Published online Jun 5, 2025. doi: 10.4292/wjgpt.v16.i2.105375
Revised: March 19, 2025
Accepted: March 20, 2025
Published online: June 5, 2025
Processing time: 134 Days and 6.9 Hours
Anemia is a prevalent and challenging complication in patients with hematologic and solid malignancies, which stems from the direct effects of malignancy, treatment-induced toxicities, and systemic inflammation. It affects patients’ survival, functional status, and quality of life profoundly. Recent literature has highlighted the emerging role of the gut microbiome in the pathogenesis of cancer-associated anemia. The gut microbiota, through its intricate interplay with iron metabolism, inflammatory pathways, and immune modulation, may either exacerbate or ameliorate anemia depending on its composition, and functional integrity. Dysbiosis, characterized by disruption in the gut microbial ecosystem, is very common in cancer patients. This microbial imbalance is implicated in anemia causation through diminished iron absorption, persistent low-grade inflammation, and suppression of erythropoiesis.
To consolidate current evidence regarding the interplay between gut microbiome and anemia in the setting of malignancies. It aims to provide a detailed exploration of the mechanistic links between dysbiosis and anemia, identifies unique challenges associated with various cancer types, and evaluates the efficacy of microbiome-focused therapies. Through this integrative approach, the review seeks to establish a foundation for innovative clinical strategies aimed at mitigating anemia and improving patient outcomes in oncology.
A literature search was performed using multiple databases, including Google Scholar, PubMed, Scopus, and Web of Science, using a combination of keywords and Boolean operators to refine results. Keywords included “cancer-associated anemia”, “gut microbiome”, “intestinal microbiota”, “iron metabolism”, “gut dysbiosis”, “short-chain fatty acids”, “hematopoiesis”, “probiotics”, “prebiotics”, and “fecal microbiota transplantation”. Articles published in English between 2000 and December 2024 were included, with a focus on contemporary and relevant findings.
Therapeutic strategies aimed at restoration of gut microbial homeostasis, such as probiotics, prebiotics, dietary interventions, and fecal microbiota transplantation (FMT), can inhibit anemia-causing pathways by enhancing microbial diversity, suppressing detrimental flora, reducing systemic inflammation and optimizing nutrient absorption.
Gut dysbiosis causes anemia and impairs response to chemotherapy in cancer patients. Microbiome-centered interventions, such as probiotics, prebiotics, dietary modifications, and FMT, have shown efficacy in restoring microbial balance, reducing inflammation, and enhancing nutrient bioavailability. Emerging approaches, including engineered probiotics and bacteriophage therapies, are promising precision-based, customizable solutions for various microbiome compositions and imbalances. Future research should focus on integrating microbiome-targeted strategies with established anemia therapies.
Core Tip: The gut microbiome is increasingly recognized as a pivotal factor in cancer-associated anemia, influencing its pathophysiology, clinical manifestations, and potential therapeutic strategies. Dysbiosis, or microbial imbalance, is common in cancer patients and exacerbates anemia through mechanisms such as impaired iron absorption, heightened systemic inflammation, and suppression of erythropoiesis. Emerging evidence highlights how gut microbiota modulates iron metabolism, inflammatory cytokine production, and immune responses, linking microbial health directly to anemia severity. Promising interventions, including probiotics, prebiotics, fecal microbiota transplantation, and dietary modifications, aim to restore microbial balance, optimize iron bioavailability, and reduce inflammation. As research advances, microbiome-targeted therapies could transform anemia management, integrating seamlessly with existing treatments and offering personalized solutions for oncology patients. Further studies are needed to refine these approaches and establish microbiome-based biomarkers for anemia prediction and therapeutic monitoring.
- Citation: Bangolo A, Amoozgar B, Habibi M, Simms E, Nagesh VK, Wadhwani S, Wadhwani N, Auda A, Elias D, Mansour C, Abbott R, Jebara N, Zhang L, Gill S, Ahmed K, Ip A, Goy A, Cho C. Exploring the gut microbiome’s influence on cancer-associated anemia: Mechanisms, clinical challenges, and innovative therapies. World J Gastrointest Pharmacol Ther 2025; 16(2): 105375
- URL: https://www.wjgnet.com/2150-5349/full/v16/i2/105375.htm
- DOI: https://dx.doi.org/10.4292/wjgpt.v16.i2.105375
Anemia is a common and debilitating complication in malignancy, affecting 30%-90% of patients depending on the cancer type, stage, and treatment regimen[1-3]. The etiology of anemia in cancer is multifactorial, encompassing systemic inflammation, bone marrow suppression, nutritional deficits, and direct tumor-mediated effects. Moreover, cancer therapies such as chemotherapy, radiation, and targeted treatments exacerbate anemia through their myelosuppressive effects[4-6]. Reduced hemoglobin levels compromises oxygen delivery to tissues, and results in fatigue, dyspnea, and an overall diminution in functional capacity and quality of life. While conventional interventions, such as erythropoiesis-stimulating agents, red blood cell transfusions, and iron supplementation remain mainstays of treatment, several limitations do exist, warranting development of alternative therapeutic strategies[7,8].
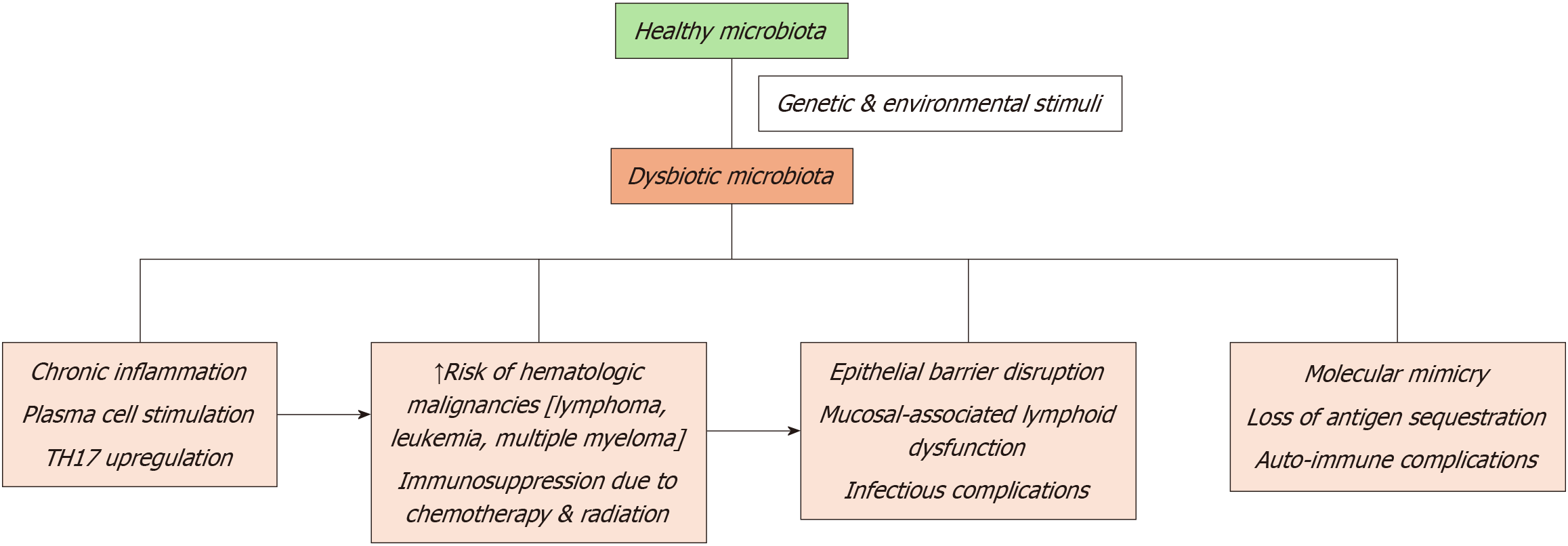
The gut microbiome has recently emerged as a significant contributor to optimal hematopoiesis and iron metabolism. The gastrointestinal tract houses trillions of microorganisms that produce bioactive metabolites, regulate nutrient absorption, and modulate immune and inflammatory pathways[9-11]. Cancer patients often experience gut dysbiosis, which is characterized by altered microbial diversity and abundance. Dysbiosis is frequently driven by chemotherapy, radiation, antibiotics, and dietary changes, and has profound implications on anemia[12,13]. Impaired iron absorption due to altered expression of critical gut-associated proteins, such as ferroportin and divalent metal transporter 1, promotes systemic inflammation through increased bacterial translocation and endotoxin production, and suppresses erythropoiesis through inflammatory cytokines[14,15].
Given the intricate relationship between the gut microbiome and cancer-associated anemia, microbiome-targeted interventions have garnered considerable attention. Strategies such as probiotics, prebiotics, and fecal microbiota transplantation (FMT) have demonstrated the potential to restore microbial diversity and mitigate systemic inflammation[16,17]. Emerging evidence further suggests that dietary modifications and microbiome-focused therapies may enhance iron bioavailability, reduce inflammation, and promote hematopoietic recovery in cancer patients[18,19]. This review integrates current evidence on the role of the gut microbiome in the pathophysiology of anemia associated with hematologic and solid malignancies, highlighting emerging therapeutic directions and laying the groundwork for innovative approaches to improve outcomes for patients with cancer.
This review was conducted using a systematic and comprehensive approach to identify, evaluate, and synthesize current evidence on the relationship between the gut microbiome and cancer-associated anemia. A literature search was performed using multiple databases, including Google Scholar, PubMed, Scopus, and Web of Science, to ensure broad and inclusive coverage. The search strategy incorporated a combination of keywords and Boolean operators to refine results. Keywords included “cancer-associated anemia”, “gut microbiome”, “intestinal microbiota”, “iron metabolism”, “gut dysbiosis”, “short-chain fatty acids”, “hematopoiesis”, “probiotics”, “prebiotics”, and “fecal microbiota transplantation”. Articles published in English between 2000 and December 2024 were included, with a focus on contemporary and relevant findings. This review was not registered, and no protocol was prepared prior.
The search process was conducted iteratively, with manual review of search results to identify additional studies through references cited in key articles. This snowballing technique ensured that no major studies were omitted from the review. The literature search and study selection were performed independently by multiple authors to minimize bias and ensure the reliability of results.
To ensure relevance and rigor, predefined inclusion and exclusion criteria were applied. Studies were included if they addressed the interplay between the gut microbiome and anemia in cancer patients, particularly regarding iron metabolism, systemic inflammation, immune modulation, or erythropoiesis. Research focusing on microbiome-targeted interventions, such as probiotics, prebiotics, dietary modifications, and FMT, was also prioritized. Preclinical studies, clinical trials, observational studies, and systematic reviews or meta-analyses with mechanistic or therapeutic insights were included.
Studies were excluded if they focused exclusively on anemia in non-cancer populations, lacked original data, or were limited to non-peer-reviewed formats such as editorials, opinion pieces, or conference abstracts. The selection process ensured that only high-quality and relevant studies were included for analysis.
A structured process was used to extract and synthesize data from the included studies. Titles and abstracts were screened first to assess relevance, followed by a full-text review of eligible articles. Data extraction focused on study characteristics, including study design, population characteristics, interventions, outcomes, and mechanistic insights into the gut microbiome’s role in anemia. Specific emphasis was placed on identifying how the gut microbiome influences key aspects of anemia pathophysiology, such as iron absorption, systemic inflammation, and immune regulation.
The extracted data were then synthesized to create a cohesive narrative. Studies were categorized thematically to explore mechanistic pathways, clinical challenges, and therapeutic interventions. For example, studies focusing on microbiome-induced changes in iron homeostasis or inflammation were grouped together, while research on interventions like probiotics and prebiotics was analyzed separately. Thematic synthesis enabled identification of common patterns and gaps in the literature, which were integrated into the discussion of findings.
This review relied solely on publicly available data from peer-reviewed studies and did not involve human or animal subjects. As a secondary research study, ethical approval was not required. The review adhered to the highest standards of scientific integrity, ensuring that all data sources were appropriately cited and credited.
The review was designed to systematically analyze the role of the gut microbiome in cancer-associated anemia from multiple perspectives, including its pathophysiology, clinical impact, and therapeutic potential. This multi-faceted framework enabled the integration of basic, translational, and clinical research findings into a comprehensive and actionable analysis. This methodology provided a robust foundation for synthesizing current evidence and formulating conclusions about the gut microbiome’s role in cancer-associated anemia. The rigorous approach ensured the inclusion of relevant, high-quality studies to inform the findings and recommendations presented in this review.
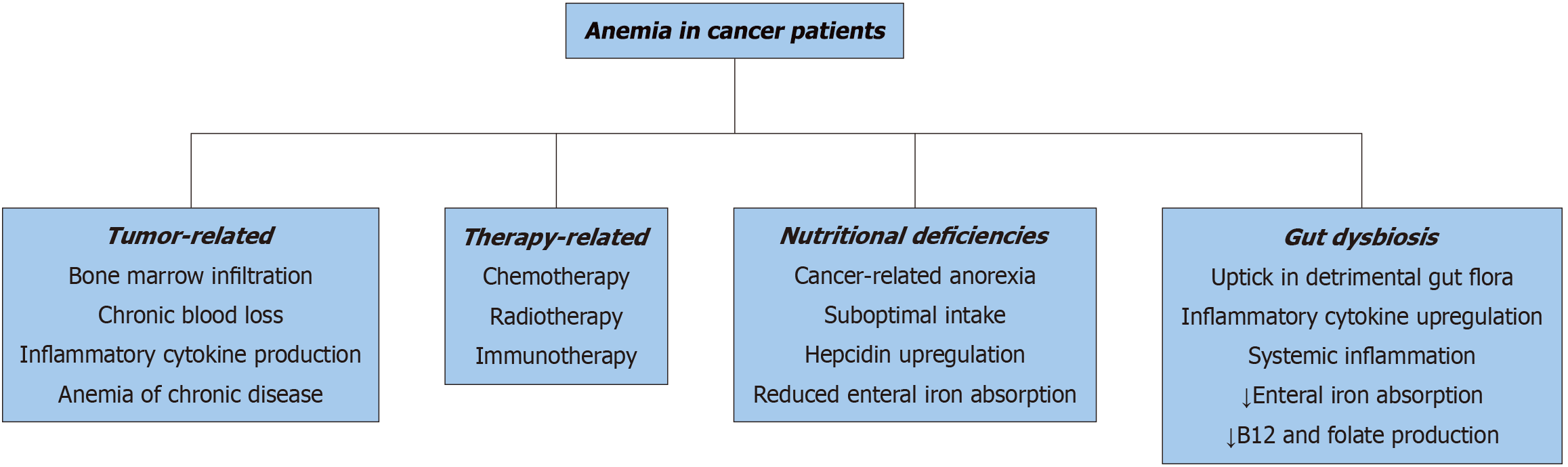
Anemia in cancer patients is the result of a complex interplay between tumor biology, therapeutic interventions, systemic inflammation, and nutritional deficits. Tumor-related mechanisms are significant contributors to anemia, particularly in cases where hematologic malignancies, such as leukemia and lymphoma, or metastatic solid tumors infiltrate the bone marrow, thereby impairing hematopoiesis[1,6,20]. Additionally, malignancies affecting the gastrointestinal or genitourinary (GU) systems often cause chronic blood loss. For instance, occult bleeding associated with colorectal and gastric cancers is a common driver of iron deficiency and subsequent anemia[2,21]. Furthermore, many tumors secrete pro-inflammatory cytokines, including tumor necrosis factor-alpha (TNF-α) and interleukin-6 (IL-6), which disrupt normal erythropoiesis by altering the bone marrow microenvironment and inducing anemia of chronic disease (ACD)[5,22].
The impact of cancer therapies on anemia cannot be overstated. Chemotherapy, particularly platinum-based compounds and alkylating agents, directly damages hematopoietic progenitor cells, resulting in myelosuppression and reduced red blood cell production[23,24]. Similarly, radiation therapy targeted at regions such as the pelvis, spine, or chest often leads to bone marrow hypoplasia, further exacerbating anemia[25,26]. Emerging treatment modalities, including immune checkpoint inhibitors (ICI) and other targeted therapies, also contribute to anemia, albeit through less direct mechanisms, such as autoimmune hemolysis or bone marrow failure[27,28]. While these therapies are pivotal in improving cancer control, their cumulative effects on anemia necessitate vigilant monitoring and proactive management to safeguard patient quality of life and outcomes[3,29].
Nutritional deficiencies represent another critical but often underappreciated factor in the pathogenesis of anemia among cancer patients. Chemotherapy and antibiotic-induced dysbiosis disrupt the gastrointestinal microbiome, im
Microcytic anemia: Microcytic anemia commonly arises from chronic blood loss or iron deficiency, both of which are prevalent in cancer patients due to tumor-associated bleeding or treatment-induced gastrointestinal toxicity[36-38]. Dysbiosis, a disruption of the gut microbiome, further exacerbates iron deficiency by impairing iron metabolism and absorption[38,39]. Beneficial gut microbes, such as Bifidobacterium and Lactobacillus, are integral in supporting intestinal iron uptake, producing short-chain fatty acids (SCFAs), and fostering an optimal gut environment for nutrient absorption[40,41]. When dysbiosis reduces these microbial populations, the delicate balance of iron homeostasis is disrupted, aggravating anemia[42,43]. Compounding this issue, inflammation-driven hepcidin overproduction—a process influenced by gut dysbiosis—blocks the release of iron from macrophages and suppresses intestinal iron absorption, creating a vicious cycle of iron depletion[44,45].
Normocytic anemia: Normocytic anemia, frequently encountered in cancer patients, is closely tied to ACD, which stems from systemic inflammation and immune dysregulation. Although often overlooked, the gut microbiome plays a significant role in this process. Dysbiosis can lead to a decrease in beneficial microbial metabolites, such as butyrate and other SCFAs, which are critical for regulating immune function[46,47]. This reduction contributes to an overproduction of inflammatory cytokines, including IL-6 and TNF-α, which suppress erythropoiesis and impair bone marrow function[48,49]. Moreover, gut barrier disruption due to dysbiosis allows endotoxins, such as lipopolysaccharides (LPS), to enter the systemic circulation, further amplifying inflammation and exacerbating ACD[50,51].
Macrocytic anemia: Macrocytic anemia results from deficiencies in folate or vitamin B12, both essential for DNA synthesis and red blood cell production. In cancer patients, these deficiencies can be attributed to chemotherapy-induced malabsorption, inadequate dietary intake, or gut microbiome imbalances[52,53]. Specific gut bacteria, including Bacteroides and Clostridium, play a critical role in synthesizing folate and vitamin B12[54,55]. Dysbiosis, by reducing the abundance of these microbial populations, diminishes the availability of these vital nutrients, thus worsening macrocytic anemia[56,57]. Chemotherapy further exacerbates this condition by damaging gut epithelial integrity, impairing the absorption of folate and vitamin B12, and contributing to anemia[58,59].
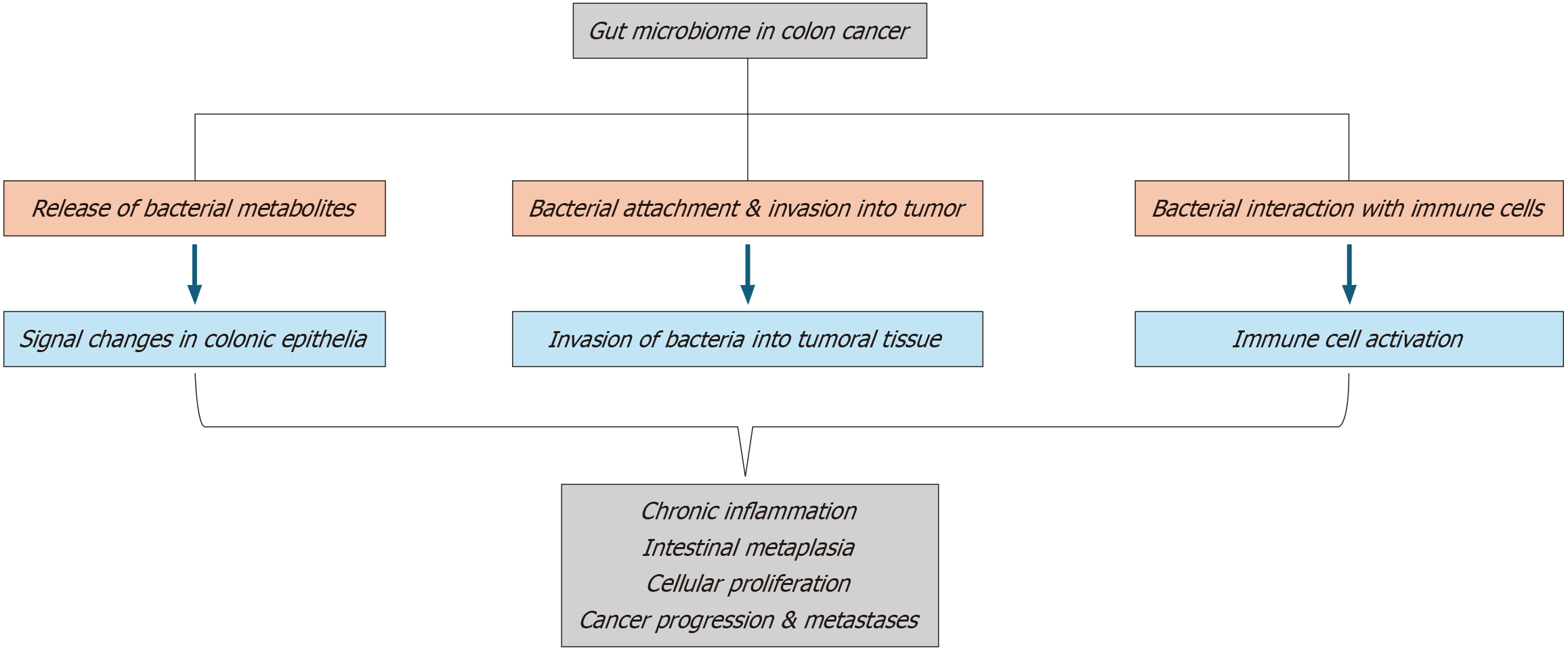
The gut microbiome is intricately involved in regulating iron metabolism, a key factor in the prevention and treatment of anemia. Specific bacterial species, such as Bacteroides, contribute to optimizing dietary iron absorption by creating a favorable gut environment and generating metabolites that enhance duodenal iron uptake[60,61]. However, a disruption in the balance of the gut microbiota, termed dysbiosis, compromises this process, leading to reduced iron bioavailability even when dietary intake is sufficient[62,63]. Furthermore, during dysbiosis, the competition between microbes for luminal iron exacerbates the host’s deficiency, amplifying the risk of microcytic anemia[64,65]. This disruption is particularly problematic in patients with malignancies, where cancer-associated inflammation and the gastrointestinal toxicity of chemotherapy further destabilize the gut microbiome, compounding iron malabsorption[66,67].
The gut microbiome also has significant influence over systemic inflammation, a central mechanism underlying anemia in malignancies. Dysbiosis increases levels of pro-inflammatory cytokines, including IL-6 and TNF-α, which suppress erythropoiesis by inhibiting erythropoietin production and inducing excessive hepcidin secretion[68-70]. Elevated hepcidin reduces iron release from macrophages and limits its absorption in the intestines, fueling anemia associated with chronic inflammation[71,72]. Compounding this issue, a weakened intestinal barrier during dysbiosis permits translocation of bacterial endotoxins, such as LPS, into systemic circulation. These endotoxins trigger immune activation and perpetuate the inflammatory cascade[71,72]. Cancer treatments, including chemotherapy, often intensify gut microbiome imbalances, creating a feedback loop of inflammation and anemia that is particularly challenging to interrupt[73,74].
Beyond iron metabolism and inflammation, the gut microbiome also plays a critical role in immune modulation, influencing hematopoiesis and anemia development. A healthy microbiome supports balanced T-cell differentiation and function, particularly through beneficial bacteria like Lactobacillus and Bifidobacterium, which promote the differentiation of regulatory T-cells that suppress inflammation and sustain hematopoietic stem cell (HSC) activity[75-78]. Dysbiosis, however, disrupts this equilibrium, pushing T-cell responses toward a pro-inflammatory phenotype that damages the bone marrow microenvironment and suppresses red blood cell production[79,80]. Additionally, microbial metabolites, such as butyrate, are essential for maintaining HSC quiescence and promoting erythropoiesis[81,82]. In states of dysbiosis, the depletion of these metabolites destabilizes hematopoiesis, further perpetuating anemia in cancer patients[83,84].
The multifaceted interplay between gut microbiota, iron metabolism, inflammation, and immune regulation highlights the importance of addressing dysbiosis in managing anemia, particularly in patients undergoing cancer treatment.
Head and neck cancers: Anemia frequently complicates head and neck cancers, driven by both the malignancy itself and its treatment regimens. Chronic blood loss and mucositis, particularly following chemoradiation, contribute to persistent anemia by impairing nutritional intake and promoting systemic inflammation[85,86]. Dysbiosis, characterized by an imbalance in the gut microbiota, further exacerbates this condition. Treatment-induced mucositis compromises the gut barrier, facilitating the translocation of microbial endotoxins into circulation and triggering heightened inflammatory responses[87,88]. Additionally, dysbiosis reduces the availability of essential nutrients, such as iron, folate, and vitamin B12, which are indispensable for erythropoiesis[89,90].
Chronic blood loss and iron deficiency are common in esophageal and gastric cancers, often resulting from tumor erosion of the gastrointestinal lining[91,92]. Reduced gastric acid production, either tumor-induced or following gastrectomy, disrupts gut microbiota composition, impairing dietary iron solubilization and absorption[93,94]. Dysbiosis worsens this imbalance, diminishing the microbiome's role in supporting iron metabolism. For instance, reductions in beneficial bacteria such as Bifidobacterium and Lactobacillus, which facilitate iron uptake, are frequently observed in gastric cancer patients, correlating with more severe anemia[95,96].
Anemia in pancreatic cancer arises from chronic inflammation, malnutrition, and systemic effects of the malignancy. Cachexia and exocrine insufficiency exacerbate deficiencies in nutrients critical for erythropoiesis, including iron, folate, and vitamin B12[97,98]. Dysbiosis amplifies this process, with microbial imbalances exacerbating systemic inflammation and impairing erythropoiesis[99,100]. Bangolo et al[101] highlight the role of inflammatory markers, including interleukins, in pancreatic cancer-associated anemia. These markers, modulated by the gut microbiota, represent potential therapeutic targets for alleviating anemia. Chemotherapy regimens, such as FOLFIRINOX, further complicate this scenario by inducing gastrointestinal toxicity and worsening dysbiosis[101,102].
Chronic bleeding and systemic inflammation are key drivers of anemia in colorectal and anal cancers[103,104]. The gut microbiota is profoundly affected, with significant reductions in beneficial bacteria such as Faecalibacterium prausnitzii and Blautia[105,106]. These microbial shifts promote a pro-inflammatory state, inhibiting erythropoiesis and exacerbating anemia[107,108]. Additionally, cancer therapies such as chemotherapy and radiation further aggravate gut dysbiosis, impairing nutrient absorption and intensifying anemia[109,110].
In GU cancers, encompassing bladder, kidney, and prostate malignancies, anemia arises from systemic inflammation, direct marrow suppression, and treatment-related toxicities[111,112]. Radiation targeting pelvic tumors frequently damages HSCs, while chemotherapy and ICI exacerbate myelosuppression. Dysbiosis in GU cancers further amplifies systemic inflammation, impairing erythropoiesis[113,114]. Reduced levels of anti-inflammatory bacteria, such as Akkermansia muciniphila, are associated with elevated inflammatory markers and worsened anemia in prostate cancer patients undergoing treatment[115].
Inflammation serves as a unifying mechanism linking cancer, dysbiosis, and anemia across solid malignancies. Pro-inflammatory cytokines such as IL-6 and TNF-α suppress erythropoiesis by reducing erythropoietin production and increasing hepcidin levels[116]. Dysbiosis exacerbates this inflammatory state by allowing microbial endotoxins, including LPS, to enter systemic circulation[117]. This creates a feedback loop where inflammation perpetuates dysbiosis, further impairing red blood cell production[118].
Dysbiosis contributes to anemia by disrupting the absorption of key nutrients essential for erythropoiesis. Beneficial bacterial species such as Bifidobacterium and Lactobacillus play pivotal roles in iron metabolism, while Bacteroides species are involved in folate production[119,120]. The loss of these bacteria in cancer patients leads to deficiencies in iron, folate, and vitamin B12, exacerbating anemia severity[121,122].
Cancer therapies, including chemotherapy, radiation, and ICI, significantly contribute to dysbiosis and its downstream effects. Damage to the gut epithelial barrier allows pathogenic bacteria to dominate the microbiome, intensifying systemic inflammation and anemia[74,123,124]. ICI, while effective in tumor control, further disrupt the microbiome, emphasizing the need for targeted interventions to manage therapy-induced anemia[125,126].
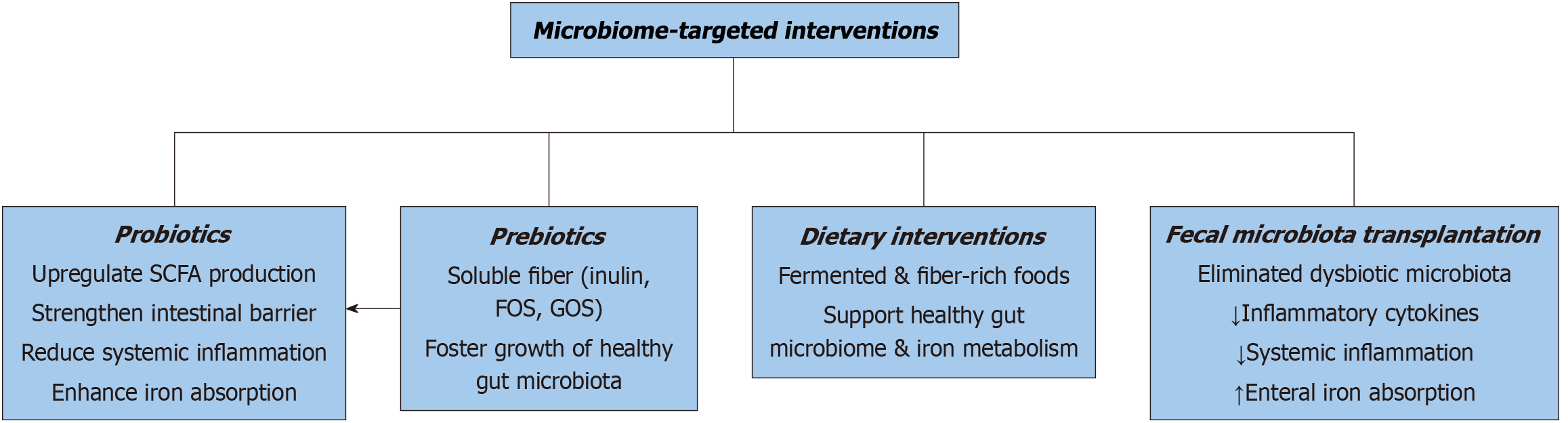
Emerging evidence underscores the potential of microbiome-targeted therapies to alleviate anemia in solid malignancies (Figures 2, 3 and 4). Probiotic supplementation with Lactobacillus rhamnosus and Bifidobacterium longum has been shown to reduce inflammation and improve iron absorption in cancer patients[127,128]. Prebiotics, including inulin and resistant starch, support the growth of beneficial bacteria, enhancing nutrient absorption and mitigating anemia severity[129,130]. FMT also holds promise as a therapeutic avenue, restoring microbial balance and improving hematopoiesis in cancer patients[131,132]. Recent findings further suggest that microbiome modulation, through dietary interventions or pharmacologic agents targeting gut inflammation, could complement existing therapies[133,134]. Additionally, ongoing research into personalized microbiome-based approaches is anticipated to refine treatment efficacy[135,136]. These interventions highlight the transformative potential of microbiome strategies in addressing anemia across oncologic settings[137].
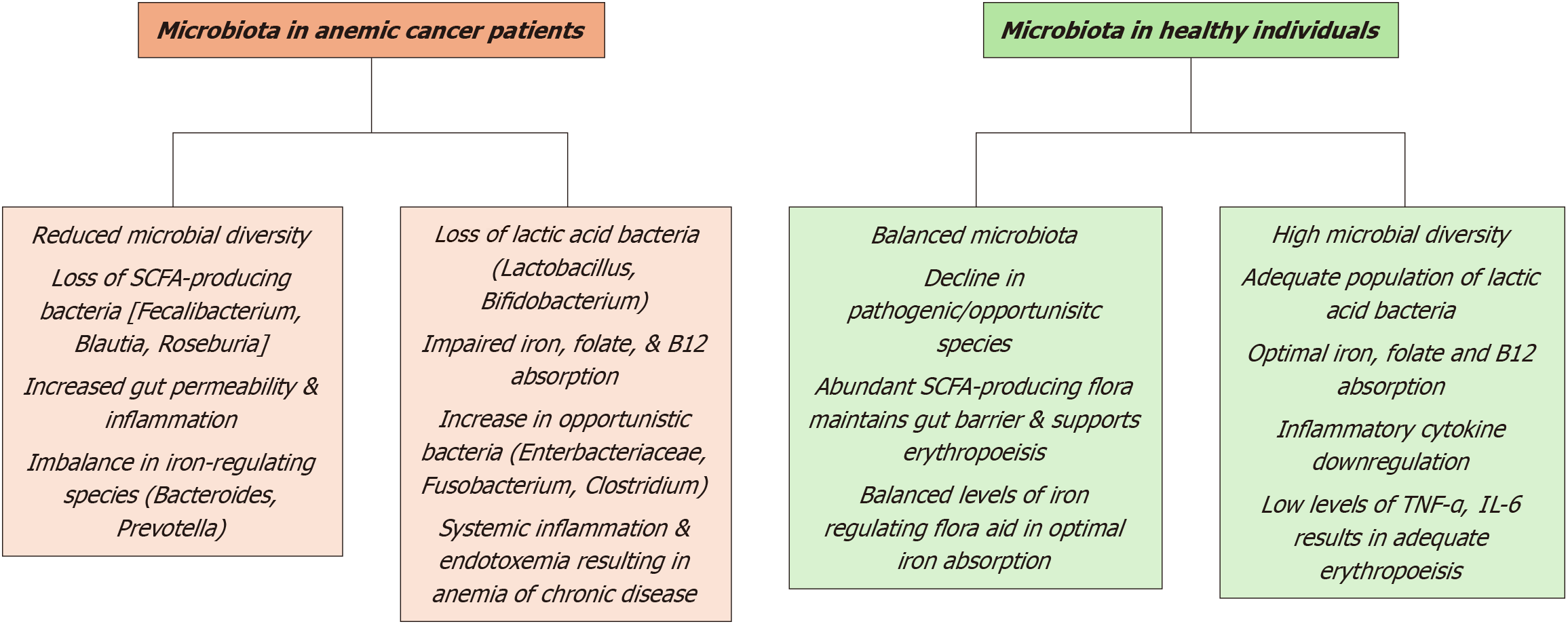
Anemia represents a significant clinical challenge in hematologic malignancies, including leukemias, lymphomas, and multiple myeloma. The pathophysiology is multifaceted, involving a combination of direct tumor effects, treatment-induced myelosuppression, and systemic inflammation. Emerging research highlights the gut microbiome's critical role in influencing anemia through its regulation of iron metabolism, inflammation, and hematopoiesis (Figure 5).
Anemia is a common complication in acute leukemias such as acute myeloid leukemia and acute lymphoblastic leukemia, primarily resulting from bone marrow infiltration by malignant cells and treatment-associated myelosuppression[138,139]. Chemotherapeutic agents like cytarabine and anthracyclines impair hematopoietic progenitor cell function, leading to disruptions in erythropoiesis[140,141]. Moreover, gut microbiome imbalances exacerbate hematologic deficiencies. Patients with reduced microbial diversity, particularly lower levels of commensal bacteria such as Faecalibacterium, exhibit delayed recovery of hematologic parameters, including prolonged neutropenia and anemia, post-induction chemo
In lymphomas, anemia is often mediated by systemic inflammation, which suppresses erythropoiesis through the overproduction of inflammatory cytokines[146,147]. Cytokines such as IL-6 and TNF-α interfere with erythropoietin signaling and promote iron sequestration through increased hepcidin levels[148,149]. Dysbiosis contributes to this inflammatory cascade by enabling gut barrier dysfunction and systemic translocation of microbial endotoxins, including LPS[150,151]. Elevated circulating LPS levels further stimulate cytokine production, compounding anemia[152,153]. Notably, patients with greater microbial diversity in the gut microbiome have demonstrated better responses to lymphoma therapies and improved hematologic profiles[154,155].
In multiple myeloma, anemia arises due to bone marrow infiltration by malignant plasma cells, impaired erythropoietin production, and resistance to erythropoietin signaling[156,157]. Dysbiosis in these patients is marked by disruptions in SCFAs, particularly butyrate and propionate, which are essential for immune and hematopoietic regulation[158,159]. Decreased SCFA levels are linked to heightened inflammation and reduced erythropoiesis[160]. Microbiota-derived metabolites also influence myeloma progression by modulating cytokine activity and T-cell differentiation[161]. Emerging preclinical studies suggest that restoring microbial balance using probiotics or FMT may improve anemia and potentially slow disease progression[162].
Targeting the gut microbiome represents a promising strategy for alleviating anemia in hematologic malignancies. Probiotic strains, including Lactobacillus and Bifidobacterium, have shown potential in restoring microbial diversity and enhancing hematologic recovery[163]. Prebiotics and dietary interventions aimed at supporting the growth of beneficial bacteria have demonstrated efficacy in reducing systemic inflammation and improving nutrient absorption (Figure 2). In more refractory cases, FMT is being investigated as a method to re-establish a healthy gut microbiome and support erythropoiesis. Although promising, these approaches require further clinical validation to optimize their application in managing anemia in this patient population[163,165].
FMT is categorized as a drug and a biologic by the Food and Drug Administration. Standardized protocols, involving rigorous donor screening, are followed to prevent transmission of infections, deleterious microbial metabolites, or antibiotic resistant pathogens to prospective recipients. Unlike synthetic microbial consortium, FMT inoculates varied microbial ecosystems into the recipients. Furthermore, there is limited, longitudinal data on its long-term safety, especially pertaining to inadvertent immune and metabolic sequelae. Systemic inflammation can drive instability in the recipient’s gut microbiome and may give rise to metabolic disorders which can take months to years to manifest. Undiagnosed disorders of iron metabolism or dysbiotic microbiome in the donor can be transmitted to the recipient through FMT. Lack of standardization in composition and efficacy of FMT inocula makes it harder to deduce anticipated benefits. Moreover, variability in patients’ microbiomes leads to heterogeneous treatment responses. Standard microbiome profiling techniques need to be developed. Additionally, data on the effect of gut microbiome on anemia in cancer patients is unclear (Figure 6). Well-powered randomised controlled trials need to be conducted to establish standardized evidence-based treatment guidelines. Microbiome-based diagnostics need to be explored in order to identify the subset of patients who can potentially benefit from this modality. Use of FMT in treatment of cancer-related anemia is largely experimental or off-label due to the absence of a fixed molecular composition, and unpredictable therapeutic effect. Therefore, regulatory bodies continue to scrutinize the efficacy of FMT in the realm of cancer-related anemia.
Microbiome variability has been shown to impact responsiveness to certain cancer therapeutics. For example, presence of Akkermansia muciniphila, Bifidobacterium, and Faecalibacterium prausnitzii correlates with increased responsiveness to ICI. Intuitively, dysbiotic microbiome often results in a suboptimal immunotherapy response. Identification of specific gut microbial signatures can help stratify patients prior to initiation of a particular therapy. For instance, low levels of Akkermansia muciniphila are predictive of suboptimal response to immunotherapy. Microbial modulation is recommended in these patients Studies have demonstrated that FMT from ICI responders to non-responders improves response to ICIs in the latter group. Gammaproteobacteria has been shown to reduce responsiveness to gemcitabine in pancreatic cancer patients. Also, SCFA-producing organisms have been shown to ameliorate chemotherapy-induced adverse effects, namely mucositis and diarrhea, in cancer patients. Symbiotic microbiome confers protection against radiation enteritis by maintaining epithelial integrity and curtailing systemic inflammation. Studies utilizing synthetic/engineered microbial consortia tailored to improve response to therapies are underway. Selective modulation of gut microbiota using CRISPR technology is being explored to enhance drug metabolism and efficacy. Nonetheless, several regulatory hurdles need to be addressed for approval of microbiome based interventions as adjunct therapies. Longitudinal data to ascertain the trajectory of gut microbiome and resultant treatment durability needs to be procured.
Microbiome overgrowth can also result in small intestinal bowel overgrowth, causing distressing gastrointestinal side effects. Microbiome based interventions, namely FMT, probiotics and live bacterial therapeutics must be utilized with caution in immunosuppressed patients. Probiotic sepsis, bacteremia, endotoxemia, and infections with multidrug resistant organisms can occur in immunocompromised recipients. Dysregulated immune responses with microbiome-based interventions can manifest in several ways. Probiotics can worsen graft-versus-host disease in transplant patients by T helper type 1 (TH1)/TH17 upregulation. FMT can seldom exacerbate the side effects of chemo/immunotherapy and/or cause a cytokine storm from excessive immune activation, worsening cancer-related inflammation. Owing to absence of regulatory oversight and quality control while utilizing live-bacterial therapies, postbiotics (SCFAs) and microbiome-derived compounds are deemed safer immuno-modulatory alternatives that do not pose serious infectious risks.
The evolving understanding of the interplay between the gut microbiome and anemia in hematologic malignancies provides a foundation for innovative therapeutic approaches. Future research should focus on integrating gut microbiome profiling into routine clinical practice to identify dysbiosis patterns unique to specific malignancies. Large-scale microbiome biomarker studies and prospective trials to assess the efficacy of microbiome-based therapies in anemia management need to be conducted. Current literature on this topic is extremely limited. Moreover, the data on dose-response relationship between prebiotic/probiotic supplementation and degree of anemia amelioration lacks granularity, and needs to be explored further. Development of machine learning (ML) algorithms can assist with analysis of complex microbiome-based data and subsequent creation of anemia risk prediction tools based on distinct microbial signatures. Supervised learning models can utilize microbial composition for anemia classification in cancer patients. Deep learning models can longitudinally analyze vast amounts of complex microbiome-anemia interactions, and scrutinize dynamic changes in treatment response and composition of iron regulating bacteria within the hosts. ML based models can combine data on microbiome sequencing, hosts’ dietary, laboratory and genomic markers to predict anemia severity, treatment response, and develop personalized dietary and microbiome-based approaches to address anemia. Personalized microbiome-based interventions, combined with standard oncologic therapies, could enhance hematologic outcomes, improve quality of life, and address the multifactorial nature of anemia in patients with hematologic cancers.
Anemia introduces significant challenges in the management of malignancies, amplifying the physical, psychological, and therapeutic burden of cancer care. Its impact extends beyond mere laboratory findings, affecting patient outcomes and complicating treatment strategies.
One of the most debilitating effects of anemia in cancer patients is its contribution to cancer-related fatigue, a condition that profoundly diminishes quality of life. Fatigue limits patients' ability to engage in daily activities, often leading to physical deconditioning and emotional distress[166]. Research underscores a direct correlation between hemoglobin levels and fatigue severity, illustrating how anemia exacerbates both physical and emotional challenges[167]. Moreover, the pervasive fatigue caused by anemia extends its impact beyond the patient, increasing caregiver strain and elevating healthcare resource demands[166-169].
The presence of anemia compromises the effectiveness and tolerability of cornerstone cancer therapies, including chemotherapy and radiotherapy. These treatments frequently worsen anemia through mechanisms such as myelosuppression and erythrocyte destruction[170]. In turn, anemia can necessitate dose reductions or delays in therapy, which may compromise oncologic outcomes[171]. Additionally, anemia-induced tumor hypoxia is a critical concern. Hypoxic conditions enhance tumor aggressiveness by promoting angiogenesis and invasion while diminishing the efficacy of therapies, particularly radiotherapy[172]. Tumor hypoxia decreases reactive oxygen species generation, thereby reducing radiation-induced cytotoxicity. This phenomenon is notably observed in malignancies like cervical and head-and-neck cancers, where anemia correlates with poor treatment responses and survival outcomes[170-175].
Anemia is increasingly recognized as an independent prognostic factor in oncology, with significant associations identified across various cancer types. Lower hemoglobin levels are linked to reduced overall survival and progression-free survival in both hematologic and solid malignancies[176,177]. Beyond cancer-specific outcomes, anemia exacerbates preexisting comorbid conditions, such as cardiovascular disease, by worsening tissue hypoxia. This contributes to elevated morbidity and mortality risks in affected patients[176-178].
The interplay between anemia and systemic inflammation perpetuates a complex cycle of fatigue, immune dysfunction, and resistance to erythropoietin-based therapies. Chronic inflammation, driven by cancer itself or its treatments, exacerbates erythropoietic suppression and disrupts iron homeostasis[179,180]. This emphasizes the need for comprehensive management strategies that address underlying mechanisms. Therapies aimed at modulating dysregulated iron metabolism and correcting gut microbiome imbalances have shown promise in mitigating anemia's multifactorial effects[181].
Probiotics and prebiotics: The potential of probiotics and prebiotics in addressing anemia lies in their ability to enhance gut microbial diversity, support SCFA production, and modulate systemic inflammation[182]. Probiotic strains such as Lactobacillus and Bifidobacterium are critical in maintaining gut barrier function and promoting iron absorption at the level of the duodenum. Clinical trials involving cancer patients undergoing chemotherapy have demonstrated the effectiveness of Lactobacillus rhamnosus GG in reducing gastrointestinal toxicity, indirectly supporting erythropoiesis and nutrient absorption[182-184]. Complementing this, prebiotics such as inulin and fructooligosaccharides serve as substrates for beneficial gut bacteria, fostering microbial activity and metabolite synthesis[185]. These effects are especially relevant in mitigating chemotherapy-induced dysbiosis, as prebiotics improve the microenvironment required for red blood cell production by reducing systemic inflammation[186].
Probiotics and prebiotics also show promise in countering the inflammatory pathways that impair erythropoiesis. Research has demonstrated that these agents can downregulate pro-inflammatory cytokines, particularly IL-6 and TNF-α, which are major contributors to iron metabolism dysregulation and erythropoietin resistance[187,188]. Specific strains, such as Bifidobacterium animalis subsp. lactis, have been linked to improved iron uptake and increased hemoglobin levels in anemic patients, offering a targeted therapeutic avenue[189-192].
FMT represents a novel therapeutic strategy for anemia, particularly in cases associated with gut dysbiosis. By transferring a healthy microbiome, FMT restores microbial diversity, enhances nutrient absorption, and modulates inflammatory responses that contribute to anemia[193,194]. This approach has shown promise in hematologic malignancies, where dysbiosis exacerbates systemic inflammation and suppresses erythropoiesis[193,194]. A case report involving refractory ACD illustrated the potential of FMT to normalize hemoglobin levels and reduce dependence on erythropoiesis-stimulating agents[195].
Emerging research suggests that the benefits of FMT extend to regulating the gut-liver axis, a critical pathway in iron metabolism and red blood cell production[196,197]. Preclinical studies have highlighted FMT's ability to enhance populations of iron-assimilating bacteria such as Bacteroides and Faecalibacterium, further supporting its role in addressing cancer-related anemia[198,199]. These findings underscore the potential of FMT as a therapeutic option, particularly for patients with treatment-resistant anemia.
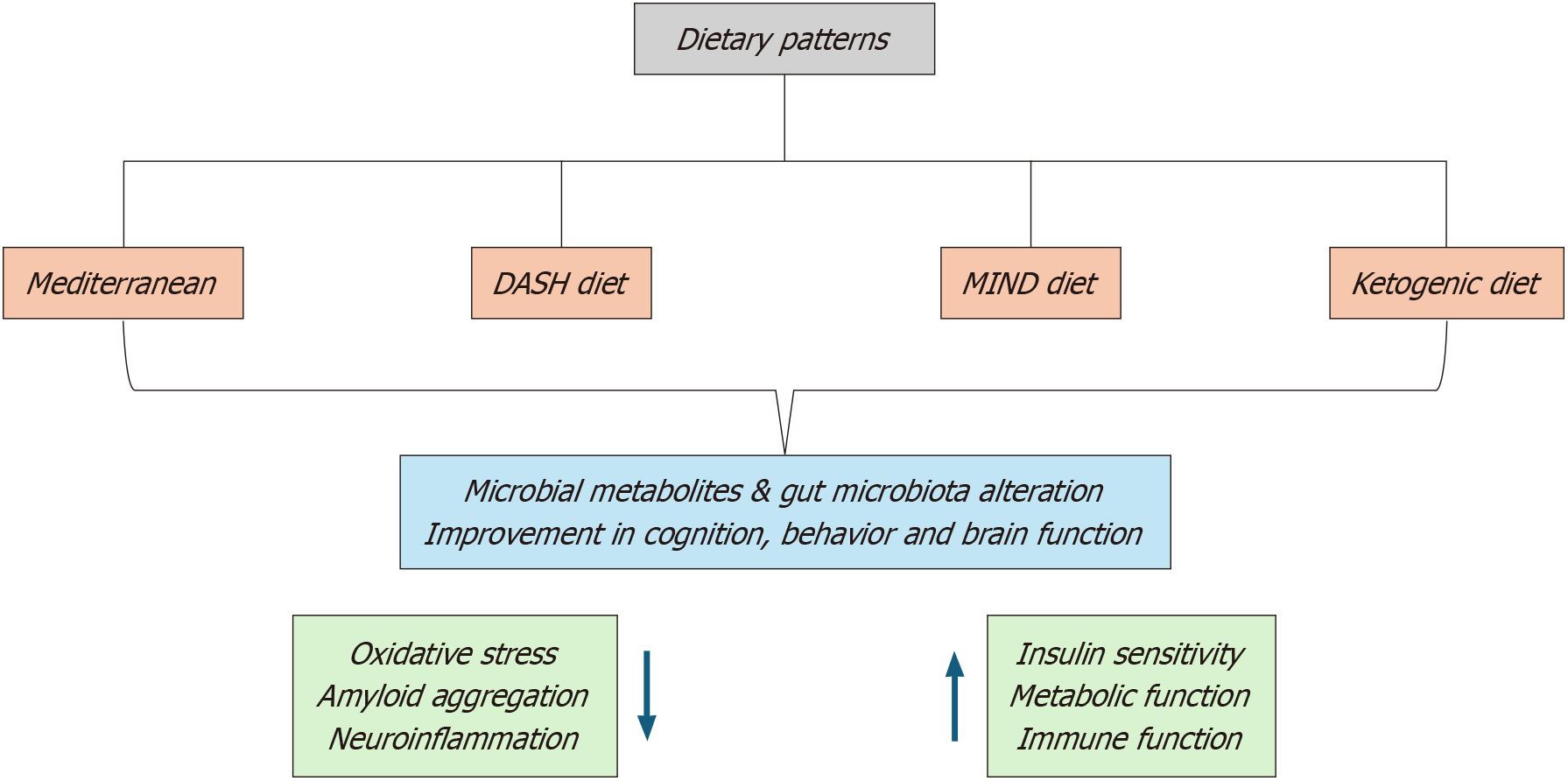
Dietary modifications aimed at promoting gut health have gained traction as adjuncts in anemia management. High-fiber diets rich in whole grains, legumes, and vegetables stimulate the growth of beneficial bacteria, thereby increasing SCFA production and improving iron absorption[200,201]. The fermentation of fiber by gut microbiota generates butyrate, a key SCFA that enhances intestinal epithelial integrity and reduces systemic inflammation—factors essential for erythropoiesis[202,203]. Studies in cancer patients have demonstrated that incorporating fiber-rich foods into their diets improves gut health and alleviates anemia-related complications.
Iron-fortified foods and supplements are commonly used in dietary interventions; however, their efficacy is contingent on the composition of the gut microbiome. Certain bacterial species facilitate iron metabolism, highlighting the importance of microbiome health in optimizing iron bioavailability[204,205]. Notably, combining iron supplementation with prebiotics such as galactooligosaccharides has been shown to enhance iron absorption and effectively address anemia[206,207]. These findings suggest that dietary strategies tailored to balance nutrient intake and promote microbial diversity offer a comprehensive approach to anemia management[208].
Integrated therapeutic strategies combining probiotics, prebiotics, FMT, and dietary interventions may yield superior outcomes in anemia management. For instance, the co-administration of probiotics and prebiotics alongside dietary modifications has been shown to improve hemoglobin levels and attenuate systemic inflammation more effectively than standalone interventions[208,209]. Similarly, pairing FMT with targeted dietary changes can restore microbial diversity while addressing specific nutritional deficiencies[210]. These combination therapies are particularly valuable in the context of chemotherapy-induced anemia, as they alleviate gastrointestinal toxicity and enhance systemic nutrient availability[211,212].
Despite the promise of microbiome-targeted interventions, several challenges remain. Patient responses to these therapies are highly variable, necessitating personalized approaches to maximize efficacy[213-216]. The lack of standardized protocols and limited data on long-term outcomes further complicates their implementation in clinical practice. Additionally, the interplay between cancer therapies and gut microbiota must be carefully monitored to avoid potential adverse interactions, such as exacerbation of dysbiosis[217,218].
To refine microbiome-targeted strategies for anemia management, future research should prioritize the identification of dysbiosis patterns specific to cancer patients. Advances in sequencing technologies can enable personalized interventions tailored to individual microbiome profiles[219,220]. Clinical trials evaluating the combined efficacy of probiotics, prebiotics, FMT, and dietary changes in conjunction with conventional treatments will be instrumental in establishing evidence-based guidelines[221-223]. Finally, elucidating the molecular pathways through which the gut microbiome influences erythropoiesis and systemic inflammation may uncover new therapeutic targets, paving the way for innovative approaches in oncology[224].
The advancing understanding of the gut microbiome’s role in anemia management has set the stage for innovative, patient-specific therapeutic strategies. By targeting dysbiosis and addressing its impacts on iron metabolism, inflammation, and immune regulation, future interventions may significantly enhance outcomes for cancer patients facing anemia.
Developments in microbiome research have enabled the customization of therapeutic approaches to address unique microbial imbalances in individual patients. Engineered probiotics capable of delivering essential metabolites, such as butyrate and folate, are under investigation for their ability to improve erythropoiesis and mitigate inflammation[225,226]. Another promising area involves bacteriophage-based therapies that specifically target pathogenic strains linked to dysbiosis. For example, bacteriophages designed for Escherichia coli strains associated with inflammation may effectively reduce cytokine activity, thereby improving iron absorption and erythropoiesis[227,229]. These personalized approaches are particularly relevant for patients undergoing chemotherapy, where gut microbial disruptions are widespread and poorly addressed by generalized treatments[230,231].
In addition, synthetic microbial consortia (SMC) designed to mimic the functions of a healthy microbiota offer a forward-looking strategy. These consortia could be customized based on the patient’s microbiota profile to encourage the growth of beneficial bacteria such as Bacteroides and Faecalibacterium, both of which are critical for efficient iron metabolism and inflammation control[232,233]. Tables 1 and 2 summarize the mechanisms and interventions in microbiome-targeted anemia management. Table 3 presents a comparative analysis of anemia prevalence and severity across different cancer types with microbiome involvement.
| Aspect | Key points | Examples or details |
| Mechanisms of dysbiosis-induced anemia | Impaired iron absorption | Decreased Lactobacillus and Faecalibacterium disrupt iron uptake mechanisms. Altered expression of ferroportin and divalent metal transporter 1 due to microbial imbalances |
| Systemic inflammation | Elevated cytokines like interleukin-6 and tumor necrosis factor-alpha drive hepcidin overproduction, reducing iron availability. Bacterial endotoxins (e.g., lipopolysaccharides) trigger systemic inflammatory responses | |
| Suppression of erythropoiesis | Inflammatory cytokines disrupt erythropoietin signaling and bone marrow microenvironment. Reduced SCFA production affects hematopoietic stem cell function | |
| Key challenges | Variable patient responses | Differences in microbiota composition impact therapy outcomes. Variability in cancer type and stage complicates standardized approaches |
| Lack of standardized protocols | Absence of uniform methodologies for microbiome-targeted interventions. Limited consensus on FMT donor screening and dietary recommendations | |
| Limited long-term outcome data | Few clinical trials evaluating long-term efficacy of combined microbiome-focused and conventional therapies. Insufficient tracking of adverse effects or durability of response | |
| Microbiome-targeted interventions | Probiotics and prebiotics | Lactobacillus and Bifidobacterium strains improve gut barrier function and support iron metabolism. Prebiotics like inulin and fructooligosaccharides promote SCFA production and microbial diversity |
| FMT | Restores microbial diversity and enhances iron absorption in treatment-resistant anemia. Potential to regulate the gut-liver axis, influencing systemic iron homeostasis | |
| Dietary modifications | High-fiber diets rich in whole grains and legumes promote SCFA-producing bacteria (e.g., Faecalibacterium). Diets tailored to individual microbiota profiles address specific nutrient deficiencies like iron and vitamin B12 | |
| Emerging technologies | CRISPR-Cas9 | Precision editing of microbial genomes to correct dysbiosis-related pathways. Application in modifying gut bacteria to enhance SCFA production or suppress inflammation |
| Machine learning models | Integrates microbiome and host genomic data for predictive modeling of therapy outcomes. Facilitates personalized treatment plans by identifying high-risk microbial patterns | |
| Integrative approaches | Combined probiotics/prebiotics with iron supplementation | Reduces gastrointestinal side effects commonly associated with iron therapy. Enhances bioavailability and absorption of iron |
| FMT with erythropoiesis-stimulating agents | Combines microbial diversity restoration with stimulation of red blood cell production for synergistic benefits. Effective in patients with refractory anemia | |
| Immunotherapy combined with microbiome modulation | Enhances antitumor immune responses by improving gut microbial balance. Addresses anemia caused by cancer therapy-induced dysbiosis |
| Therapy | Mechanism of action | Clinical utility |
| Probiotics (Lactobacillus, Bifidobacterium) | Restore gut microbial balance, enhance nutrient absorption, reduce inflammation | Improved iron absorption and downregulation of inflammatory cytokines in cancer patients |
| Prebiotics (dietary fibers, inulin, fructooligosaccharide) | Foster growth of beneficial gut bacteria, increase SCFA production, improve gut barrier function | Superior gut microbiota composition and reduction in anemia severity |
| Fecal microbiota transplantation | Replenishes beneficial gut microbiota, restores iron metabolism, and reduces inflammation | Improvement in hemoglobin levels and microbiota diversity in anemic patients |
| Synbiotics (probiotics + prebiotics) | Synergistic effect enhancing microbiota diversity, iron absorption, and immune modulation | Reduction in chemotherapy-induced anemia and gut inflammation |
| Postbiotics (SCFAs, microbial metabolites) | Modulate immune response, improve iron bioavailability, and suppress inflammation | Enhanced erythropoiesis and reduced systemic inflammation |
| Dietary interventions (fermented foods, fiber-rich diets, iron and vitamin supplementation) | Support beneficial microbiota, optimize iron and vitamin B12 absorption, reduce gut permeability | Increased erythropoiesis, superior efficacy when combined with probiotics or prebiotics |
| Cancer type | Prevalence | Microbiome involvement |
| Hematologic | 50%-90% | Gut microbiome alteration causes inflammation and nutrient malabsorption |
| Gastrointestinal | 40%-80% | Gut dysbiosis reduces iron absorption, increases iron sequestration by macrophages due to elevation in hepcidin levels |
| Gynecological | 30%-70% | Microbiome disruption impacts estrogen metabolism, inciting a dysregulated immune response and increased gut permeability |
| Genitourinary | 30%-60% | Chronic inflammation and immune activation affect erythropoiesis; renal dysfunction impacts erythropoietin production; altered microbiome composition influences systemic inflammation |
| Lung cancer | 40%-70% | Systemic inflammation leads to anemia of chronic disease; chemotherapy and radiation induce gut microbiome changes, exacerbating inflammation and iron dysregulation |
| Breast cancer | 25%-50% | Chemotherapy and hormonal therapy impact gut microbiota, leading to malabsorption of iron and vitamins; systemic inflammation contributes to anemia |
The identification of microbiome-derived biomarkers that predict anemia severity and therapeutic response is a crucial next step. Studies have pointed to specific microbial patterns, such as reductions in Lactobacillus and Faecalibacterium, that correlate with heightened anemia risk in cancer patients[234-236]. Leveraging advanced sequencing technologies and ML tools, researchers are developing predictive models based on microbiota profiles[237-239]. Notably, SCFA profiles have been associated with enhanced iron absorption and hemoglobin improvement, providing a measurable endpoint for microbiome-focused interventions[240,241].
Beyond diagnostics, microbial metabolites like butyrate, propionate and acetate are being explored as therapeutic targets. These metabolites regulate both immune activity and iron homeostasis, offering potential for use as markers of intervention efficacy[242,243]. Incorporating such biomarkers into clinical practice could lead to earlier detection and precision-targeted management of anemia in oncology.
Postbiotics are bioactive compounds produced by beneficial bacteria during fermentation. These include SCFAs, peptides, enzymes, and bacterial cell wall components that exert health benefits without the need for live bacteria[209]. SCFAs maintain the integrity of gut barrier, enhance expression of iron transporters on enterocytes, and downregulate hepcidin, thereby increasing bioavailability of iron. They also exert an antioxidant effect, reduce the levels of pro-inflammatory cytokines, and promote a favorable milieu for iron-regulating bacteria[244-248].
Bacteriophage therapy has emerged as a promising supplemental treatment for certain malignancies. Phaga-mediate alteration of gut microbiota can negate dysbiosis, and influence cancer progression and treatment outcomes. Using viruses equipped to selectively destroy detrimental strains (Escherichia coli, Enterococcus faecalis, or antibiotic-resistant strains), while preserving beneficial microbes, allows for restoration of a healthy gut microbiome. Phage cocktails can be tailored to attain specific bacterial profiles in order to enhance response to cancer therapy. Phage therapy remains in its infancy and has yet to surpass regulatory hurdles before becoming mainstream. Additionally, issues with bacteriophage delivery mechanisms and treatment resistance also need to be addressed, and this remains an ongoing area of research[61,170].
SMC essentially comprises several beneficial bacterial strains, and are administered either as multi-strain probiotics/microbial cocktails (Faecalibacterium prausnitzii, Akkermansia muciniphila), genetically engineered microbes (SCFA producing strains), or prebiotic-supported consortia. SMC offers a promising approach to modulate gut microbiome for correction of dysbiosis, improvement of treatment response, development of personalized therapies and enhance overall patient outcomes. Nevertheless, they need to be rigorously tested and validated in clinical trials prior to use in clinical settings[246].
Future strategies in anemia management will rely on combining microbiome-targeted therapies with established interventions. Probiotics and prebiotics, for instance, can enhance the absorption of iron supplements while minimizing gastrointestinal side effects[249,250]. Similarly, integrating FMT with erythropoiesis-stimulating agents may provide synergistic benefits for patients with severe anemia[251-253].
Dietary modifications tailored to the patient’s unique microbiota composition represent another promising avenue. Diets rich in fibers, whole grains, and legumes promote beneficial bacterial populations and address specific nutrient deficiencies such as iron and vitamin B12[254,255]. Figure 2 illustrates some dietary patterns that positively impact the gut microbiome. Emerging research also highlights the potential of combining microbiome modulation with immunotherapy to bolster immune responses and address anemia comprehensively in cancer patients[256,257].
Future therapies will benefit from advancements in microbiome engineering and computational technologies. CRISPR-Cas9 is being explored for its ability to edit microbial genomes, targeting pathways implicated in dysbiosis and anemia progression. Computational models that integrate microbiome and host genomic data could guide individualized treatment plans, optimizing therapeutic efficacy based on patient-specific factors. By incorporating these cutting-edge approaches, the field can move closer to delivering personalized, effective solutions for managing anemia in oncology[258-260].
The gut microbiome is an indispensable yet underappreciated factor in the pathogenesis and management of cancer-associated anemia. This review highlights the intricate interplay between dysbiosis and the mechanisms driving anemia, including disruptions in iron metabolism, immune regulation, systemic inflammation, and nutrient absorption. Gut dysbiosis causes anemia and impairs response to chemotherapy in cancer patients. It exacerbates chemotherapy-induced myelosuppression and systemic inflammation, further impairing erythropoiesis in hematologic malignancies. Similarly, in solid malignancies, chronic tumor-associated bleeding, treatment-induced gastrointestinal toxicity, and malabsorption of essential nutrients are compounded by microbiota imbalances. Microbiome-centered interventions, such as probiotics, prebiotics, dietary modifications, and FMT, have shown efficacy in restoring microbial balance, reducing inflammation, and enhancing nutrient bioavailability. Emerging approaches, including engineered probiotics and bacteriophage therapies, are promising precision-based, customizable solutions for various microbiome compositions and imbalances. Future research should focus on integrating microbiome-targeted strategies with established anemia therapies. Incorporating microbiome-based biomarkers into clinical practice could refine diagnostic precision, predict therapeutic outcomes, and enable tailored interventions. Additionally, advancing clinical trials to evaluate the efficacy of microbiome-based therapies is critical for establishing their role in standard oncology care. Understanding the intricate role of microbial metabolites in erythropoiesis and systemic inflammation will further refine our approach to anemia management. Bridging the gap between cutting-edge microbiome science and clinical oncology can provide transformative improvements in anemia management, enhancing quality of life, treatment tolerability, and overall outcomes for patients with malignancies. Continued research and innovation are imperative to fully unlock the therapeutic potential of microbiome interventions in oncology.
| 1. | Aapro M, Österborg A, Gascón P, Ludwig H, Beguin Y. Prevalence and management of cancer-related anaemia, iron deficiency and the specific role of i.v. iron. Ann Oncol. 2012;23:1954-1962. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 127] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 2. | Rodgers GM. Update on iron supplementation in patients with cancer-related anemia. Expert Rev Hematol. 2024;17:505-514. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 3. | Gilreath JA, Rodgers GM. How I treat cancer-associated anemia. Blood. 2020;136:801-813. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 70] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 4. | Aapro M, Beguin Y, Bokemeyer C, Dicato M, Gascón P, Glaspy J, Hofmann A, Link H, Littlewood T, Ludwig H, Österborg A, Pronzato P, Santini V, Schrijvers D, Stauder R, Jordan K, Herrstedt J; ESMO Guidelines Committee. Management of anaemia and iron deficiency in patients with cancer: ESMO Clinical Practice Guidelines. Ann Oncol. 2018;29:iv96-iv110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 138] [Article Influence: 19.7] [Reference Citation Analysis (1)] |
| 5. | Gilreath JA, Stenehjem DD, Rodgers GM. Diagnosis and treatment of cancer-related anemia. Am J Hematol. 2014;89:203-212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 147] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 6. | Bohlius J, Bohlke K, Castelli R, Djulbegovic B, Lustberg MB, Martino M, Mountzios G, Peswani N, Porter L, Tanaka TN, Trifirò G, Yang H, Lazo-Langner A. Management of Cancer-Associated Anemia With Erythropoiesis-Stimulating Agents: ASCO/ASH Clinical Practice Guideline Update. J Clin Oncol. 2019;37:1336-1351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 56] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 7. | Rodgers GM 3rd, Becker PS, Blinder M, Cella D, Chanan-Khan A, Cleeland C, Coccia PF, Djulbegovic B, Gilreath JA, Kraut EH, Matulonis UA, Millenson MM, Reinke D, Rosenthal J, Schwartz RN, Soff G, Stein RS, Vlahovic G, Weir AB 3rd. Cancer- and chemotherapy-induced anemia. J Natl Compr Canc Netw. 2012;10:628-653. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 131] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 8. | Abdel-Razeq H, Hashem H. Recent update in the pathogenesis and treatment of chemotherapy and cancer induced anemia. Crit Rev Oncol Hematol. 2020;145:102837. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 71] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 9. | Ludwig H, Müldür E, Endler G, Hübl W. Prevalence of iron deficiency across different tumors and its association with poor performance status, disease status and anemia. Ann Oncol. 2013;24:1886-1892. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 162] [Cited by in RCA: 161] [Article Influence: 13.4] [Reference Citation Analysis (1)] |
| 10. | Bohlius J, Schmidlin K, Brillant C, Schwarzer G, Trelle S, Seidenfeld J, Zwahlen M, Clarke MJ, Weingart O, Kluge S, Piper M, Napoli M, Rades D, Steensma D, Djulbegovic B, Fey MF, Ray-Coquard I, Moebus V, Thomas G, Untch M, Schumacher M, Egger M, Engert A. Erythropoietin or Darbepoetin for patients with cancer--meta-analysis based on individual patient data. Cochrane Database Syst Rev. 2009;2009:CD007303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 72] [Article Influence: 4.5] [Reference Citation Analysis (1)] |
| 11. | Bennett CL, Silver SM, Djulbegovic B, Samaras AT, Blau CA, Gleason KJ, Barnato SE, Elverman KM, Courtney DM, McKoy JM, Edwards BJ, Tigue CC, Raisch DW, Yarnold PR, Dorr DA, Kuzel TM, Tallman MS, Trifilio SM, West DP, Lai SY, Henke M. Venous thromboembolism and mortality associated with recombinant erythropoietin and darbepoetin administration for the treatment of cancer-associated anemia. JAMA. 2008;299:914-924. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 552] [Cited by in RCA: 501] [Article Influence: 29.5] [Reference Citation Analysis (0)] |
| 12. | Schwabe RF, Jobin C. The microbiome and cancer. Nat Rev Cancer. 2013;13:800-812. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1054] [Cited by in RCA: 1232] [Article Influence: 102.7] [Reference Citation Analysis (2)] |
| 13. | Marchesi JR, Adams DH, Fava F, Hermes GD, Hirschfield GM, Hold G, Quraishi MN, Kinross J, Smidt H, Tuohy KM, Thomas LV, Zoetendal EG, Hart A. The gut microbiota and host health: a new clinical frontier. Gut. 2016;65:330-339. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1341] [Cited by in RCA: 1559] [Article Influence: 173.2] [Reference Citation Analysis (0)] |
| 14. | Sonnenburg JL, Bäckhed F. Diet-microbiota interactions as moderators of human metabolism. Nature. 2016;535:56-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1187] [Cited by in RCA: 1502] [Article Influence: 166.9] [Reference Citation Analysis (0)] |
| 15. | Peled JU, Gomes ALC, Devlin SM, Littmann ER, Taur Y, Sung AD, Weber D, Hashimoto D, Slingerland AE, Slingerland JB, Maloy M, Clurman AG, Stein-Thoeringer CK, Markey KA, Docampo MD, Burgos da Silva M, Khan N, Gessner A, Messina JA, Romero K, Lew MV, Bush A, Bohannon L, Brereton DG, Fontana E, Amoretti LA, Wright RJ, Armijo GK, Shono Y, Sanchez-Escamilla M, Castillo Flores N, Alarcon Tomas A, Lin RJ, Yáñez San Segundo L, Shah GL, Cho C, Scordo M, Politikos I, Hayasaka K, Hasegawa Y, Gyurkocza B, Ponce DM, Barker JN, Perales MA, Giralt SA, Jenq RR, Teshima T, Chao NJ, Holler E, Xavier JB, Pamer EG, van den Brink MRM. Microbiota as Predictor of Mortality in Allogeneic Hematopoietic-Cell Transplantation. N Engl J Med. 2020;382:822-834. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 304] [Cited by in RCA: 489] [Article Influence: 97.8] [Reference Citation Analysis (1)] |
| 16. | Wong SH, Yu J. Gut microbiota in colorectal cancer: mechanisms of action and clinical applications. Nat Rev Gastroenterol Hepatol. 2019;16:690-704. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 384] [Cited by in RCA: 812] [Article Influence: 135.3] [Reference Citation Analysis (0)] |
| 17. | Ganz T, Nemeth E. Iron homeostasis in host defence and inflammation. Nat Rev Immunol. 2015;15:500-510. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 433] [Cited by in RCA: 565] [Article Influence: 56.5] [Reference Citation Analysis (0)] |
| 18. | Kassebaum NJ, Jasrasaria R, Naghavi M, Wulf SK, Johns N, Lozano R, Regan M, Weatherall D, Chou DP, Eisele TP, Flaxman SR, Pullan RL, Brooker SJ, Murray CJ. A systematic analysis of global anemia burden from 1990 to 2010. Blood. 2014;123:615-624. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1183] [Cited by in RCA: 1256] [Article Influence: 114.2] [Reference Citation Analysis (0)] |
| 19. | Vieira AT, Teixeira MM, Martins FS. The role of probiotics and prebiotics in inducing gut immunity. Front Immunol. 2013;4:445. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 154] [Cited by in RCA: 164] [Article Influence: 13.7] [Reference Citation Analysis (1)] |
| 20. | Spivak JL, Gascón P, Ludwig H. Anemia management in oncology and hematology. Oncologist. 2009;14 Suppl 1:43-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 94] [Article Influence: 5.9] [Reference Citation Analysis (1)] |
| 21. | Testa U, Castelli G, Elvira P. Experimental and investigational therapies for chemotherapy-induced anemia. Expert Opin Investig Drugs. 2015;24:1433-1445. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 0.8] [Reference Citation Analysis (1)] |
| 22. | Marques O, Weiss G, Muckenthaler MU. The role of iron in chronic inflammatory diseases: from mechanisms to treatment options in anemia of inflammation. Blood. 2022;140:2011-2023. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 61] [Reference Citation Analysis (0)] |
| 23. | Hart L, Ogbonnaya A, Boykin K, Deyoung K, Bailey R, Heritage T, Lopez-Gonzalez L, Huang H, Gordan L. Burden of chemotherapy-induced myelosuppression among patients with extensive-stage small cell lung cancer: A retrospective study from community oncology practices. Cancer Med. 2023;12:10020-10030. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 24. | Gafter-Gvili A, Steensma DP, Auerbach M. Should the ASCO/ASH Guidelines for the use of intravenous iron in cancer- and chemotherapy-induced anemia be updated? J Natl Compr Canc Netw. 2014;12:657-664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 14] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 25. | Harrison L, Blackwell K. Hypoxia and anemia: factors in decreased sensitivity to radiation therapy and chemotherapy? Oncologist. 2004;9 Suppl 5:31-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 248] [Cited by in RCA: 257] [Article Influence: 12.9] [Reference Citation Analysis (1)] |
| 26. | Zhang Y, Chen X, Wang X, Chen J, Du C, Wang J, Liao W. Insights into ionizing radiation-induced bone marrow hematopoietic stem cell injury. Stem Cell Res Ther. 2024;15:222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 27. | Michot JM, Lazarovici J, Tieu A, Champiat S, Voisin AL, Ebbo M, Godeau B, Michel M, Ribrag V, Lambotte O. Haematological immune-related adverse events with immune checkpoint inhibitors, how to manage? Eur J Cancer. 2019;122:72-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 105] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 28. | Tanios GE, Doley PB, Munker R. Autoimmune hemolytic anemia associated with the use of immune checkpoint inhibitors for cancer: 68 cases from the Food and Drug Administration database and review. Eur J Haematol. 2019;102:157-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 50] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 29. | Knight K, Wade S, Balducci L. Prevalence and outcomes of anemia in cancer: a systematic review of the literature. Am J Med. 2004;116 Suppl 7A:11S-26S. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 334] [Cited by in RCA: 367] [Article Influence: 17.5] [Reference Citation Analysis (1)] |
| 30. | Heshiki Y, Vazquez-Uribe R, Li J, Ni Y, Quainoo S, Imamovic L, Li J, Sørensen M, Chow BKC, Weiss GJ, Xu A, Sommer MOA, Panagiotou G. Predictable modulation of cancer treatment outcomes by the gut microbiota. Microbiome. 2020;8:28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 114] [Article Influence: 22.8] [Reference Citation Analysis (1)] |
| 31. | Zhu Y, Michelle Luo T, Jobin C, Young HA. Gut microbiota and probiotics in colon tumorigenesis. Cancer Lett. 2011;309:119-127. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 174] [Cited by in RCA: 159] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 32. | Ganz T. Hepcidin and its role in regulating systemic iron metabolism. Hematology Am Soc Hematol Educ Program. 2006;29-35, 507. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 122] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 33. | Muckenthaler MU, Rivella S, Hentze MW, Galy B. A Red Carpet for Iron Metabolism. Cell. 2017;168:344-361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 559] [Cited by in RCA: 915] [Article Influence: 114.4] [Reference Citation Analysis (0)] |
| 34. | Pandey H, Tang DWT, Wong SH, Lal D. Gut Microbiota in Colorectal Cancer: Biological Role and Therapeutic Opportunities. Cancers (Basel). 2023;15:866. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 70] [Reference Citation Analysis (0)] |
| 35. | Wu WKK, Yu J. Microbiota-based biomarkers and therapeutics for cancer management. Nat Rev Gastroenterol Hepatol. 2024;21:72-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 36. | Madeddu C, Gramignano G, Astara G, Demontis R, Sanna E, Atzeni V, Macciò A. Pathogenesis and Treatment Options of Cancer Related Anemia: Perspective for a Targeted Mechanism-Based Approach. Front Physiol. 2018;9:1294. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 127] [Cited by in RCA: 116] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 37. | Anand S, Burkenroad A, Glaspy J. Workup of anemia in cancer. Clin Adv Hematol Oncol. 2020;18:640-646. [PubMed] |
| 38. | Camaschella C, Nai A, Silvestri L. Iron metabolism and iron disorders revisited in the hepcidin era. Haematologica. 2020;105:260-272. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 187] [Cited by in RCA: 416] [Article Influence: 83.2] [Reference Citation Analysis (0)] |
| 39. | Dostal A, Lacroix C, Pham VT, Zimmermann MB, Del'homme C, Bernalier-Donadille A, Chassard C. Iron supplementation promotes gut microbiota metabolic activity but not colitis markers in human gut microbiota-associated rats. Br J Nutr. 2014;111:2135-2145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 63] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 40. | Kovatcheva-Datchary P, Nilsson A, Akrami R, Lee YS, De Vadder F, Arora T, Hallen A, Martens E, Björck I, Bäckhed F. Dietary Fiber-Induced Improvement in Glucose Metabolism Is Associated with Increased Abundance of Prevotella. Cell Metab. 2015;22:971-982. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 927] [Cited by in RCA: 1243] [Article Influence: 124.3] [Reference Citation Analysis (2)] |
| 41. | Yilmaz B, Li H. Gut Microbiota and Iron: The Crucial Actors in Health and Disease. Pharmaceuticals (Basel). 2018;11:98. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 106] [Cited by in RCA: 197] [Article Influence: 28.1] [Reference Citation Analysis (1)] |
| 42. | Sidaway P. Intestinal microbiota predict HSCT outcome. Nat Rev Clin Oncol. 2020;17:275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 43. | Ganz T. Hepcidin, a key regulator of iron metabolism and mediator of anemia of inflammation. Blood. 2003;102:783-788. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1016] [Cited by in RCA: 984] [Article Influence: 44.7] [Reference Citation Analysis (0)] |
| 44. | Hardang IM, Lilleholt K, Hagve TA. [Anemia of chronic disease]. Tidsskr Nor Laegeforen. 2017;137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 45. | Natalucci V, Virgili E, Calcagnoli F, Valli G, Agostini D, Zeppa SD, Barbieri E, Emili R. Cancer Related Anemia: An Integrated Multitarget Approach and Lifestyle Interventions. Nutrients. 2021;13:482. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 24] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 46. | Furusawa Y, Obata Y, Fukuda S, Endo TA, Nakato G, Takahashi D, Nakanishi Y, Uetake C, Kato K, Kato T, Takahashi M, Fukuda NN, Murakami S, Miyauchi E, Hino S, Atarashi K, Onawa S, Fujimura Y, Lockett T, Clarke JM, Topping DL, Tomita M, Hori S, Ohara O, Morita T, Koseki H, Kikuchi J, Honda K, Hase K, Ohno H. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature. 2013;504:446-450. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2951] [Cited by in RCA: 3782] [Article Influence: 315.2] [Reference Citation Analysis (0)] |
| 47. | Morrison DJ, Preston T. Formation of short chain fatty acids by the gut microbiota and their impact on human metabolism. Gut Microbes. 2016;7:189-200. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1566] [Cited by in RCA: 2366] [Article Influence: 262.9] [Reference Citation Analysis (0)] |
| 48. | Galy B, Conrad M, Muckenthaler M. Mechanisms controlling cellular and systemic iron homeostasis. Nat Rev Mol Cell Biol. 2024;25:133-155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 282] [Article Influence: 282.0] [Reference Citation Analysis (0)] |
| 49. | Ohno H. The impact of metabolites derived from the gut microbiota on immune regulation and diseases. Int Immunol. 2020;32:629-636. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 50. | Leon LR, Helwig BG. Role of endotoxin and cytokines in the systemic inflammatory response to heat injury. Front Biosci (Schol Ed). 2010;2:916-938. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 39] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 51. | Alexander JL, Wilson ID, Teare J, Marchesi JR, Nicholson JK, Kinross JM. Gut microbiota modulation of chemotherapy efficacy and toxicity. Nat Rev Gastroenterol Hepatol. 2017;14:356-365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 451] [Cited by in RCA: 663] [Article Influence: 82.9] [Reference Citation Analysis (0)] |
| 52. | Gori S, Inno A, Belluomini L, Bocus P, Bisoffi Z, Russo A, Arcaro G. Gut microbiota and cancer: How gut microbiota modulates activity, efficacy and toxicity of antitumoral therapy. Crit Rev Oncol Hematol. 2019;143:139-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 124] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 53. | Sottotetti F, Malovini A, Maccarone S, Riva G, Tibollo V, Palumbo R, Tagliaferri B, Bellazzi R, Cena H, Di Sabatino A, Locati LD, Lenti MV. Vitamin B12 status in hospitalised cancer patients: Prevalence and clinical implications of depletion and hypervitaminosis. Clin Nutr ESPEN. 2024;63:585-594. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 54. | Degnan PH, Taga ME, Goodman AL. Vitamin B12 as a modulator of gut microbial ecology. Cell Metab. 2014;20:769-778. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 264] [Cited by in RCA: 304] [Article Influence: 27.6] [Reference Citation Analysis (0)] |
| 55. | Magnúsdóttir S, Ravcheev D, de Crécy-Lagard V, Thiele I. Systematic genome assessment of B-vitamin biosynthesis suggests co-operation among gut microbes. Front Genet. 2015;6:148. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 453] [Cited by in RCA: 516] [Article Influence: 51.6] [Reference Citation Analysis (0)] |
| 56. | Weiss GA, Hennet T. Mechanisms and consequences of intestinal dysbiosis. Cell Mol Life Sci. 2017;74:2959-2977. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 393] [Cited by in RCA: 436] [Article Influence: 54.5] [Reference Citation Analysis (0)] |
| 57. | Heilfort L, Kutschan S, Dörfler J, Freuding M, Büntzel J, Münstedt K, Hübner J. A Systematic Review of the Benefit of B-Vitamins as a Complementary Treatment in Cancer Patients. Nutr Cancer. 2023;75:33-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 58. | Xu H, Xu L, Page JH, Cannavale K, Sattayapiwat O, Rodriguez R, Chao C. Incidence of anemia in patients diagnosed with solid tumors receiving chemotherapy, 2010-2013. Clin Epidemiol. 2016;8:61-71. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 38] [Article Influence: 4.2] [Reference Citation Analysis (1)] |
| 59. | Nayak RR, Alexander M, Deshpande I, Stapleton-Gray K, Rimal B, Patterson AD, Ubeda C, Scher JU, Turnbaugh PJ. Methotrexate impacts conserved pathways in diverse human gut bacteria leading to decreased host immune activation. Cell Host Microbe. 2021;29:362-377.e11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 84] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 60. | Mayneris-Perxachs J, Moreno-Navarrete JM, Fernández-Real JM. The role of iron in host-microbiota crosstalk and its effects on systemic glucose metabolism. Nat Rev Endocrinol. 2022;18:683-698. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 78] [Article Influence: 26.0] [Reference Citation Analysis (3)] |
| 61. | Liping Z, Sheng Y, Yinhang W, Yifei S, Jiaqun H, Xiaojian Y, Shuwen H, Jing Z. Comprehensive retrospect and future perspective on bacteriophage and cancer. Virol J. 2024;21:278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 62. | Deriu E, Liu JZ, Pezeshki M, Edwards RA, Ochoa RJ, Contreras H, Libby SJ, Fang FC, Raffatellu M. Probiotic bacteria reduce salmonella typhimurium intestinal colonization by competing for iron. Cell Host Microbe. 2013;14:26-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 304] [Cited by in RCA: 365] [Article Influence: 33.2] [Reference Citation Analysis (0)] |
| 63. | Nemeth E, Ganz T. Anemia of inflammation. Hematol Oncol Clin North Am. 2014;28:671-681, vi. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 243] [Cited by in RCA: 288] [Article Influence: 26.2] [Reference Citation Analysis (0)] |
| 64. | Wessling-Resnick M. Iron homeostasis and the inflammatory response. Annu Rev Nutr. 2010;30:105-122. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 377] [Cited by in RCA: 355] [Article Influence: 23.7] [Reference Citation Analysis (1)] |
| 65. | Xiao L, Tang R, Wang J, Wan D, Yin Y, Xie L. Gut microbiota bridges the iron homeostasis and host health. Sci China Life Sci. 2023;66:1952-1975. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 29] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 66. | Zhang Q, Wu W, Guo F, Li J, Jin Y, Cai G, Yang Y. Characteristics of Gut Microbiota and Fecal Metabolites in Patients with Colorectal Cancer-Associated Iron Deficiency Anemia. Microorganisms. 2024;12:1319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 67. | Cheng WY, Wu CY, Yu J. The role of gut microbiota in cancer treatment: friend or foe? Gut. 2020;69:1867-1876. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 117] [Cited by in RCA: 238] [Article Influence: 47.6] [Reference Citation Analysis (0)] |
| 68. | Weiss G, Ganz T, Goodnough LT. Anemia of inflammation. Blood. 2019;133:40-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 332] [Cited by in RCA: 632] [Article Influence: 90.3] [Reference Citation Analysis (0)] |
| 69. | Weiss G, Schett G. Anaemia in inflammatory rheumatic diseases. Nat Rev Rheumatol. 2013;9:205-215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 88] [Article Influence: 6.8] [Reference Citation Analysis (1)] |
| 70. | de Jong PR, González-Navajas JM, Jansen NJ. The digestive tract as the origin of systemic inflammation. Crit Care. 2016;20:279. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 71] [Cited by in RCA: 95] [Article Influence: 10.6] [Reference Citation Analysis (1)] |
| 71. | Malesza IJ, Bartkowiak-Wieczorek J, Winkler-Galicki J, Nowicka A, Dzięciołowska D, Błaszczyk M, Gajniak P, Słowińska K, Niepolski L, Walkowiak J, Mądry E. The Dark Side of Iron: The Relationship between Iron, Inflammation and Gut Microbiota in Selected Diseases Associated with Iron Deficiency Anaemia-A Narrative Review. Nutrients. 2022;14:3478. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 53] [Reference Citation Analysis (0)] |
| 72. | Wickert A, Schwantes A, Fuhrmann DC, Brüne B. Inflammation in a ferroptotic environment. Front Pharmacol. 2024;15:1474285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 73. | Mishra V, Mishra Y. Role of Gut Microbiome in Cancer Treatment. Indian J Microbiol. 2024;64:1310-1325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 74. | Montassier E, Gastinne T, Vangay P, Al-Ghalith GA, Bruley des Varannes S, Massart S, Moreau P, Potel G, de La Cochetière MF, Batard E, Knights D. Chemotherapy-driven dysbiosis in the intestinal microbiome. Aliment Pharmacol Ther. 2015;42:515-528. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 238] [Cited by in RCA: 351] [Article Influence: 35.1] [Reference Citation Analysis (1)] |
| 75. | Tanoue T, Morita S, Plichta DR, Skelly AN, Suda W, Sugiura Y, Narushima S, Vlamakis H, Motoo I, Sugita K, Shiota A, Takeshita K, Yasuma-Mitobe K, Riethmacher D, Kaisho T, Norman JM, Mucida D, Suematsu M, Yaguchi T, Bucci V, Inoue T, Kawakami Y, Olle B, Roberts B, Hattori M, Xavier RJ, Atarashi K, Honda K. A defined commensal consortium elicits CD8 T cells and anti-cancer immunity. Nature. 2019;565:600-605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 476] [Cited by in RCA: 794] [Article Influence: 132.3] [Reference Citation Analysis (0)] |
| 76. | Wells JM. Immunomodulatory mechanisms of lactobacilli. Microb Cell Fact. 2011;10 Suppl 1:S17. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 217] [Cited by in RCA: 272] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 77. | Lyu M, Suzuki H, Kang L, Gaspal F, Zhou W, Goc J, Zhou L, Zhou J, Zhang W; JRI Live Cell Bank, Shen Z, Fox JG, Sockolow RE, Laufer TM, Fan Y, Eberl G, Withers DR, Sonnenberg GF. ILC3s select microbiota-specific regulatory T cells to establish tolerance in the gut. Nature. 2022;610:744-751. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 181] [Article Influence: 60.3] [Reference Citation Analysis (1)] |
| 78. | Khosravi A, Yáñez A, Price JG, Chow A, Merad M, Goodridge HS, Mazmanian SK. Gut microbiota promote hematopoiesis to control bacterial infection. Cell Host Microbe. 2014;15:374-381. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 381] [Cited by in RCA: 484] [Article Influence: 44.0] [Reference Citation Analysis (0)] |
| 79. | Chaudhary S, Kaur P, Singh TA, Bano KS, Vyas A, Mishra AK, Singh P, Mehdi MM. The dynamic crosslinking between gut microbiota and inflammation during aging: reviewing the nutritional and hormetic approaches against dysbiosis and inflammaging. Biogerontology. 2024;26:1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 80. | Bhutta NK, Xu X, Jian C, Wang Y, Liu Y, Sun J, Han B, Wu S, Javeed A. Gut microbiota mediated T cells regulation and autoimmune diseases. Front Microbiol. 2024;15:1477187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 12] [Reference Citation Analysis (1)] |
| 81. | Koh A, De Vadder F, Kovatcheva-Datchary P, Bäckhed F. From Dietary Fiber to Host Physiology: Short-Chain Fatty Acids as Key Bacterial Metabolites. Cell. 2016;165:1332-1345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2533] [Cited by in RCA: 4203] [Article Influence: 467.0] [Reference Citation Analysis (0)] |
| 82. | Nastasi C, Fredholm S, Willerslev-Olsen A, Hansen M, Bonefeld CM, Geisler C, Andersen MH, Ødum N, Woetmann A. Butyrate and propionate inhibit antigen-specific CD8(+) T cell activation by suppressing IL-12 production by antigen-presenting cells. Sci Rep. 2017;7:14516. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 93] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 83. | Trompette A, Gollwitzer ES, Pattaroni C, Lopez-Mejia IC, Riva E, Pernot J, Ubags N, Fajas L, Nicod LP, Marsland BJ. Dietary Fiber Confers Protection against Flu by Shaping Ly6c(-) Patrolling Monocyte Hematopoiesis and CD8(+) T Cell Metabolism. Immunity. 2018;48:992-1005.e8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 295] [Cited by in RCA: 487] [Article Influence: 81.2] [Reference Citation Analysis (2)] |
| 84. | Zhou X, Li J, Guo J, Geng B, Ji W, Zhao Q, Li J, Liu X, Liu J, Guo Z, Cai W, Ma Y, Ren D, Miao J, Chen S, Zhang Z, Chen J, Zhong J, Liu W, Zou M, Li Y, Cai J. Gut-dependent microbial translocation induces inflammation and cardiovascular events after ST-elevation myocardial infarction. Microbiome. 2018;6:66. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 139] [Cited by in RCA: 224] [Article Influence: 32.0] [Reference Citation Analysis (0)] |
| 85. | Zagury-Orly I, Khaouam N, Noujaim J, Desrosiers MY, Maniakas A. The Effect of Radiation and Chemoradiation Therapy on the Head and Neck Mucosal Microbiome: A Review. Front Oncol. 2021;11:784457. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (1)] |
| 86. | Dorobisz K, Dorobisz T, Zatoński T. The Microbiome's Influence on Head and Neck Cancers. Curr Oncol Rep. 2023;25:163-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 87. | Al-Qadami G, Van Sebille Y, Bowen J, Wardill H. Oral-Gut Microbiome Axis in the Pathogenesis of Cancer Treatment-Induced Oral Mucositis. Front Oral Health. 2022;3:881949. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 25] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 88. | Stringer AM, Hargreaves BM, Mendes RA, Blijlevens NMA, Bruno JS, Joyce P, Kamath S, Laheij AMGA, Ottaviani G, Secombe KR, Tonkaboni A, Zadik Y, Bossi P, Wardill HR. Updated perspectives on the contribution of the microbiome to the pathogenesis of mucositis using the MASCC/ISOO framework. Support Care Cancer. 2024;32:558. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 89. | Koury MJ, Ponka P. New insights into erythropoiesis: the roles of folate, vitamin B12, and iron. Annu Rev Nutr. 2004;24:105-131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 246] [Cited by in RCA: 273] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 90. | Liu X, Zhang H, Shi G, Zheng X, Chang J, Lin Q, Tian Z, Yang H. The impact of gut microbial signals on hematopoietic stem cells and the bone marrow microenvironment. Front Immunol. 2024;15:1338178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 91. | Al-Bawardy B, Proctor DD. Gastrointestinal vascular malformations and neoplasms: arterial, venous, arteriovenous, and capillary. In: Wang TC, Camilleri M, Lebwohl B, Lok AS, Sandborn WJ, Wang KK, Wu GD, editors. Feldman M, Friedman LS, Brandt LJ, Sleisenger and Fordtran’s Gastrointestinal and Liver Disease. 11th ed. Hoboken (NJ): Wiley, 2022. [DOI] [Full Text] |
| 92. | Nandakumar S, Singh N, Tharani AR, Pankiw M, Brezden-Masley C. Intravenous iron and iron deficiency anemia in patients with gastrointestinal cancer: A systematic review. PLoS One. 2024;19:e0302964. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 93. | Erawijantari PP, Mizutani S, Shiroma H, Shiba S, Nakajima T, Sakamoto T, Saito Y, Fukuda S, Yachida S, Yamada T. Influence of gastrectomy for gastric cancer treatment on faecal microbiome and metabolome profiles. Gut. 2020;69:1404-1415. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 112] [Article Influence: 22.4] [Reference Citation Analysis (0)] |
| 94. | Zeng R, Gou H, Lau HCH, Yu J. Stomach microbiota in gastric cancer development and clinical implications. Gut. 2024;73:2062-2073. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Reference Citation Analysis (0)] |
| 95. | Pappas-Gogos G, Tepelenis K, Fousekis F, Katsanos K, Pitiakoudis M, Vlachos K. The Implication of Gastric Microbiome in the Treatment of Gastric Cancer. Cancers (Basel). 2022;14:2039. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 15] [Article Influence: 5.0] [Reference Citation Analysis (1)] |
| 96. | Liu D, Zhang R, Chen S, Sun B, Zhang K. Analysis of gastric microbiome reveals three distinctive microbial communities associated with the occurrence of gastric cancer. BMC Microbiol. 2022;22:184. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 28] [Article Influence: 9.3] [Reference Citation Analysis (1)] |
| 97. | Bartel MJ, Asbun H, Stauffer J, Raimondo M. Pancreatic exocrine insufficiency in pancreatic cancer: A review of the literature. Dig Liver Dis. 2015;47:1013-1020. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 62] [Article Influence: 6.2] [Reference Citation Analysis (1)] |
| 98. | Vujasinovic M, Valente R, Del Chiaro M, Permert J, Löhr JM. Pancreatic Exocrine Insufficiency in Pancreatic Cancer. Nutrients. 2017;9:183. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 105] [Cited by in RCA: 96] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 99. | Boicean A, Ichim C, Todor SB, Anderco P, Popa ML. The Importance of Microbiota and Fecal Microbiota Transplantation in Pancreatic Disorders. Diagnostics (Basel). 2024;14:861. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 26] [Reference Citation Analysis (0)] |
| 100. | Zhang X, Liu Q, Liao Q, Zhao Y. Pancreatic Cancer, Gut Microbiota, and Therapeutic Efficacy. J Cancer. 2020;11:2749-2758. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 46] [Article Influence: 9.2] [Reference Citation Analysis (1)] |
| 101. | Bangolo AI, Trivedi C, Jani I, Pender S, Khalid H, Alqinai B, Intisar A, Randhawa K, Moore J, De Deugd N, Faisal S, Suresh SB, Gopani P, Nagesh VK, Proverbs-Singh T, Weissman S. Impact of gut microbiome in the development and treatment of pancreatic cancer: Newer insights. World J Gastroenterol. 2023;29:3984-3998. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 6] [Cited by in RCA: 3] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 102. | Zhang B, Zhou F, Hong J, Ng DM, Yang T, Zhou X, Jin J, Zhou F, Chen P, Xu Y. The role of FOLFIRINOX in metastatic pancreatic cancer: a meta-analysis. World J Surg Oncol. 2021;19:182. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 20] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 103. | Deleemans JM, Chleilat F, Reimer RA, Henning JW, Baydoun M, Piedalue KA, McLennan A, Carlson LE. The chemo-gut study: investigating the long-term effects of chemotherapy on gut microbiota, metabolic, immune, psychological and cognitive parameters in young adult Cancer survivors; study protocol. BMC Cancer. 2019;19:1243. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 51] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 104. | Gao R, Wang Z, Li H, Cao Z, Gao Z, Chen H, Zhang X, Pan D, Yang R, Zhong H, Shen R, Yin L, Jia Z, Shen T, Qin N, Hu Z, Qin H. Gut microbiota dysbiosis signature is associated with the colorectal carcinogenesis sequence and improves the diagnosis of colorectal lesions. J Gastroenterol Hepatol. 2020;35:2109-2121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 29] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 105. | Garvey M. Intestinal Dysbiosis: Microbial Imbalance Impacts on Colorectal Cancer Initiation, Progression and Disease Mitigation. Biomedicines. 2024;12:740. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 106. | John Kenneth M, Tsai HC, Fang CY, Hussain B, Chiu YC, Hsu BM. Diet-mediated gut microbial community modulation and signature metabolites as potential biomarkers for early diagnosis, prognosis, prevention and stage-specific treatment of colorectal cancer. J Adv Res. 2023;52:45-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 10] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 107. | Quaglio AEV, Grillo TG, De Oliveira ECS, Di Stasi LC, Sassaki LY. Gut microbiota, inflammatory bowel disease and colorectal cancer. World J Gastroenterol. 2022;28:4053-4060. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 219] [Cited by in RCA: 220] [Article Influence: 73.3] [Reference Citation Analysis (115)] |
| 108. | Liu C, Fu L, Wang Y, Yang W. Influence of the gut microbiota on immune cell interactions and cancer treatment. J Transl Med. 2024;22:939. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 12] [Reference Citation Analysis (1)] |
| 109. | Oh B, Boyle F, Pavlakis N, Clarke S, Guminski A, Eade T, Lamoury G, Carroll S, Morgia M, Kneebone A, Hruby G, Stevens M, Liu W, Corless B, Molloy M, Libermann T, Rosenthal D, Back M. Emerging Evidence of the Gut Microbiome in Chemotherapy: A Clinical Review. Front Oncol. 2021;11:706331. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 31] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 110. | Yin B, Wang X, Yuan F, Li Y, Lu P. Research progress on the effect of gut and tumor microbiota on antitumor efficacy and adverse effects of chemotherapy drugs. Front Microbiol. 2022;13:899111. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 17] [Reference Citation Analysis (0)] |
| 111. | Bozzini C, Busti F, Marchi G, Vianello A, Cerchione C, Martinelli G, Girelli D. Anemia in patients receiving anticancer treatments: focus on novel therapeutic approaches. Front Oncol. 2024;14:1380358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 112. | Du Y, He C, An Y, Huang Y, Zhang H, Fu W, Wang M, Shan Z, Xie J, Yang Y, Zhao B. The Role of Short Chain Fatty Acids in Inflammation and Body Health. Int J Mol Sci. 2024;25:7379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 68] [Reference Citation Analysis (0)] |
| 113. | Zhang X, Huang JX, Tang M, Zhang Q, Deng L, Song CH, Li W, Yang M, Shi HP, Cong MH. A comprehensive analysis of the association between anemia and systemic inflammation in older patients with cancer. Support Care Cancer. 2023;32:39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 9] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 114. | Fan S, Jiang Z, Zhang Z, Xing J, Wang D, Tang D. Akkermansia muciniphila: a potential booster to improve the effectiveness of cancer immunotherapy. J Cancer Res Clin Oncol. 2023;149:13477-13494. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 8] [Article Influence: 4.0] [Reference Citation Analysis (2)] |
| 115. | Vergalito F, Bagnoli D, Maiuro L, Pannella G, Palombo V, Testa B, Coppola F, Di Marco RMA, Tremonte P, Lombardi SJ, Iorizzo M, Coppola R, Succi M. Akkermansia muciniphila: new insights into resistance to gastrointestinal stress, adhesion, and protein interaction with human mucins through optimised in vitro trials and bioinformatics tools. Front Microbiol. 2024;15:1462220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 116. | Cong J, Zhou P, Zhang R. Intestinal Microbiota-Derived Short Chain Fatty Acids in Host Health and Disease. Nutrients. 2022;14:1977. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 110] [Article Influence: 36.7] [Reference Citation Analysis (0)] |
| 117. | Manilla V, Di Tommaso N, Santopaolo F, Gasbarrini A, Ponziani FR. Endotoxemia and Gastrointestinal Cancers: Insight into the Mechanisms Underlying a Dangerous Relationship. Microorganisms. 2023;11:267. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 15] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 118. | Kandpal M, Indari O, Baral B, Jakhmola S, Tiwari D, Bhandari V, Pandey RK, Bala K, Sonawane A, Jha HC. Dysbiosis of Gut Microbiota from the Perspective of the Gut-Brain Axis: Role in the Provocation of Neurological Disorders. Metabolites. 2022;12:1064. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 60] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 119. | D'Aimmo MR, Satti M, Scarafile D, Modesto M, Pascarelli S, Biagini SA, Luiselli D, Mattarelli P, Andlid T. Folate-producing bifidobacteria: metabolism, genetics, and relevance. Microbiome Res Rep. 2024;3:11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (1)] |
| 120. | Malla MA, Dubey A, Kumar A, Yadav S, Hashem A, Abd Allah EF. Exploring the Human Microbiome: The Potential Future Role of Next-Generation Sequencing in Disease Diagnosis and Treatment. Front Immunol. 2018;9:2868. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 175] [Cited by in RCA: 178] [Article Influence: 29.7] [Reference Citation Analysis (0)] |
| 121. | Epstein-Peterson ZD, Li DG, Lavery JA, Barrow B, Chokshi I, Korenstein D. Inpatient folate testing at an academic cancer center: single-year experience. Support Care Cancer. 2020;28:4235-4240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (1)] |
| 122. | Kautz L, Jung G, Valore EV, Rivella S, Nemeth E, Ganz T. Identification of erythroferrone as an erythroid regulator of iron metabolism. Nat Genet. 2014;46:678-684. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 817] [Cited by in RCA: 823] [Article Influence: 74.8] [Reference Citation Analysis (0)] |
| 123. | Dinan TG, Cryan JF. The Microbiome-Gut-Brain Axis in Health and Disease. Gastroenterol Clin North Am. 2017;46:77-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 515] [Cited by in RCA: 680] [Article Influence: 85.0] [Reference Citation Analysis (0)] |
| 124. | Wang Z, Wang Q, Wang X, Zhu L, Chen J, Zhang B, Chen Y, Yuan Z. Gut microbial dysbiosis is associated with development and progression of radiation enteritis during pelvic radiotherapy. J Cell Mol Med. 2019;23:3747-3756. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 117] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 125. | Dubin K, Callahan MK, Ren B, Khanin R, Viale A, Ling L, No D, Gobourne A, Littmann E, Huttenhower C, Pamer EG, Wolchok JD. Intestinal microbiome analyses identify melanoma patients at risk for checkpoint-blockade-induced colitis. Nat Commun. 2016;7:10391. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 560] [Cited by in RCA: 769] [Article Influence: 85.4] [Reference Citation Analysis (0)] |
| 126. | Gomes AC, Moreira AC, Mesquita G, Gomes MS. Modulation of Iron Metabolism in Response to Infection: Twists for All Tastes. Pharmaceuticals (Basel). 2018;11:84. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 31] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 127. | Vitellio P, Celano G, Bonfrate L, Gobbetti M, Portincasa P, De Angelis M. Effects of Bifidobacterium longum and Lactobacillus rhamnosus on Gut Microbiota in Patients with Lactose Intolerance and Persisting Functional Gastrointestinal Symptoms: A Randomised, Double-Blind, Cross-Over Study. Nutrients. 2019;11:886. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 105] [Cited by in RCA: 78] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 128. | Tekin T, Dincer E. Effect of resistant starch types as a prebiotic. Appl Microbiol Biotechnol. 2023;107:491-515. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 16] [Reference Citation Analysis (0)] |
| 129. | Mousavinasab F, Karimi R, Taheri S, Ahmadvand F, Sanaaee S, Najafi S, Halvaii MS, Haghgoo A, Zamany M, Majidpoor J, Khosravifar M, Baniasadi M, Talebi M, Movafagh A, Aghaei-Zarch SM, Khorram N, Farnia P, Kalhor K. Microbiome modulation in inflammatory diseases: Progress to microbiome genetic engineering. Cancer Cell Int. 2023;23:271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 130. | Liu Y, Wang J, Wu C. Modulation of Gut Microbiota and Immune System by Probiotics, Pre-biotics, and Post-biotics. Front Nutr. 2021;8:634897. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 136] [Article Influence: 45.3] [Reference Citation Analysis (0)] |
| 131. | Cammarota G, Ianiro G, Tilg H, Rajilić-Stojanović M, Kump P, Satokari R, Sokol H, Arkkila P, Pintus C, Hart A, Segal J, Aloi M, Masucci L, Molinaro A, Scaldaferri F, Gasbarrini G, Lopez-Sanroman A, Link A, de Groot P, de Vos WM, Högenauer C, Malfertheiner P, Mattila E, Milosavljević T, Nieuwdorp M, Sanguinetti M, Simren M, Gasbarrini A; European FMT Working Group. European consensus conference on faecal microbiota transplantation in clinical practice. Gut. 2017;66:569-580. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 853] [Cited by in RCA: 784] [Article Influence: 98.0] [Reference Citation Analysis (1)] |
| 132. | Hourigan SK, Nicholson MR, Kahn SA, Kellermayer R. Updates and Challenges in Fecal Microbiota Transplantation for Clostridioides difficile Infection in Children. J Pediatr Gastroenterol Nutr. 2021;73:430-432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 133. | Cappellini MD, Comin-Colet J, de Francisco A, Dignass A, Doehner W, Lam CS, Macdougall IC, Rogler G, Camaschella C, Kadir R, Kassebaum NJ, Spahn DR, Taher AT, Musallam KM; IRON CORE Group. Iron deficiency across chronic inflammatory conditions: International expert opinion on definition, diagnosis, and management. Am J Hematol. 2017;92:1068-1078. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 187] [Cited by in RCA: 321] [Article Influence: 40.1] [Reference Citation Analysis (0)] |
| 134. | Ooi VY, Yeh TY. Recent Advances and Mechanisms of Phage-Based Therapies in Cancer Treatment. Int J Mol Sci. 2024;25:9938. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 135. | Fischbach MA, Segre JA. Signaling in Host-Associated Microbial Communities. Cell. 2016;164:1288-1300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 113] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 136. | Zitvogel L, Ayyoub M, Routy B, Kroemer G. Microbiome and Anticancer Immunosurveillance. Cell. 2016;165:276-287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 257] [Cited by in RCA: 356] [Article Influence: 39.6] [Reference Citation Analysis (0)] |
| 137. | Qiao B, Sugianto P, Fung E, Del-Castillo-Rueda A, Moran-Jimenez MJ, Ganz T, Nemeth E. Hepcidin-induced endocytosis of ferroportin is dependent on ferroportin ubiquitination. Cell Metab. 2012;15:918-924. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 217] [Cited by in RCA: 256] [Article Influence: 19.7] [Reference Citation Analysis (0)] |
| 138. | Short NJ, Rytting ME, Cortes JE. Acute myeloid leukaemia. Lancet. 2018;392:593-606. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 364] [Cited by in RCA: 548] [Article Influence: 78.3] [Reference Citation Analysis (0)] |
| 139. | Hunger SP, Mullighan CG. Acute Lymphoblastic Leukemia in Children. N Engl J Med. 2015;373:1541-1552. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1090] [Cited by in RCA: 1331] [Article Influence: 133.1] [Reference Citation Analysis (0)] |
| 140. | Minotti G, Menna P, Salvatorelli E, Cairo G, Gianni L. Anthracyclines: molecular advances and pharmacologic developments in antitumor activity and cardiotoxicity. Pharmacol Rev. 2004;56:185-229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2875] [Cited by in RCA: 2688] [Article Influence: 128.0] [Reference Citation Analysis (0)] |
| 141. | Blum W, Sanford BL, Klisovic R, DeAngelo DJ, Uy G, Powell BL, Stock W, Baer MR, Kolitz JE, Wang ES, Hoke E, Mrózek K, Kohlschmidt J, Bloomfield CD, Geyer S, Marcucci G, Stone RM, Larson RA; Alliance for Clinical Trials in Oncology. Maintenance therapy with decitabine in younger adults with acute myeloid leukemia in first remission: a phase 2 Cancer and Leukemia Group B Study (CALGB 10503). Leukemia. 2017;31:34-39. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 68] [Article Influence: 7.6] [Reference Citation Analysis (1)] |
| 142. | Taur Y, Jenq RR, Perales MA, Littmann ER, Morjaria S, Ling L, No D, Gobourne A, Viale A, Dahi PB, Ponce DM, Barker JN, Giralt S, van den Brink M, Pamer EG. The effects of intestinal tract bacterial diversity on mortality following allogeneic hematopoietic stem cell transplantation. Blood. 2014;124:1174-1182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 544] [Cited by in RCA: 671] [Article Influence: 61.0] [Reference Citation Analysis (2)] |
| 143. | Wang L, Gao R, Liu SF, Gao CJ. [Role of Intestinal Microbiota in Hematopoietic Stem Cell Transplantation - Review]. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2018;26:302-306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 144. | Smith PM, Howitt MR, Panikov N, Michaud M, Gallini CA, Bohlooly-Y M, Glickman JN, Garrett WS. The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science. 2013;341:569-573. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2937] [Cited by in RCA: 3948] [Article Influence: 329.0] [Reference Citation Analysis (1)] |
| 145. | Wang R, Yang X, Liu J, Zhong F, Zhang C, Chen Y, Sun T, Ji C, Ma D. Gut microbiota regulates acute myeloid leukaemia via alteration of intestinal barrier function mediated by butyrate. Nat Commun. 2022;13:2522. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 109] [Article Influence: 36.3] [Reference Citation Analysis (0)] |
| 146. | Rauf A, Khalil AA, Rahman UU, Khalid A, Naz S, Shariati MA, Rebezov M, Urtecho EZ, de Albuquerque RDDG, Anwar S, Alamri A, Saini RK, Rengasamy KRR. Recent advances in the therapeutic application of short-chain fatty acids (SCFAs): An updated review. Crit Rev Food Sci Nutr. 2022;62:6034-6054. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 93] [Article Influence: 23.3] [Reference Citation Analysis (0)] |
| 147. | Wang LY, He LH, Xu LJ, Li SB. Short-chain fatty acids: bridges between diet, gut microbiota, and health. J Gastroenterol Hepatol. 2024;39:1728-1736. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 21] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 148. | Franchini M, Montagnana M, Lippi G. Hepcidin and iron metabolism: from laboratory to clinical implications. Clin Chim Acta. 2010;411:1565-1569. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 44] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 149. | Schmidt PJ. Regulation of Iron Metabolism by Hepcidin under Conditions of Inflammation. J Biol Chem. 2015;290:18975-18983. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 113] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 150. | Belkaid Y, Hand TW. Role of the microbiota in immunity and inflammation. Cell. 2014;157:121-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2488] [Cited by in RCA: 3424] [Article Influence: 311.3] [Reference Citation Analysis (1)] |
| 151. | Lozupone CA, Stombaugh JI, Gordon JI, Jansson JK, Knight R. Diversity, stability and resilience of the human gut microbiota. Nature. 2012;489:220-230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3051] [Cited by in RCA: 3642] [Article Influence: 280.2] [Reference Citation Analysis (0)] |
| 152. | Turizo-Smith AD, Córdoba-Hernandez S, Mejía-Guarnizo LV, Monroy-Camacho PS, Rodríguez-García JA. Inflammation and cancer: friend or foe? Front Pharmacol. 2024;15:1385479. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 17] [Reference Citation Analysis (0)] |
| 153. | Schumann RR. Old and new findings on lipopolysaccharide-binding protein: a soluble pattern-recognition molecule. Biochem Soc Trans. 2011;39:989-993. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 122] [Article Influence: 8.7] [Reference Citation Analysis (1)] |
| 154. | Lin Z, Mao D, Jin C, Wang J, Lai Y, Zhang Y, Zhou M, Ge Q, Zhang P, Sun Y, Xu K, Wang Y, Zhu H, Lai B, Wu H, Mu Q, Ouyang G, Sheng L. The gut microbiota correlate with the disease characteristics and immune status of patients with untreated diffuse large B-cell lymphoma. Front Immunol. 2023;14:1105293. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 13] [Reference Citation Analysis (1)] |
| 155. | Chong PP, Koh AY. The gut microbiota in transplant patients. Blood Rev. 2020;39:100614. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 31] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 156. | Liu L, Yu Z, Cheng H, Mao X, Sui W, Deng S, Wei X, Lv J, Du C, Xu J, Huang W, Xia S, An G, Zhou W, Ma X, Cheng T, Qiu L, Hao M. Multiple myeloma hinders erythropoiesis and causes anaemia owing to high levels of CCL3 in the bone marrow microenvironment. Sci Rep. 2020;10:20508. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 34] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 157. | Bouchnita A, Eymard N, Moyo TK, Koury MJ, Volpert V. Bone marrow infiltration by multiple myeloma causes anemia by reversible disruption of erythropoiesis. Am J Hematol. 2016;91:371-378. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 25] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 158. | Rodríguez-García A, Arroyo A, García-Vicente R, Morales ML, Gómez-Gordo R, Justo P, Cuéllar C, Sánchez-Pina J, López N, Alonso R, Puig N, Mateos MV, Ayala R, Gómez-Garre D, Martínez-López J, Linares M. Short-Chain Fatty Acid Production by Gut Microbiota Predicts Treatment Response in Multiple Myeloma. Clin Cancer Res. 2024;30:904-917. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 21] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 159. | Mann ER, Lam YK, Uhlig HH. Short-chain fatty acids: linking diet, the microbiome and immunity. Nat Rev Immunol. 2024;24:577-595. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 308] [Article Influence: 308.0] [Reference Citation Analysis (0)] |
| 160. | Zhang L, Xiang Y, Li Y, Zhang J. Gut microbiome in multiple myeloma: Mechanisms of progression and clinical applications. Front Immunol. 2022;13:1058272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 161. | Al-Qadami GH, Secombe KR, Subramaniam CB, Wardill HR, Bowen JM. Gut Microbiota-Derived Short-Chain Fatty Acids: Impact on Cancer Treatment Response and Toxicities. Microorganisms. 2022;10:2048. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 79] [Reference Citation Analysis (0)] |
| 162. | Brevi A, Cogrossi LL, Lorenzoni M, Mattorre B, Bellone M. The Insider: Impact of the Gut Microbiota on Cancer Immunity and Response to Therapies in Multiple Myeloma. Front Immunol. 2022;13:845422. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 14] [Reference Citation Analysis (4)] |
| 163. | Thoda C, Touraki M. Probiotic-Derived Bioactive Compounds in Colorectal Cancer Treatment. Microorganisms. 2023;11:1898. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 164. | Huang F, Li S, Chen W, Han Y, Yao Y, Yang L, Li Q, Xiao Q, Wei J, Liu Z, Chen T, Deng X. Postoperative Probiotics Administration Attenuates Gastrointestinal Complications and Gut Microbiota Dysbiosis Caused by Chemotherapy in Colorectal Cancer Patients. Nutrients. 2023;15:356. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 72] [Reference Citation Analysis (0)] |
| 165. | Gilliam CH, Brazelton de Cardenas J, Carias D, Maron Alfaro G, Hayden RT, Hakim H. Lactobacillus bloodstream infections genetically related to probiotic use in pediatric hematopoietic cell transplant patients. Infect Control Hosp Epidemiol. 2023;44:484-487. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 11] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 166. | Holzner B, Kemmler G, Greil R, Kopp M, Zeimet A, Raderer M, Hejna M, Zöchbauer S, Krajnik G, Huber H, Fleischhacker WW, Sperner-Unterweger B. The impact of hemoglobin levels on fatigue and quality of life in cancer patients. Ann Oncol. 2002;13:965-973. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 103] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 167. | Barca-Hernando M, Muñoz-Martin AJ, Rios-Herranz E, Garcia-Escobar I, Beato C, Font C, Oncala-Sibajas E, Revuelta-Rodriguez A, Areses MC, Rivas-Jimenez V, Ballaz-Quincoces A, Moreno-Santos MA, Lopez-Saez JB, Gallego-Gallego I, Elias-Hernandez T, Asensio-Cruz MI, Chasco-Eguilaz L, Garcia-Gonzalez G, Estevez-Garcia P, Marin-Barrera L, Otero-Candelera R, Lopez-Ruz S, Lima-Alvarez J, Sanchez-Diaz JM, Real-Dominguez M, Borrego-Delgado MC, Marin-Romero S, Jara-Palomares L. Case-Control Analysis of the Impact of Anemia on Quality of Life in Patients with Cancer: A Qca Study Analysis. Cancers (Basel). 2021;13:2517. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (4)] |
| 168. | Taurisano P, De Feudis RL, Graziano G, Marzano N, Curci A, Fidanzio A, Annunziata MA, Antinone V, Brovelli S, Carone M, Cavanna L, Cormio C, Cuomo A, Mattei VD, Silvestre AD, Lettini A, Petrone A, Scriminaci MC, Tralongo P, Caro MFD, Lanciano T. Patient-caregiver relationship in cancer fatigue and distress. A dyadic approach. Curr Psychol. 2023;42:28167-28179. [RCA] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (1)] |
| 169. | Berger AM, Mooney K, Alvarez-Perez A, Breitbart WS, Carpenter KM, Cella D, Cleeland C, Dotan E, Eisenberger MA, Escalante CP, Jacobsen PB, Jankowski C, LeBlanc T, Ligibel JA, Loggers ET, Mandrell B, Murphy BA, Palesh O, Pirl WF, Plaxe SC, Riba MB, Rugo HS, Salvador C, Wagner LI, Wagner-Johnston ND, Zachariah FJ, Bergman MA, Smith C; National comprehensive cancer network. Cancer-Related Fatigue, Version 2.2015. J Natl Compr Canc Netw. 2015;13:1012-1039. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 412] [Cited by in RCA: 597] [Article Influence: 66.3] [Reference Citation Analysis (0)] |
| 170. | Petrov G, Dymova M, Richter V. Bacteriophage-Mediated Cancer Gene Therapy. Int J Mol Sci. 2022;23:14245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 17] [Reference Citation Analysis (1)] |
| 171. | Muz B, de la Puente P, Azab F, Azab AK. The role of hypoxia in cancer progression, angiogenesis, metastasis, and resistance to therapy. Hypoxia (Auckl). 2015;3:83-92. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 842] [Cited by in RCA: 1399] [Article Influence: 139.9] [Reference Citation Analysis (1)] |
| 172. | Mahdy MS, Azmy AF, Dishisha T, Mohamed WR, Ahmed KA, Hassan A, Aidy SE, El-Gendy AO. Irinotecan-gut microbiota interactions and the capability of probiotics to mitigate Irinotecan-associated toxicity. BMC Microbiol. 2023;23:53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 38] [Reference Citation Analysis (0)] |
| 173. | Naqvi AAT, Fatima K, Mohammad T, Fatima U, Singh IK, Singh A, Atif SM, Hariprasad G, Hasan GM, Hassan MI. Insights into SARS-CoV-2 genome, structure, evolution, pathogenesis and therapies: Structural genomics approach. Biochim Biophys Acta Mol Basis Dis. 2020;1866:165878. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 492] [Cited by in RCA: 670] [Article Influence: 134.0] [Reference Citation Analysis (1)] |
| 174. | Chamseddine AN, Ducreux M, Armand JP, Paoletti X, Satar T, Paci A, Mir O. Intestinal bacterial β-glucuronidase as a possible predictive biomarker of irinotecan-induced diarrhea severity. Pharmacol Ther. 2019;199:1-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 77] [Article Influence: 12.8] [Reference Citation Analysis (3)] |
| 175. | Tanaka H, Ono T, Manabe Y, Kajima M, Fujimoto K, Yuasa Y, Shiinoki T, Yamaji Y, Matsunaga K. Anemia is a Prognostic Factor for Overall Survival Rate in Patients with Non-Small Cell Lung Cancer Treated with Stereotactic Body Radiation Therapy. Cancer Manag Res. 2021;13:7447-7453. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 176. | Liu Z, Sun R, Li J, Cheng W, Li L. Relations of Anemia With the All-Cause Mortality and Cardiovascular Mortality in General Population: A Meta-Analysis. Am J Med Sci. 2019;358:191-199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 34] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 177. | Kaiafa G, Kanellos I, Savopoulos C, Kakaletsis N, Giannakoulas G, Hatzitolios AI. Is anemia a new cardiovascular risk factor? Int J Cardiol. 2015;186:117-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 59] [Article Influence: 5.9] [Reference Citation Analysis (1)] |
| 178. | Conde Díez S, de Las Cuevas Allende R, Conde García E. Anemia of inflammation and iron metabolism in chronic diseases. Rev Clin Esp (Barc). 2024;224:598-608. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (1)] |
| 179. | Lanser L, Fuchs D, Kurz K, Weiss G. Physiology and Inflammation Driven Pathophysiology of Iron Homeostasis-Mechanistic Insights into Anemia of Inflammation and Its Treatment. Nutrients. 2021;13:3732. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 72] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 180. | Macciò A, Madeddu C, Gramignano G, Mulas C, Tanca L, Cherchi MC, Floris C, Omoto I, Barracca A, Ganz T. The role of inflammation, iron, and nutritional status in cancer-related anemia: results of a large, prospective, observational study. Haematologica. 2015;100:124-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 152] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 181. | Guglielmi G. Gut microbiome discovery provides roadmap for life-saving cancer therapies. Nature. 2024;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 182. | Capurso L. Thirty Years of Lactobacillus rhamnosus GG: A Review. J Clin Gastroenterol. 2019;53 Suppl 1:S1-S41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 219] [Article Influence: 36.5] [Reference Citation Analysis (0)] |
| 183. | Banna GL, Torino F, Marletta F, Santagati M, Salemi R, Cannarozzo E, Falzone L, Ferraù F, Libra M. Lactobacillus rhamnosus GG: An Overview to Explore the Rationale of Its Use in Cancer. Front Pharmacol. 2017;8:603. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 100] [Article Influence: 12.5] [Reference Citation Analysis (1)] |
| 184. | Ballini A, Charitos IA, Cantore S, Topi S, Bottalico L, Santacroce L. About Functional Foods: The Probiotics and Prebiotics State of Art. Antibiotics (Basel). 2023;12:635. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 41] [Reference Citation Analysis (0)] |
| 185. | Feng W, Liu J, Ao H, Yue S, Peng C. Targeting gut microbiota for precision medicine: Focusing on the efficacy and toxicity of drugs. Theranostics. 2020;10:11278-11301. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 71] [Article Influence: 14.2] [Reference Citation Analysis (1)] |
| 186. | Kiousi DE, Kouroutzidou AZ, Neanidis K, Karavanis E, Matthaios D, Pappa A, Galanis A. The Role of the Gut Microbiome in Cancer Immunotherapy: Current Knowledge and Future Directions. Cancers (Basel). 2023;15:2101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 21] [Reference Citation Analysis (1)] |
| 187. | Rusu IG, Suharoschi R, Vodnar DC, Pop CR, Socaci SA, Vulturar R, Istrati M, Moroșan I, Fărcaș AC, Kerezsi AD, Mureșan CI, Pop OL. Iron Supplementation Influence on the Gut Microbiota and Probiotic Intake Effect in Iron Deficiency-A Literature-Based Review. Nutrients. 2020;12:1993. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 93] [Cited by in RCA: 93] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 188. | Koker G, Sahinturk Y, Ozcelik Koker G, Coskuner MA, Eren Durmus M, Catli MM, Cekin AH. Improved gastrointestinal tolerance and iron status via probiotic use in iron deficiency anaemia patients initiating oral iron replacement: a randomised controlled trial. Br J Nutr. 2024;132:1308-1316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 189. | Singh A, Alexander SG, Martin S. Gut microbiome homeostasis and the future of probiotics in cancer immunotherapy. Front Immunol. 2023;14:1114499. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 20] [Reference Citation Analysis (0)] |
| 190. | Ratajczak W, Rył A, Mizerski A, Walczakiewicz K, Sipak O, Laszczyńska M. Immunomodulatory potential of gut microbiome-derived short-chain fatty acids (SCFAs). Acta Biochim Pol. 2019;66:1-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 202] [Article Influence: 33.7] [Reference Citation Analysis (0)] |
| 191. | Maftei NM, Raileanu CR, Balta AA, Ambrose L, Boev M, Marin DB, Lisa EL. The Potential Impact of Probiotics on Human Health: An Update on Their Health-Promoting Properties. Microorganisms. 2024;12:234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 107] [Article Influence: 107.0] [Reference Citation Analysis (0)] |
| 192. | Rashidi A, Ebadi M, Rehman TU, Elhusseini H, Kazadi D, Halaweish H, Khan MH, Hoeschen A, Cao Q, Luo X, Kabage AJ, Lopez S, Holtan SG, Weisdorf DJ, Khoruts A, Staley C. Randomized Double-Blind Phase II Trial of Fecal Microbiota Transplantation Versus Placebo in Allogeneic Hematopoietic Cell Transplantation and AML. J Clin Oncol. 2023;41:5306-5319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 30] [Article Influence: 15.0] [Reference Citation Analysis (1)] |
| 193. | Zhang X, Luo X, Tian L, Yue P, Li M, Liu K, Zhu D, Huang C, Shi Q, Yang L, Xia Z, Zhao J, Ma Z, Li J, Leung JW, Lin Y, Yuan J, Meng W, Li X, Chen Y. The gut microbiome dysbiosis and regulation by fecal microbiota transplantation: umbrella review. Front Microbiol. 2023;14:1286429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 194. | Hamamah S, Gheorghita R, Lobiuc A, Sirbu IO, Covasa M. Fecal microbiota transplantation in non-communicable diseases: Recent advances and protocols. Front Med (Lausanne). 2022;9:1060581. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 23] [Reference Citation Analysis (0)] |
| 195. | Zhao Y, Gong C, Xu J, Chen D, Yang B, Chen Z, Wei L. Research Progress of Fecal Microbiota Transplantation in Liver Diseases. J Clin Med. 2023;12:1683. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 25] [Reference Citation Analysis (0)] |
| 196. | Yang YP, Zhang XY, Cui BT, Zhang H, Wu ZF, Tan X, Cheng W, Zhu X, Zhang FM, Qin HL, Wei H. Preclinical safety, effectiveness evaluation, and screening of functional bacteria for fecal microbiota transplantation based on germ-free animals. World J Meta-Anal. 2021;9:496-504. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (2)] |
| 197. | Xu B, Fu Y, Yin N, Qin W, Huang Z, Xiao W, Huang H, Mei Q, Fan J, Zeng Y, Huang C. Bacteroides thetaiotaomicron and Faecalibacterium prausnitzii served as key components of fecal microbiota transplantation to alleviate colitis. Am J Physiol Gastrointest Liver Physiol. 2024;326:G607-G621. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (1)] |
| 198. | Clarke G, Sandhu KV, Griffin BT, Dinan TG, Cryan JF, Hyland NP. Gut Reactions: Breaking Down Xenobiotic-Microbiome Interactions. Pharmacol Rev. 2019;71:198-224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 240] [Cited by in RCA: 198] [Article Influence: 33.0] [Reference Citation Analysis (2)] |
| 199. | Vinelli V, Biscotti P, Martini D, Del Bo' C, Marino M, Meroño T, Nikoloudaki O, Calabrese FM, Turroni S, Taverniti V, Unión Caballero A, Andrés-Lacueva C, Porrini M, Gobbetti M, De Angelis M, Brigidi P, Pinart M, Nimptsch K, Guglielmetti S, Riso P. Effects of Dietary Fibers on Short-Chain Fatty Acids and Gut Microbiota Composition in Healthy Adults: A Systematic Review. Nutrients. 2022;14:2559. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 99] [Cited by in RCA: 86] [Article Influence: 28.7] [Reference Citation Analysis (0)] |
| 200. | Oliver A, Chase AB, Weihe C, Orchanian SB, Riedel SF, Hendrickson CL, Lay M, Sewall JM, Martiny JBH, Whiteson K. High-Fiber, Whole-Food Dietary Intervention Alters the Human Gut Microbiome but Not Fecal Short-Chain Fatty Acids. mSystems. 2021;6:e00115-21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 95] [Article Influence: 23.8] [Reference Citation Analysis (1)] |
| 201. | Thomson C, Garcia AL, Edwards CA. Interactions between dietary fibre and the gut microbiota. Proc Nutr Soc. 2021;1-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 31] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 202. | He J, Zhang P, Shen L, Niu L, Tan Y, Chen L, Zhao Y, Bai L, Hao X, Li X, Zhang S, Zhu L. Short-Chain Fatty Acids and Their Association with Signalling Pathways in Inflammation, Glucose and Lipid Metabolism. Int J Mol Sci. 2020;21:6356. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 127] [Cited by in RCA: 546] [Article Influence: 109.2] [Reference Citation Analysis (0)] |
| 203. | Karamantziani T, Pouliakis A, Xanthos T, Ekmektzoglou K, Paliatsiou S, Sokou R, Iacovidou N. The Effect of Oral Iron Supplementation/Fortification on the Gut Microbiota in Infancy: A Systematic Review and Meta-Analysis. Children (Basel). 2024;11:231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 204. | Paganini D, Uyoga MA, Kortman GAM, Cercamondi CI, Moretti D, Barth-Jaeggi T, Schwab C, Boekhorst J, Timmerman HM, Lacroix C, Karanja S, Zimmermann MB. Prebiotic galacto-oligosaccharides mitigate the adverse effects of iron fortification on the gut microbiome: a randomised controlled study in Kenyan infants. Gut. 2017;66:1956-1967. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 133] [Article Influence: 16.6] [Reference Citation Analysis (1)] |
| 205. | Mikulic N, Uyoga MA, Stoffel NU, Derrien M, Nyilima S, Kostopoulos I, Roeselers G, Chenoll E, Mwasi E, Pironaci G, Karanja S, Bourdet-Sicard R, Zimmermann MB. Prebiotics increase iron absorption and reduce the adverse effects of iron on the gut microbiome and inflammation: a randomized controlled trial using iron stable isotopes in Kenyan infants. Am J Clin Nutr. 2024;119:456-469. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 9] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 206. | Kaewarsar E, Chaiyasut C, Lailerd N, Makhamrueang N, Peerajan S, Sirilun S. Optimization of Mixed Inulin, Fructooligosaccharides, and Galactooligosaccharides as Prebiotics for Stimulation of Probiotics Growth and Function. Foods. 2023;12:1591. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 20] [Reference Citation Analysis (0)] |
| 207. | Spencer CN, McQuade JL, Gopalakrishnan V, McCulloch JA, Vetizou M, Cogdill AP, Khan MAW, Zhang X, White MG, Peterson CB, Wong MC, Morad G, Rodgers T, Badger JH, Helmink BA, Andrews MC, Rodrigues RR, Morgun A, Kim YS, Roszik J, Hoffman KL, Zheng J, Zhou Y, Medik YB, Kahn LM, Johnson S, Hudgens CW, Wani K, Gaudreau PO, Harris AL, Jamal MA, Baruch EN, Perez-Guijarro E, Day CP, Merlino G, Pazdrak B, Lochmann BS, Szczepaniak-Sloane RA, Arora R, Anderson J, Zobniw CM, Posada E, Sirmans E, Simon J, Haydu LE, Burton EM, Wang L, Dang M, Clise-Dwyer K, Schneider S, Chapman T, Anang NAS, Duncan S, Toker J, Malke JC, Glitza IC, Amaria RN, Tawbi HA, Diab A, Wong MK, Patel SP, Woodman SE, Davies MA, Ross MI, Gershenwald JE, Lee JE, Hwu P, Jensen V, Samuels Y, Straussman R, Ajami NJ, Nelson KC, Nezi L, Petrosino JF, Futreal PA, Lazar AJ, Hu J, Jenq RR, Tetzlaff MT, Yan Y, Garrett WS, Huttenhower C, Sharma P, Watowich SS, Allison JP, Cohen L, Trinchieri G, Daniel CR, Wargo JA. Dietary fiber and probiotics influence the gut microbiome and melanoma immunotherapy response. Science. 2021;374:1632-1640. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 443] [Cited by in RCA: 539] [Article Influence: 134.8] [Reference Citation Analysis (0)] |
| 208. | Apte A, Parge A, Nimkar R, Sinha A. Effect of probiotic and prebiotics supplementation on hemoglobin levels and iron absorption among women of reproductive age and children: a systematic review and meta-analysis. BMC Nutr. 2025;11:31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (1)] |
| 209. | Zakrzewska Z, Zawartka A, Schab M, Martyniak A, Skoczeń S, Tomasik PJ, Wędrychowicz A. Prebiotics, Probiotics, and Postbiotics in the Prevention and Treatment of Anemia. Microorganisms. 2022;10:1330. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 25] [Article Influence: 8.3] [Reference Citation Analysis (1)] |
| 210. | Zhong Y, Cao J, Ma Y, Zhang Y, Liu J, Wang H. Fecal Microbiota Transplantation Donor and Dietary Fiber Intervention Collectively Contribute to Gut Health in a Mouse Model. Front Immunol. 2022;13:842669. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 7] [Article Influence: 2.3] [Reference Citation Analysis (1)] |
| 211. | Opoku-Acheampong I, McLaud T, Anderson OS. Fecal Microbiota Transplantation to Prevent and Treat Chronic Disease: Implications for Dietetics Practice. J Acad Nutr Diet. 2022;122:33-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (1)] |
| 212. | Zmora N, Suez J, Elinav E. You are what you eat: diet, health and the gut microbiota. Nat Rev Gastroenterol Hepatol. 2019;16:35-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 626] [Cited by in RCA: 1097] [Article Influence: 182.8] [Reference Citation Analysis (2)] |
| 213. | Ouwehand AC. A review of dose-responses of probiotics in human studies. Benef Microbes. 2017;8:143-151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 141] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 214. | Matson V, Fessler J, Bao R, Chongsuwat T, Zha Y, Alegre ML, Luke JJ, Gajewski TF. The commensal microbiome is associated with anti-PD-1 efficacy in metastatic melanoma patients. Science. 2018;359:104-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1390] [Cited by in RCA: 2126] [Article Influence: 303.7] [Reference Citation Analysis (1)] |
| 215. | ElRakaiby M, Dutilh BE, Rizkallah MR, Boleij A, Cole JN, Aziz RK. Pharmacomicrobiomics: the impact of human microbiome variations on systems pharmacology and personalized therapeutics. OMICS. 2014;18:402-414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 112] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 216. | Bharti R, Grimm DG. Current challenges and best-practice protocols for microbiome analysis. Brief Bioinform. 2021;22:178-193. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 297] [Cited by in RCA: 294] [Article Influence: 73.5] [Reference Citation Analysis (0)] |
| 217. | Suez J, Zmora N, Segal E, Elinav E. The pros, cons, and many unknowns of probiotics. Nat Med. 2019;25:716-729. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 405] [Cited by in RCA: 837] [Article Influence: 139.5] [Reference Citation Analysis (0)] |
| 218. | Derosa L, Iebba V, Silva CAC, Piccinno G, Wu G, Lordello L, Routy B, Zhao N, Thelemaque C, Birebent R, Marmorino F, Fidelle M, Messaoudene M, Thomas AM, Zalcman G, Friard S, Mazieres J, Audigier-Valette C, Sibilot DM, Goldwasser F, Scherpereel A, Pegliasco H, Ghiringhelli F, Bouchard N, Sow C, Darik I, Zoppi S, Ly P, Reni A, Daillère R, Deutsch E, Lee KA, Bolte LA, Björk JR, Weersma RK, Barlesi F, Padilha L, Finzel A, Isaksen ML, Escudier B, Albiges L, Planchard D, André F, Cremolini C, Martinez S, Besse B, Zhao L, Segata N, Wojcik J, Kroemer G, Zitvogel L. Custom scoring based on ecological topology of gut microbiota associated with cancer immunotherapy outcome. Cell. 2024;187:3373-3389.e16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 64] [Article Influence: 64.0] [Reference Citation Analysis (0)] |
| 219. | Kashyap PC, Chia N, Nelson H, Segal E, Elinav E. Microbiome at the Frontier of Personalized Medicine. Mayo Clin Proc. 2017;92:1855-1864. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 135] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 220. | Rivera K, Gonzalez L, Bravo L, Manjarres L, Andia ME. The Gut-Heart Axis: Molecular Perspectives and Implications for Myocardial Infarction. Int J Mol Sci. 2024;25:12465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 221. | Tan P, Li X, Shen J, Feng Q. Fecal Microbiota Transplantation for the Treatment of Inflammatory Bowel Disease: An Update. Front Pharmacol. 2020;11:574533. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 70] [Article Influence: 14.0] [Reference Citation Analysis (3)] |
| 222. | Mousa WK, Al Ali A. The Gut Microbiome Advances Precision Medicine and Diagnostics for Inflammatory Bowel Diseases. Int J Mol Sci. 2024;25:11259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 223. | Severyn CJ, Brewster R, Andermann TM. Microbiota modification in hematology: still at the bench or ready for the bedside? Hematology Am Soc Hematol Educ Program. 2019;2019:303-314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 224. | Wu J, Huang H, Wang L, Gao M, Meng S, Zou S, Feng Y, Feng Z, Zhu Z, Cao X, Li B, Kang G. A tailored series of engineered yeasts for the cell-dependent treatment of inflammatory bowel disease by rational butyrate supplementation. Gut Microbes. 2024;16:2316575. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 225. | Ma J, Lyu Y, Liu X, Jia X, Cui F, Wu X, Deng S, Yue C. Engineered probiotics. Microb Cell Fact. 2022;21:72. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 46] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 226. | Malard F, Mohty M. Let's reconstitute microbiota diversity. Blood. 2021;137:1442-1444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 227. | Bolocan AS, Callanan J, Forde A, Ross P, Hill C. Phage therapy targeting Escherichia coli-a story with no end? FEMS Microbiol Lett. 2016;363:fnw256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 31] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 228. | Bayat F, Hilal A, Thirugnanasampanthar M, Tremblay D, Filipe CDM, Moineau S, Didar TF, Hosseinidoust Z. High throughput platform technology for rapid target identification in personalized phage therapy. Nat Commun. 2024;15:5626. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 229. | Scott BM, Gutiérrez-Vázquez C, Sanmarco LM, da Silva Pereira JA, Li Z, Plasencia A, Hewson P, Cox LM, O'Brien M, Chen SK, Moraes-Vieira PM, Chang BSW, Peisajovich SG, Quintana FJ. Self-tunable engineered yeast probiotics for the treatment of inflammatory bowel disease. Nat Med. 2021;27:1212-1222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 170] [Article Influence: 42.5] [Reference Citation Analysis (0)] |
| 230. | Pesce M, Seguella L, Del Re A, Lu J, Palenca I, Corpetti C, Rurgo S, Sanseverino W, Sarnelli G, Esposito G. Next-Generation Probiotics for Inflammatory Bowel Disease. Int J Mol Sci. 2022;23:5466. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 33] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 231. | Barra M, Danino T, Garrido D. Engineered Probiotics for Detection and Treatment of Inflammatory Intestinal Diseases. Front Bioeng Biotechnol. 2020;8:265. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 53] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 232. | Phipps O, Al-Hassi HO, Quraishi MN, Kumar A, Brookes MJ. Influence of Iron on the Gut Microbiota in Colorectal Cancer. Nutrients. 2020;12:2512. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 27] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 233. | Formiga R, Sokol H. Faecalibacterium prausnitzii: one species with multiple potential implications in cancer research. Gut. 2024;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 234. | Shi J, Shen H, Huang H, Zhan L, Chen W, Zhou Z, Lv Y, Xiong K, Jiang Z, Chen Q, Liu L. Gut microbiota characteristics of colorectal cancer patients in Hubei, China, and differences with cohorts from other Chinese regions. Front Microbiol. 2024;15:1395514. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 235. | Papoutsoglou G, Tarazona S, Lopes MB, Klammsteiner T, Ibrahimi E, Eckenberger J, Novielli P, Tonda A, Simeon A, Shigdel R, Béreux S, Vitali G, Tangaro S, Lahti L, Temko A, Claesson MJ, Berland M. Machine learning approaches in microbiome research: challenges and best practices. Front Microbiol. 2023;14:1261889. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 36] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 236. | Hernández Medina R, Kutuzova S, Nielsen KN, Johansen J, Hansen LH, Nielsen M, Rasmussen S. Machine learning and deep learning applications in microbiome research. ISME Commun. 2022;2:98. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 120] [Article Influence: 40.0] [Reference Citation Analysis (1)] |
| 237. | Marcos-Zambrano LJ, López-Molina VM, Bakir-Gungor B, Frohme M, Karaduzovic-Hadziabdic K, Klammsteiner T, Ibrahimi E, Lahti L, Loncar-Turukalo T, Dhamo X, Simeon A, Nechyporenko A, Pio G, Przymus P, Sampri A, Trajkovik V, Lacruz-Pleguezuelos B, Aasmets O, Araujo R, Anagnostopoulos I, Aydemir Ö, Berland M, Calle ML, Ceci M, Duman H, Gündoğdu A, Havulinna AS, Kaka Bra KHN, Kalluci E, Karav S, Lode D, Lopes MB, May P, Nap B, Nedyalkova M, Paciência I, Pasic L, Pujolassos M, Shigdel R, Susín A, Thiele I, Truică CO, Wilmes P, Yilmaz E, Yousef M, Claesson MJ, Truu J, Carrillo de Santa Pau E. A toolbox of machine learning software to support microbiome analysis. Front Microbiol. 2023;14:1250806. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 11] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 238. | Chambers ES, Preston T, Frost G, Morrison DJ. Role of Gut Microbiota-Generated Short-Chain Fatty Acids in Metabolic and Cardiovascular Health. Curr Nutr Rep. 2018;7:198-206. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 289] [Cited by in RCA: 439] [Article Influence: 73.2] [Reference Citation Analysis (0)] |
| 239. | Soriano-Lerma A, García-Burgos M, Alférez MJM, Pérez-Carrasco V, Sanchez-Martin V, Linde-Rodríguez Á, Ortiz-González M, Soriano M, García-Salcedo JA, López-Aliaga I. Gut microbiome-short-chain fatty acids interplay in the context of iron deficiency anaemia. Eur J Nutr. 2022;61:399-412. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 28] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 240. | Haghikia A, Zimmermann F, Schumann P, Jasina A, Roessler J, Schmidt D, Heinze P, Kaisler J, Nageswaran V, Aigner A, Ceglarek U, Cineus R, Hegazy AN, van der Vorst EPC, Döring Y, Strauch CM, Nemet I, Tremaroli V, Dwibedi C, Kränkel N, Leistner DM, Heimesaat MM, Bereswill S, Rauch G, Seeland U, Soehnlein O, Müller DN, Gold R, Bäckhed F, Hazen SL, Haghikia A, Landmesser U. Propionate attenuates atherosclerosis by immune-dependent regulation of intestinal cholesterol metabolism. Eur Heart J. 2022;43:518-533. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 189] [Article Influence: 47.3] [Reference Citation Analysis (0)] |
| 241. | Parada Venegas D, De la Fuente MK, Landskron G, González MJ, Quera R, Dijkstra G, Harmsen HJM, Faber KN, Hermoso MA. Short Chain Fatty Acids (SCFAs)-Mediated Gut Epithelial and Immune Regulation and Its Relevance for Inflammatory Bowel Diseases. Front Immunol. 2019;10:277. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 970] [Cited by in RCA: 2175] [Article Influence: 362.5] [Reference Citation Analysis (0)] |
| 242. | Dai JH, Tan XR, Qiao H, Liu N. Emerging clinical relevance of microbiome in cancer: promising biomarkers and therapeutic targets. Protein Cell. 2024;15:239-260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 27] [Article Influence: 27.0] [Reference Citation Analysis (0)] |
| 243. | Stone E, Campbell K, Grant I, McAuliffe O. Understanding and Exploiting Phage-Host Interactions. Viruses. 2019;11:567. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 67] [Cited by in RCA: 138] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 244. | Qasem HH, El-Sayed WM. The bacterial microbiome and cancer: development, diagnosis, treatment, and future directions. Clin Exp Med. 2024;25:12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 10] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 245. | Islam MS, Fan J, Pan F. The power of phages: revolutionizing cancer treatment. Front Oncol. 2023;13:1290296. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 17] [Reference Citation Analysis (0)] |
| 246. | Zhou T, Wu J, Tang H, Liu D, Jeon BH, Jin W, Wang Y, Zheng Y, Khan A, Han H, Li X. Enhancing tumor-specific recognition of programmable synthetic bacterial consortium for precision therapy of colorectal cancer. NPJ Biofilms Microbiomes. 2024;10:6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 247. | Ciont C, Mesaroș A, Pop OL, Vodnar DC. Iron oxide nanoparticles carried by probiotics for iron absorption: a systematic review. J Nanobiotechnology. 2023;21:124. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 248. | Varvara RA, Vodnar DC. Probiotic-driven advancement: Exploring the intricacies of mineral absorption in the human body. Food Chem X. 2024;21:101067. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 9] [Reference Citation Analysis (1)] |
| 249. | Reddi S, Senyshyn L, Ebadi M, Podlesny D, Minot SS, Gooley T, Kabage AJ, Hill GR, Lee SJ, Khoruts A, Rashidi A. Fecal microbiota transplantation to prevent acute graft-versus-host disease: pre-planned interim analysis of donor effect. Nat Commun. 2025;16:1034. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 6.0] [Reference Citation Analysis (1)] |
| 250. | Lee JY, Kim Y, Kim J, Kim JK. Fecal Microbiota Transplantation: Indications, Methods, and Challenges. J Microbiol. 2024;62:1057-1074. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 251. | Yadegar A, Bar-Yoseph H, Monaghan TM, Pakpour S, Severino A, Kuijper EJ, Smits WK, Terveer EM, Neupane S, Nabavi-Rad A, Sadeghi J, Cammarota G, Ianiro G, Nap-Hill E, Leung D, Wong K, Kao D. Fecal microbiota transplantation: current challenges and future landscapes. Clin Microbiol Rev. 2024;37:e0006022. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 65] [Article Influence: 65.0] [Reference Citation Analysis (0)] |
| 252. | Beane KE, Redding MC, Wang X, Pan JH, Le B, Cicalo C, Jeon S, Kim YJ, Lee JH, Shin EC, Li Y, Zhao J, Kim JK. Effects of dietary fibers, micronutrients, and phytonutrients on gut microbiome: A review. Appl Biol Chem. 2021;64:36. [DOI] [Full Text] |
| 253. | Kolodziejczyk AA, Zheng D, Elinav E. Diet-microbiota interactions and personalized nutrition. Nat Rev Microbiol. 2019;17:742-753. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 333] [Cited by in RCA: 555] [Article Influence: 92.5] [Reference Citation Analysis (0)] |
| 254. | Lu Y, Yuan X, Wang M, He Z, Li H, Wang J, Li Q. Gut microbiota influence immunotherapy responses: mechanisms and therapeutic strategies. J Hematol Oncol. 2022;15:47. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 269] [Article Influence: 89.7] [Reference Citation Analysis (0)] |
| 255. | Zhang M, Liu J, Xia Q. Role of gut microbiome in cancer immunotherapy: from predictive biomarker to therapeutic target. Exp Hematol Oncol. 2023;12:84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 53] [Reference Citation Analysis (1)] |
| 256. | Bai X, Huang Z, Duraj-Thatte AM, Ebert MP, Zhang F, Burgermeister E, Liu X, Scott BM, Li G, Zuo T. Engineering the gut microbiome. Nat Rev Bioeng. 2023;1:665-679. [DOI] [Full Text] |
| 257. | Eindor-Abarbanel A, Healey GR, Jacobson K. Therapeutic Advances in Gut Microbiome Modulation in Patients with Inflammatory Bowel Disease from Pediatrics to Adulthood. Int J Mol Sci. 2021;22:12506. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 22] [Article Influence: 5.5] [Reference Citation Analysis (1)] |
| 258. | Sudhakar P, Machiels K, Verstockt B, Korcsmaros T, Vermeire S. Computational Biology and Machine Learning Approaches to Understand Mechanistic Microbiome-Host Interactions. Front Microbiol. 2021;12:618856. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 26] [Article Influence: 6.5] [Reference Citation Analysis (2)] |
| 259. | Nazir A, Hussain FHN, Raza A. Advancing microbiota therapeutics: the role of synthetic biology in engineering microbial communities for precision medicine. Front Bioeng Biotechnol. 2024;12:1511149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |