Published online Mar 18, 2021. doi: 10.4292/wjgpt.v12.i2.32
Peer-review started: December 18, 2020
First decision: January 10, 2021
Revised: January 10, 2021
Accepted: March 10, 2021
Article in press: March 10, 2021
Published online: March 18, 2021
Processing time: 89 Days and 23.5 Hours
Hepatitis B virus is a universal health problem. There are approximately 250 million people living with hepatitis B worldwide, and approximately 600000 of these people die every year due to the virus.
To compare the pretreatment and post-treatment histopathological results of patients with hepatitis be antigen (HBeAg)-negative chronic hepatitis B (CHB) who had been receiving tenofovir disoproxil fumarate (TDF) treatment at our clinic for at least 5 years.
Patients with HBeAg-negative CHB who were being treated with TDF (245 mg/d) were included in the study. Liver biopsies of patients before TDF treatment and liver biopsies after 5 years of TDF treatment were retrospectively compared.
A total of 50 HBeAg-negative CHB patients were included in the study (mean age: 47.9 ± 10.4 years, men: 27.54%). Histological improvement was observed in 78% (39) of the patients after 5 years of treatment. After the 5 years of treatment, the mean Ishak score of the patients was 1.3 ± 1.3, and the mean histologic activity index score was 4.1 ± 2.8. A 1.53 point reduction in Ishak fibrosis score was detected after long-term TDF treatment.
Liver biopsies after 5 years of TDF treatment revealed a significant histological response and a regression of the necroinflammatory score compared to pretreatment liver biopsies. To better understand the effects of antiviral treatments on the improvement of liver histology, long-term studies involving larger numbers of patients are needed.
Core Tip: Hepatitis B virus is a universal health problem. There are approximately 250 million people living with hepatitis B worldwide, and approximately 600000 of these people die every year due to the virus. Viral suppression with treatment can also lead to histological healing. In the current study, we aimed to compare the pretreatment and post-treatment histopathological results of patients with hepatitis be antigen-negative chronic hepatitis B who have been receiving tenofovir disoproxil fumarate (TDF) treatment at our clinic for at least 5 years. Liver biopsies after 5 years of TDF treatment revealed a significant histological response and a regression of the necroinflammatory score compared to pretreatment liver biopsies.
- Citation: Abayli B, Abaylı C, Gencdal G. Histopathological evaluation of long-term tenofovir disoproxil fumarate treatment in patients with hepatitis be antigen-negative chronic hepatitis B. World J Gastrointest Pharmacol Ther 2021; 12(2): 32-39
- URL: https://www.wjgnet.com/2150-5349/full/v12/i2/32.htm
- DOI: https://dx.doi.org/10.4292/wjgpt.v12.i2.32
Hepatitis B virus (HBV) is a universal health problem. There are approximately 250 million people living with hepatitis B worldwide, and approximately 600000 of these people die every year due to the virus. The treatment of chronic HBV infection depends on many factors including clinical variables (e.g., liver inflammation and/or the presence or absence of cirrhosis), the patient's immunological response to infection [e.g., hepatitis be antigen (HBeAg) status], risk factors for the rapid progression of the disease (e.g., age > 40 and family history of hepatocellular carcinoma), and virological factors (e.g., HBV viral load and genotype). Treatment strategies for chronic hepatitis B (CHB) typically include pegylated interferon or nucleos(t)ide analogues (e.g., entecavir and tenofovir). Interferon is recommended primarily for young patients with compensated liver disease who do not want to undergo long-term treatment[1-4].
For HBeAg-negative CHB patients, the predicted response to treatment is less accurate. Treatment should be started immediately after the diagnosis of HBeAg-negative CHB, because untreated spontaneous remission is rarely seen in this group. The aim of treatment in patients with CHB is to reduce the mortality and morbidity associated with the disease and to increase the quality of life and the lifetime of the patient by preventing complications such as cirrhosis, liver failure and hepatocellular carcinoma that may occur with the progression of the disease. The main goal in therapy is to achieve the long-term suppression of HBV deoxyribonucleic acid (DNA) replication. Viral suppression with treatment can also lead to histological healing. Recent studies have found histological improvements in sexually transmitted disease patients who received antiviral therapy[2-9].
