ND | ND | ND | ND | Debulking | Yes | Yes | Alive 37 months after presentation |
| Almalki et al[42], 2017 | 36 | F | Loss of INI1 | 3 months | 1 month | Hypopituitarism | Large | Debulking | Yes | Yes | Alive 3 years after presentation |
| Elsayad et al[43], 2016 | 66 | M | Loss of INI1 | ND | ND | ND | 2.2 cm × 1.6 cm × 1.4 cm | Debulking | No | Yes | Alive 4 years after presentation |
| Larran-Escandon et al[44], 2016 | 43 | F | Loss of INI1 | 3 months | 2 weeks | Hypopituitarism | 2.0 cm × 2.3 cm | Debulking | No | No | Died 1 month after presentation |
| Nobusawa et al[45], 2016 | 69 | F | Loss of INI1 (Met) | ND | Nost specified. Progressed over a few days | ND | 2.8 cm × 1.6 cm | Debulking | Yes | Yes | Alive 2 years after presentation |
| Lev et al[13], 2015 | 36 | F | Loss of INI1 | 1 months | 6 days | Hypopituitarism | 3.3 cm × 3.2 cm × 2.3 cm | Debulking | Yes | Yes | Died 2.5 years after presentation |
| Biswas et al[46], 2015 | 48 | F | Loss of INI1 | ND | 2 weeks | ND | ND | Debulking | Yes | Yes | Died 2 months after presentation |
| Regan et al[47], 2015 | 45 | F | Loss of INI1 | 9 days | 9 days | ND | Large | Debulking | No | Yes | Died 6 months after presentation |
| Park et al[48], 2014 | 42 | F | Loss of INI1 | ND | Progressive | ND | Large | Debulking | Yes | Yes | Alive 2 years after presentation |
| Shitara and Akiyama[49], 2014 | 44 | F | Loss of INI1 | ND | 2 months | ND | Large | Debulking | Yes | Yes | Alive 17 months after presentation |
| Moretti et al[50], 2013 | 60 | F | Loss of INI1 | ND | ND | ND | Large | Debulking | Yes | Yes | Alive 30 months after presentation |
| Chou et al[51], 2013 | 43 | F | Loss of INI1 | 10 days | 10 days | ND | 2 cm | Debulking | No | Yes | Alive at 2 weeks |
| Schneiderhan et al[52], 2011 | 61 | F | Loss of INI1 (Met) | ND | ND | ND | 2 cm | Debulking | No | No | Died 3 months after presentation |
| Schneiderhan et al[52], 2011 | 57 | F | Loss of INI1 | ND | ND | ND | 2 cm | Debulking | Yes | Yes | Alive 6 months after presentation |
| Las Heras and Pritzker[53], 2010 | 46 | F | Loss of INI1 | ND | ND | ND | ND | Debulking | ND | ND | ND |
| Arita et al[54], 2008 | 56 | F | Loss of INI1 (Met) | 2 months | 2 months | ND | 2.5 cm | Debulking | No | Yes | Died 23 months after presentation |
| Raisanen et al[55], 2005 | 31 | F | Loss of INI1 | ND | ND | ND | 1.6 cm | Debulking | No | Yes | Died 9 months after presentation |
| Raisanen et al[55], 2005 | 20 | F | Loss of INI1 | ND | ND | ND | 2.0 cm | Debulking | Yes | Yes | Alive 28 months after presentation |
| Acuity of sellar compression symptoms | Pituitary hormone status | Pituitary MRI | Other clinical clues | |
| Apoplexy | Hyperacute (hours) | Hypopituitarism | With hemorrhage | Known history of pituitary adenoma |
| Sellar AT/RT | Acute (days to weeks) | Hypopituitarism | Invasive mass | Predominantly in females |
| Pituitary adenoma | Chronic (months) | Pituitary hormone excess or deficiency | Noninvasive or invasive | History of genetic syndromes |
| Sellar lymphoma | Chronic (months) | Hypopituitarism | Invasive mass | Unclear |
| Sellar metastasis | Chronic (months) | Hypopituitarism | Invasive mass | Known malignancies |
| Hypophysitis | Chronic (months) | Hypopituitarism | Thickened stalk | Postpartum females |
In summary, the acuity of sellar compression symptoms is critically important to make a correct diagnosis of a non-adenoma sellar mass. In particular, rapidly progressive headache and subsequent similarly rapidly progressive visual symptoms in a female with a large sellar mass are pathognomonic of sellar AT/RT, which I term the “Yu rule” to facilitate usage and discussion. I recognize that the exact duration of sellar compression symptoms can be challenging to assess in some cases but that at least the acuteness of these symptoms is nonetheless evident in all cases of AT/RT in which these symptoms are described.
