Published online Nov 13, 2019. doi: 10.4291/wjgp.v10.i4.42
Peer-review started: April 30, 2019
First decision: September 6, 2019
Revised: September 28, 2019
Accepted: October 18, 2019
Article in press: October 18, 2019
Published online: November 13, 2019
Processing time: 196 Days and 5.3 Hours
Perianal fistulae are either primary (idiopathic) or secondary [commonly associated with Crohn’s disease, (CD)]. It is assumed, although not proven, that the pathophysiology differs.
To systematically compare the clinical phenotypes, cytokine and phosphoprotein profiles of idiopathic and CD-related perianal fistulae.
Sixty-one patients undergoing surgery for perianal fistula were prospectively recruited (48 idiopathic, 13 CD) into a cohort study. Clinical data, including the Perineal Disease Activity Index (PDAI) and EQ-5D-5L were collected. Biopsies of the fistula tract, granulation tissue, internal opening mucosa and rectal mucosa were obtained at surgery. Concentrations of 30 cytokines and 39 phosphoproteins were measured in each biopsy using a magnetic bead multiplexing instrument and a chemiluminescent antibody array respectively. Over 12000 clinical and 23500 laboratory measurements were made.
The PDAI was significantly higher (indicating more active disease) in the CD group with a mean difference of 2.40 (95%CI: 0.52-4.28, P = 0.01). Complex pathoanatomy was more prevalent in the CD group, namely more multiple fistulae, supralevator extensions, collections and rectal thickening. The IL-12p70 concentration at the internal opening specimen site was significantly higher (median difference 19.7 pg/mL, 99%CI: 0.2-40.4, P = 0.008) and the IL-1RA/IL-1β ratio was significantly lower in the CD group at the internal opening specimen site (median difference 15.0, 99%CI = 0.4-50.5, P = 0.008). However in the remaining 27 cytokines and all 39 of the phosphoproteins across the four biopsy sites, no significant differences were found between the groups.
CD-related perianal fistulae are more clinically severe and anatomically complex than idiopathic perianal fistulae. However, overall there are no major differences in cytokine and phosphoprotein profiles.
Core tip: We systematically compared idiopathic and Crohn’s perianal fistulae, but did not find major differences in their cytokine and phosphoprotein profiles. Although more research is needed, our results support the thesis that biological agents effective in Crohn’s disease-related perianal fistulae may also have a role in selected surgically-intractable idiopathic perianal fistulae.
- Citation: Haddow JB, Musbahi O, MacDonald TT, Knowles CH. Comparison of cytokine and phosphoprotein profiles in idiopathic and Crohn’s disease-related perianal fistula. World J Gastrointest Pathophysiol 2019; 10(4): 42-53
- URL: https://www.wjgnet.com/2150-5330/full/v10/i4/42.htm
- DOI: https://dx.doi.org/10.4291/wjgp.v10.i4.42
Perianal fistulae occur in 1.04 to 2.32 per 10000 people per annum[1,2], are more common in males (ratio about 2:1), and cause significant physical and psychosocial morbidity. Fistulotomy can achieve successful healing at one year in 75% of patients[3], but is not always possible when the fistula involves a significant portion of the anal sphincter complex. Sphincter-sparing operations such as a fistula plug, advancement flap and ligation of the internal fistula tract only have success rates at one year of 55%, 53% and 42% respectively[3]. In Crohn’s disease (CD), perianal fistulae can heal in response to biological agents in 68% of patients[4]. Treatments for perianal fistulae could be improved if we had a better understanding of their pathophysiology.
The aetiology and pathophysiology of perianal fistulae is still unclear. The majority (90%) are considered to be primary or idiopathic[5]. In such patients, Parks’ cryptoglandular theory prevails, supposing infection of an anal gland as the primary lesion. Suppuration then penetrates through the internal anal sphincter creating a fistula tract[6].
In contrast, secondary fistulae, which can be associated with inflammatory bowel disease, tuberculosis and human immunodeficiency virus[5,7]. are assumed to arise from inflammation of the anorectal mucosa. By far the most common association is with CD, where perianal involvement is found in around one third of patients[8]. Scharl and Rogler summarised the immunological evidence for the pathogenesis of fistulae in CD[9]. In their proposed mechanism, an epithelial defect caused by inflammation or injury allows pathogen-associated molecular patterns from the microbiota to gain entry to the lamina propria and induce various pathways mediated by TNF-α, transforming growth factor beta (TGF-β), IL-13, matrix metalloproteinases (MMPs) and integrin-αvβ6. These drive epithelial-to-mesenchymal transition, which allows cell invasion and migration, resulting in a penetrating fistula tract lined by transitional cells.
Research to date into the pathophysiology of idiopathic and CD-related perianal fistula has evolved separately due to the underlying assumption that they are fundamentally different. Some previous studies made the comparison between these two types by use of specific histological, microbiological and immunological methods[10-13]. Overall their conclusions supported this underlying assumption, but testing this assumption was not their objective. We therefore hypothesised that idiopathic and CD-related perianal fistulae are different and aimed to test this systematically by comparing their clinical phenotypes, cytokine and phosphoprotein profiles.
We conducted a prospective cohort study between March 2014 and April 2015 within a NHS University Hospital (approved by Queen’s Square Research Ethics Committee, reference number 14/LO/0071). Adults with an idiopathic or CD-related perianal fistula requiring surgical intervention were included. Rectal, intestinal and subcutaneous fistulae were excluded.
One hundred and thirty consecutive patients were identified from outpatient and inpatient referrals, multidisciplinary meetings and surgical waiting lists. Twenty-two were not approached (simply for logistic reasons), 28 were ineligible and 11 declined. Thus we recruited 61 patients, 48 (79%) idiopathic and 13 (11%) CD-related (Supplementary Figure 1).
Patient characteristics were in keeping with the general epidemiology (Supplementary Table 1). None had a stoma. In the CD group, six had previous abdominal fistulae, six had previous abdominal surgery, and seven were naïve to anti-TNF-α antibody therapy. All had a combination of radiological, endoscopic and histological features of CD. Idiopathic patients had CD excluded by radiological and endoscopic imaging in 32 (67%) and clinical assessment alone in 16 (33%). Reasons for not imaging patients were patient declined (4) and not clinically indicated (16).
For the purposes of studying the rectal biopsies, we approached nine consecutive patients undergoing diagnostic colonoscopy for non-inflammatory conditions. Two declined and one was excluded due to ileal inflammation found during colonoscopy. Thus we recruited six healthy controls for rectal biopsies (Supplementary Table 2). Five were female and the mean age was 53.5 years (SD = 20.6).