In the current study, we aimed to compare the pretreatment and post-treatment histopathological results of patients with HBeAg-negative CHB who have been receiving tenofovir disoproxil fumarate (TDF) treatment at our clinic for at least 5 years.
Patients with HBeAg-negative CHB who received TDF treatment (245 mg/d) were included in this study. Patients were included in the study if they were over 18 years, had received antiviral treatment for at least 5 years, came to the outpatient control regularly, underwent liver biopsy before and after treatment, and had available laboratory parameters. Patients were excluded if they were HBeAg-positive, were previously treated with interferon or another antiviral, had a treatment incom-patibility, or had been receiving treatment for less than 5 years. Patients were also excluded in cases of human immunodeficiency virus, hepatitis C or hepatitis D co-infection were detected. Elecsys instrument (Roche Diagnostics, Italy) was used to detect HBsAg, anti-HBs, HBeAg and anti-HBe. The real-time polymerase chain reaction AmpliPrep/COBAS TaqMan HBV test 2.0 (Roche Molecular Systems, NJ, United States) was used to quantify HBV DNA. Liver biopsies of patients were evaluated by an experienced pathologist.
In our hospital, patients are followed up in the hepatology outpatient clinic according to the 2017 hepatitis B European Association for the Study of the Liver guidelines. According to these guidelines, a decrease in necroinflammatory activity [indicated by a ≥ 2 point decrease in the histologic activity index (HAI) or in the Ishak system] without worsening fibrosis compared to the pretreatment histological findings is determined as histological response. The virological response in patients who receive NA treatment is defined as undetectable by a sensitive polymerase chain reaction assay when HBV DNA is below the 10 IU/mL limit of detection. Serological responses for HBsAg are HBsAg loss and the development of anti-HBs. Normalisation of alanine aminotransferase (ALT) levels based on the ULN (40 IU/L) is determined as biochemical response.
The data are presented as the mean, median, standard deviation and percentage. All analysis was performed using IBM Statistic Package for Social Science Statistics, V.20.0 (IBM Corp., Armonk, NY, United States). The Kolmogorov–Smirnov test was used to assess normality of quantitative variables. Differences in the variables pre and post treatment were analysed by the Wilcoxon test within groups. All tests were two-tailed, and P < 0.05 was considered to be statistically significant.
This retrospective study was organised in accordance with the Helsinki Declaration. Local ethics committee approval was obtained (No. 11.03.2020-52/756).
A total of 50 HBeAg-negative chronic HBV patients were included in the study. The demographic characteristics of the patients are presented in Table 1. The baseline mean HAI score of the patients was 7.2 ± 3.2, and the mean baseline Ishak fibrosis score was 2.2 ± 1.4. The average time from the start of treatment to liver biopsy was 60.8 ± 9.7 wk. All patients were treated with tenofovir disoproxil fumarate (245 mg/d).
| mean ± SD | P value | ||
| Age | 47.9 ± 10.4 | ||
| Male | 27 (54%) | ||
| Treatment period (wk) | 60.8 ± 9.7 | ||
| AST | BT | 82.3 ± 218.6 | < 0.001 |
| AT | 23.7 ± 14.1 | ||
| ALT | BT | 74.3 ± 118.1 | < 0.001 |
| AT | 23.7 ± 14.1 | ||
| T.Bil. | BT | 0.8 ± 0.4 | 0.024 |
| AT | 0.8 ± 1 | ||
| Albumin | BT | 3.9 ± 0.7 | > 0.5 |
| AT | 4 ± 0.8 | ||
| Creatine | BT | 1 ± 1.98 | > 0.5 |
| AT | 0.8 ± 0.2 | ||
| PLT | BT | 208000 ± 55000 | 0.007 |
| AT | 238000 ± 78000 | ||
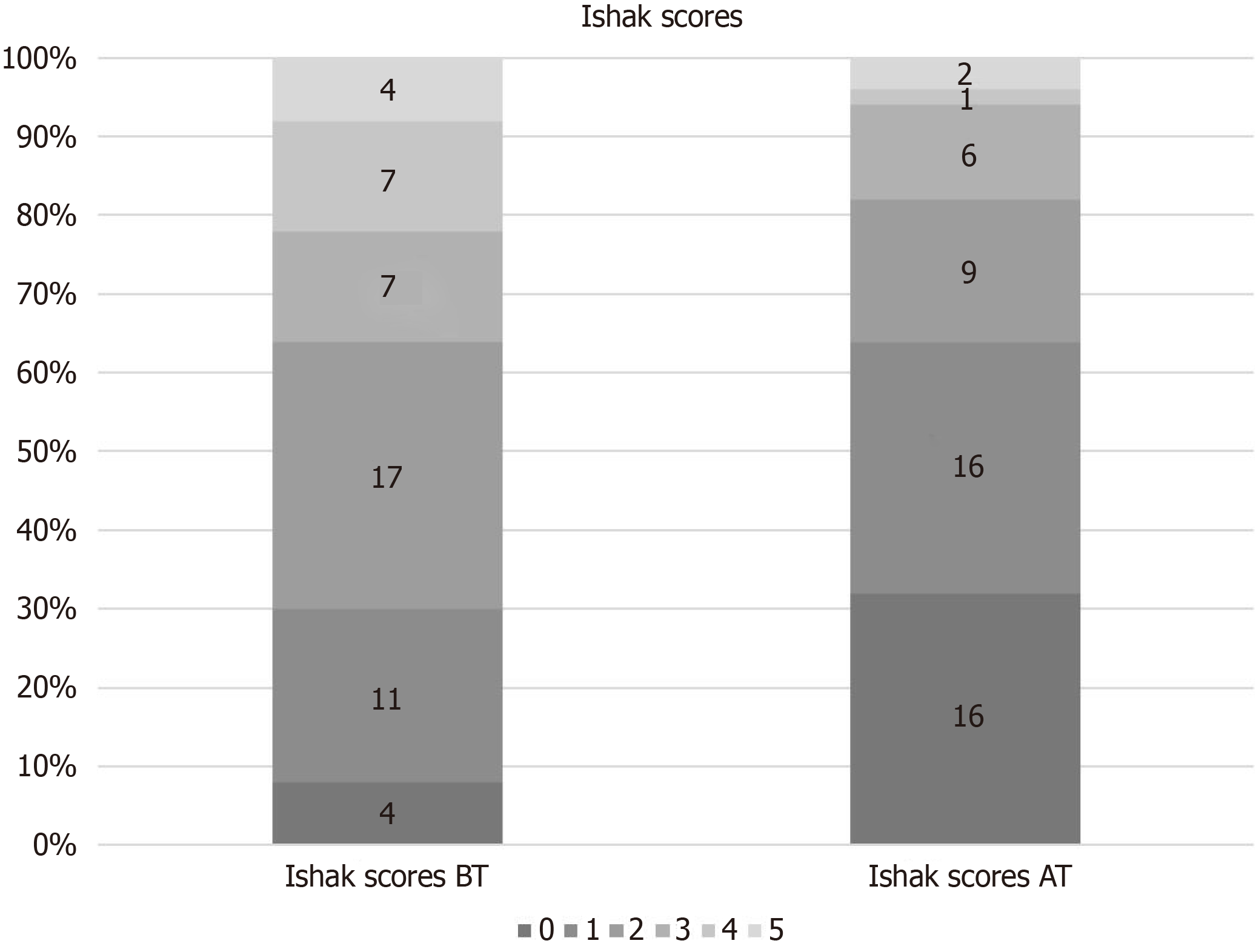
| Ishak score | BT | 2.2 ± 1.4 | 0.002 |
| AT | 1.3 ± 1.3 | ||
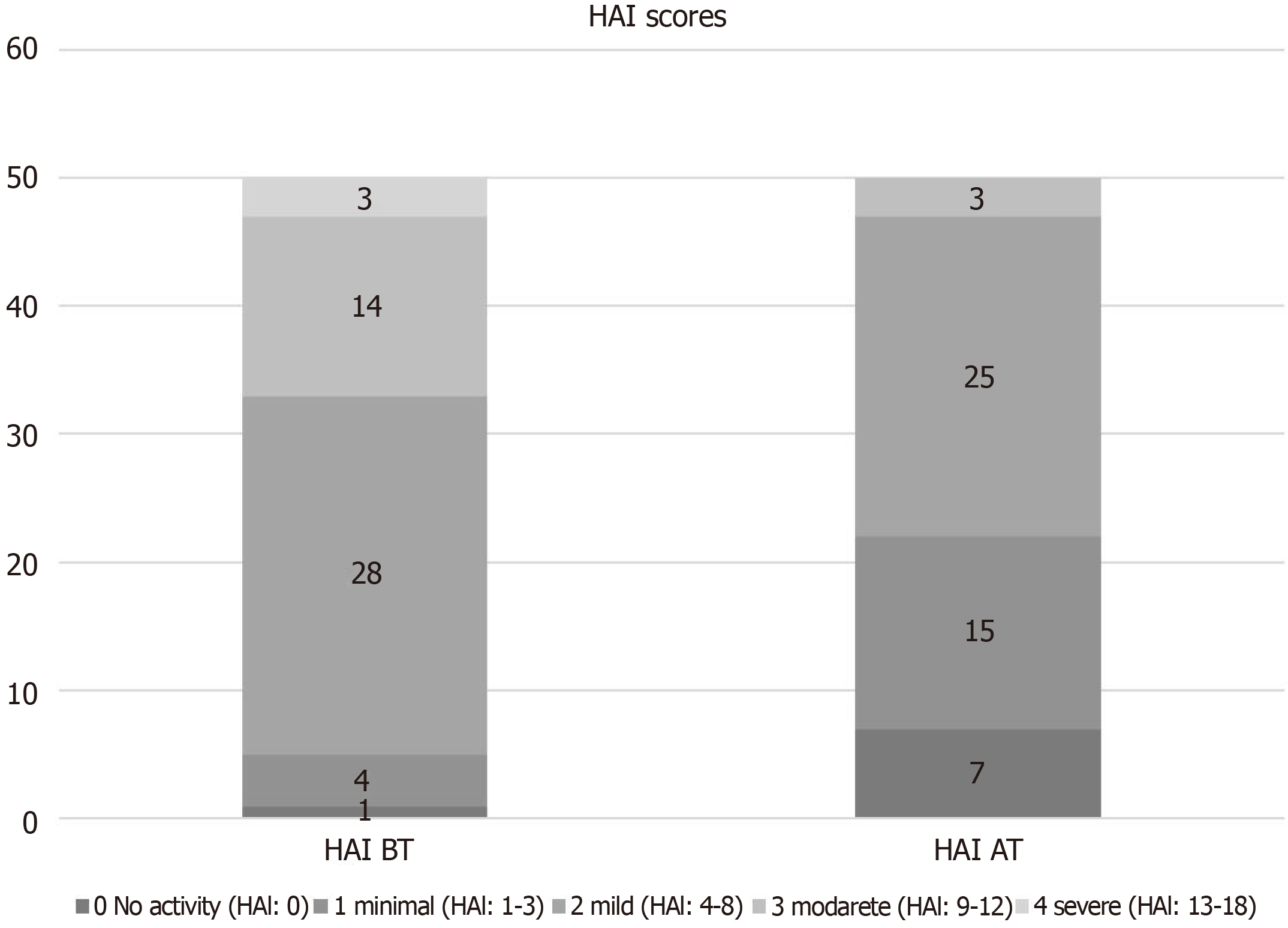
| HAI score | BT | 7.2 ± 3.2 | < 0.001 |
| AT | 4.1 ± 2.8 |
All biopsy samples were evaluated by a pathologist specialising in liver diseases. Histological improvement was observed in 78% (39) of the patients after 5 years of treatment. After 5 years of treatment, the mean Ishak score of the patients was 1.3 ± 1.3, and the mean HAI score was 4.1 ± 2.8. A 1.53 point reduction in Ishak fibrosis scores after long-term treatment was obtained. The long term treatment (60 wk) resulted in most of patients with no or minimal necroinflammation, as assessed by HAI score (Figure 1), and no or minimal fibrosis, as defined by Ishak classification (Figure 2).
Eleven patients had advanced fibrosis or cirrhosis (Ishak score ≥ 4) before the treatment. After long-term treatment, all patients demonstrated at least a 1 point reduction in the Ishak fibrosis score, with a median reduction of 2.9 points from pre-treatment values
At the time of post treatment biopsy, 100% of the patients (50/50) had an HBV DNA level < 300 copies/mL; therefore, genotypic testing for resistance was not performed.