| 1. | Famini P, Maya MM, Melmed S. Pituitary magnetic resonance imaging for sellar and parasellar masses: ten-year experience in 2598 patients. J Clin Endocrinol Metab. 2011;96:1633-1641. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 162] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 2. | Schwetye KE, Dahiya SM. Sellar Tumors. Surg Pathol Clin. 2020;13:305-329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 3. | Chen J, Gandomkar Z, Reed WM. Investigating the impact of cognitive biases in radiologists' image interpretation: A scoping review. Eur J Radiol. 2023;166:111013. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 4. | Jipa A, Jain V. Imaging of the sellar and parasellar regions. Clin Imaging. 2021;77:254-275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 19] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 5. | Ugga L, Franca RA, Scaravilli A, Solari D, Cocozza S, Tortora F, Cavallo LM, De Caro MDB, Elefante A. Neoplasms and tumor-like lesions of the sellar region: imaging findings with correlation to pathology and 2021 WHO classification. Neuroradiology. 2023;65:675-699. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 6. | Ananthakrishnan S. Hypophysitis: it's not your mother's pituitary adenoma. Endocr Pract. 2010;16:159-161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 7. | Nesvick CL, Lafay-Cousin L, Raghunathan A, Bouffet E, Huang AA, Daniels DJ. Atypical teratoid rhabdoid tumor: molecular insights and translation to novel therapeutics. J Neurooncol. 2020;150:47-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 42] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 8. | Louis DN, Perry A, Reifenberger G, von Deimling A, Figarella-Branger D, Cavenee WK, Ohgaki H, Wiestler OD, Kleihues P, Ellison DW. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: a summary. Acta Neuropathol. 2016;131:803-820. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10993] [Cited by in RCA: 10867] [Article Influence: 1207.4] [Reference Citation Analysis (0)] |
| 9. | Zin F, Cotter JA, Haberler C, Dottermusch M, Neumann J, Schüller U, Schweizer L, Thomas C, Nemes K, Johann PD, Kool M, Frühwald MC, Paulus W, Judkins A, Hasselblatt M. Histopathological patterns in atypical teratoid/rhabdoid tumors are related to molecular subgroup. Brain Pathol. 2021;31:e12967. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 21] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 10. | Johann PD, Erkek S, Zapatka M, Kerl K, Buchhalter I, Hovestadt V, Jones DTW, Sturm D, Hermann C, Segura Wang M, Korshunov A, Rhyzova M, Gröbner S, Brabetz S, Chavez L, Bens S, Gröschel S, Kratochwil F, Wittmann A, Sieber L, Geörg C, Wolf S, Beck K, Oyen F, Capper D, van Sluis P, Volckmann R, Koster J, Versteeg R, von Deimling A, Milde T, Witt O, Kulozik AE, Ebinger M, Shalaby T, Grotzer M, Sumerauer D, Zamecnik J, Mora J, Jabado N, Taylor MD, Huang A, Aronica E, Bertoni A, Radlwimmer B, Pietsch T, Schüller U, Schneppenheim R, Northcott PA, Korbel JO, Siebert R, Frühwald MC, Lichter P, Eils R, Gajjar A, Hasselblatt M, Pfister SM, Kool M. Atypical Teratoid/Rhabdoid Tumors Are Comprised of Three Epigenetic Subgroups with Distinct Enhancer Landscapes. Cancer Cell. 2016;29:379-393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 366] [Cited by in RCA: 409] [Article Influence: 45.4] [Reference Citation Analysis (0)] |
| 11. | Major K, Daggubati LC, Mau C, Zacharia B, Glantz M, Pu C. Sellar Atypical Teratoid/Rhabdoid Tumors (AT/RT): A Systematic Review and Case Illustration. Cureus. 2022;14:e26838. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 12. | Liu X, Wang R, Li M, Chen G. Pituitary Metastasis of Lung Neuroendocrine Carcinoma Mimicking Pituitary Adenoma:Case Report and Literature Review. Front Endocrinol (Lausanne). 2021;12:678947. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 13. | Lev I, Fan X, Yu R. Sellar Atypical Teratoid/Rhabdoid Tumor: Any Preoperative Diagnostic Clues? AACE Clin Case Rep. 2015;1:e2-e7. [RCA] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 9] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 14. | Yu R. Sellar Mass in 2 Patients With Acute-Onset Headache and Visual Symptoms: Not Your Usual Pituitary Adenoma. AACE Clin Case Rep. 2023;9:197-200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 15. | Raghu ALB, Flower HD, Statham PFX, Brennan PM, Hughes MA. Sellar Remodeling after Surgery for Nonfunctioning Pituitary Adenoma: Intercarotid Distance as a Predictor of Recurrence. J Neurol Surg B Skull Base. 2020;81:579-584. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 16. | Danesh-Meyer HV, Yoon JJ, Lawlor M, Savino PJ. Visual loss and recovery in chiasmal compression. Prog Retin Eye Res. 2019;73:100765. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 48] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 17. | Briet C, Salenave S, Bonneville JF, Laws ER, Chanson P. Pituitary Apoplexy. Endocr Rev. 2015;36:622-645. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 215] [Cited by in RCA: 269] [Article Influence: 26.9] [Reference Citation Analysis (0)] |
| 18. | Gondim JA, de Almeida JP, de Albuquerque LA, Schops M, Gomes E, Ferraz T. Headache associated with pituitary tumors. J Headache Pain. 2009;10:15-20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 63] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 19. | Jahangiri A, Lamborn KR, Blevins L, Kunwar S, Aghi MK. Factors associated with delay to pituitary adenoma diagnosis in patients with visual loss. J Neurosurg. 2012;116:283-289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 35] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 20. | Caputo M, Prencipe N, Bisceglia A, Bona C, Maccario M, Aimaretti G, Grottoli S, Gasco V. Primary Pituitary Lymphoma As Rare Cause Of A Pituitary Mass And Hypopituitarism In Adulthood. Endocr Pract. 2020;26:1337-1350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 21. | Javanbakht A, D'Apuzzo M, Badie B, Salehian B. Pituitary metastasis: a rare condition. Endocr Connect. 2018;7:1049-1057. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 67] [Cited by in RCA: 67] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 22. | Langlois F, Varlamov EV, Fleseriu M. Hypophysitis, the Growing Spectrum of a Rare Pituitary Disease. J Clin Endocrinol Metab. 2022;107:10-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 77] [Article Influence: 25.7] [Reference Citation Analysis (0)] |
| 23. | Zamudio-Coronado KW, Zohdy YM, Maldonado J, Pradilla G, Garzon-Muvdi T. Sellar atypical teratoid/rhabdoid tumor in adults: survival analysis of treatment strategies. Illustrative case. J Neurosurg Case Lessons. 2023;6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 24. | Aldhafeeri W, Habelrih F, Alshehri LA, Abdullah J, Alkutbi MM, Shah SM. Atypical Teratoid/Rhabdoid Tumor of the Sellar Region in an Adult Male: A Case Report. Cureus. 2023;15:e36599. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 25. | Baiano C, Della Monica R, Franca RA, Del Basso De Caro ML, Cavallo LM, Chiariotti L, Ius T, Jouanneau E, Somma T. Atypical Teratoid Rhabdoid Tumor: A Possible Oriented Female Pathology? Front Oncol. 2022;12:854437. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 7] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 26. | Duan Z, Yao K, Yang S, Qu Y, Ren M, Zhang Y, Fan T, Zhao H, Gao J, Feng J, Fan X, Qi X. Primary adult sellar SMARCB1/INI1-deficient tumor represents a subtype of atypical teratoid/rhabdoid tumor. Mod Pathol. 2022;35:1910-1920. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 27. | Fukuda N, Ogiwara M, Nakata S, Hanihara M, Kawataki T, Kawai M, Nobusawa S, Yokoo H, Kinouchi H. An Adult Case of Sellar Atypical Teratoid/Rhabdoid Tumor Presenting with Lung Metastasis, Harboring a Compound Heterozygous Mutation in INI1. NMC Case Rep J. 2021;8:267-274. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 28. | Liu F, Fan S, Tang X, Fan S, Zhou L. Adult Sellar Region Atypical Teratoid/Rhabdoid Tumor: A Retrospective Study and Literature Review. Front Neurol. 2020;11:604612. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 29. | Bokhari RA, Bafaqeeh M, Al-obaysi S, Al-aman A, Alshakweer W. Atypical Teratoid/Rhabdoid Tumor of the Sellar Region: A Case Report and Review of the Literature. J Neurol Res. 2020;10:13-16. [DOI] [Full Text] |
| 30. | Madhavan P, Schwartz P, Kantorovich V. SAT-LB304 A Rare Case of Atypical Teratoid/Rhabdoid Tumor of Sellar Region in an Adult. J Endocr Soc. 2020;4. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 31. | Siddiqui M, Thoms D, Samples D, Caron J. Atypical teratoid/rhabdoid tumor presenting with subarachnoid and intraventricular hemorrhage. Surg Neurol Int. 2019;10:139. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 32. | Asmaro K, Arshad M, Massie L, Griffith B, Lee I. Sellar Atypical Teratoid/Rhabdoid Tumor Presenting with Subarachnoid and Intraventricular Hemorrhage. World Neurosurg. 2019;123:e31-e38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 33. | Lawler K, Robertson T. A rare case of sellar region atypical teratoid/rhabdoid tumour in an adult female. Pathology. 2019;51:S89. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 34. | Su HY, Su YF. A 37-Year-Old Woman with Progressive Right Side Ptosis for One Month. Brain Pathol. 2018;28:441-442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 35. | Nishikawa A, Ogiwara T, Nagm A, Sano K, Okada M, Chiba A, Agata M, Kaneko T, Tamada H, Uehara T, Hongo K. Atypical teratoid/rhabdoid tumor of the sellar region in adult women: Is it a sex-related disease? J Clin Neurosci. 2018;49:16-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 18] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 36. | Barresi V, Lionti S, Raso A, Esposito F, Cannavò S, Angileri FF. Pituitary atypical teratoid rhabdoid tumor in a patient with prolactinoma: A unique description. Neuropathology. 2018;38:260-267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 20] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 37. | Paolini MA, Kipp BR, Sukov WR, Jenkins SM, Barr Fritcher EG, Aranda D, SantaCruz KS, Al-Dandan S, Fisher P, McDonald WC, Bondurant CP, Van Dyke Darkow G, Giannini C, Parisi JE, Jentoft ME, Raghunathan A. Sellar Region Atypical Teratoid/Rhabdoid Tumors in Adults: Clinicopathological Characterization of Five Cases and Review of the Literature. J Neuropathol Exp Neurol. 2018;77:1115-1121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 16] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 38. | Johann PD, Bens S, Oyen F, Wagener R, Giannini C, Perry A, Raisanen JM, Reis GF, Nobusawa S, Arita K, Felsberg J, Reifenberger G, Agaimy A, Buslei R, Capper D, Pfister SM, Schneppenheim R, Siebert R, Frühwald MC, Paulus W, Kool M, Hasselblatt M. Sellar Region Atypical Teratoid/Rhabdoid Tumors (ATRT) in Adults Display DNA Methylation Profiles of the ATRT-MYC Subgroup. Am J Surg Pathol. 2018;42:506-511. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 47] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 39. | Huq S, Honasoge M, Sulanc E. Adult atypical sellar teratoid tumor presenting as diabetes insipidus. Endocr Pract. 2018;24:178-179. |
| 40. | Pratt D, Mehta GU, Wang HW, Chittiboina P, Quezado M. A 47-year old female with a destructive sellar mass. Brain Pathol. 2017;27:241-242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 41. | Nakata S, Nobusawa S, Hirose T, Ito S, Inoshita N, Ichi S, Amatya VJ, Takeshima Y, Sugiyama K, Sonoda Y, Haga H, Hirato J, Nakazato Y, Yokoo H. Sellar Atypical Teratoid/Rhabdoid Tumor (AT/RT): A Clinicopathologically and Genetically Distinct Variant of AT/RT. Am J Surg Pathol. 2017;41:932-940. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 36] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 42. | Almalki MH, Alrogi A, Al-Rabie A, Al-Dandan S, Altwairgi A, Orz Y. Atypical Teratoid/Rhabdoid Tumor of the Sellar Region in an Adult With Long Survival: Case Report and Review of the Literature. J Clin Med Res. 2017;9:216-220. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 23] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 43. | Elsayad K, Kriz J, Samhouri L, Haverkamp U, Straeter R, Stummer W, Eich HT. Long-term survival following additive radiotherapy in patients with atypical teratoid rhabdoid tumors. Strahlenther Onkol. 2016;192:569-581. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 13] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 44. | Larrán-Escandón L, Mateo-Gavira I, Vilchez-López FJ, Gómez Cárdenas E, Aguilar Diosdado M. Pituitary apoplexy as presentation of atypical teratoid/rhabdoid tumor in an adult. Endocrinol Nutr. 2016;63:364-365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 12] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 45. | Nobusawa S, Nakata S, Hirato J, Kawashima T, Sato K, Fujimaki H, Okura N, Ikota H, Yokoo H. Atypical teratoid/rhabdoid tumor in the sella turcica of an elderly female with a distinct vascular pattern and genetic alterations. Virchows Arch. 2016;469:711-715. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 19] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 46. | Biswas S, Wood M, Joshi A, Bown N, Strain L, Martinsson T, Campbell J, Ashworth A, Swain A. Exome Sequencing of an Adult Pituitary Atypical Teratoid Rhabdoid Tumor. Front Oncol. 2015;5:236. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 20] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 47. | Regan JM, Tehrani M, Rodriguez FJ, Watson JC. Adult At/Rt: Predilection for the Pituitary? J Neurol Neurosurg. 2015;2:110. [DOI] [Full Text] |
| 48. | Park HG, Yoon JH, Kim SH, Cho KH, Park HJ, Kim SH, Kim EH. Adult-onset sellar and suprasellar atypical teratoid rhabdoid tumor treated with a multimodal approach: a case report. Brain Tumor Res Treat. 2014;2:108-113. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 41] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 49. | Shitara S, Akiyama Y. Atypical teratoid/rhabdoid tumor in sellar turcica in an adult: A case report and review of the literature. Surg Neurol Int. 2014;5:75. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 33] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 50. | Moretti C, Lupoi D, Spasaro F, Chioma L, Di Giacinto P, Colicchia M, Frajoli M, Mocini R, Ulisse S, Antonelli M, Giangaspero F, Gnessi L. Sella turcica atypical teratoid/rhabdoid tumor complicated with lung metastasis in an adult female. Clin Med Insights Case Rep. 2013;6:177-182. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 37] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 51. | Chou S, Lo S, Wong H, Lau P, Chan C, Tang K, Cheung Y. Atypical Teratoid / Rhabdoid Tumour in the Sella Turcica of a Female Adult. Hong Kong J Radiol. 2013;. [RCA] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 52. | Schneiderhan TM, Beseoglu K, Bergmann M, Neubauer U, Macht S, Hänggi D, Reifenberger G, Riemenschneider MJ. Sellar atypical teratoid/rhabdoid tumours in adults. Neuropathol Appl Neurobiol. 2011;37:326-329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 31] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 53. | Las Heras F, Pritzker KP. Adult variant of atypical teratoid/rhabdoid tumor: immunohistochemical and ultrastructural confirmation of a rare tumor in the sella tursica. Pathol Res Pract. 2010;206:788-791. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 38] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 54. | Arita K, Sugiyama K, Sano T, Oka H. Atypical teratoid/rhabdoid tumour in sella turcica in an adult. Acta Neurochir (Wien). 2008;150:491-5; discussion 496. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 43] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 55. | Raisanen J, Biegel JA, Hatanpaa KJ, Judkins A, White CL, Perry A. Chromosome 22q deletions in atypical teratoid/rhabdoid tumors in adults. Brain Pathol. 2005;15:23-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 84] [Article Influence: 4.2] [Reference Citation Analysis (0)] |