Published online Aug 15, 2014. doi: 10.4291/wjgp.v5.i3.304
Revised: March 19, 2014
Accepted: July 12, 2014
Published online: August 15, 2014
Processing time: 218 Days and 18 Hours
Ulcerative colitis (UC) is one of the main types of inflammatory bowel disease, which is caused by dysregulated immune responses in genetically predisposed individuals. Several genetic factors, including interleukin and interleukin receptor gene polymorphisms and other inflammation-related genes play central role in mediating and modulating the inflammation in the human body, thereby these can be the main cause of development of the disease. It is clear these data are very important for understanding the base of the disease, especially in terms of clinical utility and validity, but summarized literature is exiguous for challenge health specialist that can used in the clinical practice nowadays. This review summarizes the current literature on inflammation-related genetic polymorphisms which are associated with UC. We performed an electronic search of Pubmed Database among publications of the last 10 years, using the following medical subject heading terms: UC, ulcerative colitis, inflammation, genes, polymorphisms, and susceptibility.
Core tip: Ulcerative colitis (UC) is a disorder of the idiopathic and chronic inflammation of the colonic mucosa. Several genetics factors influence the development of the disease, especially interleukin and interleukin receptor gene polymorphisms and other inflammation-related genes. In this review we collected the current literature on PubMed Database about those genetic markers that are associated with UC, we focused on the following terminology: UC, inflammation, genes, polymorphisms, susceptibility.
- Citation: Sarlos P, Kovesdi E, Magyari L, Banfai Z, Szabo A, Javorhazy A, Melegh B. Genetic update on inflammatory factors in ulcerative colitis: Review of the current literature. World J Gastrointest Pathophysiol 2014; 5(3): 304-321
- URL: https://www.wjgnet.com/2150-5330/full/v5/i3/304.htm
- DOI: https://dx.doi.org/10.4291/wjgp.v5.i3.304
Ulcerative colitis (UC; MIM 191390) and Crohn’s disease (CD; MIM 26600) are the two main, related forms of inflammatory bowel disease (IBD) which are chronic, relapsing inflammatory disorders of the gastrointestinal tract[1]. The highest annual incidence rate of UC was reported in Europe (24.3/100000) and in North America (19.2/100000). However, in Asia and in the Middle East the rate is much lower (6.3/100000) believed to be associated with the different level of industrialization[2]. UC has a bimodal pattern of incidence, with the mean age diagnosis between ages 15 and 30 years, and a second smaller peak between ages 50 and 70 years[3]. Clinically, UC is characterized by superficial, continuous mucosal inflammation and ulcers restricted to the colon, whereas CD is a segmental, transmural disorder involving any part of the gastrointestinal tract[4].
Although the precise etiology of IBD still remains obscure, the accepted hypothesis is that in genetically susceptible individuals the commensal luminal flora trigger an inappropriate, overactive mucosal immune response causing intestinal tissue damage that is further modified by specific environmental factors (e.g., smoking)[5].
At first, observational family studies and twin studies directed the interest to genetic components in the pathogenesis of IBD[6,7]. Recently, genome-wide association studies (GWAS) have resulted in the identification of many novel single nucleotide polymorphisms (SNPs) for CD initially and latterly for UC which is thought to be more genetically heterogeneous than CD. To date, the number of known risk loci has expanded to 163, of which 110 confer common susceptibility to IBD, whereas 30 seem to be specific to CD and 23 to UC[8].
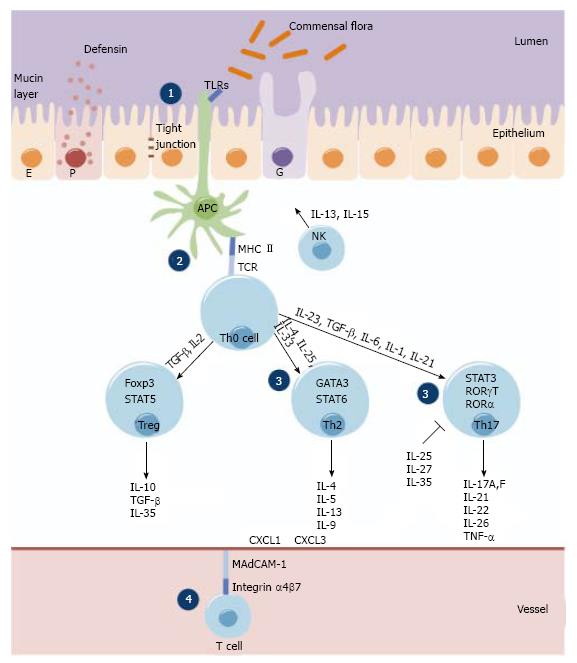
Immunologically, CD is associated with a T helper type 1 (Th1)[9] and T helper type 17 (Th17)[10] immune response, thus interferon gamma/interleukin-12 (IFNγ/IL-12) and interleukin-23/interleukin-17 (IL-23/IL-17) cytokines assign the downstream release of complex network of further pro-inflammatory cytokines (e.g., IL-18, IL-2, IL-1, IL-21, IL-22, IL-17A, IL-17F, IL-26). However, UC is thought to be the result of a Th17 (IL-17) and a modified Th2 response (IL-13, IL-5 and IL-9). In addition, interleukin-6 (IL-6) and tumor necrosis factor alpha (TNFα) are produced by both T helper type 2 (Th2) cells and Th1 cells.
The IBD-associated loci encode for genes involved in maintenance of epithelial barrier integrity, antigen pattern recognition, autophagy, innate immunological response, coordination of adaptive immune responses and leukocyte recruitment (Figure 1).
Most of the difference at molecular level between UC and CD is found in human leukocyte antigen (HLA) Class II genes and in genes related to pattern recognition [e.g., nucleotide-binding oligomerization domains (NODs), toll-like receptors (TLRs]), innate immunity (e.g., IL-23R) or autophagy pathways (e.g., ATG16L1, IRGM). The HLA class II genes DR2, DR9, and DRB1*0103 were identified as susceptibility genes for UC, whereas DR4 was a protective gene[11,12]. HLA haplotype DRB1*0103 is significantly associated with disease susceptibility, extensive disease, and an increased risk of colectomy[13]. While several genes involved in bacterial sensing [nucleotide-binding oligomerization domain 2/ caspase activation recruitment domain 15 (NOD2/CARD15)] and processing mechanisms (autophagy related genes ATG16L1 and IRGM) are defective only in CD, the Th17/IL-23 axis related cytokines [e.g., IL-23R, IL-12B and their downstream components signal transducer and activator of transcription 3 (STAT3), janus kinase 2 (JAK2)] have been associated with both CD and UC.
Dysfunction of the barrier integrity, enhanced permeability is also a main feature in UC. Recently, in a large review epithelial barrier genes were discussed in detail, namely, extracellular matrix protein 1 (ECM1), cadherin type 1 (CDH1), hepatocyte nuclear factor 4, alpha (HNF4α), and laminin beta 1 (LAMB1).
These genes were found not to be associated with CD, implying they may confer susceptibility specifically to UC[14]. Interestingly, the CDH1 locus represents the first genetic association also identified in a GWAS for colorectal cancer susceptibility[15,16].
In our review we focus on inflammation-related genes and polymorphisms including interleukin and interleukin receptor gene polymorphisms which are involved in the pathogenesis of UC.
Cytotoxic T-lymphocyte antigen 4 (CTLA4) is an inhibitory receptor expressed by activated T cells. It is an important downregulator of the T cell activation and might contribute to peripheral tolerance. CTLA4 is a good candidate gene for susceptibility to UC, because it acts as a negative regulator of T cell activation and T/B, T/monocyte-macrophage cognate interaction. The localization of CTLA4 gene is on chromosome 2q33. Several genetic polymorphisms have been reported in the human CTLA4 gene[17,18].
In a Tunisian population study, where A+49G was analyzed comparing the UC patients with the control subjects, the frequencies of the +49A allele and the homozygous +49 A/A genotype were higher in UC patients than in controls, but those differences were not statistically significant[17].
In a Dutch Caucasian and in a Han Chinese UC cohort studies the C-318T and A+49G polymorphisms of CTLA4 gene were examined. No significant differences were observed in distribution of allele, genotype and haplotype frequencies between UC and control group[19].
A Hungarian cohort was examined for the same polymorphisms and no association was found between heterozygous AG genotype, homozygous GG variant, and G allele frequency of the CTLA4 gene A+49G polymorphisms comparing the UC (IBD) group to the healthy controls. The A+49G does not represent an obligatory susceptibility factor for UC[20].
The A-1661G and the T-1722C two other SNPs in the non-exonic region were investigated in the Han Chinese population. The frequency of A/G + G/G genotype at position -1661 was statistically higher in UC patients than in healthy controls. The G allele frequency was also significantly increased in UC patients than in the controls. The A-1661G polymorphism of the CTLA4 is a risk factor for UC in Han Chinese of central China. They found no association between T-1722C polymorphism and UC[18].
Janus kinase 2 (JAK2) is a member of a family of tyrosine kinases involved in a specific subset of cytokine receptor signaling pathways. JAK2 has been found to be constitutively associated with the prolactin receptor and required for responses to gamma interferon[21-23].
In GWAS studies several UC loci were identified. The rs10758669 and the rs10974944 SNPs in the JAK2 locus were found to be strongly associated with UC in the population from the United Kingdom[14] and Netherlands[24].
In a Korean population two SNPs (rs10758669 and rs10975003) were investigated. The rs10758669 showed no significant differences in genotype and allele distribution between UC patients and controls, while it was significant on level of genotype and allele frequencies in case of rs10975003. The rs10975003 SNP plays role in the pathogenesis of UC in Koreans[25].
This protein is a member of the signal transducer and activator of transcription (STAT) protein family. It is encoded by the signal transducer and activator of transcription 3 (STAT3) gene. In response to cytokines and growth factors, STAT family members are phosphorylated by the receptor associated kinases, and then form homo- or heterodimers that translocate to the cell nucleus where they act as transcription activators. This protein is activated by phosphorylation in response to various cytokines and growth factors including interferons (IFNs), epidermal growth factor (EGF), interleukin-5 (IL-5), IL-6, hepatocyte growth factor (HGF), leukemia inhibitory factor (LIF) and bone morphogenetic protein 2 (BMP2). STAT3 relays the expression of a variety of genes in response to cell stimuli, and thus plays pivotal role in several cellular processes, such as cell growth and apoptosis[26-28].
In a large GWAS study the rs12948909 SNPs in the STAT3 locus was identified and found to be strongly associated with UC in the United Kingdom population[14].
In the Hungarian population the STAT3 rs744166 was investigated. STAT3 rs744166 TT genotype and T allele frequencies were significantly higher in patients with UC than in controls. Logistic regression analysis revealed that the TT genotype confers as an increased risk for the development of UC[23].
In a North American study the same polymorphisms were tested, but no significant differences were found between the UC group and healthy controls [29].
The pro-inflammatory cytokine, tumor necrosis factor alpha (TNFα) has important pathogenic role both in CD and in UC[30,31]. Through its ability to cause epithelial barrier disruption in colonic epithelial cells[32,33] it is responsible for tissue damage. The TNFα gene can be found at the inflammatory bowel disease 3 (IBD3) locus within the major histocompatibility complex (MHC) region. In several studies it has been found as a susceptibility locus for IBD[34-36]. Level of TNFα is elevated in serum, stools, and inflamed bowel mucosa of patients with IBD[37-41].
The polymorphism at position -308 is a point mutation, where the presence of G defines the common variant TNF1, and A the less common variant TNF2. Susceptibility to UC has been positively[42] and negatively[43] associated with carriage of TNF2 allele. Some studies suggested that this allele might have a small but significant association with greater levels of TNF transcription[44,45]. However other authors did not find any influence of TNFα bi-allelic polymorphism on UC susceptibility, although they reported a higher frequency of the TNF2 allele in women with extensive disease compared with those with distal colitis[46]. In Mexican Mestizo UC patients increased frequency of TNF2 allele and TNF 1/2 genotype was found, suggesting this could be an additional genetic marker for the susceptibility to UC[47]. Similar findings have been reported in patients with UC with Caucasian origin[42,46,48,49].
The TNFa polymorphisms A-308G and T-857C increase the TNFα production, raising the possibility of correlation with different disease course or response to therapy[50]. The A-238G and A-308G in TNFα promoter region have been found as a susceptibility factor in different autoimmune disorders, including asthma[51,52], psoriasis[53] and rheumatoid arthritis[54]. The polymorphism A-238G was associated with lower production of TNFα in Caucasian UC patients[46].
In a New Zealand Caucasian UC cohort was found, that carriers of the TNFα -308A allele may give higher risk for pancolitis and the necessity for bowel resection[55]. In Israeli Jewish patients having CD or UC, the allele and carrier frequencies of -857T allele did not differ between IBD patients and controls, suggesting this SNP in Ashkenazi Jewish patients neither determines the susceptibility, nor influences the clinical phenotype of CD or UC[56].
Different studies supported that TNFa -308 in UC may be an ethnic population-specific risk factor. Studies from East Asia suggested strong association of the TNFα -308 gene promoter polymorphism for UC in East Asians. Allele frequency of TNFα -308A was significantly higher in Han Chinese UC patients than in healthy controls. Haplotype analysis revealed 6 haplotypes including H5 (TNF 1031T/863C/857C/380G/308A/238G/) and H3 (TNF 1031C/863C/857C/ 380G/308A/238G/). Haplotype frequency of H5 was significantly higher in UC patients, suggesting that H5 is associated with UC and the TNFα gene may be a susceptibility gene to UC[57]. Interestingly, the meta-analysis did not reveal any association of the TNFα -308 gene promoter polymorphism with UC in Europeans[58]. In a Caucasoid population from the North of Spain the TNFα-308 alleles did not influence the appearance of steroid dependency either in UC or in CD[59]. In Italian pediatric patients the TNFα−308 was significantly increased in patients with UC[60].
In Czech pediatric IBD patients significant differences in TNFα -308 A polymorphism were found between UC group and controls, but no differences were noted between this polymorphism and the clinical characteristics of UC[61].
Significant correlation of the TNFα -863A variant was demonstrated with colonic disease and greater height at diagnosis[62], but in this study they could not find any significant difference for the -857 allele. In patients with UC only a trend toward an increased frequency of steroid resistance was found in carriers of the TNFα risk genotype compared to non-carriers[60].
In the Han Chinese population the TNFα C-1031T, A-863C and T-857C allele/carrier frequencies were analyzed between UC patients and healthy controls. They did not find any significant difference of the tested allele/carrier frequencies between UC patients and controls, only the TNFα -857T was increased in UC patients but did not reach statistical significance[57].
OCTN1 (SLC22A4) and OCTN2 (SLC22A5) are widely expressed [63-66], but specifically expressed in principal intestinal cell types affected by CD: epithelial cells, CD68+ macrophages and CD43+ T cells. SLC22A4 and SLC22A5 encode the polytopic transmembrane sodium-dependent carnitine and sodium-independent organic cation transporters OCTN1 and OCTN2[67]. OCTNs have important role in the maintenance of intracellular homeostasis and in the energy production of the cell [68]. Both OCTNs play important role in the maintenance of gastrointestinal health and in the prevention of gut inflammation[69,70].
CD associated variants, the OCTN1 T1672C and OCTN2 -207G were as strongly associated with UC in unrelated Caucasian subjects[71].
Homozygous patients for the OCTN1 1672T variant were significantly associated with UC in a study cohort from Italy, suggesting that OCTN1 could have a role in modulating the severity of chronic inflammation in UC[72].
The mutation that leads to L503F substitution in the OCTN1 protein can alter the transporter’s activity[73,74]. Only a weak gender-specific effect of L503F was observed at male UC patients in a cohort of familial and sporadic IBD from the central Pennsylvania, United States[75].
The multidrug-resistant transporter-1 (MDR1) gene encodes the transmembrane protein, P-glycoprotein 170 (Pgp)[76]. This gene is an excellent candidate gene for the pathogenesis of IBD[77]. Pgp functions as an ATP-dependent efflux transporter pump and is expressed in many normal tissues like in the epithelial surfaces of the intestine, biliary ductules, proximal tubules of kidneys and central nervous system[78-80].
One of the most significant MDR1 gene mutations is the C3435T polymorphism. Decreased expression of the MDR1 gene and lower Pgp activity has been associated with this variant. However, studies showed conflicting results. In a German study the T allele and TT genotype frequencies of C3435T polymorphism were significantly increased in UC patients[81]. Glas et al[82] found in a small group of UC patients partial accordance with a trend towards an increased frequency of T allele compared to controls, but a statistical difference was detected only in one of two different control groups. In a meta-analysis significant association of the 3435T allele and the 3435TT genotype has been found with UC[83]. The triallelic G2677T/A and the C3435T have been shown to correlate with Pgp expression[84-87]. Significant association of C3435T and G2677T was detected with UC: UC patients had significantly higher frequency of 2677T allele and of the 3435TT genotype. Haplotype analysis revealed that carriers of 3435T/2677T haplotype have significantly higher risk of having UC[88]. In a Japanese UC cohort the C3435T was predictive of susceptibility to later onset UC, but not for the early onset of UC[89].
Large study with German and British UC and CD patients failed to demonstrate association. It was confirmed, that this SNP is associated with UC especially in patients with extensive colitis[90]. In addition completely negative findings have been reported in large studies from North America[7], Slovenia[91] and Italy[92]. Similarly to these results, UC patients with Caucasian origin from central Poland were found that MDR1 C3435T polymorphism is not a risk factor for IBD, including both UC and CD[93].
A study with New Zealand IBD patients supported the role of MDR1 as a candidate gene for UC. Heterozygous carriers for the variants C1236T, rs2235046 and G2677T/A showed a lower risk of developing UC compared with homozygotes. Subgroup analysis revealed that C1236T and rs3789243 are associated with IBD when stratified for age of onset. The MDR1 variant rs3789243 was found to be associated with pancolitis in UC patients[94]. In the genetically heterogeneous North Indian UC cohort was found that this SNP is significantly overrepresented in UC patients.
When German IBD patients were genotyped for the two MDR1 SNPs in positions 2677G>T/A and 3435C>T it was found that the combined genotypes derived from these positions are possibly associated with young age onset of UC and severe course of disease[95]. The 2677T allele was significantly increased in British UC cases compared with controls. The TT genotype was significantly associated with severe UC. No significant association was seen with C3435T and UC or any clinical subgroup. A meta-analysis of 9 association studies of C3435T showed a significant association of the 3435T allele with UC, but not with CD. These results indicated that MDR1 sequence variants are associated with a small increase in the risk of developing UC and may influence disease behavior[96]. The MDR1 gene polymorphism G2677T/A showed significant association with CD, and the C3435T with Spanish UC patients[97]. The MDR1 3435 TT genotype and T-allelic frequencies were significantly higher in patients with UC compared with controls. The association was strongest with extensive UC, and this was also confirmed with multivariate analysis. However G2677T was not associated with UC or CD. Two-locus haplotypes showed both positive (3435T/G2677 haplotype) and negative (C3435/2677T haplotype) associations with UC. Homozygotes for the haplotype 3435T/G2677 were significantly increased in UC. Allelic variations of the MDR1 gene determined the disease extent as well as susceptibility to UC in the Scottish population[90].
Nucleotide-binding oligomerization domain1/ caspase activation recruitment domain 4 (NOD1/CARD4) is a member of the Nod-like receptor family, which is phylogenetically conserved[5,98]. It is constitutively expressed in epithelial cells throughout the gastrointestinal tract[99]. NOD1/CARD4 contains leucine-rich repeat (LRR) domain and NOD domains and has only one CARD domain[100].
Polymorphism in LRR domain of the NOD1/CARD4 gene showed association with disease severity of UC in North Indian patients. This might be due to disruption of the LRR region critical for NOD1-mediated bacterial sensing. Haplotype-based approach showed that GTTG haplotype carriers were over represented in UC patients which could increase the risk of the disease[101].
Initially, it was suggested that there is association of the deletion variant of NOD1/CARD4 +32656 (complex intronic insertion-deletion polymorphism) with susceptibility to IBD using a combination of transmission disequilibrium testing (TDT) and case-control analysis[102]. However this variant was not associated with a strong effect on susceptibility to IBD in children and adults in a Northern Europe study cohort[103]. Similar results have been found in the East Anglia IBD cohort, where no association was found between NOD1 +32656 and IBD and also no heterogeneity between UC and CD[104].
Essential components of innate immunity are the Toll-like receptors (TLRs). These are transmembrane receptors which recognize the microbial compounds from different bacteria, fungi and viruses[105-107]. TLRs are expressed by intestinal epithelial cells and immune cells in IBD patients[108,109]. TLR signaling in the intestinal sites of the colon can inhibit the inflammatory responses and maintains the colonic homeostasis[110-112]. TLRs can be found on the cell membrane (TLR1, 2, 4, 5 and 9) or on intracellular organelles (TLR3, 7 and 8)[113]. From the 10 human TLRs we review only three members regarding to their association to UC.
TLR2 is localized on the cell’s surface. With its cofactors (TLR1 and TLR6) it binds lipoproteins, which are important surface antigen of the Gram-negative outer membrane[114], TLR4 consists of a leucine-rich repeat region (LRR) and an intracellular domain homologous to IL-1 receptor[115]. It recognizes conserved pathogenic motifs of Gram-negative bacteria, mainly lipopolysaccharides (LPS). Signaling through TLR4 results in the activation of the transcriptional activator, known as nuclear factor κB (NF-κB)[116]. Similarly to TLR2, TLR9 is localized on the cell’s surface. It recognizes unmethylated CpG DNA in bacteria and viruses[117,118].
The allele and carrier frequencies of the Thr399Ile mutation in the TLR4 gene were significantly associated with UC in a Caucasian population[119]. Association of TLR4 Asp299Gly polymorphism with UC was reported first in Caucasian UC patients[120]. In a study, mentioned before[119], increased frequency of this polymorphism was observed, but it did not reach statistical significance. Similarly to Török et al study, the Asp299Gly and Thr399Ile mutations in TLR4 gene were associated with UC in Greek and in North Indian patients[121,122], but not in Dutch or Italian patients[123,124]. Interestingly, the TLR4 Asp299Gly did not show association with UC in different Asian UC populations[125-128].
The TLR2 Arg677Trp and Arg753Glu, TLR4 Asp299Gly and Thr399Ile, and TLR9 gene C1237T polymorphisms were genotyped in Chinese Han IBD patients; however none of these polymorphisms was associated with IBD. In Caucasians, both TLR4 299Gly and 399Ile conferred as a significant risk factor for developing UC and CD[127].
Three SNPs of TLR9 (C-1486T, G1174A, A2848G) were genotyped. These variations were associated with an increased risk of UC in the Japanese population. TLR9 -1486CC, 1174GG and 2848AA showed increased risk for UC, but TLR9 -1486TT, 1174AA and 2848GG decreased the risk of UC, although there were no correlations between SNPs and disease phenotype or TLR9 mRNA expression[129]. Possible associations between genetic variations in TLR9 and IBD in the German population were investigated, but no associations were detected between TLR9 gene variations and UC susceptibility[130].
Cell adhesion molecules (CAM) mediate the extravasation of leukocytes and their accumulation in inflamed intestinal mucosa. This process is controlled by a family of CAM including the intercellular cell adhesion molecule (ICAM-1), the platelet endothelial cell adhesion molecule (PECAM-1), the selectins (E, L, and P selectin) and the integrins[131,132].
ICAM-1 (CD54) is a cell surface glycoprotein belonging to the immunoglobulin superfamily. It plays key role in transendothelial migration of leukocytes, and lymphocyte activation. The membrane glycoprotein PECAM-1 (CD31) is expressed on vascular endothelial cells, platelets, some lymphocyte subsets, and monocytes[133-135]. It has important role in transendothelial migration of circulating leukocytes during inflammatory process[136], apoptosis[137] and integrin regulation[138]. The E-selectin (CD62E) is a glycoprotein, which is expressed on endothelial cells in response to pro-inflammatory cytokines (IL-1, TNF). It supports rolling of leukocytes at sites of inflammation and tissue injury. E-selectin expression is upregulated both in CD and in UC patients playing an important role in mediating of the inflammatory process in IBD[139]. L-selectin (CD62L) is expressed on normal naive T and B cells, leukocytes and on natural killer (NK) cells. It is involved in the adhesion of T cells to endothelial cells, which are regarded as crucial in the selective migration of lymphocytes to inflamed tissue sites during an inflammatory response[140].
Several mutations in ICAM1 (G241R and K469E), PECAM-1 (V125L), PECAM-1 (G98T and S128R), E-selectin (L554F) and L-selectin (F206L) were analyzed in Tunisian IBD patients and controls. A significant increase in allele frequencies of 206L of L-selectin and the associated genotype F/L was observed both in UC and in CD patients. In the subgroup analysis the L206 allele and F/L206 genotype frequencies were significantly increased in UC patients with left-sided type. No significant differences in allele or genotype frequencies were observed for ICAM-1, E-selectin, and PECAM-1 polymorphisms between UC patients, CD patients, and controls[141].
Interleukin 1 (IL-1) is pro-inflammatory cytokine, which affects cell proliferation, differentiation, and the function of many innate and specific immunocompetent cells, and acts as an endogenous pyrogen. It broadcast many inflammatory diseases by initiating and potentiating immune and inflammatory responses[142].
IL-1 is composed of two main proteins the IL-1A and the IL-1B[143]. IL-1B has major role in initiating and amplifying the inflammatory response[144]. The IL-1 receptor antagonist (IL-1RN) is an anti-inflammatory cytokine, which lacks the IL-1 receptor accessory protein (IL-1RAP) interacting domain[142].
In Mexican Mestizo UC patients five SNPs were analyzed; the rs419598, the rs315951 and the rs315952 in the IL-1RN gene, the rs16955 in the IL-1B gene and the 3811058 in the IL-1F10 gene. Significant increased frequencies of IL-1RN6/1TC (rs315952), IL-1RN6/2CC (rs315951) and decreased frequency of IL-1B-511 TC (rs16944) genotypes were found in UC patients. The patients group showed increased frequencies of IL-1RN CTC and TCG haplotypes, whereas TTG and CTG haplotypes frequencies were decreased[145].
IL-2 functions as a T cell growth factor, furthermore it supports the proliferation and differentiation of NK cells to increase their cytolytic functions. This IL plays important role in the development of Th1, Th2, Treg, and Th17 differentiation[146].
In the IL-2/IL-21 region several polymorphisms (rs6822844, rs13151961, rs13119723 and rs6840978) were studies. In a Dutch population the minor alleles of the examined SNPs were associated with IBD. The strongest association of these SNPs was found in the UC patients. In an Italian UC cohort the same strong association of the minor alleles was observed with UC. Similarly to this results, in the North American study was demonstrated, that these alleles have the strongest effect among the IBD patients in the UC subgroup [147].
IL-6 is a multifunctional, pleiotropic cytokine that is responsible for regulation of immune responses, acute-phase responses, hematopoiesis, and inflammation[148].
An Irish population study the IL-6 -174 genotype frequency showed significant difference between CD and UC group[149]. In the Caucasian population the same polymorphism was examined in CD and UC patients and found significant difference UC and CD susceptibility[150].
IL-8 is member of the CXC chemokine family[151]. Its has two receptors the CXCR1 (IL-8RA) and the CXCR2 (IL-8RB)[152]. It exerts effect mainly on the chemotaxis and migration of neutrophils, monocytes, lymphocytes, and fibroblasts[153].
IL-8 T-251A was analyzed in a Polish population. The allele frequency showed significant difference comparing the UC group to the controls[154], however this association was not observable in a Chinese UC cohort[155]. Additional polymorphisms were also tested and their effect on the serum level of IL-18. Haplotype frequency of the -353A/ -251A/ +678T haplotype was considerably higher in UC group compared to controls, suggesting this haplotype is likely to be more common in severe UC patients than in mild to moderate cases[155].
The anti-inflammatory cytokine IL-10 is produced by many cells like monocytes, T cells, B cells, NK cells, macrophages, and dendritic cells (DCs). It prevents the antigen presentation and also the subsequent release of pro-inflammatory cytokines, so it alleviates the activated immune system[156].
In a GWAS, the polymorphism rs3024505 demonstrated the most meaningful association in the combined verification UC samples, suggesting that defective IL-10 function plays important role in the pathogenesis of the UC[157]. The same polymorphisms was investigated in Australian population[158] and Danish cohort[159] and found that the rs3024505 was associated with the risk of UC.
Three promoter polymorphisms of the IL-10 gene G-1082A, C-819T, and C-592A were studied in many population but the results are contrary. In an Italian cohort the G-1082A and the C-819T SNPs were investigated. The -1082 genotype frequencies were significantly different between UC patients and controls. The frequency of the -1082A allele was also significantly higher in the UC patients than in controls. Allele and genotype frequencies of T-819C were not significantly associated with the disease. Furthermore, the frequencies of haplotypes -1082A/-819C and -1082A/-819T, which have been described to have a decreased promoter activity, were significantly increased in UC patients than in controls[160]. In a North-Eastern Mexican population the G-1082A and the C-592A SNPs were examined. The -1082 AA and -592 AA genotypes showed significantly lower frequencies in UC compared to healthy controls, while individuals heterozygous at IL-10-1082 have significantly increased occurrence of UC[161]. In a Tunisian group the A-627C and the G-1117A polymorphisms were examined and found that these two variants influencing the UC susceptibility and phenotype[162]. In the Asian population the association was confirmed between A-1082G polymorphism and UC[125,163].
IL-12 is an interleukin that is naturally produced by dendritic cells, macrophages and human B-lymphoblastoid cells in response to antigenic stimulation. It participates in the differentiation of naive T cells into Th1 cells and involved in the activities of natural killer cells and T lymphocytes. IL-12 mediates gradation of the cytotoxic activity of NK cells and CD8+ cytotoxic T lymphocytes[164]. IL-12 consist of two subunit p35 (IL-12A) and p40 (IL-12B), which is shared by IL-12 and IL-23 cytokines. The IL-12 receptor has two subunits: IL-12RB1 and IL-12RB2[165,166].
In a German population four SNPs (rs3212227, rs17860508, rs10045431 and rs6887695) of the IL-12B were investigated. Two SNPs, the rs10045431 and rs6887695 showed association with increased UC susceptibility[167]. From these SNPs the rs6887695 was investigated in a Japanese population where significant association was manifested between UC patients and controls[168].
The interleukin 17 (IL-17 or IL-17A) is a pro-inflammatory cytokine secreted by activated T cells its main tasks is inducing and mediating pro-inflammatory responses. It induces the production of many other cytokines, chemokines and prostaglandins from fibroblasts, endothelial cells, epithelial cells, keratinocytes, and macrophages[169-171].
In a Japanese population the rs2275913 SNP in the IL-17A gene and the rs763780 SNP in the IL-17F gene were investigated and found significant differences between UC group and healthy controls on level of -197A/A and 7488T/T genotype frequencies[172].
Even more recently, a GWAS in a very large European UC cohort identified an association between another IL-17 pathway gene (IL-17REL) and UC[173].
IL-18 is produced by macrophages and other cells. It functions by binding to the interleukin-18 receptor, and together with IL-12 it induces cell-mediated immunity following infection. IL-18 induces gene expression and synthesis of TNF, IL-1, Fas ligand, and several chemokines[174].
In the IL-18 gene several polymorphisms were examined. The G-137C, the C-607A and the G-656T are located in the promoter region, while the A105C, the T113G and the C127T are coding variants. In a Japanese study the G allele at 113 and the T allele at 127 were significantly higher in patients with UC compared to the control [175]. In another Japanese study allele and genotype frequency of G-137C were significantly higher in the proctitis-type UC patients than in controls[176]. The frequency of haplotype 2 (-607A, -137C), which have lower promoter activity and IFNγ-mRNA level was significantly increased in the proctitis-type patients than in the control group[176]. The C-607A and the G-137C SNPs were associated with the development of UC in Tunisian patients. The -137GG genotype frequency was significantly higher in UC than in controls and statistically significant association was found between -607AA genotype in UC patients and the distal localization of the lesions[177].
IL-23 main functions are very important in innate and adaptive immunity to regulate Th17 function and expansion[178]. This cytokine induces CD8+ memory T cells to proliferate and produce IL-17. IL-23 binds to its receptor IL-23R, which polymorphisms’ play the main role in the autoimmune diseases[179-181] especially in IBD [182].
Several independent functional SNPs of the IL-23R gene and its neighboring region were determined; several were found susceptible to (rs10889677, rs11209032, rs11465804, rs11805303, rs1495965, rs2201841, rs1004819) CD and UC in non-Jewish subjects[183].
In a Chinese cohort the rs7530511 and rs11805303 SNPs were studied and positive association was found between these variants and UC susceptibility[184]. In the Jiangsu Han population the rs11805303 was found as a susceptibility factor to UC[185].
In a Swedish population the rs10889677, the rs11209032, the rs11465804, the rs2201841 and the rs1004819 polymorphisms were investigated, and found that the rs11465804G, rs2201841C and rs1004819T allele frequencies showed significant differences between UC patient group and control. These genetic variants are individual risk factor for developing the disease[186].
In Hungarian UC patients for the IL-23R rs1004819A allele we found significantly higher allele frequency compared to control subjects and the SNP rs2201841 showed significant association with UC risk for homozygotes[187].
Expression of IL-26 seems to be restricted to memory T cells, NK cells, and Th17 cells. Thereby it could have pro-inflammatory effects in IBD[188].
Only a few markers were investigated, from these the rs2870946-G and the rs1558744-A showed association with UC[189]. Further meta-analysis study confirmed the association of rs1558744-A with UC[190].
This review shows that substantial progress has been made in the past 10 years in to find inflammatory related genetic factors and cytokines in UC. We reviewed different genes and gene polymorphisms which play role in the inflammatory process of UC. These genes could be potential targets of novel treatment strategies.
From the reviewed genes contributing to inflammation TNFα, MDR1 and TLRs were the most investigated genes. TNFα has been found as a susceptibility locus for both UC and CD[34-36]. The TNF2 allele and TNF 1/2 could be good candidate markers for the susceptibility to UC. Based on the different studies with different populations (East Asians, Han Chinese, Spanish Caucasoid, Italian, and Czech), the TNFα -308 is the most studied SNP and this may be an ethnic population-specific risk factor for UC especially for Asian populations but not for Europeans. It should be noted this polymorphism is also a susceptibility factor in other autoimmune disorders (asthma, psoriasis, and rheumatoid arthritis) too.
The MDR1 C3435T is one of the most tested SNP in UC, but with conflicting results. Some studies showed significantly increased 3435T allele and 3435TT genotype frequencies of C3435T[81,83,97], or only a trend towards an increased frequency of T allele[82], or the T allele was predictive of susceptibility to later onset UC, but not for the early onset of UC[89]. But some studies failed to demonstrate association with UC[91-93]. Other SNPs of MDR1 (C1236T and rs3789243) were associated with IBD when stratified for age of onset. The rs3789243 was found to be significantly overrepresented in genetically heterogeneous North Indian UC patients.
We reviewed 3 members from the 10 human TLRs regarding to their association to UC. Allele and carrier frequencies of the TLR4 Asp299Gly and Thr399Ile were significantly associated with UC in Caucasian[119,120], Greek and in North Indian patients[121,122], but not with Dutch, Italian[123,124] or Asian patients[125-128]. Interestingly the TLR9 polymorphisms (C-1486T, G1174A, A2848G) were associated with an increased risk of UC in the Japanese population. TLR9 -1486CC, 1174GG and 2848AA polymorphisms show increased risk for UC, but TLR9 -1486TT, 1174AA and 2848GG decrease the risk of UC[129].
From the reviewed cytokines IL-10, IL-18 and IL-23 were the most investigated genes. The IL-10 is a major anti-inflammatory cytokine, which attenuates the activated immune system with inhibiting both the antigen presentation and subsequent release of pro-inflammatory cytokines. IL-10 is a shared risk gene for CD and UC too. The promoter polymorphisms of this gene (G-1082A, C-819T, and C-592A) which are in tight linkage disequilibrium were extensively studied in many populations but with contradictory results. In the Caucasian population the carriers of G-1082A SNP were more susceptible to UC, whereas in another study carriers were associated with lower UC incidence[160,161]. In the Asian population the results strengthened the positive relationship between this SNP and UC susceptibility[125,163]. In a North-Eastern Mexican population the -592AA genotypes showed significantly decreased frequency in UC compared the results to the healthy controls[161]. In a Tunisian group the A-627C and the G-1117A variants influencing the UC susceptibility and phenotype[162]. Several other studies handle with these non-coding SNP in CD too and determine susceptibility to the disease or not.
The G-137C, the C-607A and the G-656T promoter SNPs and several others in the coding regions of IL-18 gene (A105C, the T113G and the C127T) were examined. The Japanese population is the most studies for these SNPs, significant difference was found in the allele frequency of the A105C between CD patients and controls, while this correlation could not be detected in UC patients. The 113G and 127T allele frequencies were significantly increased in patients with UC compared the results to the healthy controls[175]. In case of promoter polymorphisms, the -137CC genotype frequency was significantly increased in proctitis-type UC patients than in controls, while the other two C-607A and G-137C SNPs were associated with the development of UC in Tunisian patients[177].
The IL23R gene was identified as a CD susceptibility gene in North American non-Jewish subjects. Several independent functional SNPs in the gene and its neighboring region were determined[183]. After the primary publications, several studies have been published these SNPs in IBD and other autoimmune disease too (ankylosing spondylitis, psoriasis, Sjögren syndrome, systemic lupus erythematosus). From these SNPs (rs10889677, rs11209032, rs11465804, rs11805303, rs1495965, rs2201841, rs100481) several are risk factor to IBD both in European and Asian populations[185-187].
It can be established that these interleukin gene variants are strongly population dependent but in the given population they can be predictors for CD or UC. Despite the advances in the field of UC/IBD genetics, testing for these genetic variants is currently not recommended for clinical purposes[191].
Understanding of the detailed pathogenesis of IBD and identifying new disease associated SNPs led to the development of selective inhibitors for ILs, chemokines and their receptors. This strategies can optimize treatment efficacy and lead to personalized medicine based on the patient‘s genotype.
Biological agents are used in patients with moderate to severe disease activity who have failed conventional therapy with glucocorticoids and thiopurines. Today, the most effective and best studied anti-cytokine agents in IBD are the anti-TNFα antibodies. The mechanism of action of TNFα antagonists is based on the neutralization of both soluble TNFα and membrane TNFα and has a more global effect on inflammation than the blockade of other cytokines. Currently, three TNFα inhibitors are approved by the United States Food and Drug Administration (FDA) for inducing and maintaining clinical remission in UC: the chimeric (25% murine and 75% human sequence) monoclonal full-length IgG1 mAb infliximab[192], the fully human mAbs adalimumab[193,194] and golimumab[195]. The pegylated humanised antibody certolizumab pegol is approved only for CD (beside rheumatoid arthritis, RA and psoriatic arthritis). Etanercept, a dimeric fusion protein consisting of soluble p75-TNFR2 and the Fc portion of human IgG1, used in rheumatoid arthritis therapy, is not efficient for the treatment of intestinal inflammation[196].
Despite the expeditious development of newer biological therapies, only few have shown benefit in clinical trials in UC. Targeting of IL-23 or the IL-23 receptor or IL-23 axis is a potential therapeutic approach for autoimmune diseases including psoriasis, IBD, RA and multiple sclerosis[197]. Recently, testing of anti-IL-12/23 treatment in patients with CD has been performed. In Phase II trial, patients with moderate to severe CD that was resistant to TNF antagonists had an increased rate of response to induction with the fully human mAb ustekinumab directed against the p40, as compared with placebo[198]. However, due to the common p40 subunit and IL-12RB1 chain, the major drawback of anti-IL-23 treatment can be the simultaneous inhibition of IL-12 and a possible shutdown of the immune system. Nevertheless, it would be much more useful to design drugs that target the IL-23p19 or IL-23RA itself, so inhibiting IL-23 without modifying the effects of IL-12[197].
Treatment of CD patients with the IL-17 blocker secukinumab (anti-IL-17A) was ineffective and higher rates of adverse events were noted compared with placebo[199].
One new additional treatment for UC may be tofacitinib, an inhibitor of Janus kinases 1, 2, and 3 with in vitro functional specificity for kinases 1 and 3 over kinase 2, which is expected to block signaling involving gamma chain-containing cytokines including ILs-2, 4, 7, 9, 15, and 21. Tofacitinib, was approved for the treatment of RA in the USA, Japan and Russia in April 2013. In a double-blind, placebo-controlled, Phase II trial, patients with moderately to severely active UC treated with tofacitinib were more likely to have clinical response and remission than those receiving placebo[200].
Targeting leukocyte recruitment and cell adhesion molecules could be also an option for IBD therapy. Natalisumab, a recombinant humanised monoclonal IgG4 antibody, targets both the α4β1 heterodimer located in the central nervous system and the α4β7 integrin in the gut. The FDA approved natalizumab for both induction of remission and maintenance of remission for moderate to severe CD, though it has not been approved for this use in the European Union due to concerns over its risk/benefit ratio (risk of progressive multifocal leukoencephalopathy)[201]. Vedolizumab is a humanized mAb that specifically recognizes the α4β7 heterodimer, selectively blocks gut lymphocyte trafficking without interfering with trafficking to the central nervous system. In the Phase III study, vedolizumab was more effective than placebo as induction and maintenance therapy for UC suggesting that blockade of T cell homing in the gut may favor mucosal healing in UC[202,203].
The exact positioning of these promising new therapies in the management of UC remains uncertain currently. Additional long-term safety data and clinical experience will be needed to determine an overall benefit/harm ratio of newly developed biological agents.
The identified separate loci in IBD research individually have only modest effects on IBD susceptibility. They account together for only 20%-25% of the heritability, suggesting that gene-gene interactions as well as gene-environmental interactions could play a key role in IBD pathogenesis and fill the so called “genetic vacuum” of polygenic diseases[204]. More complete understanding of the immunopathogenic role of the various genes and ILs in intestinal inflammation will help in the development of more effective novel therapeutic strategies in UC. Next generation techniques in combination with the data analysis by systems-biology approach hopefully will contribute to the personalized therapy of the patients in the near future.
P- Reviewer: Akarsu M, Gazouli M, Yamakawa M S- Editor: Wen LL L- Editor: A E- Editor: Lu YJ
| 1. | Podolsky DK. Inflammatory bowel disease. N Engl J Med. 2002;347:417-429. [PubMed] |
| 2. | Molodecky NA, Soon IS, Rabi DM, Ghali WA, Ferris M, Chernoff G, Benchimol EI, Panaccione R, Ghosh S, Barkema HW. Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology. 2012;142:46-54.e42; quiz e30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3789] [Cited by in RCA: 3526] [Article Influence: 271.2] [Reference Citation Analysis (5)] |
| 3. | Loftus EV. Clinical epidemiology of inflammatory bowel disease: Incidence, prevalence, and environmental influences. Gastroenterology. 2004;126:1504-1517. [PubMed] |
| 4. | Silverberg MS, Satsangi J, Ahmad T, Arnott ID, Bernstein CN, Brant SR, Caprilli R, Colombel JF, Gasche C, Geboes K. Toward an integrated clinical, molecular and serological classification of inflammatory bowel disease: report of a Working Party of the 2005 Montreal World Congress of Gastroenterology. Can J Gastroenterol. 2005;19 Suppl A:5A-36A. [PubMed] |
| 5. | Van Limbergen J, Russell RK, Nimmo ER, Ho GT, Arnott ID, Wilson DC, Satsangi J. Genetics of the innate immune response in inflammatory bowel disease. Inflamm Bowel Dis. 2007;13:338-355. [PubMed] |
| 6. | Orholm M, Munkholm P, Langholz E, Nielsen OH, Sørensen TI, Binder V. Familial occurrence of inflammatory bowel disease. N Engl J Med. 1991;324:84-88. [PubMed] |
| 7. | Brant SR, Panhuysen CI, Nicolae D, Reddy DM, Bonen DK, Karaliukas R, Zhang L, Swanson E, Datta LW, Moran T. MDR1 Ala893 polymorphism is associated with inflammatory bowel disease. Am J Hum Genet. 2003;73:1282-1292. [PubMed] |
| 8. | Jostins L, Ripke S, Weersma RK, Duerr RH, McGovern DP, Hui KY, Lee JC, Schumm LP, Sharma Y, Anderson CA. Host-microbe interactions have shaped the genetic architecture of inflammatory bowel disease. Nature. 2012;491:119-124. [PubMed] |
| 9. | Fuss IJ, Neurath M, Boirivant M, Klein JS, de la Motte C, Strong SA, Fiocchi C, Strober W. Disparate CD4+ lamina propria (LP) lymphokine secretion profiles in inflammatory bowel disease. Crohn’s disease LP cells manifest increased secretion of IFN-gamma, whereas ulcerative colitis LP cells manifest increased secretion of IL-5. J Immunol. 1996;157:1261-1270. [PubMed] |
| 10. | Fujino S, Andoh A, Bamba S, Ogawa A, Hata K, Araki Y, Bamba T, Fujiyama Y. Increased expression of interleukin 17 in inflammatory bowel disease. Gut. 2003;52:65-70. [PubMed] |
| 11. | Ferguson LR, Shelling AN, Browning BL, Huebner C, Petermann I. Genes, diet and inflammatory bowel disease. Mutat Res. 2007;622:70-83. [PubMed] |
| 12. | Stokkers PC, van Aken BE, Basoski N, Reitsma PH, Tytgat GN, van Deventer SJ. Five genetic markers in the interleukin 1 family in relation to inflammatory bowel disease. Gut. 1998;43:33-39. [PubMed] |
| 13. | Bouma G, Crusius JB, Garcia-Gonzalez MA, Meijer BU, Hellemans HP, Hakvoort RJ, Schreuder GM, Kostense PJ, Meuwissen SG, Pena AS. Genetic markers in clinically well defined patients with ulcerative colitis (UC). Clin Exp Immunol. 1999;115:294-300. |
| 14. | Anderson CA, Massey DC, Barrett JC, Prescott NJ, Tremelling M, Fisher SA, Gwilliam R, Jacob J, Nimmo ER, Drummond H. Investigation of Crohn’s disease risk loci in ulcerative colitis further defines their molecular relationship. Gastroenterology. 2009;136:523-9.e3. [PubMed] |
| 15. | Houlston RS, Webb E, Broderick P, Pittman AM, Di Bernardo MC, Lubbe S, Chandler I, Vijayakrishnan J, Sullivan K, Penegar S. Meta-analysis of genome-wide association data identifies four new susceptibility loci for colorectal cancer. Nat Genet. 2008;40:1426-1435. [PubMed] |
| 16. | Barrett JC, Lee JC, Lees CW, Prescott NJ, Anderson CA, Phillips A, Wesley E, Parnell K, Zhang H, Drummond H. Genome-wide association study of ulcerative colitis identifies three new susceptibility loci, including the HNF4A region. Nat Genet. 2009;41:1330-1334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 359] [Cited by in RCA: 417] [Article Influence: 26.1] [Reference Citation Analysis (0)] |
| 17. | Ben Alaya W, Sfar I, Aouadi H, Jendoubi S, Najjar T, Filali A, Gorgi Y, Ben Abdallah T, Mouelhi L, Matri S. Association between CTLA-4 gene promoter (49 A/G) in exon 1 polymorphisms and inflammatory bowel disease in the Tunisian population. Saudi J Gastroenterol. 2009;15:29-34. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 18. | Zhou F, Xia B, Guo QS, Wang Q, Li L, Jiang L, Cheng H. [Cytotoxic T lymphocyte antigen-4 promoter gene polymorphism is significantly associated with ulcerative colitis]. Zhonghua Nei Ke Za Zhi. 2006;45:478-481. [PubMed] |
| 19. | Xia B, Crusius JB, Wu J, Zwiers A, van Bodegraven AA, Peña AS. CTLA4 gene polymorphisms in Dutch and Chinese patients with inflammatory bowel disease. Scand J Gastroenterol. 2002;37:1296-1300. [PubMed] |
| 20. | Magyari L, Faragó B, Bene J, Horvatovich K, Lakner L, Varga M, Figler M, Gasztonyi B, Mózsik G, Melegh B. No association of the cytotoxic T-lymphocyte associated gene CTLA4 +49A/G polymorphisms with Crohn’s disease and ulcerative colitis in Hungarian population samples. World J Gastroenterol. 2007;13:2205-2208. [PubMed] |
| 21. | Ghoreschi K, Laurence A, O’Shea JJ. Janus kinases in immune cell signaling. Immunol Rev. 2009;228:273-287. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 944] [Cited by in RCA: 883] [Article Influence: 55.2] [Reference Citation Analysis (0)] |
| 22. | Vera-Lastra O, Jara LJ, Espinoza LR. Prolactin and autoimmunity. Autoimmun Rev. 2002;1:360-364. |
| 23. | Polgar N, Csongei V, Szabo M, Zambo V, Melegh BI, Sumegi K, Nagy G, Tulassay Z, Melegh B. Investigation of JAK2, STAT3 and CCR6 polymorphisms and their gene-gene interactions in inflammatory bowel disease. Int J Immunogenet. 2012;39:247-252. [RCA] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 25] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 24. | Festen EA, Stokkers PC, van Diemen CC, van Bodegraven AA, Boezen HM, Crusius BJ, Hommes DW, van der Woude CJ, Balschun T, Verspaget HW. Genetic analysis in a Dutch study sample identifies more ulcerative colitis susceptibility loci and shows their additive role in disease risk. Am J Gastroenterol. 2010;105:395-402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 33] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 25. | Yang SK, Jung Y, Kim H, Hong M, Ye BD, Song K. Association of FCGR2A, JAK2 or HNF4A variants with ulcerative colitis in Koreans. Dig Liver Dis. 2011;43:856-861. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 28] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 26. | Jabalameli MR. Role of STAT3 Locus in autoimmune diseases. Genes Immun. 2011;12:155; author reply 156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 27. | Cénit MC, Alcina A, Márquez A, Mendoza JL, Díaz-Rubio M, de las Heras V, Izquierdo G, Arroyo R, Fernández O, de la Concha EG. STAT3 locus in inflammatory bowel disease and multiple sclerosis susceptibility. Genes Immun. 2010;11:264-268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 49] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 28. | Schultz KR. STAT3 mutations and persistence of autoimmunity. Blood. 2013;122:2295-2296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 29. | Plevy S, Silverberg MS, Lockton S, Stockfisch T, Croner L, Stachelski J, Brown M, Triggs C, Chuang E, Princen F. Combined serological, genetic, and inflammatory markers differentiate non-IBD, Crohn’s disease, and ulcerative colitis patients. Inflamm Bowel Dis. 2013;19:1139-1148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 101] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 30. | Nikolaus S, Bauditz J, Gionchetti P, Witt C, Lochs H, Schreiber S. Increased secretion of pro-inflammatory cytokines by circulating polymorphonuclear neutrophils and regulation by interleukin 10 during intestinal inflammation. Gut. 1998;42:470-476. [PubMed] |
| 32. | Deem RL, Shanahan F, Targan SR. Triggered human mucosal T cells release tumour necrosis factor-alpha and interferon-gamma which kill human colonic epithelial cells. Clin Exp Immunol. 1991;83:79-84. [PubMed] |
| 33. | Bruewer M, Luegering A, Kucharzik T, Parkos CA, Madara JL, Hopkins AM, Nusrat A. Proinflammatory cytokines disrupt epithelial barrier function by apoptosis-independent mechanisms. J Immunol. 2003;171:6164-6172. [PubMed] |
| 34. | Hampe J, Shaw SH, Saiz R, Leysens N, Lantermann A, Mascheretti S, Lynch NJ, MacPherson AJ, Bridger S, van Deventer S. Linkage of inflammatory bowel disease to human chromosome 6p. Am J Hum Genet. 1999;65:1647-1655. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 162] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 35. | Dechairo B, Dimon C, van Heel D, Mackay I, Edwards M, Scambler P, Jewell D, Cardon L, Lench N, Carey A. Replication and extension studies of inflammatory bowel disease susceptibility regions confirm linkage to chromosome 6p (IBD3). Eur J Hum Genet. 2001;9:627-633. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 51] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 36. | Yang H, Plevy SE, Taylor K, Tyan D, Fischel-Ghodsian N, McElree C, Targan SR, Rotter JI. Linkage of Crohn’s disease to the major histocompatibility complex region is detected by multiple non-parametric analyses. Gut. 1999;44:519-526. [PubMed] |
| 37. | Borruel N, Carol M, Casellas F, Antolín M, de Lara F, Espín E, Naval J, Guarner F, Malagelada JR. Increased mucosal tumour necrosis factor alpha production in Crohn’s disease can be downregulated ex vivo by probiotic bacteria. Gut. 2002;51:659-664. [PubMed] |
| 38. | Komatsu M, Kobayashi D, Saito K, Furuya D, Yagihashi A, Araake H, Tsuji N, Sakamaki S, Niitsu Y, Watanabe N. Tumor necrosis factor-alpha in serum of patients with inflammatory bowel disease as measured by a highly sensitive immuno-PCR. Clin Chem. 2001;47:1297-1301. [PubMed] |
| 39. | Braegger CP, Nicholls S, Murch SH, Stephens S, MacDonald TT. Tumour necrosis factor alpha in stool as a marker of intestinal inflammation. Lancet. 1992;339:89-91. [PubMed] |
| 40. | Murch SH, Braegger CP, Walker-Smith JA, MacDonald TT. Location of tumour necrosis factor alpha by immunohistochemistry in chronic inflammatory bowel disease. Gut. 1993;34:1705-1709. [PubMed] |
| 41. | Breese EJ, Michie CA, Nicholls SW, Murch SH, Williams CB, Domizio P, Walker-Smith JA, MacDonald TT. Tumor necrosis factor alpha-producing cells in the intestinal mucosa of children with inflammatory bowel disease. Gastroenterology. 1994;106:1455-1466. [PubMed] |
| 42. | Sashio H, Tamura K, Ito R, Yamamoto Y, Bamba H, Kosaka T, Fukui S, Sawada K, Fukuda Y, Tamura K. Polymorphisms of the TNF gene and the TNF receptor superfamily member 1B gene are associated with susceptibility to ulcerative colitis and Crohn’s disease, respectively. Immunogenetics. 2002;53:1020-1027. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 112] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 43. | Vatay A, Bene L, Kovács A, Prohászka Z, Szalai C, Romics L, Fekete B, Karádi I, Füst G. Relationship between the tumor necrosis factor alpha polymorphism and the serum C-reactive protein levels in inflammatory bowel disease. Immunogenetics. 2003;55:247-252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 52] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 44. | Louis E, Franchimont D, Piron A, Gevaert Y, Schaaf-Lafontaine N, Roland S, Mahieu P, Malaise M, De Groote D, Louis R. Tumour necrosis factor (TNF) gene polymorphism influences TNF-alpha production in lipopolysaccharide (LPS)-stimulated whole blood cell culture in healthy humans. Clin Exp Immunol. 1998;113:401-406. [PubMed] |
| 45. | Bouma G, Crusius JB, Oudkerk Pool M, Kolkman JJ, von Blomberg BM, Kostense PJ, Giphart MJ, Schreuder GM, Meuwissen SG, Peña AS. Secretion of tumour necrosis factor alpha and lymphotoxin alpha in relation to polymorphisms in the TNF genes and HLA-DR alleles. Relevance for inflammatory bowel disease. Scand J Immunol. 1996;43:456-463. [PubMed] |
| 46. | Koss K, Satsangi J, Fanning GC, Welsh KI, Jewell DP. Cytokine (TNF alpha, LT alpha and IL-10) polymorphisms in inflammatory bowel diseases and normal controls: differential effects on production and allele frequencies. Genes Immun. 2000;1:185-190. [PubMed] |
| 47. | Yamamoto-Furusho JK, Uscanga LF, Vargas-Alarcón G, Rodríguez-Pérez JM, Zuñiga J, Granados J. Polymorphisms in the promoter region of tumor necrosis factor alpha (TNF-alpha) and the HLA-DRB1 locus in Mexican mestizo patients with ulcerative colitis. Immunol Lett. 2004;95:31-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 28] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 48. | Wilson AG, di Giovine FS, Blakemore AI, Duff GW. Single base polymorphism in the human tumour necrosis factor alpha (TNF alpha) gene detectable by NcoI restriction of PCR product. Hum Mol Genet. 1992;1:353. [PubMed] |
| 49. | Hirv K, Seyfarth M, Uibo R, Kull K, Salupere R, Latza U, Rink L. Polymorphisms in tumour necrosis factor and adhesion molecule genes in patients with inflammatory bowel disease: associations with HLA-DR and -DQ alleles and subclinical markers. Scand J Gastroenterol. 1999;34:1025-1032. [PubMed] |
| 50. | Yap LM, Ahmad T, Jewell DP. The contribution of HLA genes to IBD susceptibility and phenotype. Best Pract Res Clin Gastroenterol. 2004;18:577-596. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 41] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 51. | Moffatt MF, Cookson WO. Tumour necrosis factor haplotypes and asthma. Hum Mol Genet. 1997;6:551-554. [PubMed] |
| 52. | Witte JS, Palmer LJ, O’Connor RD, Hopkins PJ, Hall JM. Relation between tumour necrosis factor polymorphism TNFalpha-308 and risk of asthma. Eur J Hum Genet. 2002;10:82-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 82] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 53. | Balding J, Kane D, Livingstone W, Mynett-Johnson L, Bresnihan B, Smith O, FitzGerald O. Cytokine gene polymorphisms: association with psoriatic arthritis susceptibility and severity. Arthritis Rheum. 2003;48:1408-1413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 92] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 54. | Mulcahy B, Waldron-Lynch F, McDermott MF, Adams C, Amos CI, Zhu DK, Ward RH, Clegg DO, Shanahan F, Molloy MG. Genetic variability in the tumor necrosis factor-lymphotoxin region influences susceptibility to rheumatoid arthritis. Am J Hum Genet. 1996;59:676-683. [PubMed] |
| 55. | Ferguson LR, Huebner C, Petermann I, Gearry RB, Barclay ML, Demmers P, McCulloch A, Han DY. Single nucleotide polymorphism in the tumor necrosis factor-alpha gene affects inflammatory bowel diseases risk. World J Gastroenterol. 2008;14:4652-4661. [PubMed] |
| 56. | Fidder HH, Heijmans R, Chowers Y, Bar-Meir S, Avidan B, Pena AS, Crusius JB. TNF-857 polymorphism in Israeli Jewish patients with inflammatory bowel disease. Int J Immunogenet. 2006;33:81-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 57. | Cao Q, Zhu Q, Wu ML, Hu WL, Gao M, Si JM. Genetic susceptibility to ulcerative colitis in the Chinese Han ethnic population: association with TNF polymorphisms. Chin Med J (Engl). 2006;119:1198-1203. [PubMed] |
| 58. | Lu Z, Chen L, Li H, Zhao Y, Lin L. Effect of the polymorphism of tumor necrosis factor-alpha-308 G/A gene promoter on the susceptibility to ulcerative colitis: a meta-analysis. Digestion. 2008;78:44-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 59. | Castro-Santos P, Suarez A, López-Rivas L, Mozo L, Gutierrez C. TNFalpha and IL-10 gene polymorphisms in inflammatory bowel disease. Association of -1082 AA low producer IL-10 genotype with steroid dependency. Am J Gastroenterol. 2006;101:1039-1047. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 44] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 60. | Cucchiara S, Latiano A, Palmieri O, Canani RB, D’Inca R, Guariso G, Vieni G, De Venuto D, Riegler G, De’Angelis GL. Polymorphisms of tumor necrosis factor-alpha but not MDR1 influence response to medical therapy in pediatric-onset inflammatory bowel disease. J Pediatr Gastroenterol Nutr. 2007;44:171-179. [RCA] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 64] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 61. | Sýkora J, Subrt I, Dìdek P, Siala K, Schwarz J, Machalová V, Varvarovská J, Pazdiora P, Pozler O, Stozický F. Cytokine tumor necrosis factor-alpha A promoter gene polymorphism at position -308 G--& gt; A and pediatric inflammatory bowel disease: implications in ulcerative colitis and Crohn’s disease. J Pediatr Gastroenterol Nutr. 2006;42:479-487. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 28] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 62. | Levine A, Karban A, Eliakim R, Shaoul R, Reif S, Pacht A, Wardi J, Yakir B, Silver EL. A polymorphism in the TNF-alpha promoter gene is associated with pediatric onset and colonic location of Crohn’s disease. Am J Gastroenterol. 2005;100:407-413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 38] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 63. | Lamhonwah AM, Skaug J, Scherer SW, Tein I. A third human carnitine/organic cation transporter (OCTN3) as a candidate for the 5q31 Crohn’s disease locus (IBD5). Biochem Biophys Res Commun. 2003;301:98-101. [PubMed] |
| 64. | Wu X, George RL, Huang W, Wang H, Conway SJ, Leibach FH, Ganapathy V. Structural and functional characteristics and tissue distribution pattern of rat OCTN1, an organic cation transporter, cloned from placenta. Biochim Biophys Acta. 2000;1466:315-327. [PubMed] |
| 65. | Wu X, Huang W, Prasad PD, Seth P, Rajan DP, Leibach FH, Chen J, Conway SJ, Ganapathy V. Functional characteristics and tissue distribution pattern of organic cation transporter 2 (OCTN2), an organic cation/carnitine transporter. J Pharmacol Exp Ther. 1999;290:1482-1492. [PubMed] |
| 66. | Elimrani I, Lahjouji K, Seidman E, Roy MJ, Mitchell GA, Qureshi I. Expression and localization of organic cation/carnitine transporter OCTN2 in Caco-2 cells. Am J Physiol Gastrointest Liver Physiol. 2003;284:G863-G871. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 40] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 67. | Burckhardt G, Wolff NA. Structure of renal organic anion and cation transporters. Am J Physiol Renal Physiol. 2000;278:F853-F866. [PubMed] |
| 68. | Li M, Gao X, Guo CC, Wu KC, Zhang X, Hu PJ. OCTN and CARD15 gene polymorphism in Chinese patients with inflammatory bowel disease. World J Gastroenterol. 2008;14:4923-4927. [PubMed] |
| 69. | García-Miranda P, Durán JM, Peral MJ, Ilundáin AA. Developmental maturation and segmental distribution of rat small intestinal L-carnitine uptake. J Membr Biol. 2005;206:9-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 70. | Kato Y, Sugiura M, Sugiura T, Wakayama T, Kubo Y, Kobayashi D, Sai Y, Tamai I, Iseki S, Tsuji A. Organic cation/carnitine transporter OCTN2 (Slc22a5) is responsible for carnitine transport across apical membranes of small intestinal epithelial cells in mouse. Mol Pharmacol. 2006;70:829-837. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 68] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 71. | Peltekova VD, Wintle RF, Rubin LA, Amos CI, Huang Q, Gu X, Newman B, Van Oene M, Cescon D, Greenberg G. Functional variants of OCTN cation transporter genes are associated with Crohn disease. Nat Genet. 2004;36:471-475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 572] [Cited by in RCA: 561] [Article Influence: 26.7] [Reference Citation Analysis (0)] |
| 72. | Martini M, Ferrara AM, Giachelia M, Panieri E, Siminovitch K, Galeotti T, Larocca LM, Pani G. Association of the OCTN1/1672T variant with increased risk for colorectal cancer in young individuals and ulcerative colitis patients. Inflamm Bowel Dis. 2012;18:439-448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 73. | Seth P, Wu X, Huang W, Leibach FH, Ganapathy V. Mutations in novel organic cation transporter (OCTN2), an organic cation/carnitine transporter, with differential effects on the organic cation transport function and the carnitine transport function. J Biol Chem. 1999;274:33388-33392. [PubMed] |
| 74. | Durán JM, Peral MJ, Calonge ML, Ilundáin AA. Functional characterization of intestinal L-carnitine transport. J Membr Biol. 2002;185:65-74. [PubMed] |
| 75. | Lin Z, Nelson L, Franke A, Poritz L, Li TY, Wu R, Wang Y, MacNeill C, Thomas NJ, Schreiber S. OCTN1 variant L503F is associated with familial and sporadic inflammatory bowel disease. J Crohns Colitis. 2010;4:132-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 76. | Schinkel AH. The physiological function of drug-transporting P-glycoproteins. Semin Cancer Biol. 1997;8:161-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 340] [Cited by in RCA: 318] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 77. | Ho GT, Moodie FM, Satsangi J. Multidrug resistance 1 gene (P-glycoprotein 170): an important determinant in gastrointestinal disease? Gut. 2003;52:759-766. [PubMed] |
| 78. | Juliano RL, Ling V. A surface glycoprotein modulating drug permeability in Chinese hamster ovary cell mutants. Biochim Biophys Acta. 1976;455:152-162. [PubMed] |
| 79. | Thiebaut F, Tsuruo T, Hamada H, Gottesman MM, Pastan I, Willingham MC. Cellular localization of the multidrug-resistance gene product P-glycoprotein in normal human tissues. Proc Natl Acad Sci USA. 1987;84:7735-7738. [PubMed] |
| 80. | Cordon-Cardo C, O’Brien JP, Casals D, Rittman-Grauer L, Biedler JL, Melamed MR, Bertino JR. Multidrug-resistance gene (P-glycoprotein) is expressed by endothelial cells at blood-brain barrier sites. Proc Natl Acad Sci USA. 1989;86:695-698. [PubMed] |
| 81. | Schwab M, Schaeffeler E, Marx C, Fromm MF, Kaskas B, Metzler J, Stange E, Herfarth H, Schoelmerich J, Gregor M. Association between the C3435T MDR1 gene polymorphism and susceptibility for ulcerative colitis. Gastroenterology. 2003;124:26-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 238] [Cited by in RCA: 219] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 82. | Glas J, Török HP, Schiemann U, Folwaczny C. MDR1 gene polymorphism in ulcerative colitis. Gastroenterology. 2004;126:367. [PubMed] |
| 83. | Annese V, Valvano MR, Palmieri O, Latiano A, Bossa F, Andriulli A. Multidrug resistance 1 gene in inflammatory bowel disease: a meta-analysis. World J Gastroenterol. 2006;12:3636-3644. [PubMed] |
| 84. | Hoffmeyer S, Burk O, von Richter O, Arnold HP, Brockmöller J, Johne A, Cascorbi I, Gerloff T, Roots I, Eichelbaum M. Functional polymorphisms of the human multidrug-resistance gene: multiple sequence variations and correlation of one allele with P-glycoprotein expression and activity in vivo. Proc Natl Acad Sci USA. 2000;97:3473-3478. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 557] [Cited by in RCA: 894] [Article Influence: 35.8] [Reference Citation Analysis (0)] |
| 85. | Tanabe M, Ieiri I, Nagata N, Inoue K, Ito S, Kanamori Y, Takahashi M, Kurata Y, Kigawa J, Higuchi S. Expression of P-glycoprotein in human placenta: relation to genetic polymorphism of the multidrug resistance (MDR)-1 gene. J Pharmacol Exp Ther. 2001;297:1137-1143. [PubMed] |
| 86. | Nakamura T, Sakaeda T, Horinouchi M, Tamura T, Aoyama N, Shirakawa T, Matsuo M, Kasuga M, Okumura K. Effect of the mutation (C3435T) at exon 26 of the MDR1 gene on expression level of MDR1 messenger ribonucleic acid in duodenal enterocytes of healthy Japanese subjects. Clin Pharmacol Ther. 2002;71:297-303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 199] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 87. | Kim RB, Leake BF, Choo EF, Dresser GK, Kubba SV, Schwarz UI, Taylor A, Xie HG, McKinsey J, Zhou S. Identification of functionally variant MDR1 alleles among European Americans and African Americans. Clin Pharmacol Ther. 2001;70:189-199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 760] [Cited by in RCA: 725] [Article Influence: 30.2] [Reference Citation Analysis (0)] |
| 88. | Brinar M, Cukovic-Cavka S, Bozina N, Ravic KG, Markos P, Ladic A, Cota M, Krznaric Z, Vucelic B. MDR1 polymorphisms are associated with inflammatory bowel disease in a cohort of Croatian IBD patients. BMC Gastroenterol. 2013;13:57. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 34] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 89. | Osuga T, Sakaeda T, Nakamura T, Yamada T, Koyama T, Tamura T, Aoyama N, Okamura N, Kasuga M, Okumura K. MDR1 C3435T polymorphism is predictive of later onset of ulcerative colitis in Japanese. Biol Pharm Bull. 2006;29:324-329. [PubMed] |
| 90. | Ho GT, Nimmo ER, Tenesa A, Fennell J, Drummond H, Mowat C, Arnott ID, Satsangi J. Allelic variations of the multidrug resistance gene determine susceptibility and disease behavior in ulcerative colitis. Gastroenterology. 2005;128:288-296. [PubMed] |
| 91. | Potocnik U, Ferkolj I, Glavac D, Dean M. Polymorphisms in multidrug resistance 1 (MDR1) gene are associated with refractory Crohn disease and ulcerative colitis. Genes Immun. 2004;5:530-539. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 123] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 92. | Palmieri O, Latiano A, Valvano R, D’Incà R, Vecchi M, Sturniolo GC, Saibeni S, Bossa F, Latiano T, Devoto M. Multidrug resistance 1 gene polymorphisms are not associated with inflammatory bowel disease and response to therapy in Italian patients. Aliment Pharmacol Ther. 2005;22:1129-1138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 49] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 93. | Dudarewicz M, Barańska M, Rychlik-Sych M, Trzciński R, Dziki A, Skrętkowicz J. C3435T polymorphism of the ABCB1/MDR1 gene encoding P-glycoprotein in patients with inflammatory bowel disease in a Polish population. Pharmacol Rep. 2012;64:343-350. [PubMed] |
| 94. | Huebner C, Browning BL, Petermann I, Han DY, Philpott M, Barclay M, Gearry R, McCulloch A, Demmers P, Ferguson LR. Genetic analysis of MDR1 and inflammatory bowel disease reveals protective effect of heterozygous variants for ulcerative colitis. Inflamm Bowel Dis. 2009;15:1784-1793. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 25] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 95. | Fiedler T, Büning C, Reuter W, Pitre G, Gentz E, Schmidt HH, Büttner J, Ockenga J, Gerloff T, Meisel C. Possible role of MDR1 two-locus genotypes for young-age onset ulcerative colitis but not Crohn’s disease. Eur J Clin Pharmacol. 2007;63:917-925. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 25] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 96. | Onnie CM, Fisher SA, Pattni R, Sanderson J, Forbes A, Lewis CM, Mathew CG. Associations of allelic variants of the multidrug resistance gene (ABCB1 or MDR1) and inflammatory bowel disease and their effects on disease behavior: a case-control and meta-analysis study. Inflamm Bowel Dis. 2006;12:263-271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 63] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 97. | Urcelay E, Mendoza JL, Martín MC, Mas A, Martínez A, Taxonera C, Fernandez-Arquero M, Díaz-Rubio M, de la Concha EG. MDR1 gene: susceptibility in Spanish Crohn’s disease and ulcerative colitis patients. Inflamm Bowel Dis. 2006;12:33-37. [PubMed] |
| 98. | Fritz JH, Ferrero RL, Philpott DJ, Girardin SE. Nod-like proteins in immunity, inflammation and disease. Nat Immunol. 2006;7:1250-1257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 648] [Cited by in RCA: 650] [Article Influence: 34.2] [Reference Citation Analysis (0)] |
| 99. | Hysi P, Kabesch M, Moffatt MF, Schedel M, Carr D, Zhang Y, Boardman B, von Mutius E, Weiland SK, Leupold W. NOD1 variation, immunoglobulin E and asthma. Hum Mol Genet. 2005;14:935-941. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 197] [Cited by in RCA: 206] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 100. | Ting JP, Kastner DL, Hoffman HM. CATERPILLERs, pyrin and hereditary immunological disorders. Nat Rev Immunol. 2006;6:183-195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 261] [Cited by in RCA: 238] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 101. | Verma R, Ahuja V, Paul J. Detection of single-nucleotide polymorphisms in the intron 9 region of the nucleotide oligomerization domain-1 gene in ulcerative colitis patients of North India. J Gastroenterol Hepatol. 2012;27:96-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 102. | McGovern DP, Hysi P, Ahmad T, van Heel DA, Moffatt MF, Carey A, Cookson WO, Jewell DP. Association between a complex insertion/deletion polymorphism in NOD1 (CARD4) and susceptibility to inflammatory bowel disease. Hum Mol Genet. 2005;14:1245-1250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 240] [Cited by in RCA: 238] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 103. | Van Limbergen J, Russell RK, Nimmo ER, Törkvist L, Lees CW, Drummond HE, Smith L, Anderson NH, Gillett PM, McGrogan P. Contribution of the NOD1/CARD4 insertion/deletion polymorphism +32656 to inflammatory bowel disease in Northern Europe. Inflamm Bowel Dis. 2007;13:882-889. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 104. | Tremelling M, Hancock L, Bredin F, Sharpstone D, Bingham SA, Parkes M. Complex insertion/deletion polymorphism in NOD1 (CARD4) is not associated with inflammatory bowel disease susceptibility in East Anglia panel. Inflamm Bowel Dis. 2006;12:967-971. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 105. | Aderem A, Ulevitch RJ. Toll-like receptors in the induction of the innate immune response. Nature. 2000;406:782-787. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2272] [Cited by in RCA: 2242] [Article Influence: 89.7] [Reference Citation Analysis (0)] |
| 106. | Beutler B. Inferences, questions and possibilities in Toll-like receptor signalling. Nature. 2004;430:257-263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1111] [Cited by in RCA: 1124] [Article Influence: 53.5] [Reference Citation Analysis (0)] |
| 107. | Kawai T, Akira S. Innate immune recognition of viral infection. Nat Immunol. 2006;7:131-137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1338] [Cited by in RCA: 1485] [Article Influence: 78.2] [Reference Citation Analysis (0)] |
| 108. | Cario E, Podolsky DK. Differential alteration in intestinal epithelial cell expression of toll-like receptor 3 (TLR3) and TLR4 in inflammatory bowel disease. Infect Immun. 2000;68:7010-7017. [PubMed] |
| 109. | Cario E, Rosenberg IM, Brandwein SL, Beck PL, Reinecker HC, Podolsky DK. Lipopolysaccharide activates distinct signaling pathways in intestinal epithelial cell lines expressing Toll-like receptors. J Immunol. 2000;164:966-972. [PubMed] |
| 110. | Rachmilewitz D, Katakura K, Karmeli F, Hayashi T, Reinus C, Rudensky B, Akira S, Takeda K, Lee J, Takabayashi K. Toll-like receptor 9 signaling mediates the anti-inflammatory effects of probiotics in murine experimental colitis. Gastroenterology. 2004;126:520-528. [PubMed] |
| 111. | Rakoff-Nahoum S, Paglino J, Eslami-Varzaneh F, Edberg S, Medzhitov R. Recognition of commensal microflora by toll-like receptors is required for intestinal homeostasis. Cell. 2004;118:229-241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3061] [Cited by in RCA: 3162] [Article Influence: 150.6] [Reference Citation Analysis (0)] |
| 112. | Lee J, Mo JH, Katakura K, Alkalay I, Rucker AN, Liu YT, Lee HK, Shen C, Cojocaru G, Shenouda S. Maintenance of colonic homeostasis by distinctive apical TLR9 signalling in intestinal epithelial cells. Nat Cell Biol. 2006;8:1327-1336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 457] [Cited by in RCA: 457] [Article Influence: 24.1] [Reference Citation Analysis (0)] |
| 113. | Zhang D, Zhang G, Hayden MS, Greenblatt MB, Bussey C, Flavell RA, Ghosh S. A toll-like receptor that prevents infection by uropathogenic bacteria. Science. 2004;303:1522-1526. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 733] [Cited by in RCA: 727] [Article Influence: 34.6] [Reference Citation Analysis (0)] |
| 114. | Vermeire S, Rutgeerts P, Van Steen K, Joossens S, Claessens G, Pierik M, Peeters M, Vlietinck R. Genome wide scan in a Flemish inflammatory bowel disease population: support for the IBD4 locus, population heterogeneity, and epistasis. Gut. 2004;53:980-986. |
| 115. | Medzhitov R. Toll-like receptors and innate immunity. Nat Rev Immunol. 2001;1:135-145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2861] [Cited by in RCA: 2904] [Article Influence: 121.0] [Reference Citation Analysis (0)] |
| 116. | Barton GM, Medzhitov R. Toll-like receptor signaling pathways. Science. 2003;300:1524-1525. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 963] [Cited by in RCA: 1005] [Article Influence: 45.7] [Reference Citation Analysis (0)] |
| 117. | Hemmi H, Takeuchi O, Kawai T, Kaisho T, Sato S, Sanjo H, Matsumoto M, Hoshino K, Wagner H, Takeda K. A Toll-like receptor recognizes bacterial DNA. Nature. 2000;408:740-745. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4803] [Cited by in RCA: 4821] [Article Influence: 192.8] [Reference Citation Analysis (0)] |
| 118. | Lund J, Sato A, Akira S, Medzhitov R, Iwasaki A. Toll-like receptor 9-mediated recognition of Herpes simplex virus-2 by plasmacytoid dendritic cells. J Exp Med. 2003;198:513-520. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 887] [Cited by in RCA: 903] [Article Influence: 41.0] [Reference Citation Analysis (0)] |
| 119. | Török HP, Glas J, Tonenchi L, Mussack T, Folwaczny C. Polymorphisms of the lipopolysaccharide-signaling complex in inflammatory bowel disease: association of a mutation in the Toll-like receptor 4 gene with ulcerative colitis. Clin Immunol. 2004;112:85-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 164] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 120. | Franchimont D, Vermeire S, El Housni H, Pierik M, Van Steen K, Gustot T, Quertinmont E, Abramowicz M, Van Gossum A, Devière J. Deficient host-bacteria interactions in inflammatory bowel disease? The toll-like receptor (TLR)-4 Asp299gly polymorphism is associated with Crohn’s disease and ulcerative colitis. Gut. 2004;53:987-992. [PubMed] |
| 121. | Manolakis AC, Kapsoritakis AN, Kapsoritaki A, Tiaka EK, Oikonomou KA, Lotis V, Vamvakopoulou D, Davidi I, Vamvakopoulos N, Potamianos SP. Readressing the role of Toll-like receptor-4 alleles in inflammatory bowel disease: colitis, smoking, and seroreactivity. Dig Dis Sci. 2013;58:371-380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 122. | Meena NK, Verma R, Verma N, Ahuja V, Paul J. TLR4 D299G polymorphism modulates cytokine expression in ulcerative colitis. J Clin Gastroenterol. 2013;47:773-780. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 28] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 123. | Oostenbrug LE, Drenth JP, de Jong DJ, Nolte IM, Oosterom E, van Dullemen HM, van der Linde K, te Meerman GJ, van der Steege G, Kleibeuker JH. Association between Toll-like receptor 4 and inflammatory bowel disease. Inflamm Bowel Dis. 2005;11:567-575. [PubMed] |
| 124. | Rigoli L, Romano C, Caruso RA, Lo Presti MA, Di Bella C, Procopio V, Lo Giudice G, Amorini M, Costantino G, Sergi MD. Clinical significance of NOD2/CARD15 and Toll-like receptor 4 gene single nucleotide polymorphisms in inflammatory bowel disease. World J Gastroenterol. 2008;14:4454-4461. [PubMed] |
| 125. | Kim EJ, Chung WC, Lee KM, Paik CN, Jung SH, Lee BI, Chae HS, Choi KY. Association between toll-like receptors/CD14 gene polymorphisms and inflammatory bowel disease in Korean population. J Korean Med Sci. 2012;27:72-77. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 23] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 126. | Okayama N, Fujimura K, Suehiro Y, Hamanaka Y, Fujiwara M, Matsubara T, Maekawa T, Hazama S, Oka M, Nohara H. Simple genotype analysis of the Asp299Gly polymorphism of the Toll-like receptor-4 gene that is associated with lipopolysaccharide hyporesponsiveness. J Clin Lab Anal. 2002;16:56-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 66] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 127. | Shen XY, Shi RH, Wang Y, Zhang HJ, Zhou XQ, Shen FC, Li KB. [Toll-like receptor gene polymorphisms and susceptibility to inflammatory bowel disease in Chinese Han and Caucasian populations]. Zhonghua Yi Xue Za Zhi. 2010;90:1416-1420. [PubMed] |
| 128. | Guo QS, Xia B, Jiang Y, Morré SA, Cheng L, Li J, Crusius JB, Peña AS. Polymorphisms of CD14 gene and TLR4 gene are not associated with ulcerative colitis in Chinese patients. Postgrad Med J. 2005;81:526-529. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 27] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 129. | Fuse K, Katakura K, Sakamoto N, Ohira H. Toll-like receptor 9 gene mutations and polymorphisms in Japanese ulcerative colitis patients. World J Gastroenterol. 2010;16:5815-5821. [PubMed] |
| 130. | Török HP, Glas J, Tonenchi L, Bruennler G, Folwaczny M, Folwaczny C. Crohn’s disease is associated with a toll-like receptor-9 polymorphism. Gastroenterology. 2004;127:365-366. [PubMed] |
| 131. | Salmi M, Granfors K, MacDermott R, Jalkanen S. Aberrant binding of lamina propria lymphocytes to vascular endothelium in inflammatory bowel diseases. Gastroenterology. 1994;106:596-605. [PubMed] |
| 132. | Nakamura S, Ohtani H, Watanabe Y, Fukushima K, Matsumoto T, Kitano A, Kobayashi K, Nagura H. In situ expression of the cell adhesion molecules in inflammatory bowel disease. Evidence of immunologic activation of vascular endothelial cells. Lab Invest. 1993;69:77-85. [PubMed] |
| 133. | Ohto H, Maeda H, Shibata Y, Chen RF, Ozaki Y, Higashihara M, Takeuchi A, Tohyama H. A novel leukocyte differentiation antigen: two monoclonal antibodies TM2 and TM3 define a 120-kd molecule present on neutrophils, monocytes, platelets, and activated lymphoblasts. Blood. 1985;66:873-881. [PubMed] |
| 134. | Newman PJ, Albelda SM. Cellular and molecular aspects of PECAM-1. Nouv Rev Fr Hematol. 1992;34 Suppl:S9-13. [PubMed] |
| 135. | Newman PJ, Berndt MC, Gorski J, White GC, Lyman S, Paddock C, Muller WA. PECAM-1 (CD31) cloning and relation to adhesion molecules of the immunoglobulin gene superfamily. Science. 1990;247:1219-1222. [PubMed] |
| 136. | Vaporciyan AA, DeLisser HM, Yan HC, Mendiguren II, Thom SR, Jones ML, Ward PA, Albelda SM. Involvement of platelet-endothelial cell adhesion molecule-1 in neutrophil recruitment in vivo. Science. 1993;262:1580-1582. [PubMed] |
| 137. | Gao C, Sun W, Christofidou-Solomidou M, Sawada M, Newman DK, Bergom C, Albelda SM, Matsuyama S, Newman PJ. PECAM-1 functions as a specific and potent inhibitor of mitochondrial-dependent apoptosis. Blood. 2003;102:169-179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 94] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 138. | Piali L, Hammel P, Uherek C, Bachmann F, Gisler RH, Dunon D, Imhof BA. CD31/PECAM-1 is a ligand for alpha v beta 3 integrin involved in adhesion of leukocytes to endothelium. J Cell Biol. 1995;130:451-460. [PubMed] |
| 139. | Pooley N, Ghosh L, Sharon P. Up-regulation of E-selectin and intercellular adhesion molecule-1 differs between Crohn’s disease and ulcerative colitis. Dig Dis Sci. 1995;40:219-225. [PubMed] |
| 140. | Steeber DA, Green NE, Sato S, Tedder TF. Humoral immune responses in L-selectin-deficient mice. J Immunol. 1996;157:4899-4907. [PubMed] |
| 141. | Khazen D, Jendoubi-Ayed S, Aleya WB, Sfar I, Mouelhi L, Matri S, Najjar T, Filali A, Gorgi Y, Abdallah TB. Polymorphism in ICAM-1, PECAM-1, E-selectin, and L-selectin genes in Tunisian patients with inflammatory bowel disease. Eur J Gastroenterol Hepatol. 2009;21:167-175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 142. | Akdis M, Burgler S, Crameri R, Eiwegger T, Fujita H, Gomez E, Klunker S, Meyer N, O’Mahony L, Palomares O. Interleukins, from 1 to 37, and interferon-γ: receptors, functions, and roles in diseases. J Allergy Clin Immunol. 2011;127:701-21.e1-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 499] [Cited by in RCA: 579] [Article Influence: 41.4] [Reference Citation Analysis (0)] |
| 143. | Dinarello CA. Immunological and inflammatory functions of the interleukin-1 family. Annu Rev Immunol. 2009;27:519-550. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2289] [Cited by in RCA: 2622] [Article Influence: 163.9] [Reference Citation Analysis (0)] |
| 144. | Dinarello CA. Interleukin-1, interleukin-1 receptors and interleukin-1 receptor antagonist. Int Rev Immunol. 1998;16:457-499. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 565] [Cited by in RCA: 549] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 145. | Yamamoto-Furusho JK, Santiago-Hernández JJ, Pérez-Hernández N, Ramírez-Fuentes S, Fragoso JM, Vargas-Alarcón G. Interleukin 1 β (IL-1B) and IL-1 antagonist receptor (IL-1RN) gene polymorphisms are associated with the genetic susceptibility and steroid dependence in patients with ulcerative colitis. J Clin Gastroenterol. 2011;45:531-535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 50] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 146. | Malek TR. The biology of interleukin-2. Annu Rev Immunol. 2008;26:453-479. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 733] [Cited by in RCA: 829] [Article Influence: 48.8] [Reference Citation Analysis (0)] |
| 147. | Festen EA, Goyette P, Scott R, Annese V, Zhernakova A, Lian J, Lefèbvre C, Brant SR, Cho JH, Silverberg MS. Genetic variants in the region harbouring IL2/IL21 associated with ulcerative colitis. Gut. 2009;58:799-804. [PubMed] |
| 148. | Boulanger MJ, Chow DC, Brevnova EE, Garcia KC. Hexameric structure and assembly of the interleukin-6/IL-6 alpha-receptor/gp130 complex. Science. 2003;300:2101-2104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 455] [Cited by in RCA: 510] [Article Influence: 23.2] [Reference Citation Analysis (0)] |
| 149. | Balding J, Livingstone WJ, Conroy J, Mynett-Johnson L, Weir DG, Mahmud N, Smith OP. Inflammatory bowel disease: the role of inflammatory cytokine gene polymorphisms. Mediators Inflamm. 2004;13:181-187. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 54] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 150. | Cantor MJ, Nickerson P, Bernstein CN. The role of cytokine gene polymorphisms in determining disease susceptibility and phenotype in inflammatory bowel disease. Am J Gastroenterol. 2005;100:1134-1142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 66] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 151. | Yoshimura T, Matsushima K, Tanaka S, Robinson EA, Appella E, Oppenheim JJ, Leonard EJ. Purification of a human monocyte-derived neutrophil chemotactic factor that has peptide sequence similarity to other host defense cytokines. Proc Natl Acad Sci USA. 1987;84:9233-9237. [PubMed] |
| 152. | Holmes WE, Lee J, Kuang WJ, Rice GC, Wood WI. Structure and functional expression of a human interleukin-8 receptor. Science. 1991;253:1278-1280. [PubMed] |
| 153. | Miller MD, Krangel MS. Biology and biochemistry of the chemokines: a family of chemotactic and inflammatory cytokines. Crit Rev Immunol. 1992;12:17-46. [PubMed] |
| 154. | Walczak A, Przybylowska K, Dziki L, Sygut A, Chojnacki C, Chojnacki J, Dziki A, Majsterek I. The lL-8 and IL-13 gene polymorphisms in inflammatory bowel disease and colorectal cancer. DNA Cell Biol. 2012;31:1431-1438. [PubMed] |
| 155. | Li K, Yao S, Liu S, Wang B, Mao D. Genetic polymorphisms of interleukin 8 and risk of ulcerative colitis in the Chinese population. Clin Chim Acta. 2009;405:30-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 20] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 156. | Deniz G, Erten G, Kücüksezer UC, Kocacik D, Karagiannidis C, Aktas E, Akdis CA, Akdis M. Regulatory NK cells suppress antigen-specific T cell responses. J Immunol. 2008;180:850-857. [PubMed] |
| 157. | Franke A, Balschun T, Karlsen TH, Sventoraityte J, Nikolaus S, Mayr G, Domingues FS, Albrecht M, Nothnagel M, Ellinghaus D. Sequence variants in IL10, ARPC2 and multiple other loci contribute to ulcerative colitis susceptibility. Nat Genet. 2008;40:1319-1323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 486] [Cited by in RCA: 471] [Article Influence: 27.7] [Reference Citation Analysis (0)] |
| 158. | Doecke JD, Simms LA, Zhao ZZ, Huang N, Hanigan K, Krishnaprasad K, Roberts RL, Andrews JM, Mahy G, Bampton P. Genetic susceptibility in IBD: overlap between ulcerative colitis and Crohn’s disease. Inflamm Bowel Dis. 2013;19:240-245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 36] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 159. | Andersen V, Ernst A, Christensen J, Østergaard M, Jacobsen BA, Tjønneland A, Krarup HB, Vogel U. The polymorphism rs3024505 proximal to IL-10 is associated with risk of ulcerative colitis and Crohns disease in a Danish case-control study. BMC Med Genet. 2010;11:82. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 48] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 160. | Tedde A, Laura Putignano A, Bagnoli S, Congregati C, Milla M, Sorbi S, Genuardi M, Papi L. Interleukin-10 promoter polymorphisms influence susceptibility to ulcerative colitis in a gender-specific manner. Scand J Gastroenterol. 2008;43:712-718. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 39] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 161. | Garza-González E, Pérez-Pérez GI, Mendoza-Ibarra SI, Flores-Gutiérrez JP, Bosques-Padilla FJ. Genetic risk factors for inflammatory bowel disease in a North-eastern Mexican population. Int J Immunogenet. 2010;37:355-359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 21] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 162. | Marrakchi R, Moussa A, Ouerhani S, Bougatef K, Bouhaha R, Messai Y, Rouissi K, Khadimallah I, Khodjet-el-Khil H, Najar T. Interleukin 10 promoter region polymorphisms in inflammatory bowel disease in Tunisian population. Inflamm Res. 2009;58:155-160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 163. | Ahirwar DK, Kesarwani P, Singh R, Ghoshal UC, Mittal RD. Role of tumor necrosis factor-alpha (C-863A) polymorphism in pathogenesis of inflammatory bowel disease in Northern India. J Gastrointest Cancer. 2012;43:196-204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 164. | Hsieh CS, Macatonia SE, Tripp CS, Wolf SF, O’Garra A, Murphy KM. Development of TH1 CD4+ T cells through IL-12 produced by Listeria-induced macrophages. Science. 1993;260:547-549. [PubMed] |
| 165. | Wolf SF, Sieburth D, Sypek J. Interleukin 12: a key modulator of immune function. Stem Cells. 1994;12:154-168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 128] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 166. | Parham C, Chirica M, Timans J, Vaisberg E, Travis M, Cheung J, Pflanz S, Zhang R, Singh KP, Vega F. A receptor for the heterodimeric cytokine IL-23 is composed of IL-12Rbeta1 and a novel cytokine receptor subunit, IL-23R. J Immunol. 2002;168:5699-5708. [PubMed] |
| 167. | Glas J, Seiderer J, Wagner J, Olszak T, Fries C, Tillack C, Friedrich M, Beigel F, Stallhofer J, Steib C. Analysis of IL12B gene variants in inflammatory bowel disease. PLoS One. 2012;7:e34349. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 51] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 168. | Yamazaki K, Takahashi A, Takazoe M, Kubo M, Onouchi Y, Fujino A, Kamatani N, Nakamura Y, Hata A. Positive association of genetic variants in the upstream region of NKX2-3 with Crohn’s disease in Japanese patients. Gut. 2009;58:228-232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 56] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 169. | Kobori A, Yagi Y, Imaeda H, Ban H, Bamba S, Tsujikawa T, Saito Y, Fujiyama Y, Andoh A. Interleukin-33 expression is specifically enhanced in inflamed mucosa of ulcerative colitis. J Gastroenterol. 2010;45:999-1007. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 183] [Cited by in RCA: 204] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 170. | Seiderer J, Elben I, Diegelmann J, Glas J, Stallhofer J, Tillack C, Pfennig S, Jürgens M, Schmechel S, Konrad A. Role of the novel Th17 cytokine IL-17F in inflammatory bowel disease (IBD): upregulated colonic IL-17F expression in active Crohn’s disease and analysis of the IL17F p.His161Arg polymorphism in IBD. Inflamm Bowel Dis. 2008;14:437-445. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 246] [Cited by in RCA: 272] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 171. | Schwartz S, Beaulieu JF, Ruemmele FM. Interleukin-17 is a potent immuno-modulator and regulator of normal human intestinal epithelial cell growth. Biochem Biophys Res Commun. 2005;337:505-509. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 40] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 172. | Arisawa T, Tahara T, Shibata T, Nagasaka M, Nakamura M, Kamiya Y, Fujita H, Nakamura M, Yoshioka D, Arima Y. The influence of polymorphisms of interleukin-17A and interleukin-17F genes on the susceptibility to ulcerative colitis. J Clin Immunol. 2008;28:44-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 150] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 173. | Franke A, McGovern DP, Barrett JC, Wang K, Radford-Smith GL, Ahmad T, Lees CW, Balschun T, Lee J, Roberts R. Genome-wide meta-analysis increases to 71 the number of confirmed Crohn’s disease susceptibility loci. Nat Genet. 2010;42:1118-1125. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2110] [Cited by in RCA: 2005] [Article Influence: 133.7] [Reference Citation Analysis (0)] |
| 174. | Dinarello CA. Interleukin-18. Methods. 1999;19:121-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 328] [Cited by in RCA: 337] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 175. | Aizawa Y, Sutoh S, Matsuoka M, Negishi M, Torii A, Miyakawa Y, Sugisaka H, Nakamura M, Toda G. Association of interleukin-18 gene single-nucleotide polymorphisms with susceptibility to inflammatory bowel disease. Tissue Antigens. 2005;65:88-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 26] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 176. | Takagawa T, Tamura K, Takeda N, Tomita T, Ohda Y, Fukunaga K, Hida N, Ohnishi K, Hori K, Kosaka T. Association between IL-18 gene promoter polymorphisms and inflammatory bowel disease in a Japanese population. Inflamm Bowel Dis. 2005;11:1038-1043. [PubMed] |
| 177. | Ben Aleya W, Sfar I, Habibi I, Mouelhi L, Aouadi H, Makhlouf M, Ayed-Jendoubi S, Najjar T, Ben Abdallah T, Ayed K. Interleukin-18 gene polymorphisms in tunisian patients with inflammatory bowel disease. Digestion. 2011;83:269-274. [PubMed] |
| 178. | Bin C, Zhirong Z, Xiaoqin W, Minhu C, Mei L, Xiang G, Baili C, Pinjin H. Contribution of rs11465788 in IL23R gene to Crohn’s disease susceptibility and phenotype in Chinese population. J Genet. 2009;88:191-196. [PubMed] |
| 179. | Cargill M, Schrodi SJ, Chang M, Garcia VE, Brandon R, Callis KP, Matsunami N, Ardlie KG, Civello D, Catanese JJ. A large-scale genetic association study confirms IL12B and leads to the identification of IL23R as psoriasis-risk genes. Am J Hum Genet. 2007;80:273-290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 858] [Cited by in RCA: 857] [Article Influence: 47.6] [Reference Citation Analysis (0)] |
| 180. | Sáfrány E, Pazár B, Csöngei V, Járomi L, Polgár N, Sipeky C, Horváth IF, Zeher M, Poór G, Melegh B. Variants of the IL23R gene are associated with ankylosing spondylitis but not with Sjögren syndrome in Hungarian population samples. Scand J Immunol. 2009;70:68-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 56] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 181. | Safrany E, Szell M, Csongei V, Jaromi L, Sipeky C, Szabo T, Kemeny L, Nagy J, Melegh B. Polymorphisms of the IL23R gene are associated with psoriasis but not with immunoglobulin A nephropathy in a Hungarian population. Inflammation. 2011;34:603-608. [PubMed] |
| 182. | Safrany E, Melegh B. Functional variants of the interleukin-23 receptor gene in non-gastrointestinal autoimmune diseases. Curr Med Chem. 2009;16:3766-3774. [PubMed] |
| 183. | Duerr RH, Taylor KD, Brant SR, Rioux JD, Silverberg MS, Daly MJ, Steinhart AH, Abraham C, Regueiro M, Griffiths A. A genome-wide association study identifies IL23R as an inflammatory bowel disease gene. Science. 2006;314:1461-1463. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2428] [Cited by in RCA: 2308] [Article Influence: 121.5] [Reference Citation Analysis (1)] |
| 184. | Yu P, Shen F, Zhang X, Cao R, Zhao X, Liu P, Tu H, Yang X, Shi R, Zhang H. Association of single nucleotide polymorphisms of IL23R and IL17 with ulcerative colitis risk in a Chinese Han population. PLoS One. 2012;7:e44380. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 23] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 185. | Zhao XD, Shen FC, Zhang HJ, Shen XY, Wang YM, Yang XZ, Tu HM, Tai YH, Shi RH. [Association of interleukin-23 receptor gene polymorphisms with susceptibility and phenotypes of inflammatory bowel diseases in Jiangsu Han population]. Zhonghua Nei Ke Za Zhi. 2011;50:935-941. [PubMed] |
| 186. | Einarsdottir E, Koskinen LL, Dukes E, Kainu K, Suomela S, Lappalainen M, Ziberna F, Korponay-Szabo IR, Kurppa K, Kaukinen K. IL23R in the Swedish, Finnish, Hungarian and Italian populations: association with IBD and psoriasis, and linkage to celiac disease. BMC Med Genet. 2009;10:8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 55] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 187. | Sarlos P, Varszegi D, Csongei V, Magyari L, Jaromi L, Nagy L, Melegh B. Susceptibility to ulcerative colitis in Hungarian patients determined by gene-gene interactions. World J Gastroenterol. 2014;20:219-227. [PubMed] |
| 188. | Pène J, Chevalier S, Preisser L, Vénéreau E, Guilleux MH, Ghannam S, Molès JP, Danger Y, Ravon E, Lesaux S. Chronically inflamed human tissues are infiltrated by highly differentiated Th17 lymphocytes. J Immunol. 2008;180:7423-7430. [PubMed] |
| 189. | Silverberg MS, Cho JH, Rioux JD, McGovern DP, Wu J, Annese V, Achkar JP, Goyette P, Scott R, Xu W. Ulcerative colitis-risk loci on chromosomes 1p36 and 12q15 found by genome-wide association study. Nat Genet. 2009;41:216-220. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 317] [Cited by in RCA: 320] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 190. | McGovern DP, Gardet A, Törkvist L, Goyette P, Essers J, Taylor KD, Neale BM, Ong RT, Lagacé C, Li C. Genome-wide association identifies multiple ulcerative colitis susceptibility loci. Nat Genet. 2010;42:332-337. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 525] [Cited by in RCA: 521] [Article Influence: 34.7] [Reference Citation Analysis (0)] |
| 191. | Dignass A, Eliakim R, Magro F, Maaser C, Chowers Y, Geboes K, Mantzaris G, Reinisch W, Colombel JF, Vermeire S. Second European evidence-based consensus on the diagnosis and management of ulcerative colitis part 1: definitions and diagnosis. J Crohns Colitis. 2012;6:965-990. [PubMed] |
| 192. | Rutgeerts P, Sandborn WJ, Feagan BG, Reinisch W, Olson A, Johanns J, Travers S, Rachmilewitz D, Hanauer SB, Lichtenstein GR. Infliximab for induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2005;353:2462-2476. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2744] [Cited by in RCA: 2885] [Article Influence: 144.3] [Reference Citation Analysis (2)] |
| 193. | Reinisch W, Sandborn WJ, Hommes DW, D’Haens G, Hanauer S, Schreiber S, Panaccione R, Fedorak RN, Tighe MB, Huang B. Adalimumab for induction of clinical remission in moderately to severely active ulcerative colitis: results of a randomised controlled trial. Gut. 2011;60:780-787. [PubMed] |
| 194. | Sandborn WJ, van Assche G, Reinisch W, Colombel JF, D’Haens G, Wolf DC, Kron M, Tighe MB, Lazar A, Thakkar RB. Adalimumab induces and maintains clinical remission in patients with moderate-to-severe ulcerative colitis. Gastroenterology. 2012;142:257-65.e1-3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 817] [Cited by in RCA: 943] [Article Influence: 72.5] [Reference Citation Analysis (0)] |
| 195. | Sandborn WJ, Feagan BG, Marano C, Zhang H, Strauss R, Johanns J, Adedokun OJ, Guzzo C, Colombel JF, Reinisch W. Subcutaneous golimumab maintains clinical response in patients with moderate-to-severe ulcerative colitis. Gastroenterology. 2014;146:96-109.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 442] [Cited by in RCA: 516] [Article Influence: 46.9] [Reference Citation Analysis (0)] |
| 196. | Sandborn WJ, Hanauer SB, Katz S, Safdi M, Wolf DG, Baerg RD, Tremaine WJ, Johnson T, Diehl NN, Zinsmeister AR. Etanercept for active Crohn’s disease: a randomized, double-blind, placebo-controlled trial. Gastroenterology. 2001;121:1088-1094. [PubMed] |
| 197. | Tang C, Chen S, Qian H, Huang W. Interleukin-23: as a drug target for autoimmune inflammatory diseases. Immunology. 2012;135:112-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 226] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 198. | Sandborn WJ, Gasink C, Gao LL, Blank MA, Johanns J, Guzzo C, Sands BE, Hanauer SB, Targan S, Rutgeerts P. Ustekinumab induction and maintenance therapy in refractory Crohn’s disease. N Engl J Med. 2012;367:1519-1528. [PubMed] |
| 199. | Hueber W, Sands BE, Lewitzky S, Vandemeulebroecke M, Reinisch W, Higgins PD, Wehkamp J, Feagan BG, Yao MD, Karczewski M. Secukinumab, a human anti-IL-17A monoclonal antibody, for moderate to severe Crohn’s disease: unexpected results of a randomised, double-blind placebo-controlled trial. Gut. 2012;61:1693-1700. [PubMed] |
| 200. | Sandborn WJ, Ghosh S, Panes J, Vranic I, Su C, Rousell S, Niezychowski W. Tofacitinib, an oral Janus kinase inhibitor, in active ulcerative colitis. N Engl J Med. 2012;367:616-624. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 585] [Cited by in RCA: 631] [Article Influence: 48.5] [Reference Citation Analysis (0)] |
| 201. | Targan SR, Feagan BG, Fedorak RN, Lashner BA, Panaccione R, Present DH, Spehlmann ME, Rutgeerts PJ, Tulassay Z, Volfova M. Natalizumab for the treatment of active Crohn’s disease: results of the ENCORE Trial. Gastroenterology. 2007;132:1672-1683. [PubMed] |
| 202. | Feagan BG, Greenberg GR, Wild G, Fedorak RN, Paré P, McDonald JW, Dubé R, Cohen A, Steinhart AH, Landau S. Treatment of ulcerative colitis with a humanized antibody to the alpha4beta7 integrin. N Engl J Med. 2005;352:2499-2507. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 542] [Cited by in RCA: 530] [Article Influence: 26.5] [Reference Citation Analysis (0)] |
| 203. | Feagan BG, Rutgeerts P, Sands BE, Hanauer S, Colombel JF, Sandborn WJ, Van Assche G, Axler J, Kim HJ, Danese S. Vedolizumab as induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2013;369:699-710. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1576] [Cited by in RCA: 1865] [Article Influence: 155.4] [Reference Citation Analysis (1)] |
| 204. | Zuk O, Hechter E, Sunyaev SR, Lander ES. The mystery of missing heritability: Genetic interactions create phantom heritability. Proc Natl Acad Sci USA. 2012;109:1193-1198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1086] [Cited by in RCA: 1061] [Article Influence: 81.6] [Reference Citation Analysis (0)] |