Published online Nov 6, 2013. doi: 10.4292/wjgpt.v4.i4.86
Revised: July 31, 2013
Accepted: August 5, 2013
Published online: November 6, 2013
Processing time: 142 Days and 9.9 Hours
There is an increasing appreciation for the importance of inflammation as a pathophysiologic entity that contributes to functional gastrointestinal disorders including functional dyspepsia (FD). Importantly, inflammation may serve as a mediator between psychologic and physiologic functions. This manuscript reviews the literature implicating two inflammatory cell types, mast cells and eosinophils, in the generation of dyspeptic symptoms and explores their potential as targets for the treatment of FD. There are a number of inciting events which may initiate an inflammatory response, and the subsequent recruitment and activation of mast cells and eosinophils. These include internal triggers such as stress and anxiety, as well as external triggers such as microbes and allergens. Previous studies suggest that there may be efficacy in utilizing medications directed at mast cells and eosinophils. Evidence exists to suggest that combining “anti-inflammatory” medications with other treatments targeting stress can improve the rate of symptom resolution in pediatric FD.
Core tip: Current evidence implicates gastric mast cells and duodenal eosinophils in the pathophysiology of functional dyspepsia and as mediators between psychologic and physiologic factors. Increased antral mast cell density is associated with anxiety, electromechanical dysfunction, and the postprandial distress syndrome (PDS) subtype of functional dyspepsia. Likewise, increased duodenal eosinophil density is associated with anxiety and the PDS subtype, however, effects on electromechanical function are more indirect. More importantly, mast cells and eosinophils appear to be therapeutic targets offering newer options for treating functional dyspepsia.
- Citation: Friesen CA, Schurman JV, Colombo JM, Abdel-Rahman SM. Eosinophils and mast cells as therapeutic targets in pediatric functional dyspepsia. World J Gastrointest Pharmacol Ther 2013; 4(4): 86-96
- URL: https://www.wjgnet.com/2150-5349/full/v4/i4/86.htm
- DOI: https://dx.doi.org/10.4292/wjgpt.v4.i4.86
A majority of children with chronic abdominal pain presenting to pediatric gastroenterology practices fulfill criteria for a functional gastrointestinal disorder (FGID), with the two most common being functional dyspepsia (FD) and irritable bowel syndrome (IBS)[1-4]. Prevalence estimates for FD are 3.5%-27.0% in children/adolescents and 20%-30% in adults, highlighting the pervasive nature of this disorder[5,6]. FD is defined as persistent or recurrent pain or discomfort centered in the upper abdomen (above the umbilicus) that is unrelated to a change in stool frequency or form and not exclusively relieved by defecation. A diagnosis of FD is accompanied by the lack of evidence for an inflammatory, anatomic, metabolic, or neoplastic process that explains the patient’s symptoms; however, mild, chronic inflammatory changes on mucosal biopsies do not preclude the diagnosis[5,6].
In adults, there are two recognized FD subtypes based on studies utilizing factor analysis, postprandial distress syndrome (PDS) and epigastric pain syndrome (EPS). PDS is defined as bothersome postprandial fullness occurring after ordinary sized meals and/or early satiation that prevents finishing a regular meal. EPS is defined as intermittent pain or burning of at least moderate severity localized to the epigastrium. The Rome pediatric subcommittee did not adopt the adult subtypes because of a lack data to support their existence in children and adolescents. However, there is now some data to suggest that these adult subtypes may have meaningful associations with mucosal inflammation and psychosocial functioning in children with FD[7]. For example, pediatric dyspepsia is associated with lower quality of life, increased functional disability, and increased likelihood of meeting criteria for an anxiety disorder, however, the association with anxiety appears to predominate in patients experiencing symptoms consistent with PDS[7,8]. A similar relationship between PDS and anxiety has been described in adults[9].
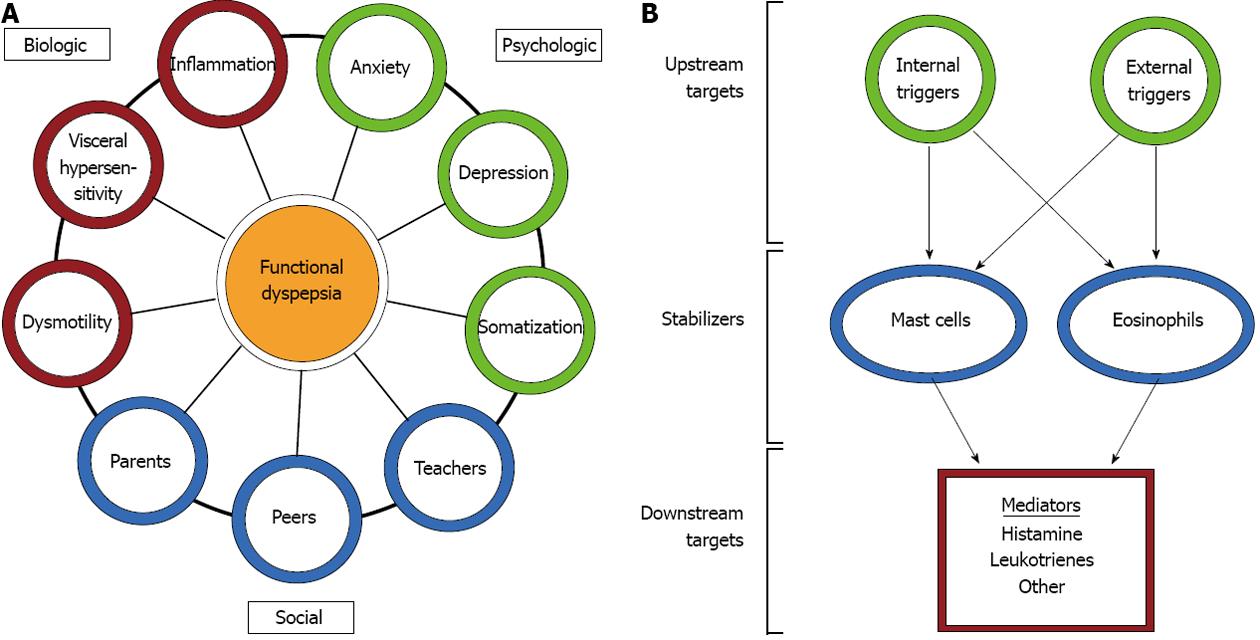
FGIDs, including FD, are probably best understood through a biopsychosocial model. This model suggests that interactions between biological/physiological factors (e.g., inflammation, mechanical disturbances, hypersensitivity), psychological factors (e.g., anxiety, depression, somatization), and social factors (e.g., interactions with parents, teachers, or peers) collectively contribute to the symptoms of FD (Figure 1). The biological factors most often implicated in FD include motility disturbances, such as delayed gastric emptying, gastric electrical disturbances, and impaired accommodation, and visceral hypersensitivity to distension, acid, and/or lipids[10-12]. Delayed gastric emptying and gastric electrical disturbances have been demonstrated in a substantial proportion of children with FD[13,14]. Similarly, water load volume, as an indicator of visceral sensitivity, differs between children with FD and healthy controls[15,16]. Consequently, electromechanical disturbances and visceral hypersensitivity represent frequent targets for therapeutic intervention in FD.
Recently, there is an increasing appreciation for the importance of inflammation as a pathophysiologic entity that contributes to FGIDs including FD. Importantly, inflammation may serve as a mediator between psychologic and physiologic functions. This manuscript reviews the literature implicating two inflammatory cell types, mast cells and eosinophils, in the generation of dyspeptic symptoms and explores their potential as targets for the treatment of FD.
In the context of FGIDs, mast cells have been studied primarily in adults with IBS where their numbers are generally elevated in the ileum and colon[17,18]. In addition, adult IBS has been associated with an increase in the density of degranulating mast cells and mast cells in close proximity to nerves which correlate with abdominal pain severity and frequency[19]. Increased mucosal mast cell density has also been demonstrated in the gastric corpus and antrum of adults with FD[20,21]. Increased mast cell density is generally isolated to the stomach of adults with FD, while increased mast cell density in the duodenum is generally associated with IBS[21,22].
Due to a lack of normal control data, it is unclear whether gastric mast cells are elevated in pediatric FD, however, antral mast cells do appear to be actively degranulating in children with FD. The mean reported degranulation index is 67% with more than 80% of patients demonstrating degranulation indices of greater than 50%[23]. Mast cells in the proximal stomach have been shown to degranulate with balloon distension of this region, demonstrating possible hypersensitivity in adults with FD[24]. Although equivalent data are not available for children, mast cell density is positively correlated with slower gastric emptying and pre-prandial dysrhythmia in children with FD[23]. Of note, the presence of pre-prandial dysrhythmia appears to be associated with increased post-prandial pain[13].
Ethical considerations preclude tissue sampling for evaluation of eosinophil density in otherwise healthy children. Existing pediatric data provide suggestive evidence of eosinophil densities that may be abnormal in the absence of established “norms”. In a pediatric autopsy study (for which presence of gastrointestinal symptoms could not be evaluated), eosinophil density was < 10/hpf in the antrum and ≤ 20/hpf in the duodenum in 100% and 82% of samples evaluated, respectively[25]. Review of biopsies from 682 presumably symptomatic children referred for endoscopy found eosinophil density was ≤ 10/hpf in the antrum and ≤ 20/hpf in the duodenum of 90% and 93% of children, respectively[26]. Maximum eosinophil density was 8/hpf in the antrum and 26/hpf in the duodenum. Thus, eosinophil density cut points of 10/hpf in the antrum and 20/hpf in the duodenum seem reasonable, but may need to be considered in tandem with measures of activation.
Eosinophil biologic activity results from mediator release with most mediators active in a concentration-dependent fashion. Thus, eosinophil effects are not just dependent on cell density, but on the extent of degranulation. However, these events may not be tightly correlated[27]. In a previous study involving 20 children with a diagnosis of FD, an eosinophil density > 20/hpf was present in only 15%, but moderate to extensive degranulation was demonstrated by electron microscopy in 95% of those evaluated[28].
Location of eosinophils also may be important to consider. Dyspeptic adults have demonstrated increased eosinophil density in the duodenum as compared with controls; however, the quantity of antral eosinophils did not differ between groups[29,30]. Duodenal biopsies from adult dyspeptics also revealed more extensive degranulation, with enhanced extracellular major basic protein (MBP)[30]. This is consistent with observations of degranulation and MBP release in pediatric patients with FD[28]. In adults, an increased eosinophil density and a higher prevalence of duodenal eosinophilia has been specifically associated with the PDS subtype of FD[30]. Eosinophilia within the upper gastrointestinal tract has been evaluated in children with unspecified (by Rome criteria) abdominal pain, as well as children with FD, providing some pediatric information regarding the association between eosinophil location and patient symptoms. In a study of 1191 children with unspecified chronic abdominal pain, eosinophilia was identified in the antrum or duodenum in 11.4%[31]. Another study found gastric eosinophilia in 19% and duodenal eosinophilia in 32% of children with unspecified chronic abdominal pain[32]. In contrast, in children specifically fulfilling FD criteria, duodenal eosinophilia has been demonstrated in 79% of patients, which closely mirrors adult findings[33].
Mast cells and eosinophils exert their biologic functions almost exclusively by the release of mediators after activation. The effects of specific mediators depend, to some extent, on the local biochemical milieu of the involved tissue[34]. As a consequence of this paracrine activity, mast cells and eosinophils interact highly with each other. In addition, mast cells and eosinophils demonstrate self-sustaining autocrine activity. For example, both eosinophils and mast cells produce interleukin (IL)-5 which augments mast cell cytokine production and is critical for the growth, chemotaxis, and activation of eosinophils[35,36]. Mast cells and eosinophils both produce eotaxin which, in conjunction with mast cell-produced histamine, serves as a chemoattractant for eosinophils[35,37]. Mast cells and eosinophils both also produce and express receptors for leukotrienes and tumor necrosis factor-α (TNF-α) which effect chemotaxis, survival, and activation of these two cell types[35,37]. Given the countless mediators that these cells produce, it is likely that activation of either cell will result in alteration of function of the other.
There are a number of triggers or inciting events which may initiate an inflammatory response, and the subsequent recruitment and activation of mast cells and eosinophils, in the gastrointestinal tract. These include internal triggers such as stress and anxiety, as well as external triggers such as microbes and allergens.
Anxiety and stress are the most highly implicated internal triggers in the development and/or maintenance of FGIDs, including FD. Children with FGIDs tend to have more concurrent symptoms of anxiety and depression than do their peers[38]. Approximately 50% of children with FD demonstrate elevated anxiety scores either in isolation or as part of more global psychosocial dysfunction[39]. Further, mucosal eosinophil density, as well as antral mast cell density, correlates with anxiety scores in children with FD[7,40].
Thus, the role of inflammation in the biopsychosocial model is probably best illustrated by examining the stress response. Corticotropin releasing hormone (CRH), produced by the hypothalamus (as well as immune cells including lymphocytes and mast cells) is a major mediator of the stress response in the hypothalamic-pituitary-adrenal (HPA) axis and, subsequently, within the brain-gut axis. The stress response results in physiologic effects which appear relevant to FGIDs including inflammation, altered gastric accommodation, gastric dysmotility, and visceral hypersensitivity. CRH also has CNS effects which may alter central processing of nociceptive messages including anxiogenic effects. Of note, the relationship between the CNS and gastrointestinal pathophysiology is bidirectional. In a rodent model, gastric irritation in the neonatal period induces a long lasting increase in depression- and anxiety-like behaviors, as well as an increased sensitivity of the HPA axis to stress[41]. CRH stress systems may be activated by afferent nerves from inflamed sites or via cytokines including TNF-α, IL-1, IL-6 and IL-12[42].
CRH receptors are widely expressed within the gastrointestinal tract and immune cells. Mast cells express both CRH1 and CRH2 receptor subtypes at their surface. Most of the inflammatory cell actions, including those on mast cells, occur via CRH-R2 receptors[43]. Once mast cells are activated, they release mediators which recruit and activate eosinophils, with both cell types interacting in a bi-directional fashion with T helper cells (Th). There also may be a direct effect for CRH on eosinophils. In a rodent model, psychologic stress results in eosinophilic expression of CRH[44]. CRH is not expressed by eosinophils in the intestines of the mice except under psychologic stress and decreases after the stress is removed[44].
Once activated by CRH, mast cells may release pro-inflammatory cytokines[45]. Adults have demonstrated selective luminal release of tryptase and histamine from jejunal mast cells under cold stress at a magnitude similar to that induced by antigen exposure in food allergic patients[46]. Once released, mast cell and eosinophil mediators can stimulate afferent nerves signaling pain, can sensitize afferent nerves resulting in visceral hypersensitivity, and can alter electromechanical function. Histamine can stimulate afferent sensory nerves via H2 receptors[47]. CRH has been shown to activate gastrointestinal mast cells with resultant mediator sensitization of afferent sensory enteric nerves[48-50]. Low grade inflammation may lead to visceral sensitivity and motility disturbances; the key appears to be a shift from a TH1 to a TH2 response, with eosinophils and mast cells as the key effector cells[51]. Stress has been shown to shift the relative proportion and trafficking of T helper lymphocytes towards a Th2 or “allergic” phenotype[42]. This shift is driven by central and peripheral CRH, catecholamines, and histamine via H2 receptors. The Th2 phenotype is associated with release of IL-4, IL-10, and IL-13 which stimulate growth and activation of mast cells and eosinophils[42].
A number of external triggers have been identified, such as microbes and allergens, which may result in eosinophil and/or mast cell recruitment and activation. The immune system may be activated by an acute infection and continue to generate symptoms after the infection resolves, resulting in so-called post-infectious FD (PI-FD). Helicobacter pylori (H. pylori) colonization represents a unique situation where symptoms may result from chronic infection and, in many patients, persist after eradication.
FD has been reported at a higher prevalence following both bacterial and parasitic infections[52]. It seems likely that FD may also be induced by viral gastroenteritis in a manner similar to that of IBS. In a study of 88 children with a previous positive bacterial stool culture, FD was present in 24% and IBS in 87%[53]. Fifty-six percent of the patients reported the onset of abdominal pain after the acute infection. Another study identified 82 adults with persistent abdominal symptoms following Giardia infection, with FD in 24.3% and IBS in 80.5%[54]. Over half of these patients reported exacerbation due to specific foods and, consistent with the biopsychosocial model, nearly half reported exacerbations with physical or mental stress[54].
PI-FD appears to represent an impaired ability to terminate the inflammatory response after elimination of the offending pathogen. It may also be associated with neuroplastic changes in visceral and central afferent pathways[55]. Duodenal eosinophilia has been described in PI-FD and gastric mast cells are significantly increased in PI-FD as compared to healthy controls[51,56]. PI-FD is associated with increased gastric release of histamine and 5-hydroxytryptamine as well as increased number of mast cells in close proximity to nerve fibers as compared to healthy controls or non-PI-FD[57].
The role of H. pylori in FD remains incompletely defined. Multiple studies have demonstrated a moderate reduction in FD symptoms with eradication of this organism while others have shown no clinical benefit[57-60]. A Cochrane review concluded that eradication was significantly better than placebo[61]. However, a large number of patients continue to experience symptoms following eradication. These may be patients in whom H. pylori had no pathologic role or may represent patients with PI-FD and prolonged submucosal inflammation[62].
H. pylori colonization in children is associated with a mixed inflammatory infiltrate including eosinophils which decrease with eradication[63]. H. pylori colonization also may be associated with increased antral mast cell density, though this appears to be H. pylori strain specific[64]. In the setting of H. pylori-associated nodular gastritis, eosinophils may be of particular significance. Patients with nodular gastritis have a higher incidence of dyspeptic symptoms which resolve with eradication therapy[62]. Nodularity is associated with an increased density of eosinophils[65]. Even in the absence of nodularity, H. pylori colonization is associated with increased antral eosinophils, as well as increased gastric fluid eosinophil cationic protein indicating that the eosinophils are actively degranulating[26,63,66]. These findings would suggest a possible pathophysiologic role for eosinophils in contributing to symptoms in patients with H. pylori colonization prior to and following eradication.
The role of allergies in the development of FD has not been well studied; however, their potential to contribute given the observed increases in and activation of mast cells and eosinophils in FD is certainly plausible. FGIDs occur more commonly in children with a history of cow’s milk allergy as infants[67]. In these children, mucosal application of cow’s milk is associated with increased eosinophils and mast cells and rapid degranulation within 10 min of application[68]. In addition, cow’s milk exposure is associated with increased mast cells in close proximity to nerves[68]. A history of allergy is associated with increased duodenal eosinophil density in adults with FD[30]. Whether food allergy accounts for a substantial portion of children with FD is not clear. We previously found no significant increase in immunoreactivity to common food allergens in FD children with duodenal eosinophilia, though it is possible that the reactions were localized to the mucosa and thus missed in our assessment[69]. It is also possible that environmental allergens may play a role in FD. Antigen exposure in adults with birch pollen allergy results not only in an increase in symptoms of FD but also an increase in mucosal MBP+ eosinophils and IgE-bearing cells in the majority of patients[70].
Treatments with the potential to impact symptoms related to inflammatory cells would primarily act by three mechanisms: (1) controlling upstream factors which recruit or activate inflammatory cells; (2) controlling the release of mediators from inflammatory cells; and (3) antagonizing the downstream effects of mediators once released from inflammatory cells (Figure 1).
Treatments directed at upstream factors would include those which interfere with activation of mast cells or eosinophils by internal triggers (e.g., CRH antagonists, selective serotonin reuptake inhibitors (SSRI) anti-depressants, anti-anxiety treatments) or external triggers (e.g., corticosteroids, anti-TNF-α, anti-IL-5 and anti-IgE).
The biopsychosocial model and CRH physiology would suggest a potential role for antagonizing CRH, or controlling its secretion by modulating anxiety and the stress response either through the use of SSRIs, anti-anxiety medications, or relaxation techniques. Though there are no previous controlled studies evaluating CRH-antagonists or SSRIs in pediatric FD, some evidence exists for the role of relaxation via biofeedback-assisted relaxation training (BART). Biofeedback is a technique whereby individuals are trained to relieve physical or emotional symptoms using signals from their bodies that are displayed visually or aurally. Biofeedback can be paired with relaxation training to yield BART. BART paired with fiber supplementation has been shown to be superior to fiber alone in children with non-specific abdominal pain[71]. The effect of BART directly on inflammation has not been studied. However, BART has been studied as an adjunctive treatment to medications directed at inflammation in children with FD in association with duodenal eosinophilia[72]. Children receiving medication plus BART demonstrated better outcomes with regard to pain intensity, duration of pain episodes, and global clinical improvement as compared to children receiving medications alone[72].
Corticosteroids represent another group of agents which may be used in the setting of mucosal eosinophilia to block upstream activation and upstream effects, although they have not been studied directly in patients with FD. Prednisone has long been considered the mainstay in the treatment of eosinophilic gastroenteritis though there are no placebo-controlled studies evaluating efficacy. The less than favorable side effect profile represents a significant draw back in considering its use as a long term agent. Budesonide may represent a safer alternative. Budesonide is a synthetic corticosteroid with high topical activity, substantial first pass elimination and relatively low systemic bioavailability. Among the commercially available preparations is an oral enteric-coated capsule formulated to optimize delivery to the ileum and colon[73]. The delivery pattern would suggest that budesonide may be less effective for proximal small bowel disease. However, the budesonide granules dissolve at an alkaline pH normally present in the proximal small bowel. Although acid suppression with omeperazole does not affect absorption, acid suppression in combination with delayed gastric emptying, as might be expected with mucosal inflammation, has not been evaluated[73]. The literature regarding budesonide and eosinophilic gastroenteritis consists of case reports where budesonide therapy has been reported to be effective against eosinophilia in the duodenum and jejunum[74-76].
TNF-α represents another theoretical “upstream” treatment target for FD. CysLTs induce TNF-α production which has been demonstrated to recruit and prolong survival of eosinophils, as well promote a TH2 response depending on other chemokines present in the microenvironment[77-79]. In a variety of allergic mouse models, anti-TNF antibodies have been shown to decrease eosinophilic infiltration and local Th2 cytokine transcription and secretion[80-82]. Pre-treatment serum TNF-α concentrations correlate negatively with the clinical response to montelukast in pediatric FD in association with duodenal eosinophilia indicating that mediation by TNF-α may represent an alternative pathway for symptom generation in these patients. Although there are no controlled studies, anti-TNF-α antibody has been reported to be effective in a series of children with resistant eosinophil disease including patients with FD[83].
Eosinophils and/or mast cells exhibit a number of cell surface markers which also serve as potential therapeutic targets in blocking upstream activation. These have been well reviewed elsewhere[84]. However, there are two of these, IL-5 and IgE, which have been targeted in humans with gastrointestinal eosinophilia and, thus, warrant specific mention.
IL-5 serves to stimulate the expression of eosinophils. In general, most clinical studies evaluating anti-IL-5 antibodies have demonstrated decreases in eosinophil density but little clinical benefit[85]. There are limited reports on the use of anti-IL-5 in patients with gastrointestinal eosinophilia and none specifically in patients with FD. In a small pilot study of adults with eosinophilic gastroenteritis, a single dose of anti-IL-5 resulted in a 50%-70% decrease in mucosal eosinophil density in 3 of the 4 patients but with minimal symptom improvement[86]. The effect of anti-IL-5 on duodenal eosinophil density was assessed in 11 adult patients treated for eosinophilic esophagitis[87]. While esophageal density decreased significantly, there was no significant effect on duodenal eosinophil density. This may simply indicate that the normal physiologic duodenal eosinophil population is unaffected.
Anti-IgE antibody has also been evaluated in a small study of adults with eosinophilic gastroenteritis but not specifically in patients with FD. In an uncontrolled, open-label study of 9 patients, anti-IgE resulted in a non-statistically significant reduction in eosinophil density in the antrum (69%) and duodenum (59%)[88]. Symptoms significantly improved but improvement had no direct relation to the decrease in mucosal eosinophil density.
Mast cell stabilizers, including cromolyn and ketotifen, represent an attractive potential therapy given data implicating mast cells in the generation of dyspeptic symptoms as previously discussed. These agents inhibit the release of mast cell mediators and, consequently, their pathophysiologic effects.
There have been no adult studies on the use of mast cell stabilizers in patients with FD. Benefit has been demonstrated in adults with IBS where it is suggested that the response may be related to blocking allergic or immunologic reactions to foods[89-91]. In an open-label observational study of oral cromolyn in children with FD in association duodenal eosinophilia, resolution of pain was demonstrated in 89% of patients who had previously failed to respond to H2 and combined H1/H2 antagonism[92] .
Ketotifen, which antagonizes the H1-receptor, in addition to stabilizing mast cells has been shown to significantly decrease pain in adults with IBS and to increase the threshold for discomfort in patients with visceral hypersensitivity though this effect could not be correlated with pain improvement[93]. Whether the observed response to this drug is related to H1-receptor antagonism or mast cell stabilization is unclear.
In general, treatments directed at antagonizing the downstream effects of mediators released by mast cells and/or eosinophils are associated with a more rapid onset of action and fewer side effects. Therefore, they should probably be viewed as first line agents in treatment directed at mast cells and eosinophils in FD. It should be noted that the two most common downstream targets, histamine and leukotrienes, also have pro-inflammatory effects that may result in further upstream activation. Further, the simple experience of symptoms may cause physical and/or emotional stress that promotes upstream activation through the pathway previously described. Thus, addressing these downstream treatment targets may have direct effect on reducing symptoms in the short-term, while also indirectly serving to reduce activation of inflammatory cells in the long-term.
Acid reduction remains the most common treatment prescribed empirically by pediatric gastroenterologists for children with dyspepsia[1]. While there are numerous adult studies to support this practice, pediatric studies are limited. In adults, H2 antagonism has been shown to improve at least some symptoms associated with FD (abdominal pain, indigestion, belching, and gastroesophageal reflux symptoms) and appear to be superior to prokinetic medications and short term use of anxiolytics[94-97]. In adults with dyspepsia, proton pump inhibitors (PPIs) are superior to placebo in symptom reduction although this appears limited to patients with ulcer-like or reflux-like dyspepsia[98-101]. Whether PPIs are superior to H2 antagonism is not completely clear. Omeperazole was found to have a modest increase in efficacy as compared to ranitidine at 4 wk (51% vs 36%) but there was no benefit at 6 mo[97].
In children with abdominal pain, famotidine was superior to placebo in global improvement with clear benefit in those with dyspepsia[102]. In a large pediatric study, omeperazole was shown to have a very modest advantage in the relief of all symptoms as compared to either famotidine or ranitidine but there was no significant difference between the three with regard to resolution of abdominal pain, epigastric pain, nausea or vomiting[103].
Given the response to PPIs, it would appear that at least some of the clinical improvement from H2 antagonism or PPIs is related directly to acid suppression. A significant portion of responders may derive benefit from treatment of overlap GER or possibly from peptic gastritis or duodenitis, however, the benefit may also be due to limiting acid exposure in patients with acid hypersensitivity. With H2 antagonism, the benefit may also be unrelated to acid reduction as histamine has direct gastric myogenic actions, modulates afferent enteric nerve excitability, and acts as an immunomodulating agent[104-108]. H2 receptors affect not only acid secretion but influence neurotransmission and immune responses[47].
There may also be additional benefit from H1 antagonism. Combining an H1 antagonist with an H2 antagonist has been reported to relieve symptoms in 50% of children with FD in association with duodenal eosinophilia and in 79% of adults with FD in association with increased antral mast cell density who had previously failed to respond to acid reduction therapy[92,109]. H1 receptors have direct affects on smooth muscle contraction and visceral sensitivity[47]. Some benefit from H1 antagonism may also be due to an anxiolytic effect. Immune modulators, such as suplatast sodium, may also indirectly inhibit H1 receptor expression by suppressing IL-4 and IL-5 production from TH2 cells[110]. Shirai et al[111], reported successful treatment with suplatast sodium in an adult with eosinophilic gastroenteritis. It has not been specifically used in patients with FD.
H4 antagonists are currently in development and may represent a treatment option in the future. H4 receptors are abundant in the small intestine, largely on hematopoietic cells including eosinophils and mast cells, as well as endocrine cells[112,113]. H4 receptor activation results in eosinophil and mast cell chemotaxis (but not degranulation) as well as T cell cytokine production[112]. Current H1 antagonists do not inhibit H4 receptors but they do share common ligands[112].
CysLTs also are a potential downstream therapeutic target. The pattern of eosinophil degranulation in pediatric FD is consistent with the release of major basic protein, which is known to enhance the synthesis of cysLT. CysLT, in turn, stimulates smooth muscle contraction and recruitment of eosinophils[114]. CysLTs have been shown to alter mast cell function via induction of IL-5 and TNF-α production in primed mast cells, an effect blocked by cysLT inhibition[115]. Leukotrienes have the potential to increase intestinal sensory nerve sensitivity during inflammation as LT receptors are expressed on spinal nerve terminals and cysLTs have been shown to increase excitability of enteric neurons and to have a pro-contactile effect on the esophagus, stomach, small intestine, colon, and gallbladder[116-123].
In a double-blind, placebo-controlled, cross-over trial of children with FD in association with duodenal eosinophilia, montelukast, a cysLT receptor antagonist, was found to be superior to placebo with regard to relief of pain[124]. The response rate was 84% in patients with eosinophil density between 20 and 29/hpf as compared to a 42% response rate with placebo. This high response rate was confirmed in a second study which also determined that the short term positive clinical response was unrelated to a decrease in eosinophil density or activation[33]. This would suggest that the effect may be mediated through an enteric nerve effect on motility or sensitivity though that remains to be demonstrated. Other leukotriene antagonists (e.g., pranlukast, zafirlukast) have not been evaluated in FD or eosinophilic gastroenteritis.
Current evidence implicates gastric mast cells and duodenal eosinophils in the pathophysiology of FD and as mediators between psychologic and physiologic factors. Increased antral mast cell density is associated with anxiety, electromechanical dysfunction, and the PDS subtype of FD. Likewise, increased duodenal eosinophil density is associated with anxiety and the PDS subtype, however, effects on electromechanical function are more indirect.
While empirical data is limited, previous studies suggest that there may be efficacy in utilizing medications directed at mast cells and eosinophils. Most current data regarding treatment response consists of case series utilizing H1/H2 antagonists, mast cell stabilizers, and anti-TNF-α, as well as a controlled trial demonstrating clinical efficacy for the use of montelukast. Evidence exists to suggest that combining “anti-inflammatory” medications with other treatments targeting stress can improve the rate of symptom resolution in pediatric FD.
There remains a need for placebo-controlled trials of the various medications and other treatments targeting mast cells and eosinophils which have been suggested to have efficacy, either alone or in combination. Likewise, there is a need to better define the upstream and downstream mediators for both mast cells and eosinophils as potential therapeutic targets for future drug development or as potential targets for agents currently available, such as lipoxygenase inhibitors, prostaglandin synthetase inhibitors, or newer drugs targeting eosinophil adhesion or Siglec-8[125,126].
P- Reviewers: Abraham P, Brogna A S- Editor: Gou SX L- Editor: A E- Editor: Wang CH
| 1. | Schurman JV, Hunter HL, Friesen CA. Conceptualization and treatment of chronic abdominal pain in pediatric gastroenterology practice. J Pediatr Gastroenterol Nutr. 2010;50:32-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 39] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 2. | Chogle A, Dhroove G, Sztainberg M, Di Lorenzo C, Saps M. How reliable are the Rome III criteria for the assessment of functional gastrointestinal disorders in children? Am J Gastroenterol. 2010;105:2697-2701. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 29] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 3. | Walker LS, Lipani TA, Greene JW, Caines K, Stutts J, Polk DB, Caplan A, Rasquin-Weber A. Recurrent abdominal pain: symptom subtypes based on the Rome II Criteria for pediatric functional gastrointestinal disorders. J Pediatr Gastroenterol Nutr. 2004;38:187-191. [PubMed] |
| 4. | Schurman JV, Friesen CA, Danda CE, Andre L, Welchert E, Lavenbarg T, Cocjin JT, Hyman PE. Diagnosing functional abdominal pain with the Rome II criteria: parent, child, and clinician agreement. J Pediatr Gastroenterol Nutr. 2005;41:291-295. [PubMed] |
| 5. | Rasquin A, Di Lorenzo C, Forbes D, Guiraldes E, Hyams JS, Staiano A, Walker LS. Childhood functional gastrointestinal disorders: child/adolescent. Gastroenterology. 2006;130:1527-1537. [PubMed] |
| 6. | Tack J, Talley NJ, Camilleri M, Holtmann G, Hu P, Malagelada JR, Stanghellini V. Functional gastroduodenal disorders. Gastroenterology. 2006;130:1466-1479. [PubMed] |
| 7. | Schurman JV, Singh M, Singh V, Neilan N, Friesen CA. Symptoms and subtypes in pediatric functional dyspepsia: relation to mucosal inflammation and psychological functioning. J Pediatr Gastroenterol Nutr. 2010;51:298-303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 56] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 8. | Rippel SW, Acra S, Correa H, Vaezi M, Di Lorenzo C, Walker LS. Pediatric patients with dyspepsia have chronic symptoms, anxiety, and lower quality of life as adolescents and adults. Gastroenterology. 2012;142:754-761. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 31] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 9. | Aro P, Talley NJ, Agréus L, Johansson SE, Bolling-Sternevald E, Storskrubb T, Ronkainen J. Functional dyspepsia impairs quality of life in the adult population. Aliment Pharmacol Ther. 2011;33:1215-1224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 165] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 10. | Timmons S, Liston R, Moriarty KJ. Functional dyspepsia: motor abnormalities, sensory dysfunction, and therapeutic options. Am J Gastroenterol. 2004;99:739-749. [PubMed] |
| 11. | Oshima T, Okugawa T, Tomita T, Sakurai J, Toyoshima F, Watari J, Yamaguchi K, Fujimoto K, Adachi K, Kinoshita Y. Generation of dyspeptic symptoms by direct acid and water infusion into the stomachs of functional dyspepsia patients and healthy subjects. Aliment Pharmacol Ther. 2012;35:175-182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 38] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 12. | Pilichiewicz AN, Feltrin KL, Horowitz M, Holtmann G, Wishart JM, Jones KL, Talley NJ, Feinle-Bisset C. Functional dyspepsia is associated with a greater symptomatic response to fat but not carbohydrate, increased fasting and postprandial CCK, and diminished PYY. Am J Gastroenterol. 2008;103:2613-2623. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 108] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 13. | Friesen CA, Lin Z, Hyman PE, Andre L, Welchert E, Schurman JV, Cocjin JT, Burchell N, Pulliam S, Moore A. Electrogastrography in pediatric functional dyspepsia: relationship to gastric emptying and symptom severity. J Pediatr Gastroenterol Nutr. 2006;42:265-269. [PubMed] |
| 14. | Riezzo G, Chiloiro M, Guerra V, Borrelli O, Salvia G, Cucchiara S. Comparison of gastric electrical activity and gastric emptying in healthy and dyspeptic children. Dig Dis Sci. 2000;45:517-524. [PubMed] |
| 15. | Schurman JV, Friesen CA, Andre L, Welchert E, Lavenbarg T, Danda CE, Cocjin JT, Hyman PE. Diagnostic utility of the water load test in children with chronic abdominal pain. J Pediatr Gastroenterol Nutr. 2007;44:51-57. [PubMed] |
| 16. | Anderson JL, Acra S, Bruehl S, Walker LS. Relation between clinical symptoms and experimental visceral hypersensitivity in pediatric patients with functional abdominal pain. J Pediatr Gastroenterol Nutr. 2008;47:309-315. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 27] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 17. | Weston AP, Biddle WL, Bhatia PS, Miner PB. Terminal ileal mucosal mast cells in irritable bowel syndrome. Dig Dis Sci. 1993;38:1590-1595. [PubMed] |
| 18. | O’Sullivan M, Clayton N, Breslin NP, Harman I, Bountra C, McLaren A, O’Morain CA. Increased mast cells in the irritable bowel syndrome. Neurogastroenterol Motil. 2000;12:449-457. [PubMed] |
| 19. | Barbara G, Stanghellini V, De Giorgio R, Cremon C, Cottrell GS, Santini D, Pasquinelli G, Morselli-Labate AM, Grady EF, Bunnett NW. Activated mast cells in proximity to colonic nerves correlate with abdominal pain in irritable bowel syndrome. Gastroenterology. 2004;126:693-702. [PubMed] |
| 20. | Hall W, Buckley M, Crotty P, O’Morain CA. Gastric mucosal mast cells are increased in Helicobacter pylori-negative functional dyspepsia. Clin Gastroenterol Hepatol. 2003;1:363-369. [PubMed] |
| 21. | Choi MG, Park SJ, Lee SY, Cho YK, Park JM, Han HW, Oh JW, Lee IS, Chung IS. Association of psychological factors with activation of mucosal immune system in functional dyspepsia. Neurogastroenterol Motil. 2004;16:668. |
| 22. | Walker MM, Talley NJ, Prabhakar M, Pennaneac’h CJ, Aro P, Ronkainen J, Storskrubb T, Harmsen WS, Zinsmeister AR, Agreus L. Duodenal mastocytosis, eosinophilia and intraepithelial lymphocytosis as possible disease markers in the irritable bowel syndrome and functional dyspepsia. Aliment Pharmacol Ther. 2009;29:765-773. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 199] [Cited by in RCA: 207] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 23. | Friesen CA, Lin Z, Singh M, Singh V, Schurman JV, Burchell N, Cocjin JT, McCallum RW. Antral inflammatory cells, gastric emptying, and electrogastrography in pediatric functional dyspepsia. Dig Dis Sci. 2008;53:2634-2640. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 36] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 24. | Hou XH, Zhu L-R, Li QX, Chen JDZ. Alterations in mast cells and 5-HT positive cells in gastric mucosa in functional dyspepsia patients with hypersensitivity. Neurogastroenterol Motil. 2001;13:398-399. |
| 25. | Lowichik A, Weinberg AG. A quantitative evaluation of mucosal eosinophils in the pediatric gastrointestinal tract. Mod Pathol. 1996;9:110-114. [PubMed] |
| 26. | Kalach N, Huvenne H, Gosset P, Papadopoulos S, Dehecq E, Decoster A, Creusy C, Dupont C. Eosinophil counts in upper digestive mucosa of Western European children: variations with age, organs, symptoms, Helicobacter pylori status, and pathological findings. J Pediatr Gastroenterol Nutr. 2011;52:175-182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 26] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 27. | Erjefält JS, Greiff L, Andersson M, Adelroth E, Jeffery PK, Persson CG. Degranulation patterns of eosinophil granulocytes as determinants of eosinophil driven disease. Thorax. 2001;56:341-344. [PubMed] |
| 28. | Friesen CA, Andre L, Garola R, Hodge C, Roberts C. Activated duodenal mucosal eosinophils in children with dyspepsia: a pilot transmission electron microscopic study. J Pediatr Gastroenterol Nutr. 2002;35:329-333. [PubMed] |
| 29. | Talley NJ, Walker MM, Aro P, Ronkainen J, Storskrubb T, Hindley LA, Harmsen WS, Zinsmeister AR, Agréus L. Non-ulcer dyspepsia and duodenal eosinophilia: an adult endoscopic population-based case-control study. Clin Gastroenterol Hepatol. 2007;5:1175-1183. [PubMed] |
| 30. | Walker MM, Salehian SS, Murray CE, Rajendran A, Hoare JM, Negus R, Powell N, Talley NJ. Implications of eosinophilia in the normal duodenal biopsy - an association with allergy and functional dyspepsia. Aliment Pharmacol Ther. 2010;31:1229-1236. [PubMed] |
| 31. | Thakkar K, Chen L, Tatevian N, Shulman RJ, McDuffie A, Tsou M, Gilger MA, El-Serag HB. Diagnostic yield of oesophagogastroduodenoscopy in children with abdominal pain. Aliment Pharmacol Ther. 2009;30:662-669. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 36] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 32. | Kokkonen J, Ruuska T, Karttunen TJ, Niinimäki A. Mucosal pathology of the foregut associated with food allergy and recurrent abdominal pains in children. Acta Paediatr. 2001;90:16-21. [PubMed] |
| 33. | Friesen CA, Neilan NA, Schurman JV, Taylor DL, Kearns GL, Abdel-Rahman SM. Montelukast in the treatment of duodenal eosinophilia in children with dyspepsia: effect on eosinophil density and activation in relation to pharmacokinetics. BMC Gastroenterol. 2009;9:32. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 37] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 34. | Buhner S, Schemann M. Mast cell-nerve axis with a focus on the human gut. Biochim Biophys Acta. 2012;1822:85-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 111] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 35. | Woodruff SA, Masterson JC, Fillon S, Robinson ZD, Furuta GT. Role of eosinophils in inflammatory bowel and gastrointestinal diseases. J Pediatr Gastroenterol Nutr. 2011;52:650-661. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 70] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 36. | Ochi H, De Jesus NH, Hsieh FH, Austen KF, Boyce JA. IL-4 and -5 prime human mast cells for different profiles of IgE-dependent cytokine production. Proc Natl Acad Sci USA. 2000;97:10509-10513. [PubMed] |
| 37. | Santos J, Alonso C, Guilarte M, Vicario M, Malagelada JR. Targeting mast cells in the treatment of functional gastrointestinal disorders. Curr Opin Pharmacol. 2006;6:541-546. [PubMed] |
| 38. | Di Lorenzo C, Colletti RB, Lehmann HP, Boyle JT, Gerson WT, Hyams JS, Squires RH, Walker LS, Kanda PT. Chronic Abdominal Pain In Children: a Technical Report of the American Academy of Pediatrics and the North American Society for Pediatric Gastroenterology, Hepatology and Nutrition. J Pediatr Gastroenterol Nutr. 2005;40:249-261. [PubMed] |
| 39. | Schurman JV, Danda CE, Friesen CA, Hyman PE, Simon SD, Cocjin JT. Variations in psychological profile among children with recurrent abdominal pain. J Clin Psychol Med Settings. 2008;15:241-251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 30] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 40. | Friesen CA, Schurman JV, Qadeer A, Andre L, Welchert E, Cocjin J. Relationship between mucosal eosinophils and anxiety in pediatric dyspepsia. Gastroenterology. 2005;129:A-158. |
| 41. | Poirier GL, Shires KL, Sugden D, Amin E, Thomas KL, Carter DA, Aggleton JP. Anterior thalamic lesions produce chronic and profuse transcriptional de-regulation in retrosplenial cortex: A model of retrosplenial hypoactivity and covert pathology. Thalamus Relat Syst. 2008;4:59-77. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 47] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 42. | Chrousos GP. Stress, chronic inflammation, and emotional and physical well-being: concurrent effects and chronic sequelae. J Allergy Clin Immunol. 2000;106:S275-S291. [PubMed] |
| 43. | Wallon C, Söderholm JD. Corticotropin-releasing hormone and mast cells in the regulation of mucosal barrier function in the human colon. Ann N Y Acad Sci. 2009;1165:206-210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 44. | Zheng PY, Feng BS, Oluwole C, Struiksma S, Chen X, Li P, Tang SG, Yang PC. Psychological stress induces eosinophils to produce corticotrophin releasing hormone in the intestine. Gut. 2009;58:1473-1479. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 99] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 45. | He SH. Key role of mast cells and their major secretory products in inflammatory bowel disease. World J Gastroenterol. 2004;10:309-318. [PubMed] |
| 46. | Santos J, Saperas E, Nogueiras C, Mourelle M, Antolín M, Cadahia A, Malagelada JR. Release of mast cell mediators into the jejunum by cold pain stress in humans. Gastroenterology. 1998;114:640-648. [PubMed] |
| 47. | Coruzzi G, Adami M, Pozzoli C. Role of histamine H4 receptors in the gastrointestinal tract. Front Biosci (Schol Ed). 2012;4:226-239. [PubMed] |
| 48. | La JH, Yang IS. Peripheral CRF mediates visceral hypersensitivity by activating mucosal mast cells in IBS rats. Neurogastroenterol Motil. 2005;17:39. |
| 49. | Wood JD. Enteric neuroimmunophysiology and pathophysiology. Gastroenterology. 2004;127:635-657. [PubMed] |
| 50. | Larauche M. Novel insights in the role of peripheral corticotropin-releasing factor and mast cells in stress-induced visceral hypersensitivity. Neurogastroenterol Motil. 2012;24:201-205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 41] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 51. | Walker MM, Warwick A, Ung C, Talley NJ. The role of eosinophils and mast cells in intestinal functional disease. Curr Gastroenterol Rep. 2011;13:323-330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 63] [Article Influence: 4.5] [Reference Citation Analysis (1)] |
| 52. | Zanini B, Ricci C, Bandera F, Caselani F, Magni A, Laronga AM, Lanzini A. Incidence of post-infectious irritable bowel syndrome and functional intestinal disorders following a water-borne viral gastroenteritis outbreak. Am J Gastroenterol. 2012;107:891-899. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 125] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 53. | Saps M, Pensabene L, Di Martino L, Staiano A, Wechsler J, Zheng X, Di Lorenzo C. Post-infectious functional gastrointestinal disorders in children. J Pediatr. 2008;152:812-816, 816.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 97] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 54. | Hanevik K, Dizdar V, Langeland N, Hausken T. Development of functional gastrointestinal disorders after Giardia lamblia infection. BMC Gastroenterol. 2009;9:27. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 106] [Cited by in RCA: 117] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 55. | Mearin F. Postinfectious functional gastrointestinal disorders. J Clin Gastroenterol. 2011;45 Suppl:S102-S105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 56. | Li X, Chen H, Lu H, Li W, Chen X, Peng Y, Ge Z. The study on the role of inflammatory cells and mediators in post-infectious functional dyspepsia. Scand J Gastroenterol. 2010;45:573-581. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 49] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 57. | Lan L, Yu J, Chen YL, Zhong YL, Zhang H, Jia CH, Yuan Y, Liu BW. Symptom-based tendencies of Helicobacter pylori eradication in patients with functional dyspepsia. World J Gastroenterol. 2011;17:3242-3247. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 25] [Reference Citation Analysis (0)] |
| 58. | Jin X, Li YM. Systematic review and meta-analysis from Chinese literature: the association between Helicobacter pylori eradication and improvement of functional dyspepsia. Helicobacter. 2007;12:541-546. [PubMed] |
| 59. | Gwee KA, Teng L, Wong RK, Ho KY, Sutedja DS, Yeoh KG. The response of Asian patients with functional dyspepsia to eradication of Helicobacter pylori infection. Eur J Gastroenterol Hepatol. 2009;21:417-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 60] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 60. | Mazzoleni LE, Sander GB, Francesconi CF, Mazzoleni F, Uchoa DM, De Bona LR, Milbradt TC, Von Reisswitz PS, Berwanger O, Bressel M. Helicobacter pylori eradication in functional dyspepsia: HEROES trial. Arch Intern Med. 2011;171:1929-1936. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 113] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 61. | Ford AC, Delaney BC, Forman D, Moayyedi P. Eradication therapy for peptic ulcer disease in Helicobacter pylori positive patients. Cochrane Database Syst Rev. 2006;19:CD003840. [PubMed] |
| 62. | Sugano K. Should we still subcategorize helicobacter pylori-associated dyspepsia as functional disease? J Neurogastroenterol Motil. 2011;17:366-371. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 38] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 63. | Ashorn M, Ruuska T, Karikoski R, Välipakka J, Mäki M. Gastric mucosal cell densities in Helicobacter pylori-positive and -negative dyspeptic children and healthy controls. J Pediatr Gastroenterol Nutr. 1994;18:146-151. [PubMed] |
| 64. | Hofman V, Lassalle S, Selva E, Kalem K, Steff A, Hébuterne X, Sicard D, Auberger P, Hofman P. Involvement of mast cells in gastritis caused by Helicobacter pylori: a potential role in epithelial cell apoptosis. J Clin Pathol. 2007;60:600-607. [PubMed] |
| 65. | Moorchung N, Srivastava AN, Gupta NK, Malaviya AK, Achyut BR, Mittal B. The role of mast cells and eosinophils in chronic gastritis. Clin Exp Med. 2006;6:107-114. [PubMed] |
| 66. | Aydemir SA, Tekin IO, Numanoglu G, Borazan A, Ustundag Y. Eosinophil infiltration, gastric juice and serum eosinophil cationic protein levels in Helicobacter pylori-associated chronic gastritis and gastric ulcer. Mediators Inflamm. 2004;13:369-372. [PubMed] |
| 67. | Saps M, Lu P, Bonilla S. Cow’s-milk allergy is a risk factor for the development of FGIDs in children. J Pediatr Gastroenterol Nutr. 2011;52:166-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 72] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 68. | Schäppi MG, Borrelli O, Knafelz D, Williams S, Smith VV, Milla PJ, Lindley KJ. Mast cell-nerve interactions in children with functional dyspepsia. J Pediatr Gastroenterol Nutr. 2008;47:472-480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 45] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 69. | Neilan NA, Dowling PJ, Taylor DL, Ryan P, Schurman JV, Friesen CA. Useful biomarkers in pediatric eosinophilic duodenitis and their existence: a case-control, single-blind, observational pilot study. J Pediatr Gastroenterol Nutr. 2010;50:377-384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 70. | Magnusson J, Lin XP, Dahlman-Höglund A, Hanson L LA, Telemo E, Magnusson O, Bengtsson U, Ahlstedt S. Seasonal intestinal inflammation in patients with birch pollen allergy. J Allergy Clin Immunol. 2003;112:45-50. [PubMed] |
| 71. | Humphreys PA, Gevirtz RN. Treatment of recurrent abdominal pain: components analysis of four treatment protocols. J Pediatr Gastroenterol Nutr. 2000;31:47-51. [PubMed] |
| 72. | Schurman JV, Wu YP, Grayson P, Friesen CA. A pilot study to assess the efficacy of biofeedback-assisted relaxation training as an adjunct treatment for pediatric functional dyspepsia associated with duodenal eosinophilia. J Pediatr Psychol. 2010;35:837-847. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 22] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 73. | Edsbäcker S, Andersson T. Pharmacokinetics of budesonide (Entocort EC) capsules for Crohn’s disease. Clin Pharmacokinet. 2004;43:803-821. [PubMed] |
| 74. | Margantinis G, Sakorafas GH, Kostopoulos P, Kontou S, Tsiakos S, Arvanitidis D. Post-ERCP/endoscopic sphincterotomy duodenal perforation is not always a surgical emergency. Dig Liver Dis. 2006;38:434-436. [PubMed] |
| 75. | Elsing C, Placke J, Gross-Weege W. Budesonide for the treatment of obstructive eosinophilic jejunitis. Z Gastroenterol. 2007;45:187-189. [PubMed] |
| 76. | Shahzad G, Moise D, Lipka S, Rizvon K, Mustacchia PJ. Eosinophilic enterocolitis diagnosed by means of upper endoscopy and colonoscopy with random biopsies treated with budenoside: a case report and review of the literature. ISRN Gastroenterol. 2011;2011:608901. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 77. | Bischoff SC, Lorentz A, Schwengberg S, Weier G, Raab R, Manns MP. Mast cells are an important cellular source of tumour necrosis factor alpha in human intestinal tissue. Gut. 1999;44:643-652. [PubMed] |
| 78. | Thomas PS, Heywood G. Effects of inhaled tumour necrosis factor alpha in subjects with mild asthma. Thorax. 2002;57:774-778. [PubMed] |
| 79. | Liu LY, Bates ME, Jarjour NN, Busse WW, Bertics PJ, Kelly EA. Generation of Th1 and Th2 chemokines by human eosinophils: evidence for a critical role of TNF-alpha. J Immunol. 2007;179:4840-4848. [PubMed] |
| 80. | Deveci F, Muz MH, Ilhan N, Kirkil G, Turgut T, Akpolat N. Evaluation of the anti-inflammatory effect of infliximab in a mouse model of acute asthma. Respirology. 2008;13:488-497. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 32] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 81. | Maillet I, Schnyder-Candrian S, Couillin I, Quesniaux VF, Erard F, Moser R, Fleury S, Kanda A, Dombrowicz D, Szymkowski DE. Allergic lung inflammation is mediated by soluble tumor necrosis factor (TNF) and attenuated by dominant-negative TNF biologics. Am J Respir Cell Mol Biol. 2011;45:731-739. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 82. | Mo JH, Kang EK, Quan SH, Rhee CS, Lee CH, Kim DY. Anti-tumor necrosis factor-alpha treatment reduces allergic responses in an allergic rhinitis mouse model. Allergy. 2011;66:279-286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 59] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 83. | Turner D, Wolters VM, Russell RK, Shakhnovich V, Muise AM, Ledder O, Ngan B, Friesen C. Anti-TNF, infliximab, and adalimumab can be effective in eosinophilic bowel disease. J Pediatr Gastroenterol Nutr. 2013;56:492-497. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 24] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 84. | Wechsler ME, Fulkerson PC, Bochner BS, Gauvreau GM, Gleich GJ, Henkel T, Kolbeck R, Mathur SK, Ortega H, Patel J. Novel targeted therapies for eosinophilic disorders. J Allergy Clin Immunol. 2012;130:563-571. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 77] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 85. | Molfino NA, Gossage D, Kolbeck R, Parker JM, Geba GP. Molecular and clinical rationale for therapeutic targeting of interleukin-5 and its receptor. Clin Exp Allergy. 2012;42:712-737. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 167] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 86. | Jawairia M, Shahzad G, Mustacchia P. Eosinophilic gastrointestinal diseases: review and update. ISRN Gastroenterol. 2012;2012:463689. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 22] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 87. | Conus S, Straumann A, Bettler E, Simon HU. Mepolizumab does not alter levels of eosinophils, T cells, and mast cells in the duodenal mucosa in eosinophilic esophagitis. J Allergy Clin Immunol. 2010;126:175-177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 49] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 88. | Foroughi S, Foster B, Kim N, Bernardino LB, Scott LM, Hamilton RG, Metcalfe DD, Mannon PJ, Prussin C. Anti-IgE treatment of eosinophil-associated gastrointestinal disorders. J Allergy Clin Immunol. 2007;120:594-601. [PubMed] |
| 89. | Lunardi C, Bambara LM, Biasi D, Cortina P, Peroli P, Nicolis F, Favari F, Pacor ML. Double-blind cross-over trial of oral sodium cromoglycate in patients with irritable bowel syndrome due to food intolerance. Clin Exp Allergy. 1991;21:569-572. [PubMed] |
| 90. | Stefanini GF, Prati E, Albini MC, Piccinini G, Capelli S, Castelli E, Mazzetti M, Gasbarrini G. Oral disodium cromoglycate treatment on irritable bowel syndrome: an open study on 101 subjects with diarrheic type. Am J Gastroenterol. 1992;87:55-57. [PubMed] |
| 91. | Stefanini GF, Saggioro A, Alvisi V, Angelini G, Capurso L, di Lorenzo G, Dobrilla G, Dodero M, Galimberti M, Gasbarrini G. Oral cromolyn sodium in comparison with elimination diet in the irritable bowel syndrome, diarrheic type. Multicenter study of 428 patients. Scand J Gastroenterol. 1995;30:535-541. [PubMed] |
| 92. | Friesen CA, Sandridge L, Andre L, Roberts CC, Abdel-Rahman SM. Mucosal eosinophilia and response to H1/H2 antagonist and cromolyn therapy in pediatric dyspepsia. Clin Pediatr (Phila). 2006;45:143-147. [PubMed] |
| 93. | Klooker TK, Braak B, Koopman KE, Welting O, Wouters MM, van der Heide S, Schemann M, Bischoff SC, van den Wijngaard RM, Boeckxstaens GE. The mast cell stabiliser ketotifen decreases visceral hypersensitivity and improves intestinal symptoms in patients with irritable bowel syndrome. Gut. 2010;59:1213-1221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 268] [Cited by in RCA: 303] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 94. | Kato M, Watanabe M, Konishi S, Kudo M, Konno J, Meguro T, Kitamori S, Nakagawa S, Shimizu Y, Takeda H. Randomized, double-blind, placebo-controlled crossover trial of famotidine in patients with functional dyspepsia. Aliment Pharmacol Ther. 2005;21 Suppl 2:27-31. [PubMed] |
| 95. | Amini M, Ghamar Chehreh ME, Khedmat H, Valizadegan G, Babaei M, Darvishi A, Taheri S. Famotidine in the treatment of functional dyspepsia: a randomized double-blind, placebo-controlled trial. J Egypt Public Health Assoc. 2012;87:29-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 96. | Seno H, Nakase H, Chiba T. Usefulness of famotidine in functional dyspepsia patient treatment: comparison among prokinetic, acid suppression and antianxiety therapies. Aliment Pharmacol Ther. 2005;21 Suppl 2:32-36. [PubMed] |
| 97. | Veldhuyzen van Zanten SJ, Chiba N, Armstrong D, Barkun A, Thomson A, Smyth S, Escobedo S, Lee J, Sinclair P. A randomized trial comparing omeprazole, ranitidine, cisapride, or placebo in helicobacter pylori negative, primary care patients with dyspepsia: the CADET-HN Study. Am J Gastroenterol. 2005;100:1477-1488. [PubMed] |
| 98. | Wang WH, Huang JQ, Zheng GF, Xia HH, Wong WM, Liu XG, Karlberg J, Wong BC. Effects of proton-pump inhibitors on functional dyspepsia: a meta-analysis of randomized placebo-controlled trials. Clin Gastroenterol Hepatol. 2007;5:178-185; quiz 140. [PubMed] |
| 99. | Talley NJ, Meineche-Schmidt V, Paré P, Duckworth M, Räisänen P, Pap A, Kordecki H, Schmid V. Efficacy of omeprazole in functional dyspepsia: double-blind, randomized, placebo-controlled trials (the Bond and Opera studies). Aliment Pharmacol Ther. 1998;12:1055-1065. [PubMed] |
| 100. | Peura DA, Kovacs TO, Metz DC, Siepman N, Pilmer BL, Talley NJ. Lansoprazole in the treatment of functional dyspepsia: two double-blind, randomized, placebo-controlled trials. Am J Med. 2004;116:740-748. [PubMed] |
| 101. | van Rensburg C, Berghöfer P, Enns R, Dattani ID, Maritz JF, Gonzalez Carro P, Fischer R, Schwan T. Efficacy and safety of pantoprazole 20 mg once daily treatment in patients with ulcer-like functional dyspepsia. Curr Med Res Opin. 2008;24:2009-2018. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 102. | See MC, Birnbaum AH, Schechter CB, Goldenberg MM, Benkov KJ. Double-blind, placebo-controlled trial of famotidine in children with abdominal pain and dyspepsia: global and quantitative assessment. Dig Dis Sci. 2001;46:985-992. [PubMed] |
| 103. | Dehghani SM, Imanieh MH, Oboodi R, Haghighat M. The comparative study of the effectiveness of cimetidine, ranitidine, famotidine, and omeprazole in treatment of children with dyspepsia. ISRN Pediatr. 2011;2011:219287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 17] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 104. | Wood JD. Neuropathophysiology of irritable bowel syndrome. J Clin Gastroenterol. 2002;35:S11-S22. [PubMed] |
| 105. | Milenov K, Todorov S, Vassileva M, Zamfirova R, Shahbazian A. Interactions between histaminergic and cholinergic pathways of gastric motility regulation. Methods Find Exp Clin Pharmacol. 1996;18:33-39. [PubMed] |
| 106. | Izzo AA, Costa M, Mascolo N, Capasso F. The role of histamine H1, H2 and H3 receptors on enteric ascending synaptic transmission in the guinea pig ileum. J Pharmacol Exp Ther. 1998;287:952-957. [PubMed] |
| 107. | Jiang W, Kreis ME, Eastwood C, Kirkup AJ, Humphrey PP, Grundy D. 5-HT(3) and histamine H(1) receptors mediate afferent nerve sensitivity to intestinal anaphylaxis in rats. Gastroenterology. 2000;119:1267-1275. [PubMed] |
| 108. | Moharana AK, Bhattacharya SK, Mediratta PK, Sharma KK. Possible role of histamine receptors in the central regulation of immune responses. Indian J Physiol Pharmacol. 2000;44:153-160. [PubMed] |
| 109. | Matter SE, Bhatia PS, Miner PB. Evaluation of antral mast cells in nonulcer dyspepsia. Dig Dis Sci. 1990;35:1358-1363. [PubMed] |
| 110. | Shahriar M, Mizuguchi H, Maeyama K, Kitamura Y, Orimoto N, Horio S, Umehara H, Hattori M, Takeda N, Fukui H. Suplatast tosilate inhibits histamine signaling by direct and indirect down-regulation of histamine H1 receptor gene expression through suppression of histidine decarboxylase and IL-4 gene transcriptions. J Immunol. 2009;183:2133-2141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 31] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 111. | Shirai T, Hashimoto D, Suzuki K, Osawa S, Aonahata M, Chida K, Nakamura H. Successful treatment of eosinophilic gastroenteritis with suplatast tosilate. J Allergy Clin Immunol. 2001;107:924-925. [PubMed] |
| 112. | Thurmond RL, Gelfand EW, Dunford PJ. The role of histamine H1 and H4 receptors in allergic inflammation: the search for new antihistamines. Nat Rev Drug Discov. 2008;7:41-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 397] [Cited by in RCA: 397] [Article Influence: 23.4] [Reference Citation Analysis (0)] |
| 113. | Leurs R, Chazot PL, Shenton FC, Lim HD, de Esch IJ. Molecular and biochemical pharmacology of the histamine H4 receptor. Br J Pharmacol. 2009;157:14-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 99] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 114. | Holgate ST, Sampson AP. Antileukotriene therapy. Future directions. Am J Respir Crit Care Med. 2000;161:S147-S153. [PubMed] |
| 115. | Mellor EA, Austen KF, Boyce JA. Cysteinyl leukotrienes and uridine diphosphate induce cytokine generation by human mast cells through an interleukin 4-regulated pathway that is inhibited by leukotriene receptor antagonists. J Exp Med. 2002;195:583-592. [PubMed] |
| 116. | Liu S, Hu HZ, Gao N, Gao C, Wang G, Wang X, Peck OC, Kim G, Gao X, Xia Y. Neuroimmune interactions in guinea pig stomach and small intestine. Am J Physiol Gastrointest Liver Physiol. 2003;284:G154-G164. [PubMed] |
| 117. | Burakoff R, Nastos E, Won S, Percy WH. Comparison of the effects of leukotrienes B4 and D4 on distal colonic motility in the rabbit in vivo. Am J Physiol. 1989;257:G860-G864. [PubMed] |
| 118. | Freedman SM, Wallace JL, Shaffer EA. Characterization of leukotriene-induced contraction of the guinea-pig gallbladder in vitro. Can J Physiol Pharmacol. 1993;71:145-150. [PubMed] |
| 119. | Goldhill JM, Finkelman FD, Morris SC, Shea-Donohue T. Neural control of mouse small intestinal longitudinal muscle: interactions with inflammatory mediators. J Pharmacol Exp Ther. 1995;274:72-77. [PubMed] |
| 120. | Goldenberg MM, Subers EM. The effect of leukotriene D4 on the isolated stomach and colon of the rat. Life Sci. 1983;33:2121-2127. [PubMed] |
| 121. | Liu S, Hu HZ, Gao C, Gao N, Wang G, Wang X, Gao X, Xia Y, Wood JD. Actions of cysteinyl leukotrienes in the enteric nervous system of guinea-pig stomach and small intestine. Eur J Pharmacol. 2003;459:27-39. [PubMed] |
| 122. | Frieling T, Becker K, Rupprecht C, Dobreva G, Häussinger D, Schemann M. Leukotriene-evoked cyclic chloride secretion is mediated by enteric neuronal modulation in guinea-pig colon. Naunyn Schmiedebergs Arch Pharmacol. 1997;355:625-630. [PubMed] |
| 123. | Kim N, Cao W, Song IS, Kim CY, Sohn UD, Harnett KM, Biancani P. Leukotriene D4-induced contraction of cat esophageal and lower esophageal sphincter circular smooth muscle. Gastroenterology. 1998;115:919-928. [PubMed] |
| 124. | Friesen CA, Kearns GL, Andre L, Neustrom M, Roberts CC, Abdel-Rahman SM. Clinical efficacy and pharmacokinetics of montelukast in dyspeptic children with duodenal eosinophilia. J Pediatr Gastroenterol Nutr. 2004;38:343-351. [PubMed] |
| 125. | Baiula M, Bedini A, Carbonari G, Dattoli SD, Spampinato S. Therapeutic targeting of eosinophil adhesion and accumulation in allergic conjunctivitis. Front Pharmacol. 2012;3:203. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 126. | Kiwamoto T, Kawasaki N, Paulson JC, Bochner BS. Siglec-8 as a drugable target to treat eosinophil and mast cell-associated conditions. Pharmacol Ther. 2012;135:327-336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 144] [Article Influence: 11.1] [Reference Citation Analysis (0)] |