We collected clinical information, Perineal Disease Activity Index (PDAI)[14], EuroQol EQ-5D-5L[15], and intra-operative findings, which were assisted by pre-operative magnetic resonance imaging in 49 (80%) patients. PDAI and EQ-5D-5L at baseline were measured in all but one participant.
Biopsy specimens were taken from: (1) The fistula tract wall; (2) Tract granulation tissue (if present); (3) Internal opening mucosa (if present); and (4) Rectal mucosa. Specimens were kept on ice and processed within four hours. One patient did not undergo biopsy due to unavailability of research staff.
Fresh specimens were prepared under a microscope. Those reserved for phosphoprotein quantification were snap frozen and stored at -80 °C. Those reserved for cytokine quantification were immediately incubated in 300 µL serum free HL-1 medium (Lonza, Cambridge, United Kingdom) containing 10 U/mL penicillin and streptokinase, 32 μg/mL gentamicin and 1 in 100 Lglutamine (Sigma-Aldrich, Gillingham, United Kingdom) at 37 °C and 5% carbon dioxide for 24 h. The supernatant was stored at -80 °C.
We used 30-plex Milliplex MAP Human Cytokine/Chemokine Magnetic Bead Panel (EMD Millipore, Billerica, MA, United States) to quantify the concentrations of 30 cytokines and chemokines: EGF, Eotaxin, G-CSF, GM-CSF, IFN-α2, IFN-γ, IL-10, IL-12P40, IL-12P70, IL-13, IL-15, IL-17, IL-1RA, IL-1α, IL-1β, IL-2, IL-3, IL-4, IL-5, IL-6, IL-7, IL-8, IP-10, MCP-1, MIP-1α, MIP-1β, TNF-α, TNF-β, RANTES, and VEGF (Supplementary Table 3). Measurements were made using a MAGPIX multiplexing instrument (Luminex Corporation, Austin, TX, United States). We followed the manufacturer’s recommended quality control procedures to ensure validity[16]. Specimen supernatants were analysed in singlet due to resource constraints, however, quality controls were measured in duplicate and 95% of these were within the accepted margin of error.
The method of phosphoprotein quantification was based on previous work within our laboratory[17]. Cells were lysed using 100 μL of radioimmunoprecipitation assay buffer containing one microlitre of Phosphatase Inhibitor Cocktail 2 and one microlitre of protease inhibitor in dimethyl sulfoxide (Sigma-Aldrich, Gillingham, United Kingdom) and a manual ultrasonic cell disruptor intermittently for 10 min at 4 °C. We then separated off the supernatant and performed a Bradford Assay (Bio-Rad Laboratories Inc., Hercules, CA, United States) to estimate the protein concentration. PathScan RTK Signaling Antibody Array, Chemiluminescent Readout (Cell Signaling Technology, Danvers, Massachusetts) was used to quantify the phosphorylation status of 28 RTKs and 11 signalling nodes: EGFR/ErbB1, HER2/ErbB2, HER3/ErbB3, FGFR1, FGFR3, FGFR4, InsR, IGF-IR, TrkA/NTRK1, TrkB/NTRK2, Met/HGFR, Ron/MST1R, Ret, ALK, PDGFR, c-Kit/SCFR, FLT3/Flk2, M-CSFR/CSF-1R, EphA1, EphA2, EphA3, EphB1, EphB3, EphB4, Tyro3/Dtk, Axl, Tie2/TEK, VEGFR2/KDR, Akt/PKB/Rac (at Thr308), Akt/PKB/Rac (at Ser473), p44/42 MAPK, S6 Ribosomal Protein, c-Abl, IRS-1, Zap-70, Src, Lck, Stat1 and Stat3 (Supplementary Table 4). A total of 113 µg of protein from each whole cell lysate was probed on the array. The chemiluminescent signals were detected on Amersham Hyperfilm ECL (GE Healthcare Life Sciences, Buckinghamshire, United Kingdom), digitised using a lightbox and camera (Nikon D70 digital camera with a Nikon 18-70 mm 1:3.5-4.5G DX lens, Nikon Corporation, Tokyo, Japan) and measured using ImageQuant TL 2005 (GE Healthcare Life Sciences, Pittsburg, PA, United States). The measurements were normalised to give pixel intensities ranging from zero to 100%. Prior experiments determined a positivity threshold of 20%.
Sample size was based on feasibility (recruitment period). Further, there is little consensus on sample size estimations for assays used in this study and no prior relevant data upon which to base a calculation.
Data were entered into a validated database using Access 2010 (Microsoft, Redmond WA, United States). Double-entering of a random 10% data sample estimated the error rate at 0.6%. Proprietary software (SPSS Statistics version 22, IBM, Armonk, NY, United States) was used to analyse data, and Excel 2010 (Microsoft, Redmond, WA, United States) to produce the heat map charts. Parametric and non-parametric methods were used for normal and non-normal data respectively. We considered P < 0.05 to be statistically significant. When comparing the cytokine and phosphoprotein data where multiple comparisons were made, using P < 0.01 as statistically significant was considered an appropriate correction. This study was reviewed by a biomedical statistician. The STROBE guidelines were followed for reporting[18].
The PDAI was significantly higher (indicating more active disease) in the CD group with a mean difference of 2.40 (95%CI: 0.52-4.28, P = 0.01). The EQ visual analogue scores (VAS) and index values were similar between the groups. Multiple fistulae were more prevalent in CD patients (23% vs 4%). The distribution of the different types of fistulae under the Parks’ classification was the same between the groups, with trans-sphincteric being by far the commonest. Prevalence of a high primary tract and horseshoe extensions were similar between groups. However, supralevator extensions and collections were commoner in the CD group (31% vs 6% and 92% vs 33% respectively). Rectal thickening, reflecting proctitis, was almost exclusively observed in the CD group (Table 1).
| Idiopathic | Crohn’s Disease | P value | |
| Mean PDAI (SD) | 5.67 (2.76) | 8.07 (3.99) | 0.01a |
| Mean EQ VAS (SD) | 71.2 (21.1) | 63.4 (20.2) | 0.22 |
| Mean EQ index (SD) | 0.734 (0.259) | 0.715 (0.214) | 0.80 |
| Seton in situ, n (%) | 21 (44) | 3 (23) | 0.22 |
| Number of fistulae, n (%) | 0.09 | ||
| Single unbranched | 31 (65) | 3 (23) | |
| Single branched | 15 (31) | 7 (54) | |
| Multiple | 2 (4) | 3 (23) | |
| Parks’ classification, n (%) | 0.54 | ||
| Inter-sphincteric | 7 (15) | 1 (8) | |
| Trans-sphincteric | 38 (79) | 10 (77) | |
| Supra-sphincteric | 3 (6) | 2 (15) | |
| High primary tract | 15 (31) | 6 (46) | 0.34 |
| Secondary tract(s), n (%) | 0.02a | ||
| None | 31 (65) | 4 (31) | |
| Infralevator | 14 (29) | 5 (38) | |
| Supralevator | 3 (6) | 4 (31) | |
| Horseshoe, n (%) extension(s) | 12 (25) | 5 (38) | 0.49 |
| Collection(s) , n (%) | 16 (33) | 12 (92) | < 0.001a |
| Thickened rectum, n (%) | 1 (2) | 7 (54) | < 0.001a |
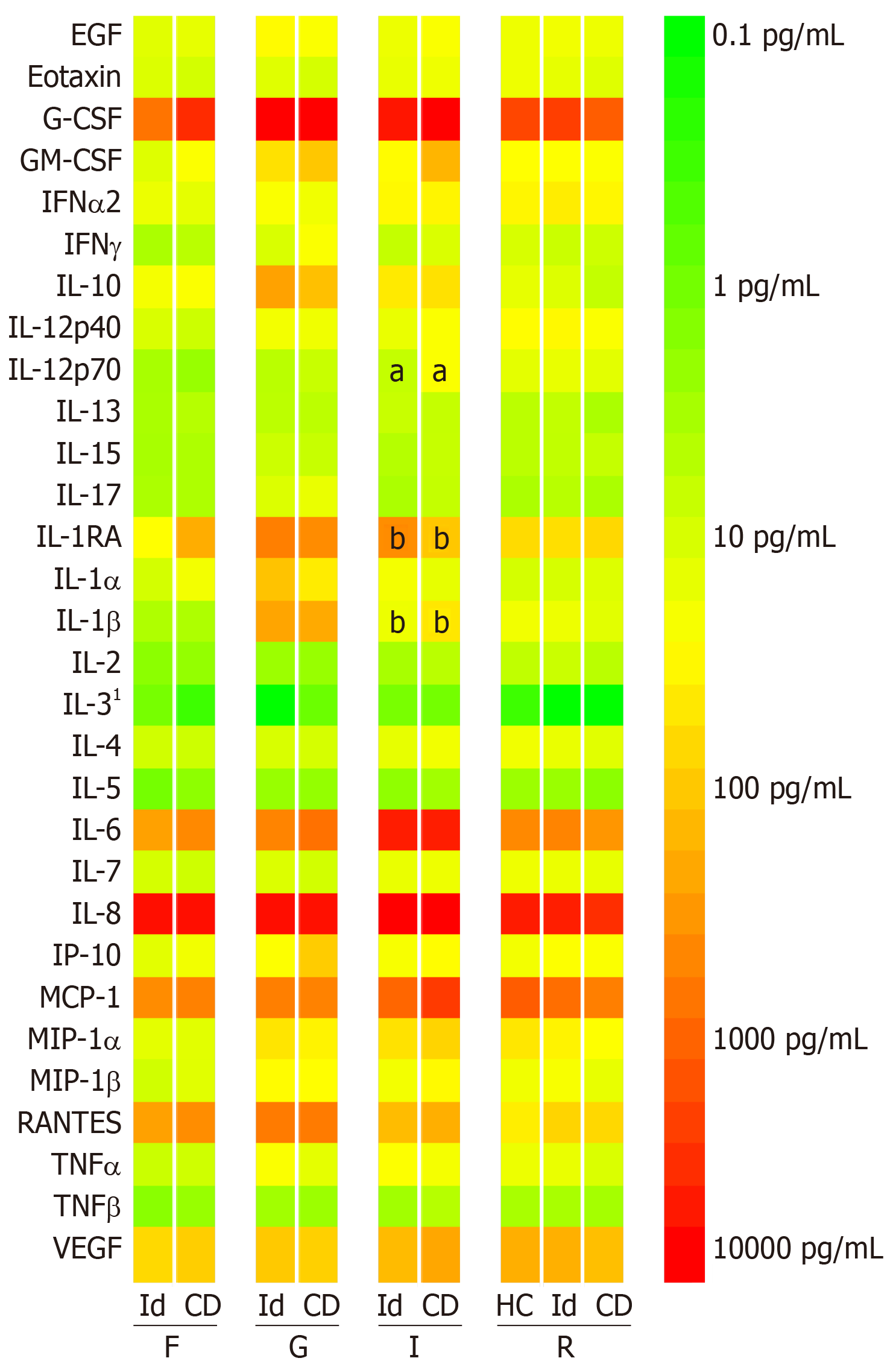
All four specimen sites yielded substantial levels of IL-1RA, IL-6, MCP-1, RANTES, VEGF, G-CSF and IL-8. GM-CSF, IFN-α2, IP-10, MIP-1α and MIP-1β were also moderately abundant in the granulation tissue, internal opening and rectal mucosa. Granulation tissue, compared to the other specimen sites, yielded higher concentrations of G-CSF, IL-10, IL-1RA, and IL-1β (Figure 1).
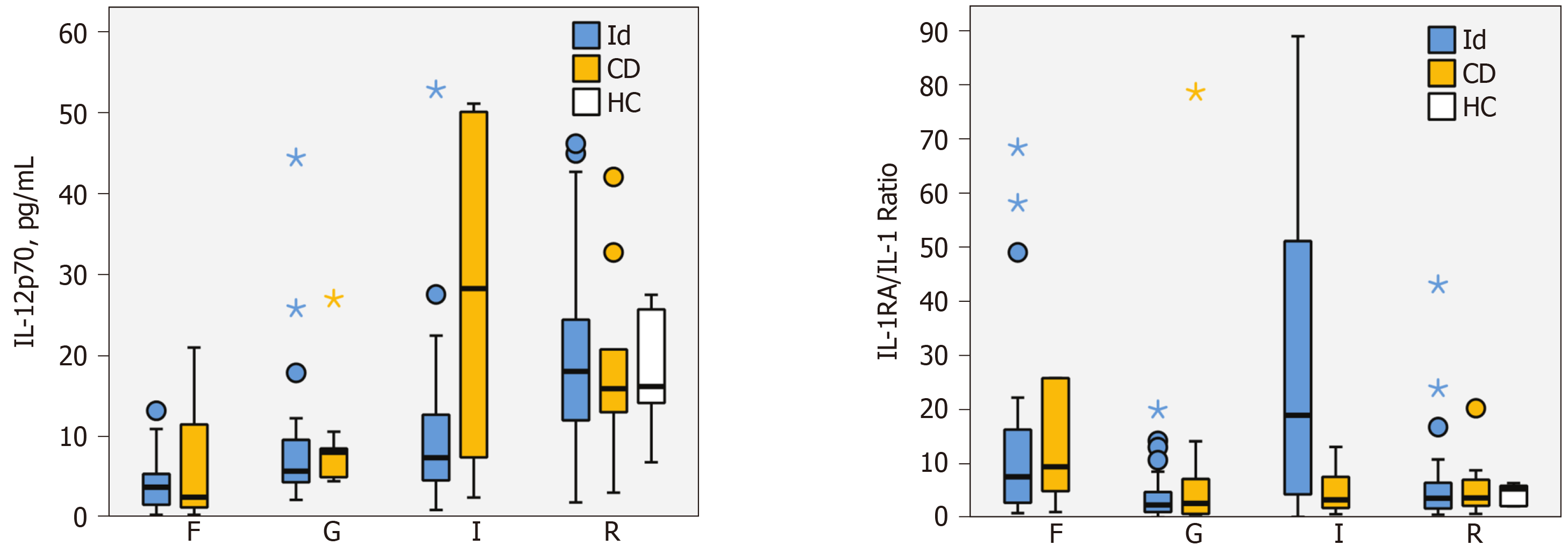
Only two cytokines demonstrated significant differences between the idiopathic and CD groups. IL-12p70 concentration at the internal opening was higher in patients with CD (28.3 pg/mL, IQR = 7.4-50.1 vs idiopathic 7.4 pg/mL, 4.6-12.7). The median difference was 19.7 pg/mL (99%CI: 0.2-40.4, P = 0.008). The IL-1RA/IL-1β ratio was significantly lower in CD group at the internal opening (3.3, IQR = 1.8-7.6 vs idiopathic 19.0, 4.3-51.2). The median difference was 15.0 (99%CI: 0.4-50.5, P = 0.008) (Figure 2).
There were no significant differences between the groups for any other cytokine concentrations at the four specimen sites. There were also no significant differences in the cytokine concentrations in the rectal mucosa between the CD group and the healthy controls (Supplementary Figure 2).
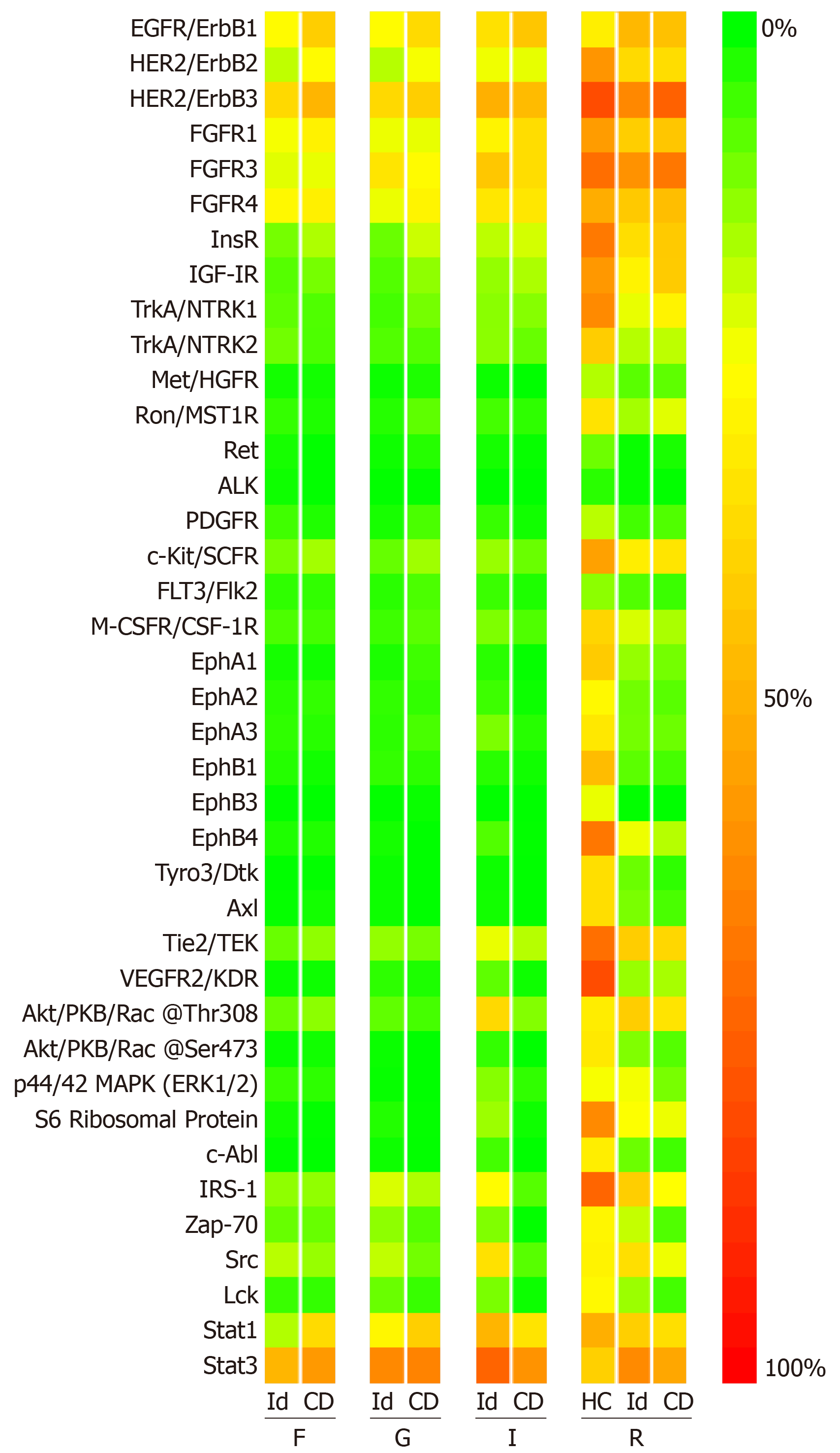
All four specimen sites yielded signals across both patient groups for EGFR, HER2, HER3, FGFR1, FGFR3, FGFR4, Stat1, and Stat3. In the rectal mucosa, we observed positive signals for InsR, IGF-1R, c-Kit, Tie2, Akt at phosphorylation site Thr308, S6 Ribosomal Protein, IRS-1 and Src in both patient groups and the healthy controls. There were no significant differences in phosphoprotein levels between the patient groups at any of specimen sites (Figure 3).
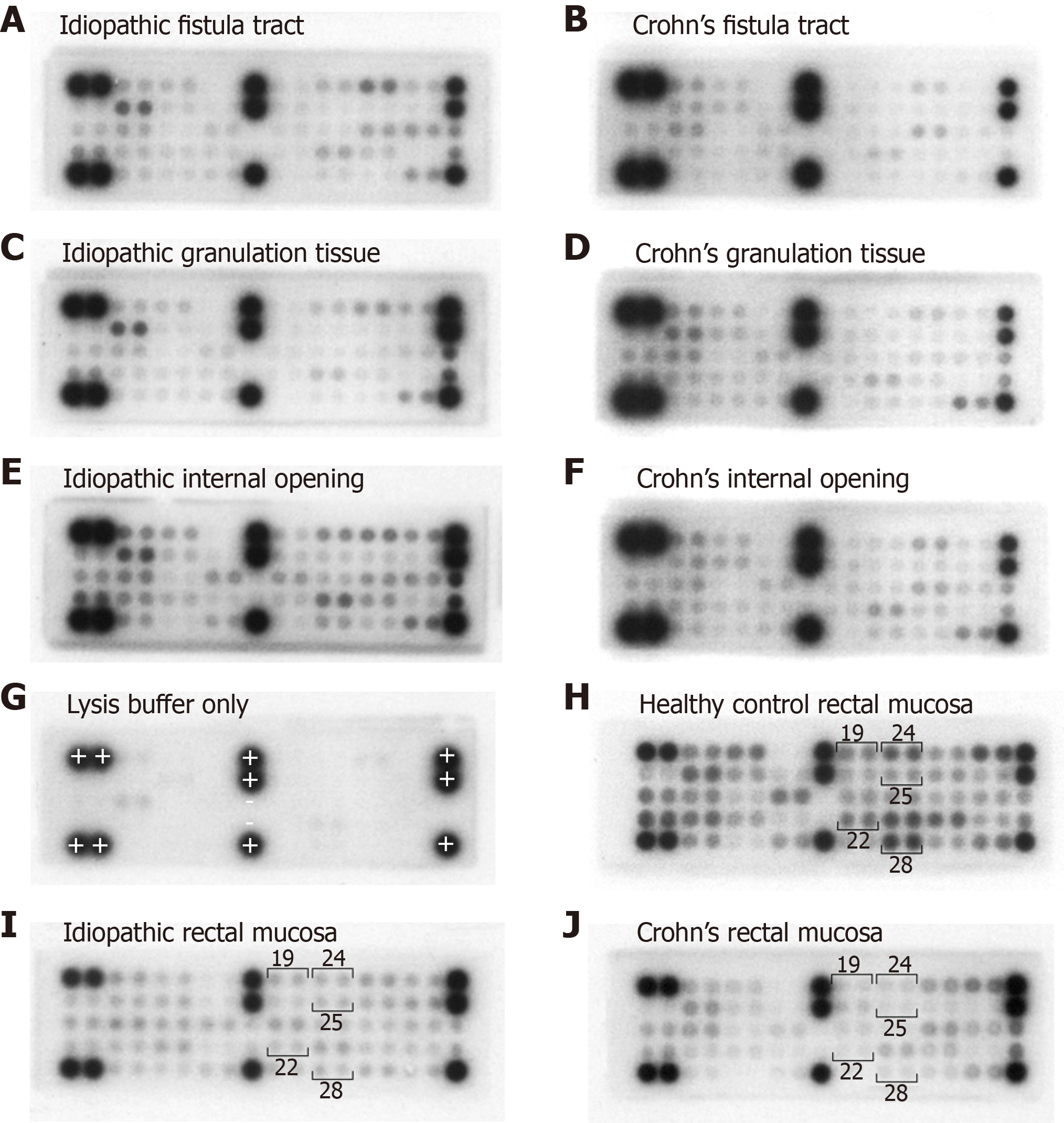
When comparing the CD group with the healthy controls at the rectal mucosa specimen site, the levels of six phosphoproteins were significantly higher in the healthy controls: EphA1, EphA2, EphB1, EphB4, Tyro3 and VEGFR2 (Supplementary Figure 3). Example microarray images are shown in Figure 4. There were no differences the remaining 33 phosphoproteins.
To our knowledge, this is the largest study to date to systematically compare idiopathic and CD-related perianal fistulae in a well-defined patient cohort. Both groups displayed a broad distribution of disease characteristics from simple to complex.
In the CD group, the PDAI was significantly higher and complex pathoanatomy was more prevalent, supporting the commonly-held belief that CD-related perianal fistulae are more severe and complex than idiopathic. However, this did not translate to differences in EQ-5D, probably because this generic health measure lacked sensitivity to demonstrate a relatively small clinical difference.
Detailed profiling of 30 different cytokines and chemokines and 39 different phosphoproteins at four different biopsy sites showed that the idiopathic and CD groups were broadly similar. Our cytokine profiling is comparable to and substantially more extensive than previous studies[13,19,20] and our phosphoprotein profiling is the first to be reported in perianal fistula disease.
Only four previous studies directly compared idiopathic and CD-related perianal fistulae. Bataille et al[10,11] found both types had a lining of granulation tissue containing histiocytes and capillaries, a lumen filled with nuclear debris, neutrophils and lymphocytes, and markers indicative of epithelial-to-mesenchymal transition. However, CD-related perianal fistulae featured more CD45R0 positive T cells and CD20 positive B cells, whilst idiopathic fistulae featured more CD68 positive macrophages. Kirkegaard et al[12] found both types had similar MMP-3 and MMP-9 upregulation. Tozer found both types had similar levels of IL-2, IL-4, IL-6, IP-10, TNF-α and IFN-γ in tissue culture supernatants, but also reported higher numbers of T cells, lower expression of dendritic cell homing markers and fewer CD65 positive macrophages in CD-related perianal fistulae[13].
It is well established that there are significant differences in the cytokine expression in CD-affected intestinal mucosa, compared with healthy mucosa, namely pro-inflammatory cytokines IL-1, TNF-α, IL-6, IL-8, IL-12, IL-17 and IL-21, and anti-inflammatory cytokines IL-10 and TGF-β[21]. Thus, notwithstanding Tozer’s results mentioned above, similar differences might be seen in idiopathic compared with CD-related perianal fistulae. To broaden this line of enquiry, we analysed the tissue culture supernatants for 30 different cytokines. Only two measurements showed a significant difference between the groups: IL-12p70 and the IL-1RA/IL-1β ratio, both at the internal opening.
IL-12 is a proinflammatory cytokine produced by dendritic cells and macrophages. It comprises two subunits, IL-12p35 and IL-12p40, which combine into IL-12p70. The IL-12 receptor is found mainly on T cells and natural killer cells. IL-12 induces production of IFN-γ, promotes T helper 1 cell differentiation and forms a link between innate resistance and acquired immunity[22]. It plays an important role in CD pathogenesis. The expression of IL-12 is upregulated in CD mucosa[23]. This study found significantly higher concentrations of IL-12p70 at the internal opening in the CD group, suggesting a similarly important role for IL-12 in CD-related perianal fistula and may represent a difference in its pathophysiology compared with idiopathic perianal fistula disease. However, these data need to be interpreted with caution, as the 99% confidence interval came close to zero.
IL-1α and IL-1β mediate immune and inflammatory responses. They are primarily produced by tissue macrophages, monocytes, fibroblasts, and dendritic cells and enable transmigration of immune cells to the site of inflammation. IL-1β concentration is increased in inflamed CD colonic mucosa[24]. We found low concentrations in the fistula tract, internal opening and rectal mucosa, with no significant differences between the groups. This might suggest that the role of IL-1 in perianal fistula differs from inflamed CD colonic mucosa. However, we found the IL-1RA/IL-1ß ratio at the internal opening was significantly lower in the CD group, which is in keeping with previous reports in inflamed CD colonic mucosa[24].
RTKs and intracellular signalling pathways control and regulate cell behaviour[25]. Many intracellular signalling pathways are upregulated in CD[17]. For example, signal transducer and activator of transcription 4 in T helper 1 cells is activated by IL-12, driving IFN-γ and TNF-α production[26]. Indeed an anti-IL-12 monoclonal antibody, ustekinumab, is of clinical benefit in CD[27]. We analysed 39 different phosphoproteins involved in epithelial cell signalling, wound healing, inflammation and T cell activation, and found no significant differences in the phosphoprotein profiles between idiopathic and CD-related perianal fistulae. We were also interested to see if fistula immunopathology changes over time. However, due to only ten patients having fistulae less than 12 mo old, this subgroup analysis was not possible.
Comparison of the rectal mucosa in the idiopathic and CD groups with healthy controls found no differences in the cytokine profiles. However, in the rectal mucosa, the signals in both disease groups appeared to be suppressed compared with the healthy controls. This difference was significant for six phosphoproteins (EphA1, EphA2, EphB1, EphB4, Tyro3 and VEGFR2) when comparing the healthy controls with the CD group.
The first four are RTKs from the Ephrin subfamily. Ephrin signalling is important in controlling cellular proliferation in the crypts and differentiation of enterocytes on the villi. Loss of ephrin receptor and ephrin signalling appears to contribute to wound healing defects as in inflammatory bowel disease[28]. Our study suggests that suppression of ephrin receptor expression in the rectal mucosa may be a pathological feature in patients with both idiopathic and CD-related perianal fistulae. The consequence of this conclusion is potentially important for two reasons: (1) Idiopathic and CD-related perianal fistulae may be similar in their immunopathology with regards to ephrin signaling; and (2) Abnormalities in those with idiopathic perianal fistulae might not be confined to the peri-fistula tissue, a notion that has not previously been reported.
Tyro3 is a RTK that, along with Axl and Mertk, and their ligands Gas6 and Protein S, makes up the Tyro3-Axl-Mertk (TAM) signalling pathway. This pathway is involved in the negative regulation of inflammation, removal of apoptotic cells and potential induction of the tissue repair response. In inflammatory bowel disease, TAM signalling is suppressed, which, in the presence of mucosal injury, leads to an accumulation of apoptotic neutrophils and a failure of macrophages to acquire an alternative activation state[29]. Our study with respect to the rectal mucosa in the CD group is consistent with this. However, it also suggests that suppression of Tyro3 may also occur in the rectal mucosa of those with idiopathic perianal fistulae, further supporting the notion described above.
VEGFR2 is the principle RTK that transmits VEGF signals in the vascular endothelium. The principle effect of VEGFR2 activation is angiogenesis, which is a feature of both health and disease. VEGFR2 may be suppressed in quiescent CD colonic mucosa, compared with healthy controls[30]. In the rectal mucosa, we found that VEGFR2 levels were lower in patients both with idiopathic and CD-related perianal fistulae, compared with healthy controls. This suggests that expression of this RTK may be suppressed in these diseases.
Only 13 patients were recruited to the CD group. Previous comparative studies have reported with similar sample sizes[10-13]. Preferential medical management in CD-related perianal fistulae could also have introduced selection bias, and referral patterns could have skewed the types of fistulae included to the more severe (especially in relation to the idiopathic group where many were tertiary referrals).
Techniques and assays were taken from similar experiments on intestinal mucosa published by our group[17]. The assays have not previously been used on fistula tract and granulation tissue, however their performance was likely to be similar as the cellular compositions of these tissues are similar.
The majority of the CD group were receiving immunomodulatory therapy, and half had received anti-TNF-α. This may have attenuated differences between the groups. However, the effect of anti-TNF-α therapy at the molecular level is unknown. One study found that mucosal TNF-α concentrations are unaltered by anti-TNF-α therapy[19]. Including only treatment-naïve patients would have mitigated this, but recruitment would have been difficult into given that most are primarily managed medically.
CD-related perianal fistulae may often be clinically more severe and complex. However, they do not substantially differ in their expression of a large panel of cytokines and phosphoproteins (Table 2).
| Similarities | Differences | |
| PDAI | Significantly higher PDAI in Crohn’s disease | |
| EQ-5D-5L | Similar EQ VAS and EQ index | |
| Morphology | Similar distribution of types by Parks’ classification | More multiple fistulae in Crohn’s disease |
| Similar prevalence of high fistulae and horseshoe extensions | Significantly more supralevator extensions, collections and rectal thickening in Crohn’s disease | |
| Cytokine concentrations | Similar concentrations of 27 cytokines at all four biopsy sites | Significantly higher IL-12p70 concentration at internal opening in Crohn’s disease |
| (EGF, eotaxin, G-CSF, GM-CSF, IFN-α2, IFN-γ, IL-10, IL-12p40, IL-13, IL-15, IL-17, IL-1α, IL-2, IL-3, IL-4, IL-5, IL-6, IL-7, IL-8, IP-10, MCP-1, MIP-1α, MIP-1β, TNF-α, TNF-β, RANTES and VEGF) | Significantly lower IL-1RA/IL-1β ratio concentration at internal opening in Crohn’s disease | |
| Phosphoprotein concentrations | Similar levels of 39 phosphoproteins at the four specimen sites | |
| (EGFR/ErbB1, HER2/ErbB2, HER3/ErbB3, FGFR1, FGFR3, FGFR4, InsR, IGF-IR, TrkA/NTRK1, TrkB/NTRK2, Met/HGFR, Ron/MST1R, Ret, ALK, PDGFR, c-Kit/SCFR, FLT3/Flk2, M-CSFR/CSF-1R, EphA1, EphA2, EphA3, EphB1, EphB3, EphB4, Tyro3/Dtk, Axl, Tie2/TEK, VEGFR2/KDR, Akt/PKB/Rac (at Thr308), Akt/PKB/Rac (at Ser473), p44/42 MAPK, S6 Ribosomal Protein, c-Abl, IRS-1, Zap-70, Src, Lck, Stat1 and Stat3) |
Despite the acknowledged limitations (particularly regarding sample size in the CD group and the fact that some CD patients were receiving immunomodulatory therapy), our data contributes to an emerging theory that idiopathic and CD-related perianal fistulae may not be as immunologically distinct as previously supposed. This line of reasoning opens the possibility that biological agents effective in CD-related perianal fistulae may also have a role in selected idiopathic perianal fistulae especially when recent randomised trial data have exposed the general limitations of surgery[3]. We acknowledge however that research is warranted.
Perianal fistulae are common and cause significant physical and psychosocial morbidity. Current treatments come with a significant failure rate. Idiopathic perianal fistulae are thought to arise from a primary infection of an anal gland, which leads to penetrating suppuration and fistula formation. Crohn’s disease (CD)-related perianal fistulae is thought to arise from altered inflammatory pathways within the mucosa.
The aetiology and pathophysiology of perianal fistulae is still unclear. A better understanding could lead to better treatments. Most research to date has assumed idiopathic and CD-related fistulae to be fundamentally different. However this assumption has never been tested.
We hypothesised that idiopathic and CD-related perianal fistulae are different and aimed to test this systematically by comparing their clinical phenotypes, cytokine and phosphoprotein profiles.
We conducted a prospective cohort study within a university hospital. Sixty-one consecutive patients undergoing surgery for perianal fistula were recruited. Clinical data, pre- and post-operative Perineal Disease Activity Index (PDAI) and EQ-5D-5L scores were measured. Biopsies of the fistula tract, granulation tissue, internal opening mucosa and rectal mucosa were obtained at surgery. These were processed in our laboratory to measure 30 cytokines and 39 phosphoproteins. To our knowledge, this is the largest study to date to systematically compare idiopathic and CD-related perianal fistulae in a well-defined patient cohort.
The PDAI was significantly higher and complex pathoanatomy was more prevalent in the CD group, supporting the commonly-held belief that CD-related perianal fistulae are more severe and complex than idiopathic. IL-12p70 concentration at the internal opening was higher and the IL-1RA/IL-1β ratio was significantly lower at the internal opening in patients with CD. There were no significant differences between the groups for any other cytokine concentrations at the four specimen sites. There were also no significant differences in phosphoprotein levels between the patient groups at any specimen site.
CD-related perianal fistulae are often clinically more severe and complex. However, they do not substantially differ in their expression of a large panel of cytokines and phosphoproteins.
Our data contributes to an emerging theory that idiopathic and CD-related perianal fistulae may not be as immunologically distinct as previously supposed. This line of reasoning opens the possibility that biological agents effective in CD-related perianal fistulae may also have a role in selected idiopathic perianal fistulae especially when recent randomised trial data have exposed the general limitations of surgery. Further research is warranted.
Thank you to Ellie McAlees for recruitment support, Anna Vossenkamper, Nadia Ahmad, Paolo Biancheri and Bob Hardcastle for laboratory support, and Amira Shamsiddinova for data-entry support.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: United Kingdom
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Abdolghaffari AH, Rath T S-Editor: Ma RY L-Editor: A E-Editor: Qi LL
| 1. | Zanotti C, Martinez-Puente C, Pascual I, Pascual M, Herreros D, García-Olmo D. An assessment of the incidence of fistula-in-ano in four countries of the European Union. Int J Colorectal Dis. 2007;22:1459-1462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 138] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 2. | Adamo K, Sandblom G, Brännström F, Strigård K. Prevalence and recurrence rate of perianal abscess--a population-based study, Sweden 1997-2009. Int J Colorectal Dis. 2016;31:669-673. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 35] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 3. | Jayne DG, Scholefield J, Tolan D, Gray R, Edlin R, Hulme CT, Sutton AJ, Handley K, Hewitt CA, Kaur M, Magill L. Anal fistula plug versus surgeon's preference for surgery for trans-sphincteric anal fistula: the FIAT RCT. Health Technol Assess. 2019;23:1-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 4. | Present D, Rutgeerts P, Targan S, Hanauer S, Mayer L, van Hogezand R, Podolsky D, Sands B, Braakman T, DeWoody K, Schaible T, van Deventer S. Infliximab for the treatment of fistulas in patients with Crohn’s disease. J Med. 1999;340:1398-1405. |
| 5. | Sainio P. Fistula-in-ano in a defined population. Incidence and epidemiological aspects. Ann Chir Gynaecol. 1984;73:219-224. [PubMed] |
| 6. | Parks AG. Pathogenesis and treatment of fistuila-in-ano. Br Med J. 1961;1:463-469. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 405] [Cited by in RCA: 320] [Article Influence: 11.9] [Reference Citation Analysis (1)] |
| 7. | Rivera M, Angelucci E, Crispino P, Pronio AM, Marcheggiano A, Vernia P, Badiali D. Perianal fistulas mimicking Crohn's disease in HIV-infected male patient. Am J Gastroenterol. 2009;104:793-794. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 8. | Aguilera-Castro L, Ferre-Aracil C, Garcia-Garcia-de-Paredes A, Rodriguez-de-Santiago E, Lopez-Sanroman A. Management of complex perianal Crohn's disease. Ann Gastroenterol. 2017;30:33-44. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 34] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 9. | Scharl M, Rogler G. Pathophysiology of fistula formation in Crohn's disease. World J Gastrointest Pathophysiol. 2014;5:205-212. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 68] [Cited by in RCA: 86] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 10. | Bataille F, Klebl F, Rümmele P, Schroeder J, Farkas S, Wild PJ, Fürst A, Hofstädter F, Schölmerich J, Herfarth H, Rogler G. Morphological characterisation of Crohn's disease fistulae. Gut. 2004;53:1314-1321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 70] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 11. | Bataille F, Rohrmeier C, Bates R, Weber A, Rieder F, Brenmoehl J, Strauch U, Farkas S, Fürst A, Hofstädter F, Schölmerich J, Herfarth H, Rogler G. Evidence for a role of epithelial mesenchymal transition during pathogenesis of fistulae in Crohn's disease. Inflamm Bowel Dis. 2008;14:1514-1527. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 114] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 12. | Kirkegaard T, Hansen A, Bruun E, Brynskov J. Expression and localisation of matrix metalloproteinases and their natural inhibitors in fistulae of patients with Crohn's disease. Gut. 2004;53:701-709. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 162] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 13. | Tozer P. Clinical and experimental studies in idiopathic and Crohn’s-related anal fistula [MD(Res)]. London: Imperial College; 2011; . |
| 14. | Irvine EJ. Usual therapy improves perianal Crohn's disease as measured by a new disease activity index. McMaster IBD Study Group. J Clin Gastroenterol. 1995;20:27-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 238] [Cited by in RCA: 236] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 15. | Herdman M, Gudex C, Lloyd A, Janssen M, Kind P, Parkin D, Bonsel G, Badia X. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L). Qual Life Res. 2011;20:1727-1736. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4003] [Cited by in RCA: 6487] [Article Influence: 463.4] [Reference Citation Analysis (0)] |
| 16. | Merck Millipore. MILLIPLEX MAP Human Cytokine/Chemokine Magnetic Bead Panel - Immunology Multiplex Assay [Internet]. Merck Millipore [cited. 2019;Sep 21] Available from: http://www.merckmillipore.com/NZ/en/product/MILLIPLEX-MAP-Human-Cytokine-Chemokine-Magnetic-Bead-Panel-Immunology-Multiplex-Assay. |
| 17. | Vossenkämper A, Hundsrucker C, Page K, van Maurik A, Sanders TJ, Stagg AJ, Das L, MacDonald TT. A CD3-specific antibody reduces cytokine production and alters phosphoprotein profiles in intestinal tissues from patients with inflammatory bowel disease. Gastroenterology. 2014;147:172-183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 31] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 18. | von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP; STROBE Initiative. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. BMJ. 2007;335:806-808. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3438] [Cited by in RCA: 6144] [Article Influence: 341.3] [Reference Citation Analysis (0)] |
| 19. | Ruffolo C, Scarpa M, Faggian D, Pozza A, Navaglia F, D'Incà R, Hoxha P, Romanato G, Polese L, Sturniolo GC, Plebani M, D'Amico DF, Angriman I. Cytokine network in rectal mucosa in perianal Crohn's disease: relations with inflammatory parameters and need for surgery. Inflamm Bowel Dis. 2008;14:1406-1412. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 18] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 20. | Ruffolo C, Scarpa M, Faggian D, Romanato G, De Pellegrin A, Filosa T, Prando D, Polese L, Scopelliti M, Pilon F, Ossi E, Frego M, D'Amico DF, Angriman I. Cytokine network in chronic perianal Crohn's disease and indeterminate colitis after colectomy. J Gastrointest Surg. 2007;11:16-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 14] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 21. | Műzes G, Molnár B, Tulassay Z, Sipos F. Changes of the cytokine profile in inflammatory bowel diseases. World J Gastroenterol. 2012;18:5848-5861. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 194] [Cited by in RCA: 196] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 22. | Trinchieri G. Interleukin-12 and the regulation of innate resistance and adaptive immunity. Nat Rev Immunol. 2003;3:133-146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2687] [Cited by in RCA: 2873] [Article Influence: 130.6] [Reference Citation Analysis (0)] |
| 23. | Monteleone G, Biancone L, Marasco R, Morrone G, Marasco O, Luzza F, Pallone F. Interleukin 12 is expressed and actively released by Crohn's disease intestinal lamina propria mononuclear cells. Gastroenterology. 1997;112:1169-1178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 409] [Cited by in RCA: 394] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 24. | Dionne S, D'Agata ID, Hiscott J, Vanounou T, Seidman EG. Colonic explant production of IL-1and its receptor antagonist is imbalanced in inflammatory bowel disease (IBD). Clin Exp Immunol. 1998;112:435-442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 68] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 25. | Gilbert SF. Cell Surface Receptors and Their Signal Transduction Pathways. 2000;[cited 2016 May 10] Available from: http://www.ncbi.nlm.nih.gov/books/NBK10043/. |
| 26. | Watford WT, Hissong BD, Bream JH, Kanno Y, Muul L, O'Shea JJ. Signaling by IL-12 and IL-23 and the immunoregulatory roles of STAT4. Immunol Rev. 2004;202:139-156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 396] [Cited by in RCA: 413] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 27. | Kotze PG, Ma C, Almutairdi A, Panaccione R. Clinical utility of ustekinumab in Crohn's disease. J Inflamm Res. 2018;11:35-47. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 31] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 28. | Perez White BE, Getsios S. Eph receptor and ephrin function in breast, gut, and skin epithelia. Cell Adh Migr. 2014;8:327-338. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 64] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 29. | Rothlin CV, Leighton JA, Ghosh S. Tyro3, Axl, and Mertk receptor signaling in inflammatory bowel disease and colitis-associated cancer. Inflamm Bowel Dis. 2014;20:1472-1480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 36] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 30. | Algaba A, Linares PM, Fernández-Contreras ME, Ordoñez A, Trápaga J, Guerra I, Chaparro M, de la Poza G, Gisbert JP, Bermejo F. Relationship between levels of angiogenic and lymphangiogenic factors and the endoscopic, histological and clinical activity, and acute-phase reactants in patients with inflammatory bowel disease. J Crohns Colitis. 2013;7:e569-e579. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 27] [Article Influence: 2.3] [Reference Citation Analysis (0)] |