All patients were HBeAg-negative. After long-term tenofovir treatment, two of the 50 patients showed HBsAg seroconversion.
Many studies have shown a statistically significant and consistent correlation between the viral load level or viral load change and the histological grade and biochemical and serological responses over the course of chronic HBV.
With antiviral therapy, viral replication is brought under control, thereby suppressing necroinflammatory activity in the vast majority of patients. In this way, progressive liver damage is avoided, and there is a decreased risk of hepatocellular carcinoma. Untreated spontaneous recovery is rare in patients with HBeAg-negative CHB. Therefore, after the diagnosis is made, non-cirrhotic patients who meet the criteria should immediately begin treatment and continue it until HBsAg seroconversion occurs. Entecavir and tenofovir are frequently used for this treatment because of their low resistance profile. In patients with hepatitis B with cirrhosis, lifetime treatment is recommended[2-9].
According to recent studies, tenofovir and entecavir are frequently used in cirrhotic and non-cirrhotic patients with CHB, and these drugs have been shown to be safe and effective. These two molecules are frequently used owing to their low resistance profiles[9-11]. According to initial studies, tenofovir treatment results in fibrosis improvement, including cirrhosis regression, in the majority of patients. In one study, 51% of the 348 patients with paired biopsies (at baseline and at week 240) showed fibrosis regression in their follow-up biopsy. Interestingly, 71 of 96 patients (74%) with Ishak stage 5 or 6 were found to have no cirrhosis at week 240[12]. In their review, Pol et al[13] reported the safety and efficacy data from two real-world cohorts in the United Kingdom and Europe (362 NA-naïve patients, follow up time: 9-28 mo). In this report, virological suppression was detected in 80%-89% of patients; breakthrough was detected in 2% of patients without any corresponding resistance mutations. HBeAg seroconversion was seen in 7%-18% of patients, and HBsAg loss occurred in 2% of the European cohort. ALT normalisation was detected in 87% of patients by week 30 wk. Pan et al[14] reported the real-world safety and efficacy of TDF (90 Asian–American patients, 48 wk period). Ten percent of the patients had a prior treatment history with lamivudine or adefovir. The authors detected virological suppression in 82% of patients, HBeAg seroconversion was detected in 12% and ALT normalisation was detected in 66% of the patients by the end of follow up. No TDF resistance was detected, and the treatment was considered well-tolerated. In the study conducted by Buti et al[15], after 5 years of tenofovir treatment, improvement in the Knodell score (≥ 1 point decrease) was found in 93.8% of cirrhotic patients and in 90.8% of non-cirrhotic patients, and no difference was found between the groups. A similar histological response with tenofovir treatment, regardless of the presence of cirrhosis, has been reported[15]. In a study, Tatar et al[16] reported remarkably good HBV DNA suppression, good biochemical response rate and improvement of liver necroinflammation in 52 CHB patients who were terated with TDF (The mean follow-up: 33 ± 11 mo). In accordance with these studies, our results showed HBV DNA to be negative in 100% of the patients after treatment; 38 (76%) had an improved Ishak fibrosis score, 34 (68%) had an improved necroinflammatory score (≥ 2 point improvement of HAI score), and HBsAg seroconversion was detected in 2 patients.
Some studies have reported a strong histological response and fibrosis regression in patients with advanced fibrosis/cirrhosis. In a study with patients who received tenofovir treatment for 5 years, a decline in fibrosis score was observed in 51%. The authors stated that this rate increased to 74% in patients with Ishak fibrosis scores of 5 and 6 before treatment, and histological improvement was greater than 91% in those with Ishak fibrosis scores >2[12]. In our study, 11 of the 50 patients had advanced fibrosis or cirrhosis (Ishak score ≥ 4) at baseline. After long-term tenofovir therapy, all 11 patients demonstrated at least a 1 point reduction in the Ishak fibrosis score, with a median reduction from baseline of 2.9 points.
This study is limited by its retrospective nature, which did not allow for the initial data to be diversified. Many pretreatment demographic characteristics that could affect the response to treatment could not be verified, and the effect of these factors on the treatment response could not be investigated.
Compared to the initial liver biopsies, the liver biopsies performed at least 5 years after the initiation of TDF treatment revealed a significant histological response and regression of the necroinflammatory score. These promising findings should be verified in a larger population by conducting a multicentre, prospective study.
Hepatitis B virus is a universal health problem. There are approximately 250 million people living with hepatitis B worldwide, and approximately 600000 of these people die every year due to the virus. Viral suppression with treatment can also lead to histological healing.
Recent studies have found histological improvements in sexually transmitted disease patients who received antiviral therapy.
In the current study, we aimed to compare the pretreatment and post-treatment histopathological results of patients with hepatitis be antigen (HBeAg)-negative chronic hepatitis B (CHB) who have been receiving tenofovir disoproxil fumarate (TDF) treatment at our clinic for at least 5 years.
Patients with HBeAg-negative CHB who were being treated with TDF (245 mg/d) were included in the study. Liver biopsies of patients before TDF treatment and liver biopsies after 5 years of TDF treatment were retrospectively compared.
A total of 50 HBeAg-negative CHB patients were included in the study (mean age: 47.9 ± 10.4 years, men: 27.54%). Histological improvement was observed in 78% (39) of the patients after 5 years of treatment. After the 5 years of treatment, the mean Ishak score of the patients was 1.3 ± 1.3, and the mean histologic activity index score was 4.1 ± 2.8. A 1.53 point reduction in Ishak fibrosis score was detected after long-term TDF treatment.
Liver biopsies after 5 years of TDF treatment revealed a significant histological response and a regression of the necroinflammatory score compared to pretreatment liver biopsies.
These promising findings should be verified in a larger population by conducting a multicentre, prospective study.
Manuscript source: Invited manuscript
Corresponding Author's Membership in Professional Societies: European Association for the Study of the Liver.
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Turkey
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, B
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Manrai M S-Editor: Zhang L L-Editor: A P-Editor: Liu JH
| 1. | World Health Organization. Hepatitis B fact sheet. [cited 4 December 2020]. Available from: http://www.who.int/mediacentre/factsheets/fs204/en/. |
| 2. | Terrault NA, Lok ASF, McMahon BJ, Chang KM, Hwang JP, Jonas MM, Brown RS Jr, Bzowej NH, Wong JB. Update on Prevention, Diagnosis, and Treatment of Chronic Hepatitis B: AASLD 2018 Hepatitis B Guidance. Clin Liver Dis (Hoboken). 2018;12:33-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 223] [Article Influence: 31.9] [Reference Citation Analysis (0)] |
| 3. | Sarin SK, Kumar M, Lau GK, Abbas Z, Chan HL, Chen CJ, Chen DS, Chen HL, Chen PJ, Chien RN, Dokmeci AK, Gane E, Hou JL, Jafri W, Jia J, Kim JH, Lai CL, Lee HC, Lim SG, Liu CJ, Locarnini S, Al Mahtab M, Mohamed R, Omata M, Park J, Piratvisuth T, Sharma BC, Sollano J, Wang FS, Wei L, Yuen MF, Zheng SS, Kao JH. Asian-Pacific clinical practice guidelines on the management of hepatitis B: a 2015 update. Hepatol Int. 2016;10:1-98. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1985] [Cited by in RCA: 1961] [Article Influence: 217.9] [Reference Citation Analysis (0)] |
| 4. | European Association for the Study of the Liver. EASL 2017 Clinical Practice Guidelines on the management of hepatitis B virus infection. J Hepatol. 2017;67:370-398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3745] [Cited by in RCA: 3802] [Article Influence: 475.3] [Reference Citation Analysis (1)] |
| 5. | Gish RG, Given BD, Lai CL, Locarnini SA, Lau JY, Lewis DL, Schluep T. Chronic hepatitis B: Virology, natural history, current management and a glimpse at future opportunities. Antiviral Res. 2015;121:47-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 196] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 6. | Iloeje UH, Yang HI, Su J, Jen CL, You SL, Chen CJ; Risk Evaluation of Viral Load Elevation and Associated Liver Disease/Cancer-In HBV (the REVEAL-HBV) Study Group. Predicting cirrhosis risk based on the level of circulating hepatitis B viral load. Gastroenterology. 2006;130:678-686. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1164] [Cited by in RCA: 1174] [Article Influence: 61.8] [Reference Citation Analysis (0)] |
| 7. | Sanai FM, Helmy A, Bzeizi KI, Babatin MA, Al-Qahtani A, Al-Ashgar HA, Al-Mdani AS, Al-Akwaa A, Almutharea S, Khan MQ, Alghamdi AS, Farah T, Al-Hamoudi W, Saadeh M, Abdo AA. Discriminant value of serum HBV DNA levels as predictors of liver fibrosis in chronic hepatitis B. J Viral Hepat. 2011;18:e217-e225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 8. | Dienstag JL, Goldin RD, Heathcote EJ, Hann HW, Woessner M, Stephenson SL, Gardner S, Gray DF, Schiff ER. Histological outcome during long-term lamivudine therapy. Gastroenterology. 2003;124:105-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 602] [Cited by in RCA: 574] [Article Influence: 26.1] [Reference Citation Analysis (0)] |
| 9. | Schiff ER, Lee SS, Chao YC, Kew Yoon S, Bessone F, Wu SS, Kryczka W, Lurie Y, Gadano A, Kitis G, Beebe S, Xu D, Tang H, Iloeje U. Long-term treatment with entecavir induces reversal of advanced fibrosis or cirrhosis in patients with chronic hepatitis B. Clin Gastroenterol Hepatol. 2011;9:274-276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 138] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 10. | Chang TT, Liaw YF, Wu SS, Schiff E, Han KH, Lai CL, Safadi R, Lee SS, Halota W, Goodman Z, Chi YC, Zhang H, Hindes R, Iloeje U, Beebe S, Kreter B. Long-term entecavir therapy results in the reversal of fibrosis/cirrhosis and continued histological improvement in patients with chronic hepatitis B. Hepatology. 2010;52:886-893. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 721] [Cited by in RCA: 772] [Article Influence: 51.5] [Reference Citation Analysis (0)] |
| 11. | Papachrysos N, Hytiroglou P, Papalavrentios L, Sinakos E, Kouvelis I, Akriviadis E. Antiviral therapy leads to histological improvement of HBeAg-negative chronic hepatitis B patients. Ann Gastroenterol. 2015;28:374-378. [PubMed] |
| 12. | Marcellin P, Gane E, Buti M, Afdhal N, Sievert W, Jacobson IM, Washington MK, Germanidis G, Flaherty JF, Aguilar Schall R, Bornstein JD, Kitrinos KM, Subramanian GM, McHutchison JG, Heathcote EJ. Regression of cirrhosis during treatment with tenofovir disoproxil fumarate for chronic hepatitis B: a 5-year open-label follow-up study. Lancet. 2013;381:468-475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1228] [Cited by in RCA: 1369] [Article Influence: 114.1] [Reference Citation Analysis (0)] |
| 13. | Pol S, Lampertico P. First-line treatment of chronic hepatitis B with entecavir or tenofovir in 'real-life' settings: from clinical trials to clinical practice. J Viral Hepat. 2012;19:377-386. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 94] [Cited by in RCA: 112] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 14. | Pan CQ, Trinh H, Yao A, Bae H, Lou L, Chan S; Study 123 Group. Efficacy and safety of tenofovir disoproxil fumarate in Asian-Americans with chronic hepatitis B in community settings. PLoS One. 2014;9:e89789. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 15. | Buti M, Fung S, Gane E, Afdhal NH, Flisiak R, Gurel S, Flaherty JF, Martins EB, Yee LJ, Dinh P, Bornstein JD, Mani Subramanian G, Janssen HL, George J, Marcellin P. Long-term clinical outcomes in cirrhotic chronic hepatitis B patients treated with tenofovir disoproxil fumarate for up to 5 years. Hepatol Int. 2015;9:243-250. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 44] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 16. | Tatar B, Gül S, Köse Ş, Pala E. Long-Term Effects of Tenofovir on Liver Histopathology in Patients with Chronic Viral Hepatitis B Infection. Turk Patoloji Derg. 2020;1:154-158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |