Published online Aug 6, 2016. doi: 10.4292/wjgpt.v7.i3.453
Peer-review started: February 29, 2016
First decision: June 16, 2016
Revised: June 20, 2016
Accepted: July 11, 2016
Article in press: July 13, 2016
Published online: August 6, 2016
Processing time: 154 Days and 7.3 Hours
AIM: To evaluate the efficacy and safety of botulinum toxin type A (BTX-A) in the management of patients with anismus.
METHODS: An organized search of published literature was conducted using electronic databases including: PubMed/MEDLINE, and Cochrane Central Register of Controlled Trials, also an internet-based search using “Google Scholar” service was conducted. Both comparative and observational studies were included. We excluded irrelevant articles, editorials, case reports, reviews, and meta-analyses. The studies that followed the patients less than 6 mo were excluded. Variables collected were demographic data of the patients, technique of BTX-A injection and number of sessions, short-term and long-term clinical improvement, post-injection changes in electromyography (EMG), defecography, manometry, and balloon expulsion test, and complications recorded after BTX-A injection.
RESULTS: Seven studies comprising 189 patients were included in the review. The median age of the patients was 41.2 years and female-to-male ratio was 1.3:1. The median dose of BTX-A injected per procedure was 100 IU (range, 20-100 IU). Lateral injection was done in five trails and combined lateral and posterior injections in two trials. Three studies used endorectal ultrasonography-guided technique, one study used EMG-guided technique, whereas the remaining three studies used manual palpation with the index finger. The median percentage of patients who reported initial improvement of symptoms was 77.4% (range 37.5%-86.7%), this percentage declined to a median of 46% (range 25%-100%) at 4 mo after injection of BTX-A. Rates of improvement evaluated by balloon expulsion test, EMG, and defecography ranged between (37.5%-80%), (54%-86.7%), and (25%-86.6%), respectively. Fourteen (7.4%) patients developed complications after injection of BTX-A. Complication rates across the studies ranged from 0% to 22.6%.
CONCLUSION: Initial satisfactory improvement of symptoms after BTX-A injection remarkably deteriorated after 3 mo of the procedure. However, repeated injection may provide better sustained results with no additional morbidities. Further analysis of more patients is necessary to conclude the safety of BTX-A for the treatment of anismus.
Core tip: Injection of botulinum toxin type A (BTX-A) is a simple, technically feasible outpatient procedure. The initial satisfactory improvement of symptoms after BTX-A injection remarkably deteriorates after three months of the procedure from a median rate of 77.4% to 46%. However, repeated injections may provide better sustained results with no additional morbidities. The endorectal ultrasonography- and electromyography-guided techniques do not add significant value regarding both initial and long-term improvement. Combined lateral and posterior injections do not offer better results than lateral injection alone, on the contrary they can lead to higher complication rates. Although most of the studies reported very low complication rates after BTX-A injection; further studies on a larger number of patients are necessary to conclude the safety of this treatment.
- Citation: Emile SH, Elfeki HA, Elbanna HG, Youssef M, Thabet W, Abd El-Hamed TM, Said B, Lotfy A. Efficacy and safety of botulinum toxin in treatment of anismus: A systematic review. World J Gastrointest Pharmacol Ther 2016; 7(3): 453-462
- URL: https://www.wjgnet.com/2150-5349/full/v7/i3/453.htm
- DOI: https://dx.doi.org/10.4292/wjgpt.v7.i3.453
Anismus is a functional disorder of the defecation process that entails failure of relaxation or even paradoxical contraction of the puborectalis muscle and external anal sphincter (EAS) during defecation[1]. The term “Anismus” was first described by Preson and Lennard-Jones[2] in 1985. Anismus, also known as pelvic floor dyssynergia and puborectalis syndrome[3], commonly affects young and middle-aged females. The exact incidence of anismus is still unknown; however, it ranges between 20% and 70% of the general population[4].
The pathophysiology of anismus is not clearly defined yet. Certain predisposing factors as physical and emotional stress, previous anorectal surgery or hysterectomy, and psychological disorders are associated with anismus[5]. Sexual assault or abuse in childhood may also contribute to the development of anismus[6].
Patients with anismus typically complain of symptoms of outlet obstruction and defecation difficulties. Frequent attempts of evacuation, prolonged straining, anal pain, and sense of incomplete evacuation are the common presenting features of this condition[7]. On digital rectal examination (DRE), the puborectalis muscle and EAS fail to relax during straining, and sometimes a paradoxical contraction may occur. Although DRE can preliminarily diagnose anismus, additional physiologic tests such as anorectal manometry[8], balloon expulsion test[2], electromyography (EMG) of the puborectalis muscle and EAS[9], and defecography[10] are required to establish the diagnosis.
Anismus is initially managed in a conservative manner, starting with dietary modification focusing on high fiber diet, then using enemas and laxatives in increasing doses. However, conservative measures usually fail to solve the problem. Biofeedback (BFB) retraining was introduced by Bleijenberg and Kuijpers[11] for the treatment of anismus. Results of BFB were conflicting with efficacy rates ranging from 31% to 89%[3]. Surgical treatment in the form of partial miotomy of the puborectalis muscle has been described in a few reports with long-term success reaching up to 67% of patients[12].
Botulinum toxin, the product of Clostridium botulinum anaerobic bacterium, divides into seven subtype (A-G) that share similar structure, yet have different antigenic properties. Botulinum toxin type A (BTX-A) functions through extracellular binding to glycoprotein structures on the presynaptic cholinergic nerve endings which prevents the secretion of acetylcholine causing neuromuscular blockage and muscle paralysis. In addition, BTX-A blocks the efferent autonomic fibers to the smooth muscles and to the exocrine glands. While BTX-A does not induce direct central nervous system effects, some indirect effects as reflex inhibition and intra-cortical inhibition have been observed[13].
Injection of BTX-A neurotoxin directly into the puborectalis muscle is a non-operative method for the treatment of anismus[14]. Similar to BFB, the results of BTX-A injection were also conflicting. While the short-term results were highly satisfactory, the long-term outcome was disappointing with success rates of around 50% necessitating repeated injections in order to maintain the initial clinical improvement[15].
The primary objective of the current review was to analyze all the eligible articles that have evaluated the efficacy of BTX-A with regard to short and long-term outcomes. The secondary objective was to assess the side effects and complications encountered after the procedure to establish an evidence-based approach of treatment of anismus with BTX-A injection.
This systematic review was registered online in the PROSPERO project under the registration number of CRD42016033892.
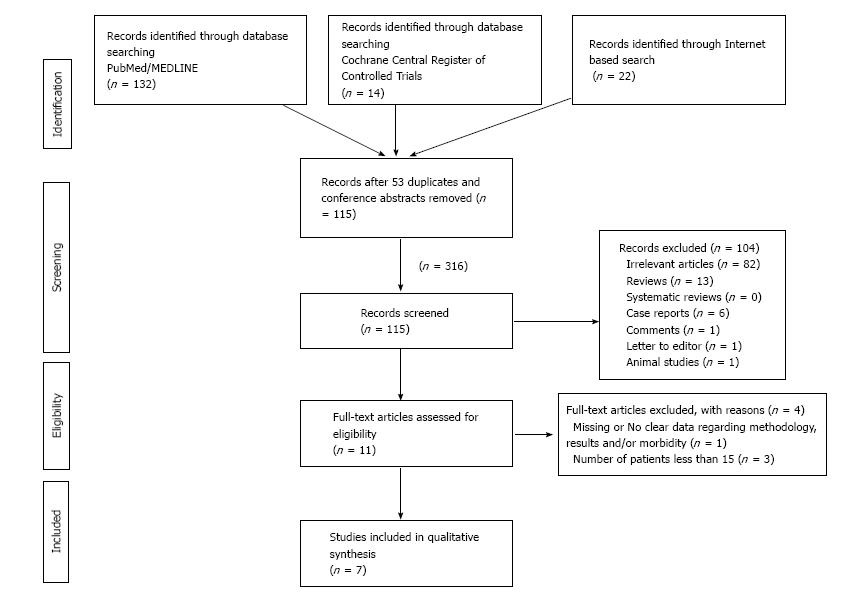
A systematic review of the literature for the role of BTX-A in the treatment of anismus was conducted following the screening guidelines established by the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) (Figure 1)[16]. Electronic databases including: PubMed/MEDLINE, and Cochrane Central Register of Controlled Trials were searched for published studies until October 2015. A parallel internet-based search using “Google Scholar” service was also conducted. The PubMed function “related articles” was used to search further articles.
The keywords used in the preliminary search process included: Anismus, puborectalis, botulinum toxin, BTA-A, puborectalis syndrome, efficacy, and safety. The following keywords syntax was utilized in the search process: (Botulinum toxin OR BTX-A) AND (Anismus OR Paradoxical contraction of puborectalis OR puborectalis syndrome).
Relevant articles mentioned in the references section of the initial publications were obtained and the related articles were also screened to add any relevant publications to the results. The full text versions of the selected articles were screened by two independent reviewers (Emile SH and Elfeki HA) to check eligibility.
This systematic review included studies that involved patients with anismus who were treated with BTX-A injection. We included both comparative and non-comparative trails that evaluated BTX-A therapy for treatment of anismus with a sample size of at least 15 patients. No language restrictions were applied.
We have excluded irrelevant articles, editorials, case reports, reviews, and meta-analyses. The studies that followed the patients less than six months were excluded. Duplicate reports were identified and excluded from this review. Articles that did not report the aim, methodology, demographic data of patients, final results, and conclusion clearly were excluded after second thorough revision.
After reviewing the full text of 11 articles, seven of them[12,17-22] met the eligibility criteria of the review. Two studies were randomized comparative trials[12,20], comparing BTX-A injection with BFB or partial division of the puborectalis muscle. The remaining five trials were observational cohort studies assessing the efficacy and complications of BTX-A injection.
Unfortunately, due to the high degree of heterogeneity among the studies included a formal meta-analysis could not be conducted in this review as two studies were comparative and five were case series studies. In addition, the studies reviewed had different methods for assessment of improvement of anismus and variable follow-up durations.
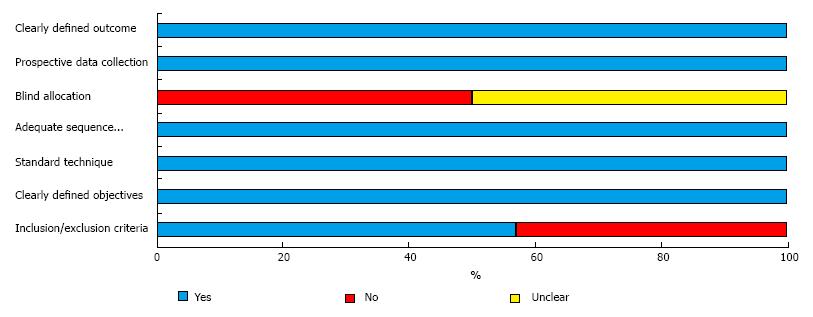
Two reviewers (Emile SH and Elfeki HA) have independently assessed the methodological quality and risk of bias in each study. The revised grading system of the Scottish Intercollegiate Guidelines Network (SIGN)[23] was used to assess comparative studies. The checklist for the quality of case series of the National Institute for Health and Clinical Excellence (NICE)[24] was used for assessment of case series studies. Six of the assessed studies were of fair quality and one study[18] was of good quality (Tables 1 and 2, Figure 2).
| Items | Farid et al[12], 2009 | Farid et al[20], 2009 |
| Inclusion criteria | 1 | 1 |
| Exclusion criteria | 1 | 1 |
| Comparable demographics | 1 | 1 |
| Number of participating centers is stated? | 1 | 1 |
| The number of surgeons is stated | 0 | 0 |
| Reporting where the authors were on the learning curve | 0 | 0 |
| Clearly stated diagnostic criteria for clinical outcomes | 1 | 1 |
| Adequate description of surgical technique | 1 | 1 |
| Standard surgical technique | 1 | 1 |
| Standard perioperative care | 0 | 0 |
| Age and range are given for patients in BTX-A group | 1 | 1 |
| Statement about any missing data | 0 | 0 |
| Age and range are given for patients in the comparative group | 1 | 1 |
| Patients in each group treated along similar timelines | 1 | 1 |
| The patients asking to enter the study, did they actually take part to it? | 0 | 0 |
| Statement about drop-out rates | 0 | 0 |
| Clearly defined outcomes | 1 | 1 |
| Availability of blind assessors | 0 | 0 |
| Assessment tools were standardized | 1 | 1 |
| Was the analysis by intention to treat? | 0 | 0 |
| Score | 12 | 12 |
| Items | Shafik et al[17] | Ron et al[18] | Maria et al[19] | Hompes et al[21] | Zhang et al[22] |
| Multi-center study | 0 | 0 | 0 | 0 | 0 |
| Clearly defined objective | 1 | 1 | 1 | 1 | 1 |
| Reported inclusion exclusion criteria | 0 | 1 | 1 | 0 | 0 |
| Clearly defined outcomes | 0 | 1 | 1 | 1 | 1 |
| Prospective data collection | 1 | 1 | 1 | 1 | 1 |
| Patients were recruited consecutively | 0 | 1 | 0 | 0 | 0 |
| Clearly described results of the study? | 1 | 1 | 1 | 1 | 1 |
| Stratified outcomes | 1 | 1 | 1 | 1 | 1 |
| Total Score | 4 | 7 | 6 | 5 | 5 |
We have extracted the following data from each study: The demographic data of the patients, technique of BTX-A injection and number of sessions, short-term and long-term clinical improvement, post-injection changes in EMG, defecography, manometry, and balloon expulsion test, and complications recorded after BTX-A injection.
The clinical diagnostic criteria of anismus in the studies included were based on the established Rome criteria[25]. The short-term and long-term clinical improvements were defined as the subjective feeling of improvement of symptoms within one month, and four months after injection, respectively.
The statistical methods of this study were reviewed by Professor Basem Eldeek, PhD, Mansoura University, Faculty of medicine. Data were extracted from the original articles into fields of Microsoft Excel spreadsheet. SPSS (Statistical Package for Social Science) version 22 under Microsoft Windows was used in the analysis of the collected data. Variables were expressed as median, normal range, and percentage of patients reported in each variable. P value less than 0.05 was considered significant.
Seven studies met the inclusion criteria of this review and were included. Two studies were retrospective and five were prospective. The median duration of follow-up was 14.6 (range, 6-19.2) mo.
The studies comprised 189 patients who were 108 (57%) female and 81 (43%) male with a female-to-male ratio of 1.3:1. The median age of the patients was 41.2 (range, 23.7-56) years. All patients complained of symptoms of outlet obstruction constipation for a median duration of 69.1 (range, 28-105.6) mo. The characteristics of each study are shown in Table 3. Only in two studies[17,22] patients completed a course of BFB retraining before they were considered unresponsive and were shifted to BTX-A injection.
| Ref. | Country | Type | n | Male | Mean age (yr) | Duration of complaint (mo) | Follow up (mo) | Dose of BTX-A (IU) | Site of injection |
| Shafik et al[17] | Egypt | Prospective | 15 | 2 | 41.2 | 105.6 | 14.6 | 25 | Lateral (3, 9 o’clock) |
| Ron et al[18] | Israel | Prospective | 24 | 9 | 23.7 | Not reported | 61.0 | 10-20 | Lateral and posterior |
| Maria et al[19] | Italy | Prospective | 24 | 10 | 56.0 | 28.0 | 39.0 | 60 | Lateral (3, 9 o’clock) |
| Farid et al[12] | Egypt | Prospective RCT | 15 | 15 | 34.7 | 71.1 | 14.7 | 100 | Lateral (5, 7 o’clock) |
| Farid et al[20] | Egypt | Prospective RCT | 24 | 7 | 34.7 | Not reported | 12.0 | 100 | Lateral (5, 7 o’clock) |
| Hompes et al[21] | United Kingdom | Retrospective | 56 | 20 | 47.5 | Not reported | 19.2 | 100 | Lateral (3, 9 o’clock) |
| Zhang et al[22] | China | Retrospective | 31 | 18 | 50.1 | 67.2 | 8.4 | 100 | Lateral and posterior (3, 6, 9 o’clock) |
The studies used BTX-A under variable commercial names (Dysport®, Botox® and Allergan®). Injection of BTX-A was performed as a day-case procedure, except in one study[22] where patients were hospitalized after BTX-A injection. The injection was conducted under local anesthesia in one study[21], caudal anesthesia in one study[23], sedation in one study[18], and without anesthesia in four studies[12,17,19,20].
The median dose of BTX-A injected per procedure was 100 IU (range, 20-100 IU). The site of injection varied; five trials employed lateral injection either at 5 and 7 o’clock[12,20], or at 3 and 9 o’clock[17,19,21]. The remaining two trials used a combination of lateral and posterior injections[18,22].
Three studies[18,19,22] used endorectal ultrasonography-guided technique for injection, one study[17] used an EMG-guided technique, whereas the remaining three studies used manual palpation with the index finger.
A single session of BTX-A injection was conducted in four studies, two sessions were conducted in two studies[17,18], and more than two injection sessions were required in one study[19]. Auxiliary pelvic floor rehabilitation (BFB) program was employed after BTX-A injection in one study[22].
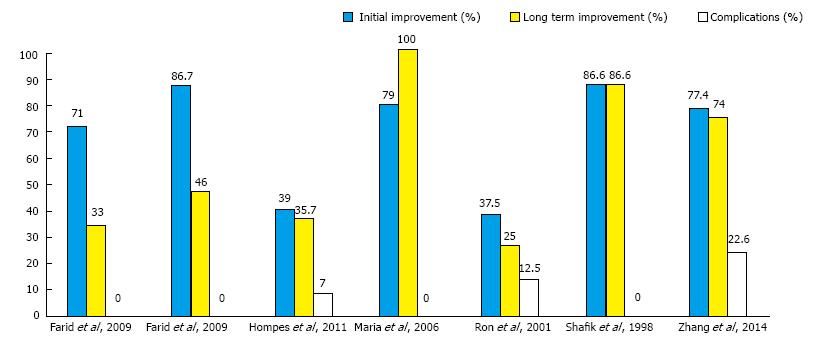
Clinical improvement: The clinical improvement of symptoms was classified into initial and long-term improvement. The median percentage of patients who reported initial improvement of symptoms was 77.4% (range, 37.5%-86.7%). This percentage declined to a median of 46% (range 25%-100%) at 4 mo after injection of BTX-A (Figure 3). One study[19] that employed repeated injections of BTX-A at two and four months reported long-term improvement in all patients.
Symptom assessment scores were not routinely used as only two studies[12,22] submitted patients to Wexner constipation scale[22] before and after BTX-A injection. The mean Wexner scores dropped from 11.2 and 14.3 before injection to 8.2 and 6.4 after injection, respectively. None of the studies used any of the scores designated for obstructed defecation syndrome.
Improvement according to anorectal manometry: Two studies[18,20] reported post-injection manometric relaxation in 28.5% and 70.8% of patients, respectively. No significant changes in anal pressures after BTX-A injection were observed according to two studies[12,21]. Conversely, two studies[19,22] reported significant decrease in the mean resting and squeeze anal pressures 3 mo after injection.
Improvement according to balloon expulsion test: Positive balloon expulsion was reported in four studies[12,18,20,22], with a median rate of 74.6% ranging from 37.5%-80%. Ninety-four patients were subjected to balloon expulsion test before and after injection, all of them failed the test before injection and 62 (66%) of them had a positive test within three month after BTX-A injection.
Improvement according to EMG: Based on EMG, three studies[12,17,20] reported post-injection improvement of anismus ranging between 54% and 86.7%. Fifty-four patients were subjected to EMG before and after injection, 39 (72%) of them showed improvement in their post-injection EMG.
Improvement according to defecography: Four studies[12,17,19,20] reported improvement of 25%-86.6% of patients in the post-injection defecogram. Seventy-eight patients underwent defecography before and after injection, 50 (64%) of them showed resolution of signs of anismus in the post-injection defecogram. One study[19] evaluated the anorectal angle (ARA) before and after injection, and reported a significant increase of the ARA during straining from 97°± 11° to 127°± 10° at two months after injection.
Fourteen (7.4%) patients developed complications after the injection of BTX-A. The median rate of complications across the studies was zero ranging from 0%-22.6% (Figure 3). Eleven (5.8%) patients developed minor transient fecal incontinence (FI) as reported by two studies[21,22]. Two patients developed acute posterior anal fissure[18], and one patient developed complete rectal prolapse[18].
Anismus is a complex functional disorder with unclear pathophysiology and elusive diagnosis rendering it a difficult condition to treat. Conservative measures for treatment of constipation usually fail to provide any significant improvement to the patients with anismus. BFB retraining, surgical division of puborectalis muscle, and BTX-A injection are the main options for treatment of anismus that were described in literature. Nevertheless, the optimal treatment of anismus is still debatable.
BFB retraining depends on the concept of operant conditioning. During BFB patients learn how to control an unconscious physiologic function with the aid of an instrument that provides visual, auditory or verbal feedback of an action that can be reinforced until a satisfactory response is accomplished[26,27].
Since the first report[11] that concluded the utility of BFB in pelvic floor disorders, several other studies tried to evaluate the efficacy of BFB in the treatment of anismus. Gilliland et al[28] reported a success rate of 63% in patients who completed their training programs. Similarly, Rhee et al[29] reported that about 69% of anismus patients showed complete response on completion of their BFB training program. However, most of these studies were small non-controlled series with short follow-up durations.
A meta-analysis of randomized controlled trials[30] evaluating BFB in the treatment of pelvic floor disorders concluded that symptomatic relief of anismus after BFB was six fold that obtained with other methods. Moreover, clinical improvement of symptoms after EMG-BFB was seven times higher than that after non-EMG BFB.
Surgical miotomy of the puborectalis muscle was described since 1960s with initial satisfactory results[1,31]. However, subsequent trials reported disappointing outcomes and unacceptably high rates of FI following division of the puborectalis muscle[32,33]. A recent pilot study[34] devised a modified semi-closed technique in dividing puborectalis muscle stating that symptomatic improvement occurred in 75% of patients without encountering any significant postoperative complications. Nonetheless, the authors recommended conducting more studies before considering this technique a validated procedure.
Hallan et al[35] described direct injection of BTX-A into the puborectalis muscle. BTX-A is a potent neurotoxin that causes muscle paralysis by inhibition of release of acetylcholine at the presynaptic region[13,14]. Injection of BTX-A emerged as a promising option in the treatment of anismus with the advantages of being less costly and technically easier than BFB retraining[19]. BTX-A injection, unlike BFB, does not depend on patient’s cooperation and compliance which are merely subjective.
As the effect of BTX-A is temporary for around three months after administration, BTX-A injection therapy was considered successful in terms of short-term symptomatic improvement of anismus. Longer term improvement necessitates repeated injections in order to maintain the achieved clinical improvement[18].
The objective of the current review was to assess the efficacy and safety of BTX-A injection in the management of anismus. Only seven studies were eligible to be included which reflects the paucity of trials in this regard. Patients were mostly middle-aged females coping with the literature[4]. Most of the studies used BTX-A injection as a primary treatment except two studies[18,23] that resorted to BTX-A after failure of BFB therapy.
Despite the availability of designated scores for obstructed defecation[36-38], none of the studies reviewed employed any of these scores to assess patients with anismus. Instead, two studies used Wexner constipation score which is not specific for obstructed defecation syndrome. The clinical utility of the obstructed defecation scores in anismus remains debatable and needs further studies to be ascertained.
Some studies used endorectal ultrasonography- or EMG-guided techniques for BTX-A injection, yet none obtained superior results compared to the studies that used manual guidance, concluding no clear benefits for the guided techniques. Although Zhang et al[22] found ultrasonography-guided injection simplified the localization of the injection site which led to a long-term improvement rate of 74%; the adjuvant BFB course they have applied to the patients after BTX-A injection could have contributed to this good outcome, rather than the guided technique of injection.
Only two studies[18,22] used combined lateral and posterior injections technique which was associated with higher complication rates with almost the same efficacy obtained by lateral injection alone. We can explain this phenomenon that posterior injection potentially affects part of EAS at the anorectal ring, subsequently this will lead to weakening of the sphincter complex and development of FI. While the site of injection played an important role in the development of complications, the dose of BTX-A did not have any special significance since the studies that used the least dose[17,18] reported conflicting results with an efficacy close to that of higher doses.
The median rate of initial improvement of symptoms after injection was 77.4% reaching up to 86%. Unfortunately, these initial good results did not last longer as they dropped to a median of 46% after three months necessitating repeated injections of BTX-A in three studies. The studies that reported satisfactory long- term results had to repeat the injection twice or more. The reason why repeated injections attained better long-term results can be attributed to the cumulative effect of BTX-A on the puborectalis muscle. Interestingly, we found that the repeated injections do not necessarily induce higher complication rates, therefore repeated BTX-A injection can potentially provide sustained improvement in cases where BFB fails and surgical miotomy is contraindicated or refused by the patient.
Improvement of anismus as assessed by the physiologic tests was variable and rather confounding. Anorectal manometry reported a decrease in anal pressures in two studies[19,21]. Conversely, the remaining studies showed no significant change in the anal pressures, although clinical improvement was evident. The rate of improvement of anismus evaluated by balloon expulsion test, EMG, and defecography ranged between (37.5%-80%), (54%-86.7%), and (25%-86.6%), respectively. Interestingly, the highest rates of improvement according to clinical examination, EMG, and defecography were the same (86%) implying the harmony of these tests with the clinical examination.
Complications after BTX-A injection were detected in 7.4% of patients. The most common complication was FI which was only transient, for two weeks, and of a minor grade. FI was reported in two studies[18,22], both applied combined lateral and posterior injections. Other morbidities as posterior anal fissure and complete rectal prolapse were observed only by one study[18] that also used posterior injection in addition to lateral injection, hence demonstrating the negative impact of posterior injection that induces further weakness to the sphincter muscles.
In summary, BTX-A injection has distinct advantages as technical feasibility, lack of need for general or spinal anesthesia, being an outpatient procedure, and excellent initial results. On the other hand, BTX-A injection proved to be a temporary short-term solution with disappointing outcome on the long term. However, longer term results can be improved further by repeated injections, although satisfactory results are still not guaranteed.
The heterogeneity of the studies included was a major limitation during the analysis and interpretation of their results, thus, a meta-analysis could not be conducted. Another limitation was the lack of data of some investigations that were not reported by some studies. In addition, most of the studies were observational with low grade of evidence; only two studies were randomized controlled trials which may influence the final outcome of the review.
The injection of BTX-A is a simple, technically feasible outpatient procedure. The initial satisfactory improvement of symptoms after BTX-A injection remarkably deteriorated after three months of the procedure. However, repeated injections may provide better sustained results with no additional morbidities.
The endorectal ultrasonography- and EMG-guided techniques did not add significant value regarding both initial and long-term improvement. Combined lateral and posterior injections technique did not achieve better results than lateral injection alone, on the contrary the studies that employed the combined injections technique reported higher complication rates. Overall, further analysis of more patients is necessary to conclude the safety of BTX-A in the treatment of anismus.
The present review suggests that injection of BTX-A in the puborectalis muscle is an effective short-term method for treatment of anismus, hence in case of deterioration of the initial satisfactory amelioration of clinical symptoms we recommend further sessions of BTX-A injection in order to maintain the clinical improvement.
From the results we obtained, we don’t recommend combined lateral and posterior injections since this technique can result in higher complication rates, yet with no substantial benefits.
Anismus is considered one of the most important causes of obstructed defecation syndrome. The precise diagnosis and management of anismus have been a challenging problem for surgeons. While biofeedback (BFB) confers excellent results in many patients; some patients fail to respond to BFB, hence alternative methods for treatment are indicated. The injection of botulinum toxin type A (BTX-A) in the puborectalis muscle provided satisfactory short-term results, yet these good results tend to deteriorate with time. The aim of this review was to determine the overall efficacy and safety of BTX-A in treatment of anismus
BTX-A has various indications in surgery as cervical dystonia, severe axillary hyperhidrosis, strabismus, and upper limb spasticity. Earlier attempts of using BTX-A for treatment of anismus date back to the nineties. BTX-A prevents the release of acetylcholine by binding to glycoprotein structures on the cholinergic nerve terminals, inducing neuromuscular blockage.
A number of trials have used BTX-A for treating anismus and pelvic floor dyssynergia using different approaches and dosage of BTX-A. Some authors used endorectal ultrasonography and EMG as a guide for the injection process. While some authors used lateral injection method; others tried combined lateral and posterior injections. The studies evaluating the efficacy and safety of BTX-A were reviewed by the authors and the data were extracted using a standardized collection tool.
This review suggests that BTX-A can be an effective method for treatment of anismus; however, the remarkable deterioration of symptom improvement may necessitate injection of further doses of BTA-X within an interval of 3-6 mo after the first injection.
BTX-A stands for botulinum toxin type A, EMG stands for electromyography, and BFB stands for biofeedback.
This is a short review about the botulinum toxin treatment for patients with anismus. This review is well written.
Manuscript Source: Invited manuscript
Specialty Type: Gastroenterology and Hepatology
Country of Origin: Egypt
Peer-Review Report Classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Amornyotin S, Onda M, Soriano-Ursua MA S- Editor: Ji FF L- Editor: A E- Editor: Lu YJ
| 1. | Wasserman IF. Puborectalis syndrome (rectal stenosis due to anorectal spasm). Dis Colon Rectum. 1964;7:87-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 87] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 2. | Preston DM, Lennard-Jones JE. Anismus in chronic constipation. Dig Dis Sci. 1985;30:413-418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 335] [Cited by in RCA: 278] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 3. | Park UC, Choi SK, Piccirillo MF, Verzaro R, Wexner SD. Patterns of anismus and the relation to biofeedback therapy. Dis Colon Rectum. 1996;39:768-773. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 33] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 4. | Rao SS, Tuteja AK, Vellema T, Kempf J, Stessman M. Dyssynergic defecation: demographics, symptoms, stool patterns, and quality of life. J Clin Gastroenterol. 2004;38:680-685. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 148] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 5. | Gosselink MJ, Schouten WR. Rectal sensory perception in females with obstructed defecation. Dis Colon Rectum. 2001;44:1337-1344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 37] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 6. | Shelton AA, Welton ML. The pelvic floor in health and disease. West J Med. 1997;167:90-98. [PubMed] |
| 7. | Kuijpers HC, Bleijenberg G. The spastic pelvic floor syndrome. A cause of constipation. Dis Colon Rectum. 1985;28:669-672. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 118] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 8. | Weber J, Ducrotte P, Touchais JY, Roussignol C, Denis P. Biofeedback training for constipation in adults and children. Dis Colon Rectum. 1987;30:844-846. [PubMed] |
| 9. | Johansson C, Nilsson BY, Mellgren A, Dolk A, Holmström B. Paradoxical sphincter reaction and associated colorectal disorders. Int J Colorectal Dis. 1992;7:89-94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 28] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 10. | Kuijpers HC, Bleijenberg G, de Morree H. The spastic pelvic floor syndrome. Large bowel outlet obstruction caused by pelvic floor dysfunction: a radiological study. Int J Colorectal Dis. 1986;1:44-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 51] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 11. | Bleijenberg G, Kuijpers HC. Treatment of the spastic pelvic floor syndrome with biofeedback. Dis Colon Rectum. 1987;30:108-111. [PubMed] |
| 12. | Farid M, Youssef T, Mahdy T, Omar W, Moneim HA, El Nakeeb A, Youssef M. Comparative study between botulinum toxin injection and partial division of puborectalis for treating anismus. Int J Colorectal Dis. 2009;24:327-334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 30] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 13. | Dressler D, Saberi FA, Barbosa ER. Botulinum toxin: mechanisms of action. Arq Neuropsiquiatr. 2005;63:180-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 144] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 14. | Jankovic J, Brin MF. Therapeutic uses of botulinum toxin. N Engl J Med. 1991;324:1186-1194. [PubMed] |
| 15. | Maria G, Brisinda G, Bentivoglio AR, Cassetta E, Albanese A. Botulinum toxin in the treatment of outlet obstruction constipation caused by puborectalis syndrome. Dis Colon Rectum. 2000;43:376-380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 47] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 16. | Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J, Moher D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339:b2700. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13930] [Cited by in RCA: 13346] [Article Influence: 834.1] [Reference Citation Analysis (0)] |
| 17. | Shafik A, El-Sibai O. Botulin toxin in the treatment of nonrelaxing puborectalis syndrome. Dig Surg. 1998;15:347-351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 41] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 18. | Ron Y, Avni Y, Lukovetski A, Wardi J, Geva D, Birkenfeld S, Halpern Z. Botulinum toxin type-A in therapy of patients with anismus. Dis Colon Rectum. 2001;44:1821-1826. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 64] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 19. | Maria G, Cadeddu F, Brandara F, Marniga G, Brisinda G. Experience with type A botulinum toxin for treatment of outlet-type constipation. Am J Gastroenterol. 2006;101:2570-2575. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 51] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 20. | Farid M, El Monem HA, Omar W, El Nakeeb A, Fikry A, Youssef T, Yousef M, Ghazy H, Fouda E, El Metwally T. Comparative study between biofeedback retraining and botulinum neurotoxin in the treatment of anismus patients. Int J Colorectal Dis. 2009;24:115-120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 46] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 21. | Hompes R, Harmston C, Wijffels N, Jones OM, Cunningham C, Lindsey I. Excellent response rate of anismus to botulinum toxin if rectal prolapse misdiagnosed as anismus (‘pseudoanismus’) is excluded. Colorectal Dis. 2012;14:224-230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 25] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 22. | Zhang Y, Wang ZN, He L, Gao G, Zhai Q, Yin ZT, Zeng XD. Botulinum toxin type-A injection to treat patients with intractable anismus unresponsive to simple biofeedback training. World J Gastroenterol. 2014;20:12602-12607. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 19] [Cited by in RCA: 16] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 23. | Baird AG, Lawrence JR. Guidelines: is bigger better? A review of SIGN guidelines. BMJ Open. 2014;4:e004278. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 18] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 24. | National Institute for Health and Clinical Excellence. NICE clinical guidelines, Appendix 4 Quality of case series form. Available from: http://www.nice.org.uk/nicemedia/pdf/Appendix_04_qualityofcase_series_form_preop.pdf. |
| 25. | Rome Foundation. Rome III Diagnostic Questionnaires. Constipation Module. [accessed 2016 Jan 30]. Available from: http://www.romecriteria.org/pdfs/ConstMode.pdf. |
| 26. | Agachan F, Chen T, Pfeifer J, Reissman P, Wexner SD. A constipation scoring system to simplify evaluation and management of constipated patients. Dis Colon Rectum. 1996;39:681-685. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 818] [Cited by in RCA: 851] [Article Influence: 29.3] [Reference Citation Analysis (0)] |
| 27. | Chiarioni G, Heymen S, Whitehead WE. Biofeedback therapy for dyssynergic defecation. World J Gastroenterol. 2006;12:7069-7074. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 66] [Cited by in RCA: 57] [Article Influence: 3.0] [Reference Citation Analysis (2)] |
| 28. | Gilliland R, Heymen S, Altomare DF, Park UC, Vickers D, Wexner SD. Outcome and predictors of success of biofeedback for constipation. Br J Surg. 1997;84:1123-1126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 41] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 29. | Rhee PL, Choi MS, Kim YH, Son HJ, Kim JJ, Koh KC, Paik SW, Rhee JC, Choi KW. An increased rectal maximum tolerable volume and long anal canal are associated with poor short-term response to biofeedback therapy for patients with anismus with decreased bowel frequency and normal colonic transit time. Dis Colon Rectum. 2000;43:1405-1411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 25] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 30. | Koh CE, Young CJ, Young JM, Solomon MJ. Systematic review of randomized controlled trials of the effectiveness of biofeedback for pelvic floor dysfunction. Br J Surg. 2008;95:1079-1087. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 66] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 31. | Wallace WC, Madden WM. Experience with partial resection of the puborectalis muscle. Dis Colon Rectum. 1969;12:196-200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 27] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 32. | Barnes PR, Hawley PR, Preston DM, Lennard-Jones JE. Experience of posterior division of the puborectalis muscle in the management of chronic constipation. Br J Surg. 1985;72:475-477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 102] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 33. | Kamm MA, Hawley PR, Lennard-Jones JE. Lateral division of the puborectalis muscle in the management of severe constipation. Br J Surg. 1988;75:661-663. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 63] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 34. | Asciore L, Pescatori LC, Pescatori M. Semi-closed bilateral partial miotomy of the puborectalis for anismus: a pilot study: Partial miotomy of the puborectalis for anismus. Int J Colorectal Dis. 2015;30:1729-1734. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 6] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 35. | Hallan RI, Williams NS, Melling J, Waldron DJ, Womack NR, Morrison JF. Treatment of anismus in intractable constipation with botulinum A toxin. Lancet. 1988;2:714-717. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 131] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 36. | Altomare DF, Spazzafumo L, Rinaldi M, Dodi G, Ghiselli R, Piloni V. Set-up and statistical validation of a new scoring system for obstructed defaecation syndrome. Colorectal Dis. 2008;10:84-88. [PubMed] |
| 37. | Renzi A, Brillantino A, Di Sarno G, d’Aniello F. Five-item score for obstructed defecation syndrome: study of validation. Surg Innov. 2013;20:119-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 48] [Article Influence: 3.7] [Reference Citation Analysis (0)] |