Published online Nov 5, 2022. doi: 10.4292/wjgpt.v13.i6.88
Peer-review started: March 17, 2022
First decision: June 16, 2022
Revised: August 2, 2022
Accepted: September 21, 2022
Article in press: September 21, 2022
Published online: November 5, 2022
Processing time: 228 Days and 17.9 Hours
Obscure small bowel bleeding is defined as gastrointestinal bleeding (GIB) that is unidentifiable with esophagogastroduodenoscopy and a colonoscopy with video capsule endoscopy (VCE) being the next gold standard step for evaluation. Small bowel transit time (SBTT) is a metric of a VCE study that is defined as the time the capsule takes to travel through the small intestine.
To determine if SBTT within the VCE study, correlates to overall detection of obscure small bowel bleeds. Furthermore, we attempted to identify any existing correlation between SBTT and re-bleeding after a negative VCE study.
This is a single center retrospective analysis of VCE studies performed for overt and occult GIB at Einstein Medical Center, Philadelphia, between 2015 and 2019. Inclusion criteria primarily consisted of patients 18 years or older who had a VCE study done as part of the workup for a GIB. Patients with incomplete VCEs, poor preparation, or with less than 6 mo of follow up were excluded. A re-bleeding event was defined either as overt or occult within a 6-mo timeframe. Overt re-bleeding was defined as Visible melena or hematochezia with > 2 gm/dL drop in hemoglobin defined an overt re-bleeding event; whereas an unexplained > 2 gm/dL drop in hemoglobin with no visible bleeding defined an occult re-bleed.
Results indicated that there was a significant and positive point biserial correlation between SBTT of 220 min and detection of a bleeding focus with a statistically significant p value of 0.008. However, the area under the curve was negligible when trying to identify a threshold time for SBTT to discriminate between risk of re-bleeding events after a negative VCE.
In terms of SBTT and association with accuracy of VCE finding a bleeding focus, 220 min was found to be adequate transit time to accurately find a bleeding focus, when present. It was found that no threshold SBTT could be identified to help predict re-bleeding after a negative VCE.
Core Tip: Understanding the utility of Small bowel transit time (SBTT) in Video Capsule Endoscopies can help with assessing accuracy and yield of video capsule endoscopy studies as well as whether a certain SBTT exists to achieve appropriate accuracy in detecting small bowel bleeding acutely or if a threshold exists in order to predict future small bowel bleeding.
- Citation: Mohan N, Jarrett S, Pop A, Rodriguez D, Dudnick R. Effect of small bowel transit time on accuracy of video capsule endoscopy in evaluating suspected small bowel bleeding. World J Gastrointest Pharmacol Ther 2022; 13(6): 88-95
- URL: https://www.wjgnet.com/2150-5349/full/v13/i6/88.htm
- DOI: https://dx.doi.org/10.4292/wjgpt.v13.i6.88
Video capsule endoscopy (VCE) has become the standard of investigation for obscure gastrointestinal (GI) bleeding[1]. Suspected small bowel bleeding is defined as recurrent and/or persistent GI bleeding from a source that remains unidentified after bidirectional GI endoscopy, both esophagogastroduodenoscopy (EGD) and a colonoscopy. This bleeding may be further subcategorized as occult or overt. Obscure-overt GI bleeding requires persistent or recurrent visible evidence of bleeding, hematochezia, or melena, whereas obscure-occult GI bleeding is defined as persistently positive fecal occult blood testing, iron deficiency anemia, or both without evidence of gross GI hemorrhage[2].
VCE is a method of colonic investigation that has been available to gastroenterologists since 2001, with multiple capsule endoscopy systems evolving over the years. While capsule endoscopy has been successfully used to investigate colonic pathologies ranging from inflammatory bowel disease, small intestinal tumors and polyposis syndromes, and celiac disease, the main indication for its use is to identify an obscure GI bleeding source[3]. While colonoscopy and EGD remain the gold standards to identify lower gastrointestinal and upper GI bleeding (GIB) sources, respectively, these methods are not physically capable of reaching the small bowel, without the help of the push enteroscopy method, but even then, as many as 21% of patients end up with an undetected bleeding site[4,5]. This is where the value of VCE studies predominate with studies showing that VCE has been found to be superior to other methods such as push enteroscopy and small bowel barium enema for the diagnosis of obscure GI bleeding[6].
Studies have found that small bowel bleeding is the most common indication for VCE, which accounts for approximately 5%-10% of all GI bleeding[7]. The most common small bowel cause of obscure GI bleeding has been found to be arteriovenous malformations or angioectasias, dominating over 20%-55% of cases[8].
The diagnostic yield is approximately 60% for overt small bowel bleeding and 30% for obscure small bowel bleeding. The negative predictive value of a normal VCE is high[1]. This has been shown in studies where there are higher re-bleeding rates in patients with occult obscure GI bleeding who had a positive VCE as compared with those with a negative VCE[9]. A recent meta-analysis demonstrated that the overall odds ratio for re-bleeding was lower after a negative VCE for obscure GI bleeding as compared with a positive VCE, especially with a short follow-up duration of 2 years. Approximately 80% of patients will not re-bleed during long term follow-up after a negative VCE[10]. This meta-analysis also did not find a statistically significant difference in re-bleeding between patients with overt vs occult GI bleeding on presentation.
VCE studies generate a small bowel transit time (SBTT), which is defined as the time that the video capsule takes to travel from the first duodenal image to the first cecal image[11]. The goal of VCE studies is to find obscure GI bleeding sources that other methods are unable to identify, but without anywhere close to a 100% detection rate, follow up for patients with negative VCE studies become critical to monitor for rebleeding events. Many studies have investigated rebleeding rates after a negative VCE study where patients will rebleed at rates between 4% and 27%[12,13]. While these studies investigated rebleeding events, they failed to investigate any tangible data points within the VCE studies to associate with rebleeding rates. With SBTT being one of the primary metrics used in VCE studies, we aimed to determine whether SBTT influences VCE yield and whether any correlation between SBTT and rebleeding after a negative VCE might exist.
Study design, participants, and data collection: This study was a single center retrospective analysis of all negative VCE studies for GIB, including both obscure-overt GI bleeding and obscure-occult GI bleeding between 2015 and 2019. Pill Cam SB2/3 capsule systems were used in all studies. Inclusion criteria included adult patients aged 18 years above whom underwent VCE for indication of suspected small bowel GI bleeding which included occult GI bleeding, overt/obscure GI bleeding, heme positive stools, melena, rectal bleeding, hematochezia, blood loss anemia and were found to have a negative VCE study. Patients were excluded from the study if they were found to have an incomplete VCE (the capsule did not reach the cecum, prohibiting a full visualization and calculation of the SBTT), poor bowel preparation resulting in an inadequate study, and if they were lost to follow up (Table 1).
| Discrete variables | |||
| Variable | Level | n | % |
| Sex | Male | 124 | 39 |
| Female | 191 | 61 | |
| Race/ethnicity | African American | 177 | 58 |
| White | 71 | 23 | |
| Other | 67 | 19 | |
| Positive VCE | Yes | 115 | 37 |
| No | 200 | 63 | |
| Continuous variables | |||
| Mean | SD | ||
| Age | 65.19 | 12.673 | |
| Small bowel transit time | 201.35 | 97.016 | |
VCE Rapid Reader was utilized to identify studies performed for obscure GI bleeding. Study indication was determined using diagnosis/indication listed for the VCE in the system. Key phrases used include GI bleeding, rectal bleeding, hematochezia, melena, heme positive stools, occult GI bleeding, obscure GI bleeding and blood loss anemia of unknown etiology. After identification of studies for the above stated indications, the negative VCE reports from this database were identified. VCE reports were deemed negative once the results were reported as normal and/or absent pathology identified to explain blood loss. All studies were read by gastroenterologists with at least 10 years of experience in capsule study interpretation.
The hospital electronic medical records were reviewed for patients with a negative VCE for GI bleeding to determine all re-bleeding rates. Re-bleeding events were defined either as overt re-bleeding or occult re-bleeding. Overt re-bleeding was defined as melena or hematochezia with > 2 gm/dL drop in hemoglobin. Occult re-bleeding was defined as unexplained > 2 gm/dL drop in hemoglobin after the initial VCE or the failure of initial hemoglobin to improve throughout the hospital stay.
We used means and standard deviations for continuous variables, and frequencies and proportions for discrete variables. We computed a point biserial correlation (PBC) to assess the strength of association between SBTT (continuous variable) and VCE yield or post VCE re-bleeding rates (binary variable)[14]. We used PBC instead of Pearson or Spearman correlations because one of our variables compared is a binary discrete variable. To identify the optimal threshold time, we used a receiver operator characteristic (ROC) curve. The ROC curve plots the sensitivity (true positive rate; probability of testing positive given one is positive) against 1-Specifiity (False positive rate; probability of testing positive given one is negative), with sensitivity on the vertical axis and False Positive on the horizontal axis[15,16]. A diagonal line is drawn to indicate equal sensitivity and specificity across all possible values. An empirical curve is drawn plotting sensitivity by specificity at different values (potential cut points) of the measure in question (transit time). Ideally, this empirical curve will be higher than the diagonal line, as the diagonal line indicates random assignment of a patient to having an abnormal result. The higher the empirical curve, the greater the likelihood of a true versus false positive result. Assessment is conducted by calculating the area under the curve (AUC). In the ROC analysis, the AUC represents the probability of being a true versus a false positive. AUC’s close to 1.0 are better, as they indicate a greater sensitivity to false positive ratio, with AUC’s around 0.5[17] being equivocal. A statistical test is employed to compare the calculated AUC to an AUC of 0.5.
A total of 316 VCE studies were reviewed by experienced gastroenterologists (Table 2). Of note, two patients were lost to follow up following VCE or did not get 6-mo re-bleeding data in the form of overt bleeding or occult bleeding, and therefore could not be included in the final analysis to assess for re-bleeding rates after a negative VCE study, giving a total cohort consisting of 314 patients. Our final resulting cohort used for analysis was 99.4% of the initial evaluated studies. Further analysis of the data revealed that 99 out of the 314 patients (31.5%) underwent initial VCE in order to evaluate overt or occult bleeding; whereas the other 215 patients (68.5%) underwent initial VCE to evaluate for unclear causes of iron deficiency anemia.
| Cases | ||||||
| Included | Excluded | Total | ||||
| n | Percent | n | Percent | n | Percent | |
| Small bowel transit time (min); rebleeding in 6 mo from VCE (Y = 0/N = 1) | 314 | 99.4% | 2 | 0.6% | 316 | 100.0% |
The mean (SD) SBTT for capsule studies in which re-bleeding was not identified was 199.56 min (95.818) with 95% confidence interval (CI) = 188.458 to 210.668. In those in whom re-bleeding was seen, the mean (SD) for SBTT was 218.14 (110.260 with 95%CI = 177.302, 258.984).
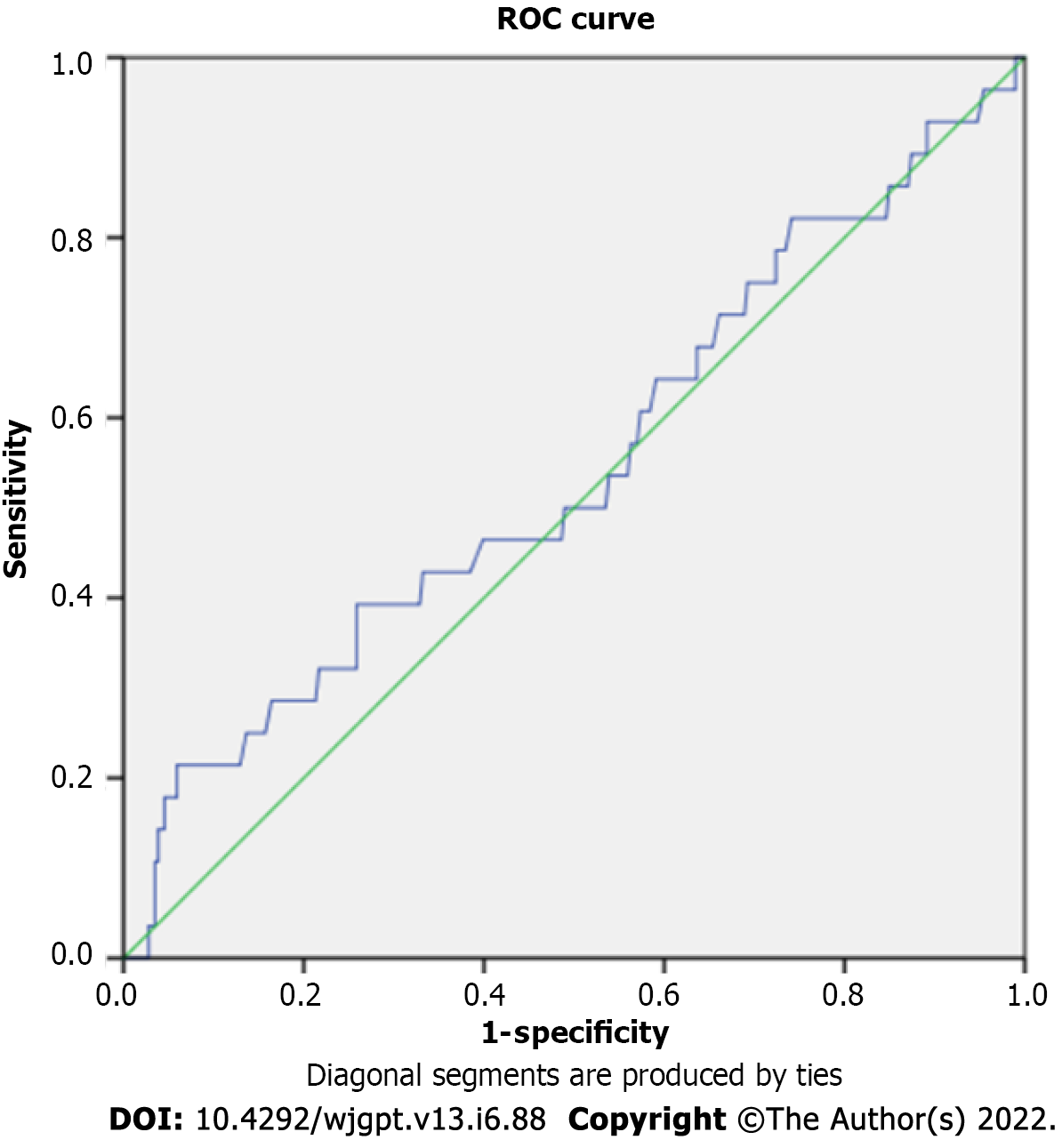
AUC was not significantly different from 0.5 (AUC = 0.545, P = 0.429), suggesting no relationship between SBTT and the risk of re-bleeding after a negative VCE (Figure 1).
There was a significant and positive point biserial correlation between SBTT of 220 min and detection of a bleeding focus (rpbc = 0.149, P = 0.008).
VCE is the gold standard for identifying obscure GIB[18]. Our study aimed to assess whether SBTT in VCE studies correlated with VCE yield in detecting obscure GIB as well as whether SBTT had any correlation with re-bleeding rates after a negative VCE.
The literature reports that the average SBTT is approximately 245 min with ranges anywhere between 18 min and 522 min[19]. Our study demonstrated that there was a significant correlation between SBTT and VCE yield in identifying an obscure GIB. We found that an average SBTT of 220 min correlated with identification of a small bowel source of bleeding on VCE. A study conducted by Girelli et al[20] in 2017 investigated the correlation between SBTT and the detection of significant small bowel lesions using capsule endoscopy. They found that the longer the SBTT (283 min and above), the higher the detection rate using capsule endoscopy. The initial part of our study also investigated this correlation to detect the rates within our institution and we found that a shorter SBTT than the average was able to yield significant results in terms of detecting an obscure GIB. This variation may speak to differences in capsule endoscopy type and technique, proficiency of detecting bleeding by the physician reviewing the endoscopy, or cause and severity of the bleed[20]. While many studies such as Girelli et al[20] show that SBTT has a significant impact on diagnostic yield of capsule endoscopy studies, there is virtually no literature that investigates those same SBTT timings and its specific correlations to re-bleeding rates in a patient with an unidentified obscure GIB which could suggest that further research into this area is needed.
VCE studies with significant findings tend to lead to specific treatments applications such as device-assisted enteroscopy which aims to treat identified lesions. The main goal at investigating an obscure GIB and identifying a treatable lesion is to assure prevention of future re-bleeding events[21]. A study by Carey et al[22] which included a trial of 260 patients demonstrated that re-bleeding events and overall outcomes improved in the subsequent months after VCE was able to identify a lesion that was then successfully treated. Viazis et al[23] also reported that VCE affects long-term outcome (i.e., resolution of bleeding) in patients with positive VCE (65.2% vs 35.4% of negative VCE group), since these patients are prone to undergo aggressive interventions. Angiodysplasia was the most common finding among patients with positive VCE (70%) and most of them received a mode of treatment that lead to resolution of bleeding (69%)[23].
Long term outcomes and re-bleeding events after a positive VCE study has been extensively studied, however few reports in the literature report the long-term outcomes as well as rebleeding rates of a negative VCE study. This may be due to the fact that when a bleed is identifiable, VCE has quite a good diagnostic yield and therefore the numbers do not favor looking into long term effects of negative VCE studies. Khamplod et al[24] found that out of 142 overt GIB patients, only 9 patients had small bowel lesions missed by VCE and they found that almost all those patients re-bled months after their negative VCE study. This is where the importance of our study comes in. VCE studies are reliable in finding an obscure GIB, but in those with negative VCE studies, with them being at high risk for re-bleeding events, finding a measure to predict the rate of re-bleeding would allow for closer monitoring and likely prevention of a re-bleeding event. With SBTT being such a good and well-studied measure, in terms of correlating with diagnostic yield of VCE studies, it only makes sense to try and use SBTT as the measure to help predict re-bleeding events after a negative VCE, which is exactly what our study set out to accomplish.
Our study was unable identify any correlation between SBTT and re-bleeding rates after a negative VCE. Considering this we were unable to identify a threshold SBTT that could be used as a predictive index for re-bleeding after a negative VCE. This is likely due to the limitation of the sample size of the study. With capsule endoscopy being such a refined and reliable technique, gathering enough negative VCE studies under an already small overall patient population could have resulted in the numbers being far from statistically significant. To find a correlation between SBTT and re-bleeding after a negative VCE, a larger study population (possibly via a multicenter study) that consists of more negative VCE studies would be necessary to find a statistically significant SBTT threshold number. Having a predictive threshold SBTT would be extremely beneficial to the general clinician. If a threshold SBTT could be found during the study, the general clinician could then know how at risk a patient will be for re-bleeding and possibly hasten follow up in higher risk individuals and therefore prevent re-hospitalization.
One of the biggest limitations to our study is the statistical power due to sample size. Our study only looked at a total of 315 VCE studies across a single institution and therefore future studies should include a much larger cohort. Another limitation of this study was the number of patients lost to follow up, as 28 of 343 patients did not follow up after the initial evaluation to then be able to assess for re-bleeding rates. One way to remedy this in future studies is to expand patient population by conducting a multi-institutional study, which would not only allow for a much larger sample size, but also compensate for patients lost to follow up, and allow for greater generalizability as the patient population would be more representative of the general population. Future studies looking into more correlational data for SBTT is recommended as being able to correlate SBTT with newer parameters such as types and severity of lesions in initially positive VCE as well as in negative VCE with later re-rebleeding events, would allow for clinicians to have more tools at hand in order to predict clinical severity for their patients with obscure GIB.
In this single center retrospective study, we were unable to determine a relationship between SBTT and the risk of re-bleeding following a negative VCE. However, we were able to identify an average SBTT of 220 min as correlational to VCE studies being able to identify a small bowel source of bleeding. We also identified a re-bleeding rate of 8.9% at 6 mo within our institution, which demonstrates the low likelihood of re-bleeding after a negative video capsule study.
Identified and evaluated the various use case scenarios and benefits of video capsule endoscopy (VCE) and used prior existing data to identify a metric within the existing technology to be used for improved and more accurate medical care.
To find a metric within already existing VCE studies to help predict re-bleeding episodes when the study comes back negative. Finding an appropriate metric to use to be able to predict such a clinical outcome would allow for improved and early clinical intervention.
To assess whether small bowel transit time (SBTT) influences VCE yield in detecting a small bowel bleed and if there was any correlation between SBTT and re-bleeding rates when VCE study was negative. If a correlation to re-bleeding rates could be found, it could allow for earlier follow-up with the patients and possible earlier interventions prior to re-bleeding events.
Single center retrospective electronic health record-based analysis of VCE studies performed for overt and occult GIB at Einstein Medical Center, Philadelphia. To identify the optimal threshold time, receiver operator characteristic (ROC) curve was used. area under the curve (AUC) was also used to assess the measure’s ability to discriminate between variables.
Our study found that there was no valuable threshold time for SBTT that could be linked to re-bleeding rates in negative VCE studies. While our study may have been too small in scale to truly see a correlational link in the predictive value of SBTT, we were able to show that SBTT is a good metric to follow in detection of the initial bleed, therefore highlighting the importance of the metric itself as a clinical tool that can aid in improvement of overall clinical care. The major problem that remains to be solved is that, with a larger amount of statistical power and the backing of multi-institutional data, will a correlational threshold for SBTT be found? If so, what is that threshold and how can it be implemented directly to benefit patient care?
While we were unable to find a correlation between SBTT and re-bleeding rates in negative VCE studies, our study was able to identify a threshold value of 220 min for SBTT in order to accurately identify a small bowel source of bleeding. This study proposes that SBTT, a tangible metric within VCE studies, due to its ability to correlate to identification of obscure gastrointestinal bleeds, should be a major metric used to predict valuable clinical outcomes.
Future studies are needed to further look into correlational data for SBTT along with newer parameters such as types and severity of lesions in initially positive VCE as well as in negative VCE with later re-rebleeding events. This would allow for clinicians to have more tools at hand in order to predict clinical severity for their patients with obscure GIB.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C, C
Grade D (Fair): D
Grade E (Poor): E
P-Reviewer: Cabezuelo AS, Spain; Chen SY, China; Costache RS, Romania; Triantafyllou K, Greece S-Editor: Chen YL L-Editor: A P-Editor: Chen YL
| 1. | Gerson LB, Fidler JL, Cave DR, Leighton JA. ACG Clinical Guideline: Diagnosis and Management of Small Bowel Bleeding. Am J Gastroenterol. 2015;110:1265-87; quiz 1288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 368] [Cited by in RCA: 448] [Article Influence: 44.8] [Reference Citation Analysis (1)] |
| 2. | Wetwittayakhlang P, Wonglhow J, Netinatsunton N, Chamroonkul N, Piratvisuth T. Re-bleeding and its predictors after capsule endoscopy in patients with obscure gastrointestinal bleeding in long-term follow-up. BMC Gastroenterol. 2019;19:216. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 3. | Bouchard S, Ibrahim M, Van Gossum A. Video capsule endoscopy: perspectives of a revolutionary technique. World J Gastroenterol. 2014;20:17330-17344. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 31] [Cited by in RCA: 22] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 4. | Tacheci I, Devière J, Kopacova M, Douda T, Bures J, Van Gossum A. The importance of upper gastrointestinal lesions detected with capsule endoscopy in patients with obscure digestive bleeding. Acta Gastroenterol Belg. 2011;74:395-399. |
| 5. | Liu K, Kaffes AJ. Review article: the diagnosis and investigation of obscure gastrointestinal bleeding. Aliment Pharmacol Ther. 2011;34:416-423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 51] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 6. | Raju GS, Gerson L, Das A, Lewis B; American Gastroenterological Association. American Gastroenterological Association (AGA) Institute technical review on obscure gastrointestinal bleeding. Gastroenterology. 2007;133:1697-1717. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 379] [Cited by in RCA: 338] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 7. | Liao Z, Gao R, Xu C, Li ZS. Indications and detection, completion, and retention rates of small-bowel capsule endoscopy: a systematic review. Gastrointest Endosc. 2010;71:280-286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 561] [Cited by in RCA: 476] [Article Influence: 31.7] [Reference Citation Analysis (0)] |
| 8. | Rondonotti E, Marmo R, Petracchini M, de Franchis R, Pennazio M. The American Society for Gastrointestinal Endoscopy (ASGE) diagnostic algorithm for obscure gastrointestinal bleeding: eight burning questions from everyday clinical practice. Dig Liver Dis. 2013;45:179-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 52] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 9. | Yung DE, Koulaouzidis A, Avni T, Kopylov U, Giannakou A, Rondonotti E, Pennazio M, Eliakim R, Toth E, Plevris JN. Clinical outcomes of negative small-bowel capsule endoscopy for small-bowel bleeding: a systematic review and meta-analysis. Gastrointest Endosc. 2017;85:305-317.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 55] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 10. | O'Grady J, Murphy CL, Barry L, Shanahan F, Buckley M. Defining gastrointestinal transit time using video capsule endoscopy: a study of healthy subjects. Endosc Int Open. 2020;8:E396-E400. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 26] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 11. | Lai LH, Wong GL, Chow DK, Lau JY, Sung JJ, Leung WK. Long-term follow-up of patients with obscure gastrointestinal bleeding after negative capsule endoscopy. Am J Gastroenterol. 2006;101:1224-1228. [PubMed] |
| 12. | Kim JB, Ye BD, Song Y, Yang DH, Jung KW, Kim KJ, Byeon JS, Myung SJ, Yang SK, Kim JH. Frequency of rebleeding events in obscure gastrointestinal bleeding with negative capsule endoscopy. J Gastroenterol Hepatol. 2013;28:834-840. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 33] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 13. | Iwamoto J, Mizokami Y, Shimokobe K, Yara S, Murakami M, Kido K, Ito M, Hirayama T, Saito Y, Honda A, Ikegami T, Ohara T, Matsuzaki Y. The clinical outcome of capsule endoscopy in patients with obscure gastrointestinal bleeding. Hepatogastroenterology. 2011;58:301-305. [PubMed] |
| 14. | Emura T, Liao YT. Critical review and comparison of continuity correction methods: The normal approximation to the binomial distribution. Commun Stat Simul Comput. 47:2266-2285. [RCA] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 15. | Mandrekar JN. Receiver operating characteristic curve in diagnostic test assessment. J Thorac Oncol. 2010;5:1315-1316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1283] [Cited by in RCA: 2560] [Article Influence: 170.7] [Reference Citation Analysis (0)] |
| 16. | Janssens ACJW, Martens FK. Reflection on modern methods: Revisiting the area under the ROC Curve. Int J Epidemiol. 2020;49:1397-1403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 71] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 17. | Søreide K. Receiver-operating characteristic curve analysis in diagnostic, prognostic and predictive biomarker research. J Clin Pathol. 2009;62:1-5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 209] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 18. | van Tuyl SA, Kuipers EJ, Timmer R, Stolk MF. Video capsule endoscopy: procedure, indications and diagnostic yield. Neth J Med. 2004;62:225-228. [PubMed] |
| 19. | Flemming J, Cameron S. Small bowel capsule endoscopy: Indications, results, and clinical benefit in a University environment. Medicine (Baltimore). 2018;97:e0148. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 19] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 20. | Girelli CM, Soncini M, Rondonotti E. Implications of small-bowel transit time in the detection rate of capsule endoscopy: A multivariable multicenter study of patients with obscure gastrointestinal bleeding. World J Gastroenterol. 2017;23:697-702. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 11] [Cited by in RCA: 19] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 21. | Tziatzios G, Gkolfakis P, Dimitriadis GD, Triantafyllou K. Long-term effects of video capsule endoscopy in the management of obscure gastrointestinal bleeding. Ann Transl Med. 2017;5:196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 22. | Carey EJ, Leighton JA, Heigh RI, Shiff AD, Sharma VK, Post JK, Fleischer DE. A single-center experience of 260 consecutive patients undergoing capsule endoscopy for obscure gastrointestinal bleeding. Am J Gastroenterol. 2007;102:89-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 214] [Cited by in RCA: 212] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 23. | Viazis N, Papaxoinis K, Vlachogiannakos J, Efthymiou A, Theodoropoulos I, Karamanolis DG. Is there a role for second-look capsule endoscopy in patients with obscure GI bleeding after a nondiagnostic first test? Gastrointest Endosc. 2009;69:850-856. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 71] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 24. | Khamplod S, Limsrivilai J, Kaosombatwattana U, Pausawasdi N, Charatcharoenwitthaya P, Pongprasobchai S, Leelakusolvong S. Negative Video Capsule Endoscopy Had a High Negative Predictive Value for Small Bowel Lesions, but Diagnostic Capability May Be Lower in Young Patients with Overt Bleeding. Can J Gastroenterol Hepatol. 2021;2021:8825123. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |