Published online Sep 5, 2025. doi: 10.4292/wjgpt.v16.i3.108257
Revised: May 6, 2025
Accepted: July 17, 2025
Published online: September 5, 2025
Processing time: 148 Days and 10.2 Hours
Alagille syndrome (ALGS) is a rare genetic disorder that affects the liver causing, cholestasis, jaundice and intractable pruritus which can significantly impair health-related quality of life (QoL). The treatment of liver involvement is mainly supportive with the majority eventually requiring liver transplant. Ileal bile acid transporter inhibition is a novel therapeutic concept for cholestatic pruritus and cholestatic liver disease. We report the effects of odevixibat treatment in a patient ALGS over 12 months.
A male patient presented with jaundice at 3 months. ALGS was suspected clini
This case demonstrates the use of ileal bile acid transporter inhibitors treating pruritus and improving patient QoL. It has potential to delay the need for liver transplantation. However more long-term studies are needed to help clinicians better understand the benefit of this new treatment.
Core Tip: Alagille syndrome is a rare genetic disorder that affects the liver causing, cholestasis, jaundice and intractable pruritus which can significantly impair health-related quality of life. The treatment is mainly supportive with majority eventually requiring liver transplant. Ileal bile acid transporter inhibition is a novel therapeutic concept for cholestatic pruritus and cholestatic liver disease. We report the effects of odevixibat treatment in a patient Alagille syndrome over 12 months. This case demonstrates the use of ileal bile acid transporter inhibitors treating pruritus and improving patient quality of life and biochemical parameters. This allowed for the delay in the need for liver transplantation. It has potential to delay the need for liver transplantation. However more long-term studies are needed to help clinicians better understand the benefit of this new treatment.
- Citation: Al Atrash E, Bitar R. Odevixibat improves pruritus and bile acid level in Alagille syndrome: A case report. World J Gastrointest Pharmacol Ther 2025; 16(3): 108257
- URL: https://www.wjgnet.com/2150-5349/full/v16/i3/108257.htm
- DOI: https://dx.doi.org/10.4292/wjgpt.v16.i3.108257
Alagille syndrome (ALGS) also known as Alagille-Watson syndrome, is a rare genetic disorder that primarily affects the liver, heart and other systems of the body. It was first described by Dr. Daniel Alagille in 1969[1]. This condition is typically inherited in an autosomal dominant manner, is caused by mutations in JAG1 or NOTCH2 genes, has variable presentation and may affect multiple organs[2]. The clinical phenotype varies within the same family, including facial abnormalities, hepatic (cholestasis, characterized by bile duct paucity on liver biopsy), skeletal (butterfly vertebrae), cardiac (primarily involving the pulmonary arteries), and ophthalmologic (posterior embryotoxon) abnormalities. One of the hallmark features of ALGS is cholestasis resulting in jaundice and intractable pruritus which can significantly impair health-related quality of life (QoL)[3].
Liver tests may manifest direct hyperbilirubinemia, transaminitis, elevated gamma-glutamyl transpeptidase levels and elevated serum bile acid levels. The treatment of liver involvement is mainly supportive, trying to improve severe pruritus with choleretic agents like ursodeoxycholic acid, naltrexone, rifampin, and cholestyramine. Surgical ileal exclusion through performing internal biliary diversion has been effective in preventing the progression of liver disease[4]. Ten to thirty percent of patients with ALGS will eventually need a liver transplant[5]. The progression of liver disease in patients with ALGS is usually slow allowing sufficient time for comprehensive evaluation and appropriate timing of liver transplantation, if needed. One of the primary indications for transplantation is impending hepatic failure, typically manifested by clinical signs such as worsening jaundice and the development of portal hypertension. This is particularly true if complicated by gastrointestinal bleeding, refractory hypersplenism or ascites unresponsive to conservative medical treatment. Additionally, non-life threatening but debilitating manifestations, such as severe pruritus or failure to thrive, may significantly impair the QoL and warrant liver transplantation[4,5].
Ileal bile acid transporter (IBAT) inhibition is a novel therapeutic concept for cholestatic pruritus and cholestatic liver disease. As of the time of writing this article, two IBAT inhibitors received Food and Drug Administration approval for the treatment of cholestasis pruritus in patients with ALGS[6]. Maralixibat is an orally-administered IBAT inhibitor. Maralixibat received its first approval in 2021, in the United States, for use in the treatment of cholestatic pruritus in patients with ALGS one year of age and older[7]. Odevixibat, a selective, reversible IBAT inhibitor, decreases enteric bile acid reuptake with minimal systemic exposure[8]. Odevixibat is indicated for treatment of pruritus in patients 3 months of age and older with progressive familial intrahepatic cholestasis in the United States and for patients ≥ 6 months old in the European Union, in 2021[9-11]. The Food and Drug Administration has approved odevixibat for the treatment of cholestatic pruritus in patients 12 months of age and older with ALGS in June 2023. We report the effects of odevixibat treatment over 16 months in a pediatric patient with genetic confirmation of ALGS who had severe pruritus and was planned to undergo liver transplantation.
A male patient born in September 2017, born at full term following uncomplicated pregnancy. At age of three months, he was noted to have jaundice by his pediatrician.
The patient had deep-set eyes, straight nose and pointed chin and no skeletal abnormalities. Further evaluation revealed cholestasis, pulmonary valve stenosis, ventricular septal defect, multicystic kidney dysplasia and posterior embryotoxon of both eyes. ALGS was highly suspected clinically. Genetic testing identified JAG1 mutation and ALGS diagnosis was confirmed. He was referred to our facility at age of 2 years for multidisciplinary care. Mother reported severe pruritus since the age of 18 months. Mother reported history of intense pruritus and patient was noted to have scratching of the face, ears, feet, and abdomen. The itching disturbed the child’s daily activity including sleeping. He was treated with ursodiol at 10 mg/kg three times a day as well as rifampin at 5 mg/kg twice daily without any significant clinical improvement he also was on fat soluble vitamin supplementation. At 3 years of age, he was prescribed cetirizine 5 mg once daily as needed for his persistent itching.
The patient had no past history of other illness.
There is no family history of liver disease or ALGS. Parents are non consanguineous.
The patient had deep-set eyes, straight nose and pointed chin and no skeletal abnormalities. Further evaluation revealed cholestasis, pulmonary valve stenosis, ventricular septal defect, multicystic kidney dysplasia and posterior embryotoxon of both eyes. ALGS was highly suspected clinically. Genetic testing identified JAG1 mutation and ALGS diagnosis was confirmed.
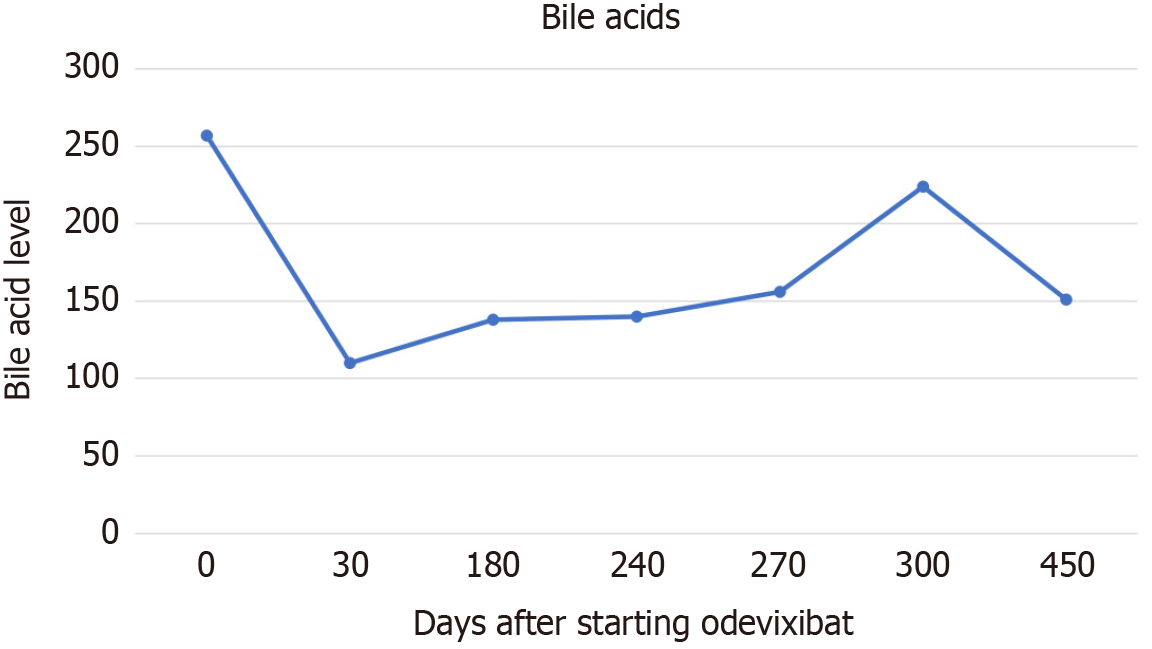
After 16 months of initiating treatment, he remained stable, no abdominal distention, abdominal pain or diarrhea reported. His liver enzymes, international normalized ratio, bile acids and bilirubin are described in Table 1.
| Blood test | Result before initiating treatment | Result 8 months after initiating treatment | Result 12 months after initiating treatment | Result 16 months after initiating treatment |
| Total bilirubin (normal range < 17 μmol/L) | 208.4 | 145.6 | 192 | 251 |
| Direct bilirubin (normal range < 5 μmol/L) | 192.2 | 135.5 | 172 | 214 |
| Alanine aminotransferase (normal range < 41 IU/L) | 183 | 113 | 146 | 194 |
| Aspartate aminotransferase (normal range < 41 IU/L) | 245 | 160 | 200 | 277 |
| Bile acids (normal range < 10 μmol/L) | 257 | 139 | 223 | 115 |
| International normalized ratio (normal range 0.7-1.1 seconds) | 1.3 | 1.2 | 1.2 | 1.2 |
| Albumin (normal range 35-52 g/L) | 31 | 35 | 35 | 37 |
| Platelet [normal range (140-400) × 109/L] | 182 | 328 | 267 | 165 |
There is none imaging examination.
The patient was ALGS.
The patient continued to be monitored and had persistent itching affecting the quality of his life, elevated serum bile acids, direct hyperbilirubinemia and transaminitis, blood workup was getting progressively worsening, and poor weight gain. Blood workup in May 2023 showed alanine aminotransferase 183 IU/L, aspartate aminotransferase 245 IU/L, gamma-glutamyl transpeptidase 122 IU/L, bilirubin total 208 μmol/L, direct bilirubin was 192 μmol/L, albumin was 31 g/L, international normalized ratio was 1.3 seconds, platelets were 188 × 109/L, bile acids level was 257.8 μmol/L with pediatric end-stage liver disease score of 15. Abdominal ultrasound June 2023 showed enlarged coarse liver, normal portal vein flow and massive splenomegaly measuring 14 cm in sagittal span (spleen size was 11.2 cm on previous scan 4 months earlier). In view of the increasing spleen size within 4 months and persistent itching affecting the patient QoL a discussion regarding the need for liver transplantation was initiated. However, family refused to proceed for liver transplantation due to social issues.
Odevixibat was trialed for the patient at initial dose of 40 μg/kg/day once a day in June 2023, increasing to 80 μg/kg/day in December 2023 and 120 μg/kg/day in March 2024. The dose was increased slowly to ameliorate gastrointestinal side effects. The patient itching improved very shortly after administration on the lower dose of 40 μg/kg/day, he was able to sleep overnight for the very first time after initiating treatment. The patients’ serum bile acids continued to drop over 16 months (Figure 1). However, serum bilirubin and alanine transferase improved initially but increased as the medication dose was increased. There was no reported side effect.
The Global Alagille Alliance is a multicenter global initiative involving 67 centers, they retrospectively collect data on children with ALGS. The study demonstrated that the 10- and 18-year native liver survival rates were 54.4% and 40.3%[12]. Therefore, the majority of patients with ALGS require a transplantation by 18 years of age. Pruritus is one of the most debilitating symptom in ALGS to a point where liver transplant is sometimes indicated to improve the patient QoL. A systematic review of ALGS reported pruritus to be present in 59%-88% with up to 45% having severe pruritus, the pruritus presented in the first 10 years of life. In the review, children with ALGS had significantly impaired health related QoL compared to controls and children with other diseases[3]. Our patient had severe pruritus with associated chronic liver disease. The patient’s pruritus was challenging and was treated with off-label medications such as rifampin and ursodeoxycholic acid. These medications were not successful in controlling pruritus. The first effective medication to improve the patient pruritus was the IBAT inhibitor which resulted in the cessation of pruritus and significant reduction in serum bile acid levels. Additionally, the patient was considered for transplantation for management of severe pruritus and deteriorating clinical condition, treatment with odevixibat improved the patient’s clinical and biochemical picture, and most importantly delayed the need for liver transplantation.
The first case report published on the use of odevixibat in a child with ALGS was in 2023[13]. The report describes the positive effect of odevixibat on a 4-year-old with ALGS in resolving pruritus and normalizing bile acid levels. We observed a good response to itching and drop in bile acid levels with no side effects in our patient, and odevixibat was well tolerated with no side effects. An additional noteworthy finding is that the patient was able to stop taking cetirizine but continued on rifampin and ursodeoxycholic acid. Data from a phase three double-blinded randomized placebo-controlled trial on 52 patients with ALGS treated with placebo or odevixibat supports our clinical findings. The study demonstrated significantly improved scratch score in the treatment group (P = 0.0024), and significantly improved bile acid level in the patients treated with odevixibat (P = 0.0012). Diarrhea was the most common adverse event seen in 29%[14].
This case reports real life clinical evidence on the benefit of odevixibat in ALGS by improving pruritus, bile acid levels and patient overall clinical condition. The disease burden is also highlighted. Pruritus was troublesome to the patient and affected his sleeping and QoL. Neither ursodeoxycholic acid nor rifampin succeeded in treating the pruritus. The patient tolerated odevixibat with no reported side effect. More data is still needed to understand the safety, the long-term effect of IBAT inhibitor on the progression of liver disease, and ability to avoid the need of liver transplantation in patients with cholestatic liver disease.
| 1. | Turnpenny PD, Ellard S. Alagille syndrome: pathogenesis, diagnosis and management. Eur J Hum Genet. 2012;20:251-257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 233] [Cited by in RCA: 274] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 2. | Spinner NB, Loomes KM, Krantz ID, Gilbert MA. Alagille Syndrome. 2000 May 19. In: GeneReviews® [Internet]. Seattle (WA): University of Washington, Seattle; 1993. [PubMed] |
| 3. | Kamath BM, Baker A, Houwen R, Todorova L, Kerkar N. Systematic Review: The Epidemiology, Natural History, and Burden of Alagille Syndrome. J Pediatr Gastroenterol Nutr. 2018;67:148-156. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 80] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 4. | Diaz-Frias J, Kondamudi NP. Alagille Syndrome. 2023 Aug 12. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025. [PubMed] |
| 5. | Alagille D, Odièvre M, Gautier M, Dommergues JP. Hepatic ductular hypoplasia associated with characteristic facies, vertebral malformations, retarded physical, mental, and sexual development, and cardiac murmur. J Pediatr. 1975;86:63-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 502] [Cited by in RCA: 414] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 6. | Baumann U, Sturm E, Lacaille F, Gonzalès E, Arnell H, Fischler B, Jørgensen MH, Thompson RJ, Mattsson JP, Ekelund M, Lindström E, Gillberg PG, Torfgård K, Soni PN. Effects of odevixibat on pruritus and bile acids in children with cholestatic liver disease: Phase 2 study. Clin Res Hepatol Gastroenterol. 2021;45:101751. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 51] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 7. | Shirley M. Maralixibat: First Approval. Drugs. 2022;82:71-76. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 30] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 8. | Graffner H, Gillberg PG, Rikner L, Marschall HU. The ileal bile acid transporter inhibitor A4250 decreases serum bile acids by interrupting the enterohepatic circulation. Aliment Pharmacol Ther. 2016;43:303-310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 78] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 9. | Riely CA. Familial intrahepatic cholestatic syndromes. Semin Liver Dis. 1987;7:119-133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 49] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 10. | European Medicines Agency. Summary of product characteristics. 2021. [cited 6 March 2021]. Available from: https://www.ema.europa.eu/en/documents/product-information/bylvay-epar-product-information_en.pdf. |
| 11. | Food and Drug Administration. Highlights of Prescribing Information of Bylvay, 2021. [cited 6 March 2021]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/215498s000lbl.pdf. |
| 12. | Vandriel SM, Li LT, She H, Wang JS, Gilbert MA, Jankowska I, Czubkowski P, Gliwicz-Miedzińska D, Gonzales EM, Jacquemin E, Bouligand J, Spinner NB, Loomes KM, Piccoli DA, D'Antiga L, Nicastro E, Sokal É, Demaret T, Ebel NH, Feinstein JA, Fawaz R, Nastasio S, Lacaille F, Debray D, Arnell H, Fischler B, Siew S, Stormon M, Karpen SJ, Romero R, Kim KM, Baek WY, Hardikar W, Shankar S, Roberts AJ, Evans HM, Jensen MK, Kavan M, Sundaram SS, Chaidez A, Karthikeyan P, Sanchez MC, Cavalieri ML, Verkade HJ, Lee WS, Squires JE, Hajinicolaou C, Lertudomphonwanit C, Fischer RT, Larson-Nath C, Mozer-Glassberg Y, Arikan C, Lin HC, Bernabeu JQ, Alam S, Kelly DA, Carvalho E, Ferreira CT, Indolfi G, Quiros-Tejeira RE, Bulut P, Calvo PL, Önal Z, Valentino PL, Desai DM, Eshun J, Rogalidou M, Dezsőfi A, Wiecek S, Nebbia G, Pinto RB, Wolters VM, Tamara ML, Zizzo AN, Garcia J, Schwarz K, Beretta M, Sandahl TD, Jimenez-Rivera C, Kerkar N, Brecelj J, Mujawar Q, Rock N, Busoms CM, Karnsakul W, Lurz E, Santos-Silva E, Blondet N, Bujanda L, Shah U, Thompson RJ, Hansen BE, Kamath BM; Global ALagille Alliance (GALA) Study Group. Natural history of liver disease in a large international cohort of children with Alagille syndrome: Results from the GALA study. Hepatology. 2023;77:512-529. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 43] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 13. | Ganschow R, Maucksch C. Odevixibat Treatment of Alagille Syndrome: A Case Report. JPGN Rep. 2023;4:e301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 14. | Ovchinsky N, Aumar M, Baker A, Baumann U, Bufler P, Cananzi M, Czubkowski P, Durmaz Ö, Fischer R, Indolfi G, Karnsakul WW, Lacaille F, Lee WS, Maggiore G, Rosenthal P, Ruiz M, Sokal E, Sturm E, van der Woerd W, Verkade HJ, Wehrman A, Clemson C, Yu Q, Ni Q, Ruvido J, Manganaro S, Mattsson JP. Efficacy and safety of odevixibat in patients with Alagille syndrome (ASSERT): a phase 3, double-blind, randomised, placebo-controlled trial. Lancet Gastroenterol Hepatol. 2024;9:632-645. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 14.0] [Reference Citation Analysis (0)] |