Published online Jun 28, 2016. doi: 10.4329/wjr.v8.i6.635
Peer-review started: September 5, 2015
First decision: October 8, 2015
Revised: February 26, 2016
Accepted: March 17, 2016
Article in press: March 17, 2016
Published online: June 28, 2016
Processing time: 294 Days and 19.2 Hours
AIM: To review the benefits of single photon emission computed tomography (SPECT)/computed tomography (CT) hybrid imaging for diagnosis of various endocrine disorders.
METHODS: We performed MEDLINE and PubMed searches using the terms: “SPECT/CT”; “functional anatomic mapping”; “transmission emission tomography”; “parathyroid adenoma”; “thyroid cancer”; “neuroendocrine tumor”; “adrenal”; “pheochromocytoma”; “paraganglioma”; in order to identify relevant articles published in English during the years 2003 to 2015. Reference lists from the articles were reviewed to identify additional pertinent articles. Retrieved manuscripts (case reports, reviews, meta-analyses and abstracts) concerning the application of SPECT/CT to endocrine imaging were analyzed to provide a descriptive synthesis of the utility of this technology.
RESULTS: The emergence of hybrid SPECT/CT camera technology now allows simultaneous acquisition of combined multi-modality imaging, with seamless fusion of three-dimensional volume datasets. The usefulness of combining functional information to depict the bio-distribution of radiotracers that map cellular processes of the endocrine system and tumors of endocrine origin, with anatomy derived from CT, has improved the diagnostic capability of scintigraphy for a range of disorders of endocrine gland function. The literature describes benefits of SPECT/CT for 99mTc-sestamibi parathyroid scintigraphy and 99mTc-pertechnetate thyroid scintigraphy, 123I- or 131I-radioiodine for staging of differentiated thyroid carcinoma, 111In- and 99mTc- labeled somatostatin receptor analogues for detection of neuroendocrine tumors, 131I-norcholesterol (NP-59) scans for assessment of adrenal cortical hyperfunction, and 123I- or 131I-metaiodobenzylguanidine imaging for evaluation of pheochromocytoma and paraganglioma.
CONCLUSION: SPECT/CT exploits the synergism between the functional information from radiopharmaceutical imaging and anatomy from CT, translating to improved diagnostic accuracy and meaningful impact on patient care.
Core tip: The emergence of hybrid single photon emission computed tomography (SPECT)/computed tomography (CT) camera technology now allows simultaneous acquisition of multi-modality imaging with seamless fusion of three-dimensional volume datasets; combining functional information from radionuclide scintigraphy and anatomy derived from CT. SPECT/CT imaging exploits the synergism between “function” and “form” for investigation of endocrine disorders, translating to improved diagnostic accuracy and meaningful impact on patient care.
- Citation: Wong KK, Gandhi A, Viglianti BL, Fig LM, Rubello D, Gross MD. Endocrine radionuclide scintigraphy with fusion single photon emission computed tomography/computed tomography. World J Radiol 2016; 8(6): 635-655
- URL: https://www.wjgnet.com/1949-8470/full/v8/i6/635.htm
- DOI: https://dx.doi.org/10.4329/wjr.v8.i6.635
The history of nuclear medicine imaging has been intimately tied to the investigation of endocrine disorders using radioisotopes labeled to ligands specific for molecular processes within cells of the endocrine system. This approach depicts physiological and pathological biodistributions of these radiotracers, creating “in vivo functional maps”, that can be used to aid diagnosis and management of thyroid, parathyroid and adrenal disorders. Radionuclide thyroid scintigraphy is the prototypic test of endocrine dysfunction via determination of thyroid trapping and organification of radioiodine. On a molecular level radioiodine uptake measurements with either radioiodine-131 (131I) or radioiodine-123 (123I) are mediated by the sodium-iodide symporter (NIS) expressed on thyroid cells[1,2]. These radioisotopes are also used following thyroidectomy for staging of well-differentiated thyroid cancers (DTC), and in the case of 131I to ablate normal thyroid remnant tissues and to treat thyroid cancer metastases[3,4].
Other commonly used endocrine radionuclide studies are parathyroid scintigraphy with 99mTc-methoxyisobutylisonitrile (sestamibi) for detection of hyperfunctioning parathyroid adenomas, somatostatin receptor scintigraphy (SRS) with radiolabelled somatostatin analogues for imaging of neuroendocrine tumors (NETs) and metaiodobenzylguanidine (MIBG) scans for imaging of chromaffin-cell tumors of the neural crest. Less widely available are adrenal cortical imaging agents, including 131I-6-β-iodomethyl-19-norcholesterol (NP-59) for evaluation of cortisol, aldosterone and androgen secreting adrenal adenomas, and 123I-metomidate (MTO), a selective β11-hydroxylase inhibitor used to image adrenocortical adenomas and some adrenocortical cancers.
The development of hybrid single photon emission computed tomography (SPECT)/computed tomography (CT) cameras that allow combined multi-modality imaging in a single diagnostic session, with fusion of three-dimensional (3D) volume datasets, represents a significant technological advance in the field of diagnostic imaging[5,6]. There is growing literature describing the advantages that contemporary SPECT/CT technology affords, when applied to the evaluation of endocrine disorders, improving diagnostic accuracy with subsequent impact on patient management[7,8].
We performed a PubMed literature search using the terms: “SPECT/CT”, “functional anatomic mapping”, “transmission emission tomography”, “parathyroid adenoma”, “thyroid cancer”, “neuroendocrine tumor”, “adrenal”, “pheochromocytoma”, “paraganglioma”, and critically reviewed those articles published in English during the years 2003 to 2015 describing the utility of endocrine SPECT/CT scintigraphy. Reference lists from the articles were reviewed to identify additional pertinent articles. We provide a synthesis of the clinical usage and benefits of SPECT/CT and conclude that the combination of function and form, in a synergistic and complementary manner, has improved diagnostic imaging (Table 1). Institutional board review approval was obtained for the figures in this manuscript.
| Reported benefits of hybrid SPECT/CT imaging | 99mTc-sestamibi parathyroid scan | 99mTc-pertechnetate or 123I-thyroid scan | 131I- or 123I-thyroid cancer scan | 111In-DTPA octreotide scan | 131I-NP-59 adrenal cortical scan | 131I- or 123I-MIBG scan |
| Precise localization of the radioactivity | + + + + | + + | + + + + | + + + + | + + + | + + + + |
| Characterization of the radioactivity | + + + + | + + | + + + + | + + + + | + + + | + + + + |
| Clarification of equivocal radiotracer uptake | + + | NA | + + + + | + + + + | + + | + + + |
| Incremental sensitivity over planar/SPECT | + + | NA | + | + + | + | + |
| Incremental specificity over planar/SPECT | + + + + | NA | + + + + | + + + + | + + + | + + + |
| Additional information on the non-diagnostic CT | + + + | NA | + + + | + + + | + + | + + + |
| Improved interobserver agreement | + + + | NA | + + | + + + + | NA | + + + |
| Improved reader confidence | + + + | NA | + + + + | + + + + | NA | + + + |
| Attenuation correction | + | NA | + | + + | NA | + + |
| Staging (TNM) of tumor | NA | NA | + + + + | + + | NA | + |
| Surveillance and detection of recurrent tumor | NA | NA | + + + + | + + + + | NA | + + |
| Preoperative localization of target for resection | + + + + | NA | + | + | + + + | + + + |
| Confirming of radiotracer avidity as a prerequisite to radionuclide based therapy1 | NA | NA | + + + + | + + + + | NA | + + + |
| Exclusion of radiotracer avidity to recommend alternative imaging2 | NA | NA | + + + | + + + | NA | + + + |
| Correlation to outcomes and prognosis | + + + | NA | + + + | + + | + + + + | + + |
| Radiation dosimetry (tumor- or organ-specific) | NA | NA | + + + | + + + | NA | NA |
| Radionuclide imaging times reduced | NA | NA | + + | + + + | NA | NA |
| Ordering of additional CT/MRI imaging reduced | NA | NA | + + + | + + + | + | + |
| Change in management | + + + + | NA | + + + + | + + + + | + + + + | + + + + |
| Surgical times reduced | + + + + | NA | NA | NA | NA | NA |
| Cost-effectiveness | + + | NA | NA | NA | NA | NA |
Radionuclide scans are performed after administration of a radiolabelled ligand, with the resultant gamma photons emitted by radioactive decay detected using Anger gamma cameras equipped with collimated sodium (Tl) iodide scintillation crystals. Historically the first images were planar acquisitions, and as such were limited by adjacent radioactivity from overlying tissues, low spatial resolution and paucity of correlative anatomic data. Subsequently, the introduction of SPECT was a major advancement, providing 3D tomography datasets initially by the process of filtered back-projection and more recently with, iterative reconstruction techniques. SPECT imaging markedly improves contrast resolution and eliminates interfering background activity, however continues to suffer from low spatial resolution and paucity of anatomic data.
The advantages of hybrid 18F-fluorodeoxyglucose (FDG) PET/CT led to an interest in the development of hybrid cameras that combined a conventional gamma camera with an inline CT scanner, with the goal of simultaneous acquisition of SPECT and CT datasets. Co-acquisition of the functional and anatomic data with hybrid scanners removed major barriers of software-based co-registration, such as issues relating to patient positioning between imaging sessions, co-integration of data, and the need for fiducial body markers. Hybrid SPECT/CT cameras were first developed by Lang et al[9] in 1992 with early models using single slice CT scanners. Later models have incorporated multi-slice helical CT with the SPECT/CT usually performed using a so-called “non-diagnostic”, non-intravenous contrast mode with the CT portion contributing an estimated radiation doses of 2-4 mSv to the patient[5,6,10,11]. SPECT/CT has been successfully applied to radionuclide cardiac imaging, bone scanning, endocrine scintigraphy, lymphoscintigraphy and liver-spleen imaging[5,6]. In our experience since 2005, we have found that SPECT/CT has incremental diagnostic value for endocrine scintigraphy, improving diagnostic interpretation and accuracy with meaningful impact on management decisions. The functional and anatomical images are viewed in a tandem side-by-side mode using a localizer tool, or in a “fusion” mode that allows comparative color blending of both 3D datasets as a single image.
An analogy for SPECT/CT for localization of radioactivity is likening its use to global positioning satellite (GPS) technology in the automobile industry. Radionuclide scintigraphy is akin to driving with only a rough idea of the location of the automobile relative to prominent landmarks. Conversely, CT is like a comprehensive street map of a city without an index; finding a specific street or location requires a detailed search by the reader. The combination of imaging modalities in the form of fusion SPECT/CT, a type of “medical GPS”, allows the interpreting physician or radiologist to navigate imaging findings with relative ease. The surgeon who is familiar with cross-sectional modalities such as CT and magnetic resonance imaging (MRI), gains more precise knowledge of the anatomical location of disease targeted for resection, leading to shortened surgical times, shorter hospital stays and faster patient recovery.
The advantages of hybrid SPECT/CT can be broadly categorized as: (1) precise localization of the radioactivity; (2) improved characterization of radioactivity resulting higher specificity, improved sensitivity and overall higher diagnostic accuracy; (3) additional anatomic information derived from the CT component with improved preoperative work-up; (4) CT-based attenuation correction and potential for volumetric dosimetry calculations; (5) increased reader confidence and interobserver agreement; and (6) impact on patient management (e.g., change in treatment plans, shortened surgical operating times)[7,8].
Primary hyperparathyroidism presents as hypercalcemia due to excess parathyroid hormone secretion that affects 1 in 2000 males and 2-3 in 500 females, and is due to parathyroid adenoma (85%), hyperplasia (10%) or parathyroid carcinoma (< 1)[12]. As a result of routine laboratory screening tests, the majority of patients with primary hyperparathyroidism are asymptomatic at presentation[13,14]. Secondary hyperparathyroidism occurs in the setting of renal dysfunction often associated with multiple hyperplastic parathyroid glands and when autonomous is referred to as tertiary hyperparathyroidism.
Surgical management of primary hyperparathyroidism with traditional four-gland exploration is curative in greater than 90% of patients[13,14]. Indications for surgery are serum calcium > 1 mg/dL above the upper limit of normal, GFR < 60 mL/min, age < 50 years, symptomatic hypercalcemia, or osteoporosis on bone mineral densitometry[12,15]. The shift to minimally invasive parathyroidectomy has advantages of shorter operation times, decreased length of hospital stays and smaller incision/scar, with similar high cure rates. This technique requires accurate preoperative localization of the parathyroid adenoma, with 99mTc-sestamibi parathyroid scintigraphy and neck ultrasound being the most commonly used imaging tests. 4D-CT is an emerging imaging modality that is promising, although it does impart higher radiation exposure[16,17]. Emphasis is for cure at initial surgery due to difficulties related to reoperation in the neck. When surgical procedures fail there may be problems related to multiglandular hyperplasia or ectopic parathyroid locations[13,14]. The introduction of intra-operative parathyroid hormone monitoring has helped to address some of the issues with these challenging cases, where a 50% decline predicts successful resection of the “culprit” adenoma responsible for hypersecretion of parathyroid hormone.
Parathyroid scintigraphy uses 99mTc-sestamibi which is accumulated by both thyroid and parathyroid tissues. Dual time-point imaging protocols use the different retention and washout rates between thyroid and parathyroid tissues at early 10-20 min and delayed 2 h time-points to identify parathyroid adenomas[12,15]. The reported sensitivity varies between 54% to 100% depending on the cohort reported[18]. Numerous studies confirm incrementally utility and diagnostic value of parathyroid SPECT/CT compared to either planar or planar/SPECT techniques for preoperative localization of parathyroid adenoma using a minimally invasive surgical approach in patients with primary hyperparathyroidism, secondary hyperparathyroidism, ectopic or multiglandular disease, and at reoperation[19-43]. We performed a meta-analysis of parathyroid SPECT/CT in 12233 patients in 24 studies and found a pooled sensitivity (identification rate) of 86%. Using pooled unpaired analysis there was a step-up in diagnostic sensitivity between planar (70%), SPECT (74%) and SPECT/CT (86%) techniques[44]. Specificity of parathyroid SPECT/CT could not be analyzed as most studies reported surgically treated cohorts without clinical follow-up of negative studies to determine actual false-negative rates. In a more recent study of 154 patients undergoing dual-phase parathyroid imaging, SPECT/CT detected more lesions (97.8%) compared to planar imaging (87.6%)[45]. SPECT/CT was superior to planar imaging particularly for the detection of small adenomas and dual-phase imaging was superior to single-phase studies regardless of the imaging technique employed.
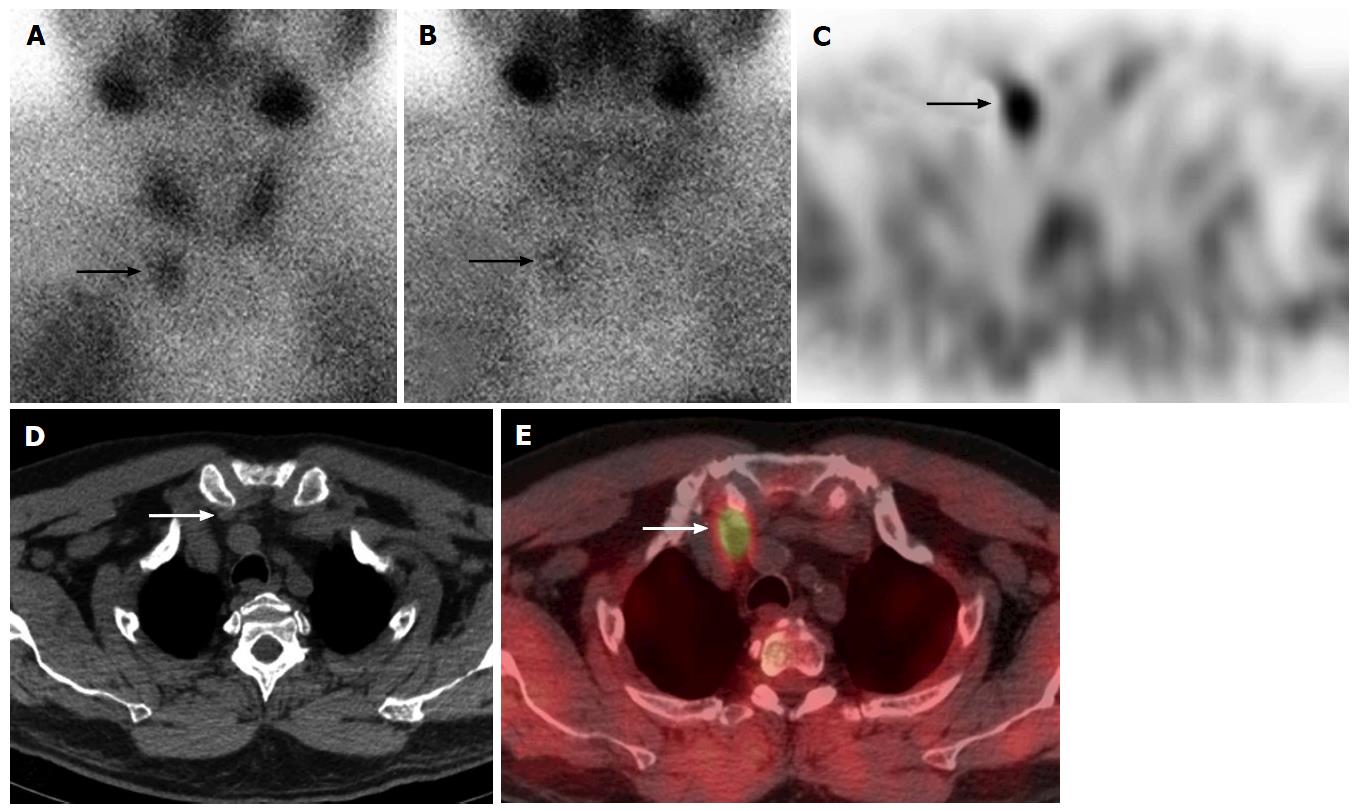
In addition to being more sensitive than planar imaging for detection of adenomas, SPECT/CT is better at lateralization and quadrant localization of the parathyroid adenoma. The CT portion may visualize the adenoma in a eutopic position posterior to the thyroid gland, or for ectopic glands, can demonstrate the superior gland located more posteriorly and the inferior gland more located anteriorly[46] (Figure 1). SPECT/CT excels for the evaluation and preoperative localization of ectopic parathyroid glands[40]. This has been universally reported as a major benefit of the technique[44], particularly for the inferior parathyroid glands that are more likely to migrate during embryological development to ectopic sites including high cervical, intra-thyroidal, intra-thymic, anterior mediastinal and mediastinal locations. Another group of patients that had improved diagnostic accuracy with SPECT/CT were patients with concurrent multinodular thyroid, which often causes false-positive results due to retention of sestamibi in thyroid nodules. SPECT/CT can distinguish eutopic adenomas from false-positive uptake in patients with multinodular goiter[30,38].
There are a few studies comparing SPECT/CT with neck ultrasound or neck CT. In a comparative study of 38 patients with primary hyperparathyroidism whose work-up included 4D-CT, neck ultrasound and MIBI SPECT/CT, 4D-CT had sensitivity of 92.1%, superior to ultrasound (84.2%), and SPECT/CT (84.2%)[47]. 4D-CT had better specificity of 95.6%, ultrasound (86.8%) and SPECT/CT (90.4%), although there were no ectopic adenomas included in this cohort. 4D-CT could be cost-effective, given its similar costs to parathyroid scintigraphy, although the main limitation of 4D-CT is the radiation dose exposure, of particular concern in younger patients[16]. Comparison of parathyroid SPECT/CT to neck ultrasound in 64 patients reported SPECT/CT sensitivity 70.6% and specificity 94.4%, was higher than ultrasound, 60.3% and 72.2% respectively[48]. In another study SPECT/CT had a sensitivity of 88.5%, planar scintigraphy 75.4% and ultrasound 77%[49]. A major limitation of neck ultrasound is the inability to detect ectopic adenomas[50].
Benefits of SPECT/CT for surgical management of hyperparathyroidism include shorter surgical times, preoperative localization of eutopic retrotracheal and ectopic adenomas and improvement in diagnostic accuracy. At surgery, the adenoma is usually found within a close distance (< 1.9 cm) from the expected location predicted by imaging[31]. More recently SPECT/CT has been used intraoperatively with a hand-held gamma-probe to locate parathyroid adenomas, similar to the technique to identify lymph nodes in breast lymphoscintigraphy[51]. SPECT/CT has the potential to avoid false-positive results, with unique cases reported due to an inflamed lymph node, metabolically active brown adipose tissue or from pulmonary emboli, uncommon though important diagnostic pitfalls[52-55]. The management of patients considering reoperation remains challenging where SPECT/CT continues to demonstrate low sensitivity, necessitating other diagnostic strategies that may include selective venous sampling or, if available, positron emission tomography (PET) imaging[36].
Thyroid pinhole imaging with either 99mTc-pertechnetate or 123I-radioiodine distinguishes hyperthyroidism due to Graves’ disease or toxic multinodular goiter, from conditions with decreased thyroid gland uptake, such as subacute thyroiditis[56,57]. Quantitative assessment of radioactive iodine thyroid uptake at 24 h provides an objective measure of thyroid function[57,58]. Another indication for thyroid imaging is to identify dominant so-called “cold” or hypofunctioning thyroid nodules to assess thyroid cancer risk. In these settings thyroid scans are usually correlated with high-resolution thyroid ultrasound. A few authors have reported cases of thyroid SPECT/CT to assist the diagnosis of co-existent Graves’ disease in the presence of a multinodular thyroid gland (Marine-Lenhart syndrome)[59].
Thyroid scans are performed in the neonatal period for diagnosis of hypothyroidism to distinguish thyroid gland agenesis from dyshormonogenesis in which the thyroid gland is present, though non-functioning. Thyroid gland ectopia is a rare disorder, the most common site being at or near the tongue base (lingual thyroid), which in 70% of cases is associated with agenesis of the eutopic thyroid[60,61]. Lingual thyroid may present with hypothyroidism and subsequent thyroid hormone replacement usually results in atrophy of the thyroid tissue. Rarely in adulthood the lingual thyroid may cause obstructive or compressive symptoms such as dysphagia, odynophagia, bleeding, coughing and obstructive sleep apnea[62,63]. The utility of thyroid imaging in cases of hypothyroidism where ultrasound cannot identify a normal thyroid gland is obvious, and thyroid agenesis should be differentiated from ectopic thyroid tissue (lingual thyroid) that may later be misdiagnosed as a soft tissue neoplasm. SPECT/CT has shown to be of diagnostic value with either 99mTc-pertechnetate[64-66] or 123I radiotracers[67,68]. An emerging therapy option for obstructive lingual thyroid is radioactive 131I[67,69,70]. While the conventional diagnostic planar thyroid scan will guide management of patients with thyroid agenesis, SPECT/CT will allow identification of ectopic thyroid (lingual thyroid) confirming its ability to accumulate radioiodine, a prerequisite for radioiodine therapy. Advantages include avoidance of surgery and associated post-operative bleeding and infection[67]. SPECT/CT has also been used to evaluate for struma ovarii, thyrotoxicosis due to ectopic thyroid tissue at adnexal sites, and has been reported to detect metastatic peritoneal deposits in a rare strumal malignancy[71].
DTC has a favorable prognosis with a greater than 90% 10-year survival for papillary thyroid cancer (PTC) and an 85% 10-year survival for follicular thyroid cancer. Standard managment of DTC centers on total or near-total thyroidectomy with central and lateral compartment dissection performed for removal of palpable, enlarged, diseased lymph nodes. Surgery removes the primary tumor, and any possible co-exisiting multifocal disease, addresses cervical lymph node involvement and prepares the patient for radioiodine remnant ablation, and whole body radioiodine imaging[72-74]. Currently, there is a trend towards performing risk stratification of thyroid cancer designed to predict recurrence risk that more readily aids management decisions, rather than simply using prognostic staging classification systems[75,76]. As thyroid microcarcinomas (tumors size ≤ 1 cm) are becoming increasingly diagnosed, the role of radioiodine in the management of DTC has become somewhat controversial in low-risk patients without extra-thyroidal tumor extension or high-risk histopathological features[77-79]. There is consensus for the use of 131I in the treatment of regional and distant metastases of DTC[72,73], however evidence is lacking that it decreases recurrence rates or increases survival in stage I, low-risk disease[72-74,80,81]. Recently there has been an exploration of reducing the administered doses of 131I for thyroid remnant ablation with excellent efficacy demonstrated using doses at a lower threshold of 1.1 GBq (30 mCi). Further, recombinant human thyrotropin (rhTSH Thyrogen®) stimulation for radioiodine imaging is now an acceptable alternative to endogenous hypothyroidism for diagnostic imaging and to perform thyroid remnant ablation[82,83], although it’s use for treatment of iodine-avid metastases is still under investigation.
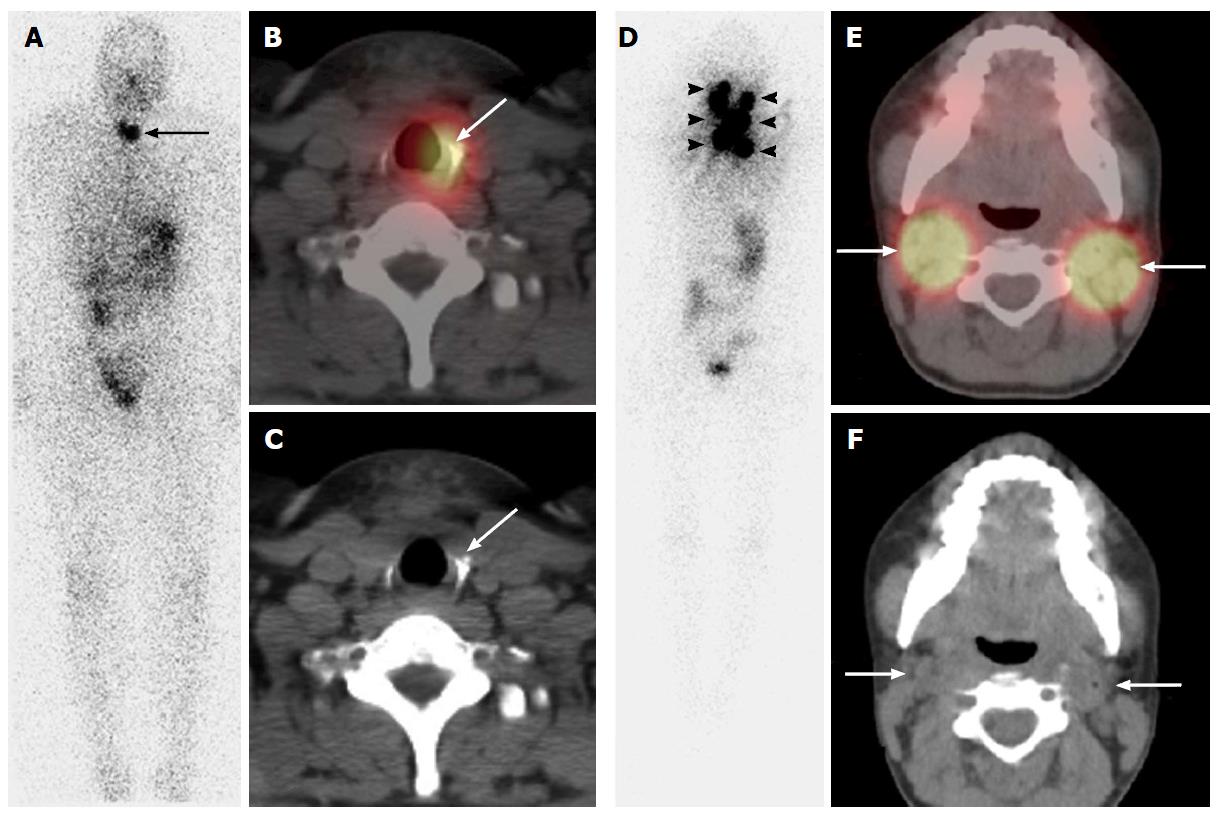
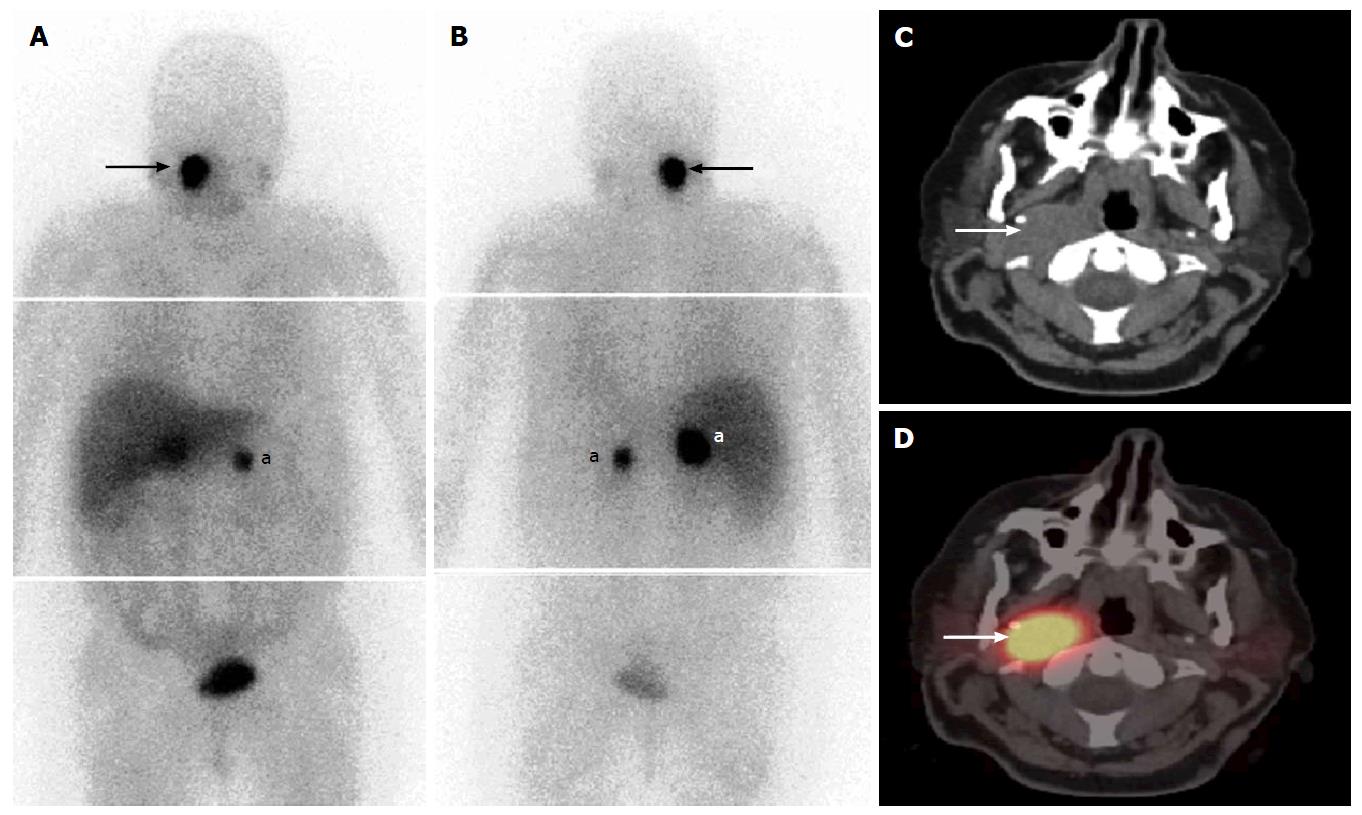
Radioiodine imaging using planar techniques has sensitivity of between 45% to 75% and excellent specificity of 90% to 100% for detection of thyroid tissues, with post-therapy 131I scans considered to have the highest sensitivity[84-86]. Planar imaging detects functioning thyroid tissue, but characterization of benign vs malignant etiology is limited by a lack of anatomic information. Focal neck uptake is often indeterminate and identification of iodine-avid metastases may be hampered by their small size, especially in locations where the anatomy has been altered by prior surgery, or in close proximity to sites of physiological radioiodine accumulation. Also, cervical lymph node metastases or other metastatic sites may go undetected on the post-ablation whole-body scan because of the much higher radioiodine avidity in thyroid remnant tissue. 131I SPECT/CT improves diagnostic accuracy by better distinguishing cervical lymph node metastases from remnant thyroid tissue in the neck[87,88] (Figure 2). SPECT/CT allows reliable diagnosis of lymph node involvement as early as at the time of thyroid remnant ablation, thus opening a new avenue for more accurate staging of patients with DTC, and guiding the most appropriate therapeutic options[89].
Reports of 131I- and 123I-SPECT/CT have studied patients prepared with either exogenous rhTSH stimulation or endogenous hypothyroidism, and imaged with diagnostic doses between 37 MBq - 430 MBq (1 mCi - 11 mCi)[85,86,90-93] and therapeutic doses from 1.1 GBq - 9.7+ GBq (30 mCi - 260+ mCi)[86,94-109]. Radioiodine SPECT/CT has been used for surveillance of thyroid cancer recurrence, in the immediate post-surgical setting prior to 131I therapy[85,86,96,110,111], at the time of first radioiodine ablation[90,91,93] and in post-therapy imaging[94-99,101,102,105,106,108,109,112-114]. An important advantage of 131I SPECT/CT is that it decreases equivocal findings frequently encountered on planar imaging[97]. Diagnostic 131I SPECT/CT performed at the time of the first radioiodine ablation correctly classified equivocal findings and had incremental diagnostic value in 41% foci, compared to planar imaging[91,92]. Thyroid remnant tissue in proximity to the recurrent laryngeal nerves is typically not removed during total thyroidectomy due to concern for nerve damage. Thyroglossal duct remnant is a midline focus in the central neck compartment often near the hyoid bone, representing some residual thyroid tissue along the tract of embryological descent[93,94]. SPECT/CT allows confident identification of benign uptake within thyroglossal duct and thyroid bed remnants and can distinguish them from nodal regional metastases, even disease within anatomically normal-sized lymph nodes, that are otherwise not easily identified on neck ultrasound after thyroidectomy[115].
Barwick et al[110] reported 123I SPECT/CT imaging for thyroid cancer recurrence with a higher sensitivity of 50% and specificity of 100%, compared to SPECT (sensitivity 45% and specificity 89%) and planar imaging (sensitivity 41% and specificity 68%). They concluded that 123I SPECT/CT primarily led to increase in imaging specificity. Patients with intermediate-to-high risk histopathological features; such as tall cell, columnar or poorly differentiated (insular) thyroid cancer have a greater risk for non-iodine-avid disease, which can be identified even in the face of negative scintigraphy, on the non-contrast enhanced CT portion of the imaging examination. 131I SPECT/CT found sites of non-iodine-avid disease in 21.6% of patients with intermediate-to-high risk stratification of their thyroid cancer[109]. The identification of non-iodine-avid disease due to loss of sodium iodide symporter expression can occur in between 20%-30% of patients with DTC and implies that radioiodine will be ineffective for imaging and therapy therefore requiring alternative FDG PET/CT imaging and consideration of external beam radiation therapy or reoperation if appropriate.
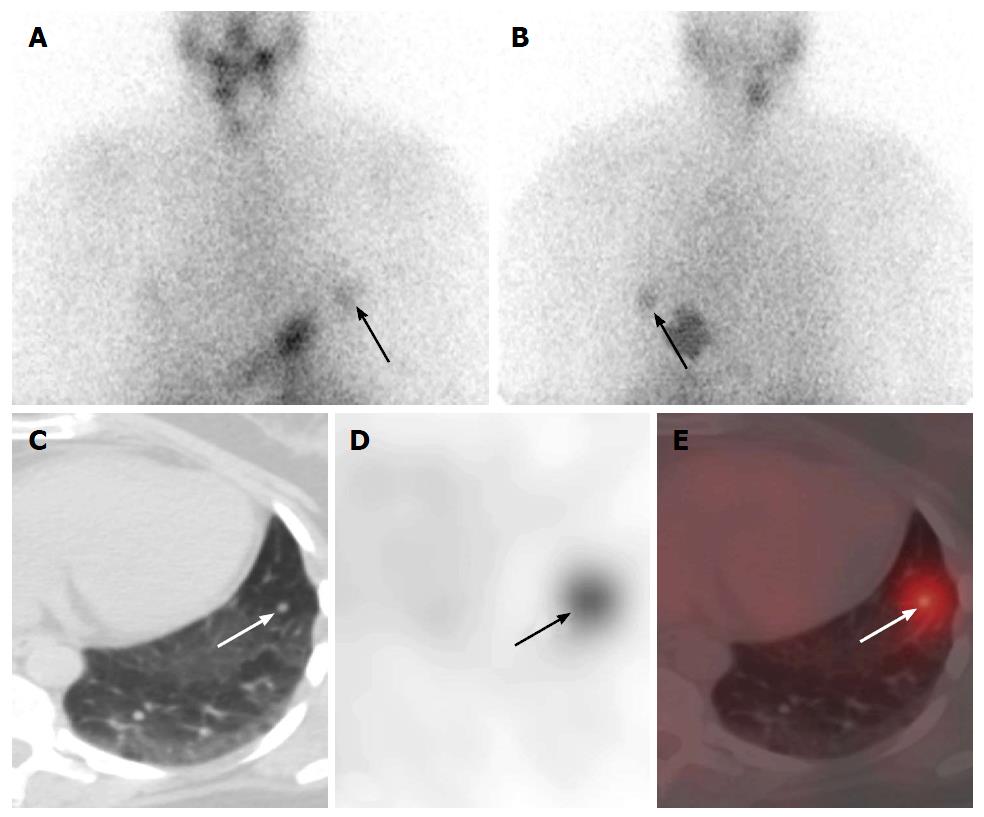
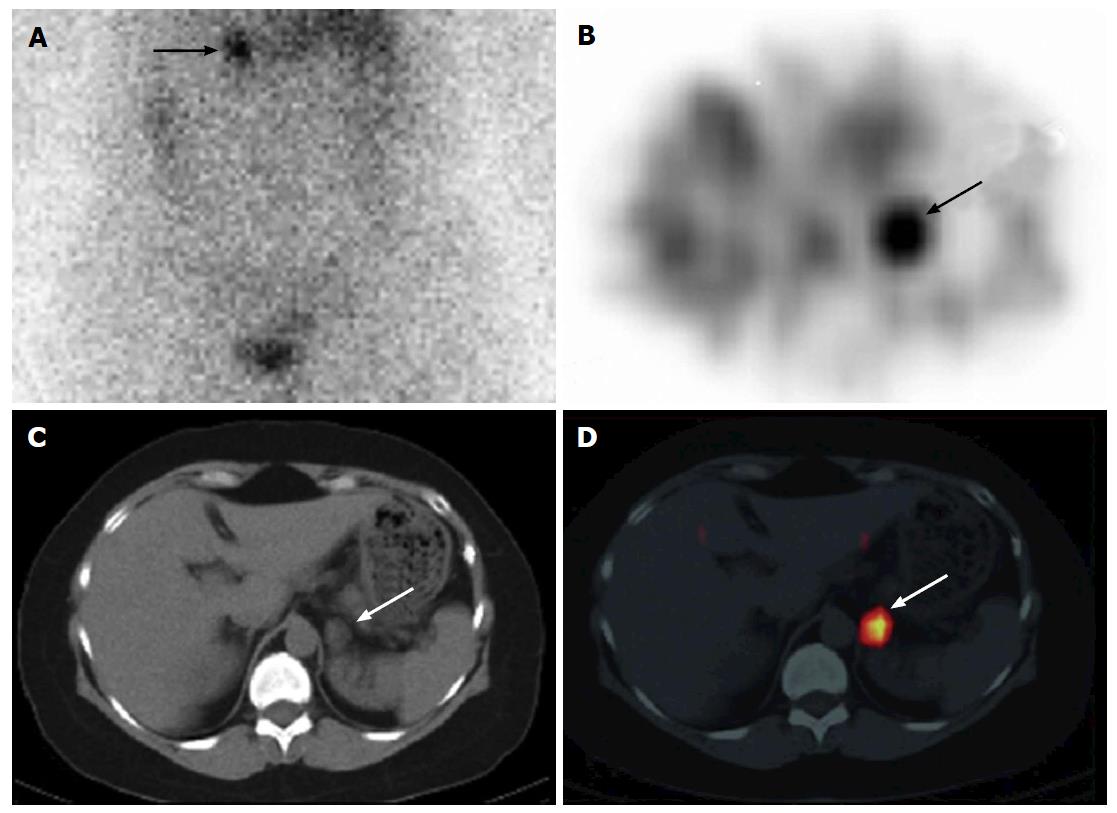
Whole body screening with 131I SPECT/CT has utility to evaluate for distant metastatic disease[85,90,91,96-98,106,108,110]. The pattern is usually metastatic spread to the lungs or bone, or rarely to liver, skin, brain or other sites (Figure 3). 131I SPECT/CT exhibits significantly higher sensitivity than 99mTc-methylene diphosphonate (99mTc-MDP) bone scans for detecting skeletal thyroid cancer metastases[113]. 131I SPECT/CT has utility for depicting distant metastatic disease including unusual sites leading to changes in staging and risk stratification[103,104]. 131I SPECT/CT has been reported to localize unanticipated thyroid cancer metastases to liver, kidney, muscle and trachea[116-125]. Numerous benign variants and physiogical disease mimics are well described and routinely identified on radioiodine scintigraphy[126-128], estimated to occur in up to 22% of radioiodine scans[111]. NIS activity or iodide-chloride channels can be found in extra-thyroidal tissues; salivary glands, lactating and non-lactating breasts, and in gastric mucosa[129]. Other sites of benign radioiodine uptake include the choroid plexus, ciliary body of the eye, lacrimal glands, placenta, thymus and skin[1,129-133]. Pattern recognition allows most of these normal variants to be correctly diagnosed on planar imaging, though the use of SPECT/CT increases interpretation confidence and resolves diagnostic uncertainty[134,135]. Case reports confirming this utility of SPECT/CT for evaluation of uncommon benign mimics of disease that include lingual thyroid(s), nasolacrimal duct(s), thymus, renal cysts, menstruating uterus, struma ovarii, and ovarian teratomas[68,116-125,136-139]. Oral radioiodine uptake that was present in 57% of scans localized to high-density dental material on SPECT/CT. Authors have postulated a novel physiologic mechanism for radioiodine uptake that radioiodine has an affinity for metals in dental amalgam via a chemical reaction[140,141].
Management decisions for thyroid cancer are based on accurate staging and risk stratification. The impact of 131I SPECT/CT on tumor node metastasis (TNM) staging was assessed in 320 patients prior to receiving their first radioiodine ablation. In those < 45 years, SPECT/CT disclosed distant sites of metastases in 4% and regional nodal metastases in 44%. In patients 45 years or older, SPECT/CT found distant metastases occurred in 10% and nodal metastases in 28%. The overall change to TNM staging contributed from 131I SPECT/CT was 4% patients < 45 years, and 25% of patients, 45 years or older[93]. In a study of 308 DTC patients with post-therapy SPECT/CT, evaluation of post-surgical, preablation thyroglobulin thresholds were examined, comparing rhTSH in 123 patients to thyroid hormone withdrawal in 185 patients[142]. For prediction of residual structural disease a cut-off thyroglobulin level of 2.8 ng/mL for rhTSH and 28 ng/mL for thyroid hormone withdrawal was established. In a study of the incremental detection rate of SPECT/CT over planar images, SPECT/CT found additional disease in 8.6% of cases. Patients with additional disease on SPECT/CT had significantly higher thyroglobulin levels (4.3 ng/mL) compared to those without additional SPECT/CT findings (1.6 ng/mL)[143].
123I or 131I SPECT/CT was studied in a pediatric population and accurately localized radioactivity foci in the neck including benign variants. It changed clinical decision-making by determining that 131I therapy was unnecessary in two children[144]. This avoidance of unnecessary 131I radiation exposure is important in those cases where SPECT/CT imaging has reliable excluded residual or metastatic disease[145,146]. Authors have found that lymph nodes > 1.0 cc are less likely to be effectively treated by radioactive iodine therapy without surgical intervention[99]. The presence of 131I uptake on post-therapy SPECT/CT better predict short-term outcome and failure of 131I therapy than planar imaging[94]. A study of 170 patients found that post-therapy SPECT/CT was the only independent prognostic indicator of treatment failure at 2 years[95]. SPECT/CT can be used to provide more accurate tumor dosimetry by better defining the volume of metastatic disease. This approach to select a calculated delivered radiation tumor-absorbed dose has been used to treat a large skull metastasis from follicular-variant PTC[147] and in a patient with diffuse bone metastases[148].
NETs are rare neoplasms derived from the amine precursor uptake and decarboxylase system, that have potential for hypersecretion of hormones and malignancy risk[149,150]. They have heterogenous biological behavior and prognosis. After diagnosis the management of NETs depends upon histopathological classification by differentiation and grade. Differentiation is used to confirm neuroendocrine origin and cell type. Ki-67 indices and mitotic rates are used to determine the grade and malignant potential of the NET. These data when combined with staging schema such as American Joint Committee on Cancer staging systems more closely reflect prognosis, and guide imaging strategies and management decisions. First-line treatment is surgical excision of the primary tumor and solitary metastatic lesions, with chemotherapy and systemic treatments reserved for widespread distant disease. As surgical resection remains the only curative treatment, accurate pre-operative staging is of paramount importance.
Depending on their classification, NETs present a clinical challenge for therapy, with anatomic and functional imaging offering complementary and unique advantages that can affect management and potentially reduce morbidity and/or mortality. Although standard imaging techniques (CT and MRI) can be useful in a search for disease, in many situations NETs are small and inconspicuous. SRS depicts NETs via overexpression of somatostatin receptors (SSTR) expression by these tumors, particularly SSTR2. 111In-DTPA-pentetreotide is the most commonly used somatostatin analogue which has the highest sensitivity and specificity for carcinoid tumors that present with a syndrome of flushing, hypotension and other symptoms of tryptophan excess. Sensitivity of 111In-DTPA-pentetreotide scintrigraphy is notably lower in poorly differentiated NETs and also in insulinomas that do not express SSTR2. Overall SRS for NET staging has a sensitivity (80%-100%) that exceeds that of CT[151,152]. 111In-DTPA-pentetreotide may be used to image pheochromocytoma and paragangliomas, although MIBG is preferred due to higher specificity. 111In-DTPA-pentetreotide is also useful for imaging of head and neck paragangliomas, which are of parasympathetic origin, including carotid body tumors and glomus vagale and jugulare.
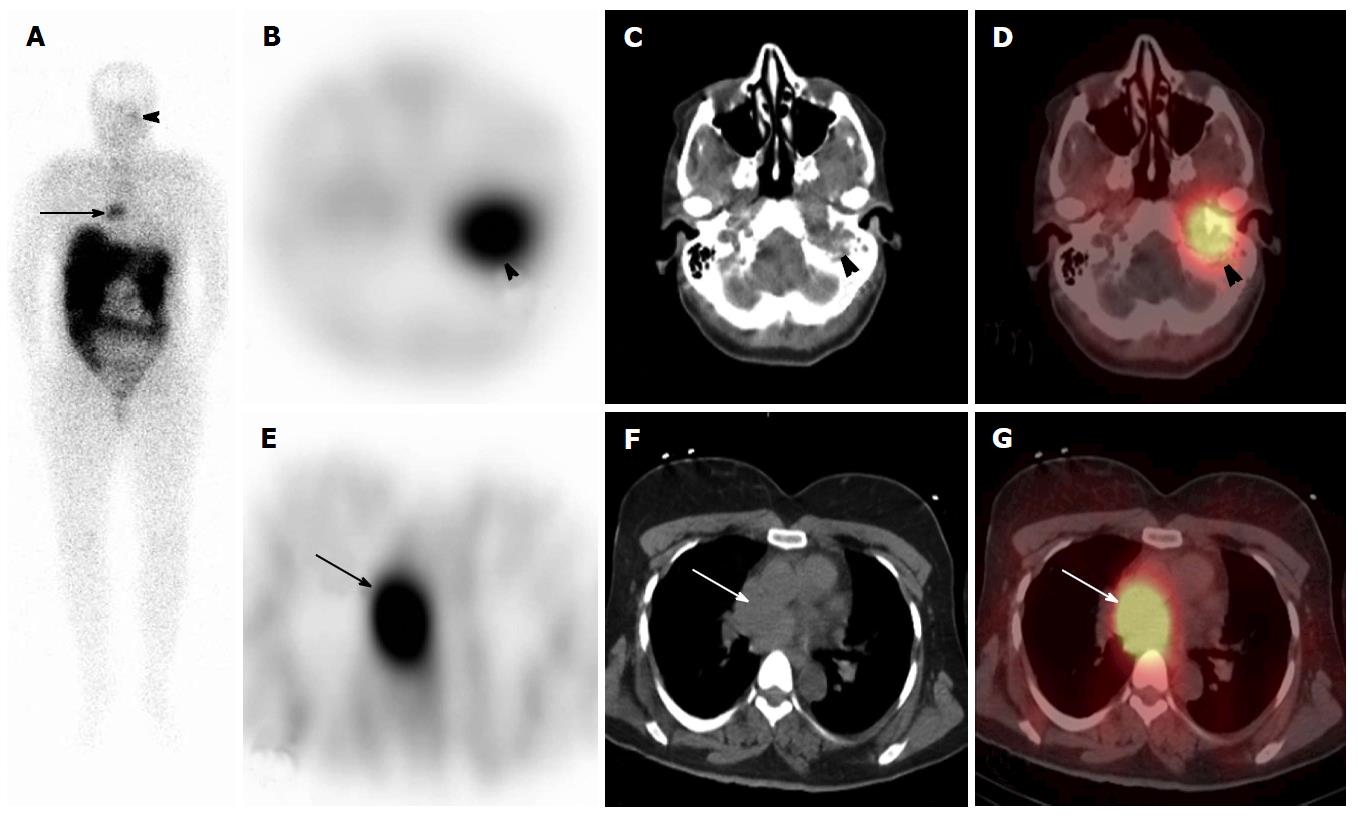
Studies have reported that 111In-DTPA-pentetreotide SPECT/CT imaging provides incremental diagnostic value over planar and SPECT imaging, achieved through superior lesion localization, identification of physiologic radioactivity distributions, and additional anatomic information derived from the non-diagnostic CT portion[153-165]. Numerous studies have found that 111In-DTPA-pentetreotide SPECT/CT fusion images had superior diagnostic accuracy compared to side-by-side analysis or software co-registration[153,155-158,161,162,165]. Typically, physiological sites of uptake in pituitary, thyroid, liver, spleen, gastrointestinal and genitourinary sites (kidneys and bladder) can be distinguished from benign inflammatory processes, infections, sarcoidosis and other granulomatous diseases that may be a source of false-positives. In a study of 107 patients 111In-DTPA-pentetreotide SPECT/CT had an overall sensitivity of 87.8% and specificity of 96.6%, compared to planar imaging, correctly characterizing and reducing the number of equivocal findings from 14 (13%) to 1 (1%)[166]. SPECT/CT was able to detected lesions unsuspected on planar and SPECT, and had an overall, low rate of false-positive findings (only 12 of 160 lesions) (Figure 4). Additional benefits of the SPECT/CT are improved interobserver variation, reader confidence, and the ability to perform shorter imaging protocols at 24-h time-points obviating the need for later 48 h imaging[162,167].
A comparative study of 99mTc-HYNIC octreotate SPECT/CT and 111In-DTPA-pentetreotide SPECT/CT was perfromed in 14 patients with NETs. 99mTc-HYNIC detected 40/43 (93%) lesions compared to 111In-DTPA-pentetreotide 36/43 (83%), although this did not reach statistical significance. This study found 99mTc-HYNIC octreotate has better image quality, lower radiation dose and a trend towards better diagnostic performance[168]. In a study looking at the ability of SRS to detect primary NETs with 68Ga-DOTATOC PET/CT in 52 patients was compared to 111In-DTPA-pentetreotide SPECT/CT in 71 patients. PET/CT was able to detect the primary tumor in 15 patients (29%) compared to only 4 (8%) with SPECT/CT[169]. Another comparative study of 68Ga-DOTATATE PET, 99mTc-HYNIC-octreotide and whole body MRI found that PET had sensitivity of 96%, compared to SPECT/CT 60% and diffusion weighted MRI of 72%[170]. The specificities for PET, SPECT/CT and MRI were 97%, 99% and 100% respectively. Therefore 68Ga-DOTA peptide PET is preferable to SRS with SPECT/CT whenever available. An interesting study comparing 111In-DTPA-pentetreotide SPECT/CT with FDG PET/CT in 15 patients with 45 metastatic lesions reported an inverse relationship between the SRS uptake and FDG uptake, supporting the hypothesis that more proliferative metastatic disease is less differentiated with respect to somatostatin receptor expression, though conversely having greater dependence upon glucose metabolism[171].
Quantitative SPECT/CT allows tumor dosimetry calculations in patients receiving radiolabeled therapies for metastatic NETs[172,173]. Automated dosimetry tested in 17 patients allowed calculation of absorbed doses to solid organs, the liver, kidneys and spleen[174]. Using SPECT/CT derived dosimetric calculations, the greatest exposure to the kidneys occurred at 1-2 h, allowing shortening of reno-protective amino-acid infusion times[175].
Adrenal cortical scintigraphy has usually been performed in 2 settings: (1) for preoperative evaluation of hyperfunctioning adrenal adenomas in which surgery is being contemplated; and (2) for characterization of incidentally discovered adrenal masses. In this setting of adrenocorticotropic hormone (ACTH)-independent Cushing’s disease, Conn’s syndrome or rarely hyperandrogenism, with biochemically proven adrenocortical hypersecretion, 131I-6-beta-iodomethyl-19-norcholesterol NP-59, a cholesterol precursor of LDL (a component of the corticosteroid synthesis pathway) has been used to lateralize the responsible adrenal adenoma[176-180]. It is important in ACTH-independent Cushing’s syndrome to distinguish a hypersecretory adenoma that is surgically curable from bilateral adrenal macrohyperplasia that responds poorly to surgical resection. Similarly hyperaldosteronism presenting with hypokalemia and hypertension with lateralizing signs on dexamethasone suppression 131I-NP-59 scintigraphy will have excellent sensitivity for diagnosis of aldosteronoma and subsequent surgical cure[177-179], whereas marohyperplasia will not be cured by surgery. Despite the exquisite detail provided by morphological imaging, CT performs less perfectly for this indication because of non-functioning adenomas that confound diagnosis, which are anatomically abnormal but may not be the source of hyperfunction.
SPECT/CT used with the NP-59 scan allows direct evaluation of the hyperfunctioning cortisol secreting tumor to lateralize disease before surgery predicting successful response to adrenalectomy[181] (Figure 5). In 49 patients with primary hyperaldosteronism undergoing surgery, two semi-quantitative parameters derived from NP-59 SPECT/CT, the adrenal: Liver ratio and the lesion to contralateral gland ratio both predicted surgical cure and correctly distinguished adenoma from macrohyperplasia[182]. Similar findings were reported for SPECT/CT in stage 1, atypical hyperaldosteronism[183]. Currently, NP-59 is not available in North America, although it continues to be commercially available in Europe and Asia.
Incidentally detected adrenal nodules are being discovered more frequently owing to widespread use of cross-sectional imaging for evaluation of disease outside the adrenal gland. The wide range of pathologies, include malignant (adrenal metastases, adrenocortical cancer) or benign (cortical adenoma, pheochromocytoma, inflammatory diseases, myelolipoma, macrohyperplasia and hemorrhage) processes. The most common benign etiology is a non-functioning adrenocortical adenoma. Unenhanced CT with Hounsfield units < 10 and size < 4 cm are highly predictive of adenoma with sensitivity of around 70% and specificity of > 95%. NP-59 scintigraphy has been used to distinguish benign cortical adenoma, from adrenocortical cancer, when the mass is inconclusive on adrenal washout CT protocol or chemical-shift MRI. Adrenocortical cancer is usually “cold” due to less-avid uptake per gram of tissue[177]; although rare false-positive have been documented. To our knowledge no SPECT/CT studies for this indication have been published. Evaluation of hyperfunctioning adenomas has also been performed with 11C-metomidate (MTO), a selective inhibitor of β11-hydroxylase. 11C MTO PET and PET/CT distinguishes tissue of adrenocortical origin with high specificity, although it is not well suited to distinguish adenoma from adrenocortical cancer.
Recently efforts have been made to radiolabel MTO with 123I[184]. The half-life of 13 h for 123I is more practical compared to the positron emitting 11C half-life of 20 min allowing SPECT/CT imaging, outside of the research setting. 123I MTO SPECT/CT was used to prospectively study a pilot set of 12 patients with adrenocortical cancer, and found to have a low diagnostic sensitivity, although there was a subset of adrenocortical cancer metastases that did have increased MTO uptake[185]. This was confirmed in 55 patients with adrenocortical cancer with M1 disease, 22 of whom had functioning disease. 123I MTO SPECT/CT detected just over a third, 164/430 lesions, with the scan being positive in only 34/54 patients, sensitivity of 59%. Planar imaging performed worse than SPECT, with SPECT/CT the being most accurate modality. The subset of 21 patients with metastatic adrenocortical cancer that displayed MTO uptake went on to have 131I radiolabeled-MTO therapy with some encouraging early responses[186]. Therefore 123I MTO SPECT/CT may have a role for confirming MTO-avidity in M1 adrenocortical cancer patients and allowing the option of a radionuclide based systemic therapy.
Pheochromocytoma is a sympathicoadrenal medullary tumor derived from chromaffin cells of the neural crest. It presents with symptoms of biological catecholamine excess, hypertension, chest pain, anxiety and abdominal discomfort. It may be discovered incidentally (3% of adrenal “incidentalomas”) or as a secondary cause of hypertension (3%-5% of cases)[187,188]. Biochemical diagnosis is made with elevated serum and urinary catecholamines and metanephrines. Chromaffin tumors that are extra-adrenal in location are termed paragangliomas and may be found anywhere in the sympathetic nervous system, most commonly at intra-abdominal sites (organ of Zuckerkandl) and less commonly in the thorax[187,188]. Up to 25% of pheochromocytomas and paragangliomas are hereditary with associated genetic syndromes that include multiple endocrine neoplasia (MEN) type 2B, neurofibromatosis Type 1, von Hippel-Lindau syndrome, familial succinate dehydrogenase (SDH-B, C, D) syndromes and Carney’s triad. Genetic testing and counseling should be considered in younger patients and when there is bilateral adrenal, multifocal or malignant disease as these are more commonly found in syndromic tumors.
Surgical management of sporadic, benign pheochromocytoma is curative and preoperative diagnosis has important implications for surgery and anesthesiology. Cross-sectional imaging with either CT or MRI has a high sensitivity (> 90%) for the detection of adrenal medullary tumors, with a lower specificity (around 80%). MIBG scintigraphy is based upon a false neurotransmitter analog of noradrenaline, with 131I MIBG and 123I MIBG imaging having pooled sensitivity of 93%-100% for detection of pheochromocytoma and 90% for paraganglioma[189]. Due to its high specificity MIBG has been used for confirmation that an adrenal mass detected on either CT or MRI is a pheochromocytoma. As a whole-body imaging technique MIBG can screen for contralateral disease, extra-adrenal tumors, occult metastases, and help guide suitability for surgical resection. Although the use of MIBG scans has been suggested in patients with genetic predispositions to screen for co-existing paraganglioma or metastases, MIBG scans have been reported to have lower sensitivity for detection of these entities, requiring alternative metabolic imaging with FDG PET/CT.
SPECT/CT use with 123I-MIBG scans has been promising for imaging pheochromocytoma and paraganglioma[190]. Several small early studies of SPECT/CT reported incremental diagnostic accuracy compared to planar imaging[191-193]. 123I-MIBG SPECT/CT compared favorably to CT and led to changes in patient management[191-193]. SPECT/CT had a distinct advantage for evaluation of focal radioactivity in close proximity to the liver or spleen, correctly confirming physiologic patterns of uptake or excretion and allowing accurate anatomic localization of focal areas of avid MIBG uptake[191,192]. Strengths of SPECT/CT compared to planar and SPECT, were improved detection of local recurrences, small extra-adrenal pheochromocytomas, multifocal tumors, and metastatic disease[193] (Figure 6).
More recently, in 126 adrenal lesions evaluated with 123I MIBG SPECT/CT, there was incremental step-up in sensitivity between planar (63.3%), SPECT (86.6%) and SPECT/CT (90%) modalities[194]. Specificity remained high on all modalities; planar 100%, SPECT 96.8% and SPECT/CT of 100%. The interobserver agreement between 2 readers was also higher with SPECT/CT; Cohen’s kappa value for planar was 0.815, SPECT was 0.826 and SPECT/CT was 0.966. In particular, SPECT/CT was helpful in reducing false-positive interpretations from known pitfalls such as compensatory post-resection uptake from the contralateral adrenal and sympathetic innervation at sites of brown fat. Recently 123I MIBG SPECT/CT was compared to MRI concluding that the highest diagnostic sensitivity occurred with a combination of both SPECT/CT and MRI modalities[195].
Similar to SRS, there are several alternative PET radioisotopes, namely 68Ga-DOTA peptides, 18F-DOPA, 18F-dopamine, 11C-hydroxyephedrine and FDG PET that can also be used for imaging of pheochromocytoma and paraganglioma. 68Ga-DOTATOC PET/CT was compared to 123I MIBG SPECT in 11 patients with either pheochromocytoma or neuroblastoma[196]. PET had a sensitivity of 94.4% compared to 76.9% with 123I -MIBG SPECT. A comparative between 68Ga-DOTATOC PET and 123I MIBG SPECT/CT study in 12 patients with extraadrenal paraganglioma found 100% sensitivity for PET compared to only 20% for MIBG[197]. PET found more lesions in 100% patients and MIBG in only 6.9%. PET was better for head and neck sites and also for detection of bone lesions[198]. A comparative study between 18F-DOPA PET and 123I MIBG SPECT/CT in 12 patients with recurrent paraganglioma found 98% sensitivity for PET compared to only 38% for MIBG[197]. 18F-DOPA found more lesions in 8 patients and MIBG found more lesions in 2 patients. Despite the lower sensitivity in these studies, MIBG imaging retains a unique role in patients being considered for 131I-MIBG radionuclide therapy.
Neuroblastoma is a rare malignant tumor of childhood originating from the neural crest with frequent metastases to the bones, adrenals, lungs and lymph nodes. A meta-analysis of MIBG scans for neuroblastoma demonstrated sensitivity of 97% and specificity of 100%[189]. The use of SPECT/CT has not been described extensively in the pediatric patient group due to concerns for radiation exposure. In cases of metastatic stage IV disease on the MIBG scan, further evaluation of sites of uptake with SPECT/CT would not be required. However, authors describing small series of SPECT/CT in neuroblastoma recommend that these concerns should be balanced against the useful diagnostic information from CT scans in guiding appropriate management of a life-threatening malignancy[199-201]. Furthermore, MIBG imaging to confirm uptake and trapping of this radiotracer via the noradrenaline receptor is a prerequisite when contemplating systemic therapeutic 131I MIBG therapy.
Medullary thyroid cancers (MTC) are derived from parafollicular (C cells) of neural crest origin, comprising 3%-12% of all thyroid cancers. MTC requires aggressive surgical management as after initial resection up to 40% of patients will have residual or recurrent disease, as indicated by elevated serum calcitonin or carcinoembryonic antigen tumor biomarkers[202]. Molecular imaging techniques used for MTC include; 99mTc(V)DMSA (sensitivity 50%-80%), 201Tl-thallium (19%), 99mTc-sestamibi (47%), 123I- or 131I-MIBG (25%-30%), 111In-DTPA-pentetreotide (50%-75%)[203] and 99mTc-EDDA/HYNIC-TOC (80%)[204]. To our knowledge, due to the rarity of MTC, no dedicated cohorts have been studied with SPECT/CT, although it is potentially useful[8,205]. 111In-DTPA-pentetreotide SPECT/CT was able to depict a paratracheal MTC recurrence in one case[206]. Another report described a patient MEN2B in whom 99mTc-(V)DMSA SPECT/CT was used to demonstrate bone metastases[207]. SPECT/CT may have a limited role for staging of MTC as the investigation of elevated calcitonin usually requires multimodality imaging with CT, MRI, CT angiography and FDG PET/CT or 18F-DOPA PET/CT, when available, which have higher sensitivity compared to SPECT/CT techniques.
A novel use of SPECT/CT has been to guide aspiration biopsy in DTC, when the ultrasound fine-needle aspiration biopsy is negative for malignant cells but the lymph node aspirate is positive for thyroglobulin[208,209]. 99mTc-sestamibi SPECT/CT has been in DTC to predict nodal metastatic status prior to surgery[210]. Graves’ eye disease is another area where SPECT/CT could have diagnostic value, with localization of 99mTc-EDDA/HYNIC-TOC to the orbits on SPECT/CT being positively related to the disease activity. SPECT/CT may be a feasible technique to assess treatment response to corticosteroids in Graves orbitopathy[211]. Novel preoperative 99mTc-folate SPECT/CT was used to investigate nonfunctioning pituitary adenomas[212].
Future use of these interesting novel applications of SPECT/CT remains to be elucidated. Hybrid SPECT/CT is a rapidly emerging, combined dual-modality imaging technique with incremental diagnostic utility over its individual components when applied to nuclear medicine imaging of endocrine disorders. SPECT/CT exploits the synergism between the functional information provided by radiopharmaceutical imaging and the anatomic data available from CT, translating to improved diagnostic accuracy and meaningful impact on patient care.
Radionuclide scintigraphy has played an integral role in the investigation of endocrine disorders providing unique diagnostic functional information. These are often combined with traditional anatomic imaging modalities such as ultrasound, computed tomography (CT) and magnetic resonance imaging to arrive at the correct clinical diagnosis. The introduction of novel hybrid imaging single photon emission computed tomography (SPECT)/CT cameras allows multimodality imaging during the same imaging session, via co-registration of three-dimensional volume datasets.
Following the success of hybrid imaging with PET/CT cameras for cancer staging, there had been growing interest in SPECT/CT with the first hybrid camera developed in 1992. Since then, there has been increasing utilization of this technology for a wide range of radionuclide studies, with accumulating evidence during the 2000’s for improved diagnostic performance when applied to endocrine scintigraphic studies.
The ability to localize the exact site of origin of a radioactivity focus via SPECT/CT now allows the interpreting radiologist or clinician to precisely characterize this radioactivity to a degree previously not possible.
SPECT/CT has improved preoperative parathyroid adenoma detection and localization, particularly ectopic locations which are challenging to resect. It has improved the staging of neuroendocrine tumors with somatostatin receptor scintigraphy and pheochromocytoma/paraganglioma using metaiodobenzylguanidine scans. It has advatanges for radioiodine imaging of thyroid cancer and for adrenal scintigraphy.
SPECT/CT refers to imaging performed on a hybrid gamma camera with an in-line CT. This produces co-registered scintigraphic and CT images that can be viewed with a localizing software tool or in “fusion” mode.
In this systematic review, the authors have presented a thorough and critical analysis of the utility of SPECT/CT for investigation of endocrine disorders. The improved accuracy of endocrine scintigraphic imaging translates to meaningful changes in patient management and improved clinical care.
P- Reviewer: Bal C, Rubello D S- Editor: Kong JX L- Editor: A E- Editor: Li D
| 1. | Chung JK. Sodium iodide symporter: its role in nuclear medicine. J Nucl Med. 2002;43:1188-1200. [PubMed] |
| 2. | Dai G, Levy O, Carrasco N. Cloning and characterization of the thyroid iodide transporter. Nature. 1996;379:458-460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 786] [Cited by in RCA: 702] [Article Influence: 24.2] [Reference Citation Analysis (0)] |
| 3. | Daniels GH. Radioactive iodine: a slice of history. Thyroid. 2013;23:253-258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 4. | Means JH. Historical background of the use of radioactive iodine in medicine. N Engl J Med. 1955;252:936-940. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 8] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 5. | Buck AK, Nekolla S, Ziegler S, Beer A, Krause BJ, Herrmann K, Scheidhauer K, Wester HJ, Rummeny EJ, Schwaiger M. SPECT/CT. J Nucl Med. 2008;49:1305-1319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 220] [Cited by in RCA: 191] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 6. | Mariani G, Bruselli L, Kuwert T, Kim EE, Flotats A, Israel O, Dondi M, Watanabe N. A review on the clinical uses of SPECT/CT. Eur J Nucl Med Mol Imaging. 2010;37:1959-1985. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 260] [Cited by in RCA: 225] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 7. | Wong KK, Fig LM, Youssef E, Ferretti A, Rubello D, Gross MD. Endocrine scintigraphy with hybrid SPECT/CT. Endocr Rev. 2014;35:717-746. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 8. | Patel CN, Chowdhury FU, Scarsbrook AF. Clinical utility of hybrid SPECT-CT in endocrine neoplasia. AJR Am J Roentgenol. 2008;190:815-824. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 9. | Lang TF, Hasegawa BH, Liew SC, Brown JK, Blankespoor SC, Reilly SM, Gingold EL, Cann CE. Description of a prototype emission-transmission computed tomography imaging system. J Nucl Med. 1992;33:1881-1887. [PubMed] |
| 10. | Histed SN, Lindenberg ML, Mena E, Turkbey B, Choyke PL, Kurdziel KA. Review of functional/anatomical imaging in oncology. Nucl Med Commun. 2012;33:349-361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 118] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 11. | Yap KS, Patel CN, Chowdhury FU, Scarsbrook AF. Less commonly used and emerging clinical applications of SPECT-CT in benign and malignant disease. Nucl Med Commun. 2012;33:808-818. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 12. | Hindié E, Ugur O, Fuster D, O’Doherty M, Grassetto G, Ureña P, Kettle A, Gulec SA, Pons F, Rubello D. 2009 EANM parathyroid guidelines. Eur J Nucl Med Mol Imaging. 2009;36:1201-1216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 197] [Cited by in RCA: 176] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 13. | Chien D, Jacene H. Imaging of parathyroid glands. Otolaryngol Clin North Am. 2010;43:399-415, x. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 18] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 14. | Taubman ML, Goldfarb M, Lew JI. Role of SPECT and SPECT/CT in the Surgical Treatment of Primary Hyperparathyroidism. Int J Mol Imaging. 2011;2011:141593. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 22] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 15. | Greenspan BS, Dillehay G, Intenzo C, Lavely WC, O’Doherty M, Palestro CJ, Scheve W, Stabin MG, Sylvestros D, Tulchinsky M. SNM practice guideline for parathyroid scintigraphy 4.0. J Nucl Med Technol. 2012;40:111-118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 91] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 16. | Kunstman JW, Kirsch JD, Mahajan A, Udelsman R. Clinical review: Parathyroid localization and implications for clinical management. J Clin Endocrinol Metab. 2013;98:902-912. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 127] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 17. | Cheung K, Wang TS, Farrokhyar F, Roman SA, Sosa JA. A meta-analysis of preoperative localization techniques for patients with primary hyperparathyroidism. Ann Surg Oncol. 2012;19:577-583. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 248] [Cited by in RCA: 253] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 18. | Ruda JM, Hollenbeak CS, Stack BC. A systematic review of the diagnosis and treatment of primary hyperparathyroidism from 1995 to 2003. Otolaryngol Head Neck Surg. 2005;132:359-372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 511] [Cited by in RCA: 488] [Article Influence: 24.4] [Reference Citation Analysis (0)] |
| 19. | Ciappuccini R, Morera J, Pascal P, Rame JP, Heutte N, Aide N, Babin E, Reznik Y, Bardet S. Dual-phase 99mTc sestamibi scintigraphy with neck and thorax SPECT/CT in primary hyperparathyroidism: a single-institution experience. Clin Nucl Med. 2012;37:223-228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 58] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 20. | Gayed IW, Kim EE, Broussard WF, Evans D, Lee J, Broemeling LD, Ochoa BB, Moxley DM, Erwin WD, Podoloff DA. The value of 99mTc-sestamibi SPECT/CT over conventional SPECT in the evaluation of parathyroid adenomas or hyperplasia. J Nucl Med. 2005;46:248-252. [PubMed] |
| 21. | Harris L, Yoo J, Driedger A, Fung K, Franklin J, Gray D, Holliday R. Accuracy of technetium-99m SPECT-CT hybrid images in predicting the precise intraoperative anatomical location of parathyroid adenomas. Head Neck. 2008;30:509-517. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 32] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 22. | Hassler S, Ben-Sellem D, Hubele F, Constantinesco A, Goetz C. Dual-isotope 99mTc-MIBI/123I parathyroid scintigraphy in primary hyperparathyroidism: comparison of subtraction SPECT/CT and pinhole planar scan. Clin Nucl Med. 2014;39:32-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 33] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 23. | Im HJ, Lee IK, Paeng JC, Lee KE, Cheon GJ, Kang KW, Chung JK, Lee DS. Functional evaluation of parathyroid adenoma using 99mTc-MIBI parathyroid SPECT/CT: correlation with functional markers and disease severity. Nucl Med Commun. 2014;35:649-654. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 22] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 24. | Kim YI, Jung YH, Hwang KT, Lee HY. Efficacy of 99mTc-sestamibi SPECT/CT for minimally invasive parathyroidectomy: comparative study with 99mTc-sestamibi scintigraphy, SPECT, US and CT. Ann Nucl Med. 2012;26:804-810. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 45] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 25. | Krausz Y, Bettman L, Guralnik L, Yosilevsky G, Keidar Z, Bar-Shalom R, Even-Sapir E, Chisin R, Israel O. Technetium-99m-MIBI SPECT/CT in primary hyperparathyroidism. World J Surg. 2006;30:76-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 88] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 26. | Lavely WC, Goetze S, Friedman KP, Leal JP, Zhang Z, Garret-Mayer E, Dackiw AP, Tufano RP, Zeiger MA, Ziessman HA. Comparison of SPECT/CT, SPECT, and planar imaging with single- and dual-phase (99m)Tc-sestamibi parathyroid scintigraphy. J Nucl Med. 2007;48:1084-1089. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 227] [Cited by in RCA: 191] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 27. | Neumann DR, Obuchowski NA, Difilippo FP. Preoperative 123I/99mTc-sestamibi subtraction SPECT and SPECT/CT in primary hyperparathyroidism. J Nucl Med. 2008;49:2012-2017. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 82] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 28. | Oksüz MO, Dittmann H, Wicke C, Müssig K, Bares R, Pfannenberg C, Eschmann SM. Accuracy of parathyroid imaging: a comparison of planar scintigraphy, SPECT, SPECT-CT, and C-11 methionine PET for the detection of parathyroid adenomas and glandular hyperplasia. Diagn Interv Radiol. 2011;17:297-307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 20] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 29. | Okuda I, Nakajima Y, Miura D, Maruno H, Kohno T, Hirata K. Diagnostic localization of ectopic parathyroid lesions: developmental consideration. Jpn J Radiol. 2010;28:707-713. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 30. | Pata G, Casella C, Besuzio S, Mittempergher F, Salerni B. Clinical appraisal of 99m technetium-sestamibi SPECT/CT compared to conventional SPECT in patients with primary hyperparathyroidism and concomitant nodular goiter. Thyroid. 2010;20:1121-1127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 45] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 31. | Pata G, Casella C, Magri GC, Lucchini S, Panarotto MB, Crea N, Giubbini R, Salerni B. Financial and clinical implications of low-energy CT combined with 99m Technetium-sestamibi SPECT for primary hyperparathyroidism. Ann Surg Oncol. 2011;18:2555-2563. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 32. | Patel CN, Salahudeen HM, Lansdown M, Scarsbrook AF. Clinical utility of ultrasound and 99mTc sestamibi SPECT/CT for preoperative localization of parathyroid adenoma in patients with primary hyperparathyroidism. Clin Radiol. 2010;65:278-287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 126] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 33. | Profanter C, Wetscher GJ, Gabriel M, Sauper T, Rieger M, Kovacs P, Bale R, Prommegger R. CT-MIBI image fusion: a new preoperative localization technique for primary, recurrent, and persistent hyperparathyroidism. Surgery. 2004;135:157-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 51] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 34. | Prommegger R, Wimmer G, Profanter C, Sauper T, Sieb M, Kovacs P, Bale R, Putzer D, Gabriel M, Margreiter R. Virtual neck exploration: a new method for localizing abnormal parathyroid glands. Ann Surg. 2009;250:761-765. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 33] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 35. | Ruf J, Lopez Hänninen E, Steinmüller T, Rohlfing T, Bertram H, Gutberlet M, Lemke AJ, Felix R, Amthauer H. Preoperative localization of parathyroid glands. Use of MRI, scintigraphy, and image fusion. Nuklearmedizin. 2004;43:85-90. [PubMed] |
| 36. | Schalin-Jäntti C, Ryhänen E, Heiskanen I, Seppänen M, Arola J, Schildt J, Väisänen M, Nelimarkka L, Lisinen I, Aalto V. Planar scintigraphy with 123I/99mTc-sestamibi, 99mTc-sestamibi SPECT/CT, 11C-methionine PET/CT, or selective venous sampling before reoperation of primary hyperparathyroidism? J Nucl Med. 2013;54:739-747. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 43] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 37. | Serra A, Bolasco P, Satta L, Nicolosi A, Uccheddu A, Piga M. Role of SPECT/CT in the preoperative assessment of hyperparathyroid patients. Radiol Med. 2006;111:999-1008. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 41] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 38. | Shafiei B, Hoseinzadeh S, Fotouhi F, Malek H, Azizi F, Jahed A, Hadaegh F, Salehian M, Parsa H, Javadi H. Preoperative 99mTc-sestamibi scintigraphy in patients with primary hyperparathyroidism and concomitant nodular goiter: comparison of SPECT-CT, SPECT, and planar imaging. Nucl Med Commun. 2012;33:1070-1076. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 52] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 39. | Sharma J, Mazzaglia P, Milas M, Berber E, Schuster DM, Halkar R, Siperstein A, Weber CJ. Radionuclide imaging for hyperparathyroidism (HPT): which is the best technetium-99m sestamibi modality? Surgery. 2006;140:856-863; discussion 863-865. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 47] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 40. | Tardin L, Prats E, Andrés A, Razola P, Deus J, Gastaminza R, Santapau A, Parra A, Banzo J. [Ectopic parathyroid adenoma: Scintigraphic detection and radioguided surgery]. Rev Esp Med Nucl. 2011;30:19-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 41. | Wimmer G, Bale R, Kovacs P, Gabriel M, Putzer D, Sauper T, Sieb M, Profanter C, Margreiter R, Prommegger R. Virtual neck exploration in patients with hyperparathyroidism and former cervical operations. Langenbecks Arch Surg. 2008;393:687-692. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 42. | Wimmer G, Profanter C, Kovacs P, Sieb M, Gabriel M, Putzer D, Bale R, Margreiter R, Prommegger R. CT-MIBI-SPECT image fusion predicts multiglandular disease in hyperparathyroidism. Langenbecks Arch Surg. 2010;395:73-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 33] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 43. | Zhen L, Li H, Liu X, Ge BH, Yan J, Yang J. The application of SPECT/CT for preoperative planning in patients with secondary hyperparathyroidism. Nucl Med Commun. 2013;34:439-444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 25] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 44. | Wong KK, Fig LM, Gross MD, Dwamena BA. Parathyroid adenoma localization with 99mTc-sestamibi SPECT/CT: a meta-analysis. Nucl Med Commun. 2015;36:363-375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 91] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 45. | Tokmak H, Demirkol MO, Alagöl F, Tezelman S, Terzioglu T. Clinical impact of SPECT-CT in the diagnosis and surgical management of hyper-parathyroidism. Int J Clin Exp Med. 2014;7:1028-1034. [PubMed] |
| 46. | Eslamy HK, Ziessman HA. Parathyroid scintigraphy in patients with primary hyperparathyroidism: 99mTc sestamibi SPECT and SPECT/CT. Radiographics. 2008;28:1461-1476. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 94] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 47. | Suh YJ, Choi JY, Kim SJ, Chun IK, Yun TJ, Lee KE, Kim JH, Cheon GJ, Youn YK. Comparison of 4D CT, ultrasonography, and 99mTc sestamibi SPECT/CT in localizing single-gland primary hyperparathyroidism. Otolaryngol Head Neck Surg. 2015;152:438-443. [PubMed] |
| 48. | Berner AM, Haroon A, Nowosinska E, Offiah C, Luqman M, Newell M, Jan H. Localization of parathyroid disease with ‘sequential multiphase and dual-tracer’ technique and comparison with neck ultrasound. Nucl Med Commun. 2015;36:45-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 49. | Noda S, Onoda N, Kashiwagi S, Kawajiri H, Takashima T, Ishikawa T, Yoshida A, Higashiyama S, Kawabe J, Imanishi Y. Strategy of operative treatment of hyperparathyroidism using US scan and (99m)Tc-MIBI SPECT/CT. Endocr J. 2014;61:225-230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 21] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 50. | Qiu ZL, Wu B, Shen CT, Zhu RS, Luo QY. Dual-phase (99m)Tc-MIBI scintigraphy with delayed neck and thorax SPECT/CT and bone scintigraphy in patients with primary hyperparathyroidism: correlation with clinical or pathological variables. Ann Nucl Med. 2014;28:725-735. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 51. | García-Talavera P, González-Selma ML, Ruiz M, Gamazo C, Sainz-Esteban A, Villanueva JG, Olmos R. The value of early SPECT/CT and hand-held γ-camera in radio-guided surgery: a case of a hard-to-locate parathyroid adenoma. Clin Nucl Med. 2014;39:1009-1011. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 8] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 52. | Aras M, Erdil TY, Ones T, Dede F, Turoglu HT. (99m)Tc-MIBI emboli in the lungs detected on SPECT/CT: a pitfall in parathyroid scan. Clin Nucl Med. 2014;39:196-197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 53. | Calò PG, Pisano G, Tatti A, Loi G, Furcas S, Nicolosi A. Cervical lymph node sarcoidosis mimicking a parathyroid adenoma: a clinical case. Clin Med Insights Case Rep. 2013;6:159-163. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 54. | Treglia G, Dambra DP, Bruno I, Mulè A, Giordano A. Costal brown tumor detected by dual-phase parathyroid imaging and SPECT-CT in primary hyperparathyroidism. Clin Nucl Med. 2008;33:193-195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 55. | Wong KK, Brown RK, Avram AM. Potential false positive Tc-99m sestamibi parathyroid study due to uptake in brown adipose tissue. Clin Nucl Med. 2008;33:346-348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 56. | Sarkar SD. Benign thyroid disease: what is the role of nuclear medicine? Semin Nucl Med. 2006;36:185-193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 34] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 57. | Intenzo CM, dePapp AE, Jabbour S, Miller JL, Kim SM, Capuzzi DM. Scintigraphic manifestations of thyrotoxicosis. Radiographics. 2003;23:857-869. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 31] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 58. | Ross DS. Syndromes of thyrotoxicosis with low radioactive iodine uptake. Endocrinol Metab Clin North Am. 1998;27:169-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 47] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 59. | Harisankar CN, Preethi GR, Chungath BB. Hybrid SPECT/CT evaluation of Marine-Lenhart syndrome. Clin Nucl Med. 2013;38:e89-e90. [PubMed] |
| 60. | Guerra G, Cinelli M, Mesolella M, Tafuri D, Rocca A, Amato B, Rengo S, Testa D. Morphological, diagnostic and surgical features of ectopic thyroid gland: a review of literature. Int J Surg. 2014;12 Suppl 1:S3-S11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 94] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 61. | Meng Z, Lou S, Tan J, Jia Q, Zheng R, Liu G, Zhu M, He Q, Li D. Scintigraphic detection of dual ectopic thyroid tissue: experience of a Chinese tertiary hospital. PLoS One. 2014;9:e95686. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 62. | Kang HC. Lingual thyroid: marked response to suppression therapy. Thyroid. 2004;14:401-402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 8] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 63. | Mussak EN, Kacker A. Surgical and medical management of midline ectopic thyroid. Otolaryngol Head Neck Surg. 2007;136:870-872. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 26] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 64. | Dolezal J, Vizda J, Horacek J, Spitalnikova S. Lingual thyroid: diagnosis using a hybrid of single photon emission computed tomography and standard computed tomography. J Laryngol Otol. 2013;127:432-434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 65. | Harisankar CN. Dual ectopic thyroid in the presence of atrophic orthotopic thyroid gland in a patient with acquired hypothyroidism: Evaluation with hybrid Single-Photon Emission Computed Tomography/Computed Tomography. Indian J Nucl Med. 2013;28:26-27. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 66. | Harisankar CN, Preethi GR, George M. Hybrid SPECT/CT evaluation of dual ectopia of thyroid in the absence of orthotopic thyroid gland. Clin Nucl Med. 2012;37:602-603. [PubMed] |
| 67. | Patel Z, Johnson L. Iodine 131 ablation of an obstructive lingual thyroid. J Radiol Case Rep. 2009;3:3-6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 68. | Vercellino L, Alaoui NI, Faugeron I, Bérenger N, de Labriolle-Vaylet C, Hindié E, Toubert ME. Lingual thyroid imaging with ¹²³I SPECT/CT. Eur J Nucl Med Mol Imaging. 2011;38:1173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 69. | Danner C, Bodenner D, Breau R. Lingual thyroid: iodine 131: a viable treatment modality revisited. Am J Otolaryngol. 2001;22:276-281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 26] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 70. | Schilling JA, Karr JW, Hursh JB. The treatment of a lingual thyroid with radioactive iodine. Surgery. 1950;27:130-18, illust. [PubMed] |
| 71. | Koo PJ, Klingensmith WC, Bagrosky BM, Haugen BR. SPECT/CT of metastatic struma ovarii. Clin Nucl Med. 2014;39:186-187. [PubMed] |
| 72. | Hay ID, McConahey WM, Goellner JR. Managing patients with papillary thyroid carcinoma: insights gained from the Mayo Clinic’s experience of treating 2,512 consecutive patients during 1940 through 2000. Trans Am Clin Climatol Assoc. 2002;113:241-260. [PubMed] |
| 73. | Jonklaas J, Sarlis NJ, Litofsky D, Ain KB, Bigos ST, Brierley JD, Cooper DS, Haugen BR, Ladenson PW, Magner J. Outcomes of patients with differentiated thyroid carcinoma following initial therapy. Thyroid. 2006;16:1229-1242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 460] [Cited by in RCA: 459] [Article Influence: 24.2] [Reference Citation Analysis (0)] |
| 74. | Mazzaferri EL, Jhiang SM. Long-term impact of initial surgical and medical therapy on papillary and follicular thyroid cancer. Am J Med. 1994;97:418-428. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1879] [Cited by in RCA: 1726] [Article Influence: 55.7] [Reference Citation Analysis (0)] |
| 75. | Cooper DS, Doherty GM, Haugen BR, Kloos RT, Lee SL, Mandel SJ, Mazzaferri EL, McIver B, Pacini F, Schlumberger M. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid. 2009;19:1167-1214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5299] [Cited by in RCA: 4742] [Article Influence: 296.4] [Reference Citation Analysis (0)] |
| 76. | British Thyroid Association RCOP. Guidelines for the mana-gement of thyroid cancer (2nd ed). [accessed 2009 Mar 24]. Available from: http: //www.british-thyroidassociation org/Guidelines/2007. |
| 77. | Davies L, Welch HG. Increasing incidence of thyroid cancer in the United States, 1973-2002. JAMA. 2006;295:2164-2167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2425] [Cited by in RCA: 2405] [Article Influence: 126.6] [Reference Citation Analysis (0)] |
| 78. | Chen AY, Jemal A, Ward EM. Increasing incidence of differentiated thyroid cancer in the United States, 1988-2005. Cancer. 2009;115:3801-3807. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 676] [Cited by in RCA: 719] [Article Influence: 44.9] [Reference Citation Analysis (0)] |
| 79. | Bourgeois P. A proposition for the use of radioiodine in WDTC management. J Nucl Med. 2009;50:328-329; author reply 329-330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 80. | Hay ID. Management of patients with low-risk papillary thyroid carcinoma. Endocr Pract. 2007;13:521-533. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 112] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 81. | Mazzaferri EL. Management of low-risk differentiated thyroid cancer. Endocr Pract. 2007;13:498-512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 178] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 82. | Mallick U, Harmer C, Yap B, Wadsley J, Clarke S, Moss L, Nicol A, Clark PM, Farnell K, McCready R. Ablation with low-dose radioiodine and thyrotropin alfa in thyroid cancer. N Engl J Med. 2012;366:1674-1685. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 414] [Cited by in RCA: 394] [Article Influence: 30.3] [Reference Citation Analysis (0)] |
| 83. | Schlumberger M, Catargi B, Borget I, Deandreis D, Zerdoud S, Bridji B, Bardet S, Leenhardt L, Bastie D, Schvartz C. Strategies of radioiodine ablation in patients with low-risk thyroid cancer. N Engl J Med. 2012;366:1663-1673. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 462] [Cited by in RCA: 450] [Article Influence: 34.6] [Reference Citation Analysis (0)] |
| 84. | Delbeke D, Schöder H, Martin WH, Wahl RL. Hybrid imaging (SPECT/CT and PET/CT): improving therapeutic decisions. Semin Nucl Med. 2009;39:308-340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 66] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 85. | Spanu A, Solinas ME, Chessa F, Sanna D, Nuvoli S, Madeddu G. 131I SPECT/CT in the follow-up of differentiated thyroid carcinoma: incremental value versus planar imaging. J Nucl Med. 2009;50:184-190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 104] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 86. | Tharp K, Israel O, Hausmann J, Bettman L, Martin WH, Daitzchman M, Sandler MP, Delbeke D. Impact of 131I-SPECT/CT images obtained with an integrated system in the follow-up of patients with thyroid carcinoma. Eur J Nucl Med Mol Imaging. 2004;31:1435-1442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 106] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 87. | Barwick TD, Dhawan RT, Lewington V. Role of SPECT/CT in differentiated thyroid cancer. Nucl Med Commun. 2012;33:787-798. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 17] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 88. | Wong KK, Wale DJ, Fig LM, Gross MD. SPET-CT in Thyroid Cancer: A Systematic Review. Clin Transl Imaging. 2014;2:459-475. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 89. | Xue YL, Qiu ZL, Song HJ, Luo QY. Value of ¹³¹I SPECT/CT for the evaluation of differentiated thyroid cancer: a systematic review of the literature. Eur J Nucl Med Mol Imaging. 2013;40:768-778. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 38] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 90. | Wong KK, Sisson JC, Koral KF, Frey KA, Avram AM. Staging of differentiated thyroid carcinoma using diagnostic 131-I SPECT-CT. AJR Am J Roentgenol. 2010;195:730-736. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 43] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 91. | Wong KK, Zarzhevsky N, Cahill JM, Frey KA, Avram AM. Incremental value of diagnostic 131I SPECT/CT fusion imaging in the evaluation of differentiated thyroid carcinoma. AJR Am J Roentgenol. 2008;191:1785-1794. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 50] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 92. | Wong KK, Zarzhevsky N, Cahill JM, Frey KA, Avram AM. Hybrid SPECT-CT and PET-CT imaging of differentiated thyroid carcinoma. Br J Radiol. 2009;82:860-876. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 28] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 93. | Avram AM, Fig LM, Frey KA, Gross MD, Wong KK. Preablation 131-I scans with SPECT/CT in postoperative thyroid cancer patients: what is the impact on staging? J Clin Endocrinol Metab. 2013;98:1163-1171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 74] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 94. | Aide N, Heutte N, Rame JP, Rousseau E, Loiseau C, Henry-Amar M, Bardet S. Clinical relevance of single-photon emission computed tomography/computed tomography of the neck and thorax in postablation (131)I scintigraphy for thyroid cancer. J Clin Endocrinol Metab. 2009;94:2075-2084. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 67] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 95. | Ciappuccini R, Heutte N, Trzepla G, Rame JP, Vaur D, Aide N, Bardet S. Postablation (131)I scintigraphy with neck and thorax SPECT-CT and stimulated serum thyroglobulin level predict the outcome of patients with differentiated thyroid cancer. Eur J Endocrinol. 2011;164:961-969. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 45] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 96. | Kohlfuerst S, Igerc I, Lobnig M, Gallowitsch HJ, Gomez-Segovia I, Matschnig S, Mayr J, Mikosch P, Beheshti M, Lind P. Posttherapeutic (131)I SPECT-CT offers high diagnostic accuracy when the findings on conventional planar imaging are inconclusive and allows a tailored patient treatment regimen. Eur J Nucl Med Mol Imaging. 2009;36:886-893. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 59] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 97. | Maruoka Y, Abe K, Baba S, Isoda T, Sawamoto H, Tanabe Y, Sasaki M, Honda H. Incremental diagnostic value of SPECT/CT with 131I scintigraphy after radioiodine therapy in patients with well-differentiated thyroid carcinoma. Radiology. 2012;265:902-909. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 45] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 98. | Menges M, Uder M, Kuwert T, Schmidt D. 131I SPECT/CT in the follow-up of patients with differentiated thyroid carcinoma. Clin Nucl Med. 2012;37:555-560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 16] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 99. | Mustafa M, Kuwert T, Weber K, Knesewitsch P, Negele T, Haug A, Linke R, Bartenstein P, Schmidt D. Regional lymph node involvement in T1 papillary thyroid carcinoma: a bicentric prospective SPECT/CT study. Eur J Nucl Med Mol Imaging. 2010;37:1462-1466. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 39] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 100. | Ruf J, Lehmkuhl L, Bertram H, Sandrock D, Amthauer H, Humplik B, Ludwig Munz D, Felix R. Impact of SPECT and integrated low-dose CT after radioiodine therapy on the management of patients with thyroid carcinoma. Nucl Med Commun. 2004;25:1177-1182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 78] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 101. | Schmidt D, Linke R, Uder M, Kuwert T. Five months’ follow-up of patients with and without iodine-positive lymph node metastases of thyroid carcinoma as disclosed by (131)I-SPECT/CT at the first radioablation. Eur J Nucl Med Mol Imaging. 2010;37:699-705. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 36] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 102. | Schmidt D, Szikszai A, Linke R, Bautz W, Kuwert T. Impact of 131I SPECT/spiral CT on nodal staging of differentiated thyroid carcinoma at the first radioablation. J Nucl Med. 2009;50:18-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 99] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 103. | Song HJ, Xue YL, Qiu ZL, Luo QY. Uncommon metastases from differentiated thyroid carcinoma. Hell J Nucl Med. 2012;15:233-240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 104. | Song HJ, Xue YL, Xu YH, Qiu ZL, Luo QY. Rare metastases of differentiated thyroid carcinoma: pictorial review. Endocr Relat Cancer. 2011;18:R165-R174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 91] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 105. | Wakabayashi H, Nakajima K, Fukuoka M, Inaki A, Nakamura A, Kayano D, Kinuya S. Double-phase (131)I whole body scan and (131)I SPECT-CT images in patients with differentiated thyroid cancer: their effectiveness for accurate identification. Ann Nucl Med. 2011;25:609-615. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 17] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 106. | Wang H, Fu HL, Li JN, Zou RJ, Gu ZH, Wu JC. The role of single-photon emission computed tomography/computed tomography for precise localization of metastases in patients with differentiated thyroid cancer. Clin Imaging. 2009;33:49-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 34] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 107. | Yamamoto Y, Nishiyama Y, Monden T, Matsumura Y, Satoh K, Ohkawa M. Clinical usefulness of fusion of 131I SPECT and CT images in patients with differentiated thyroid carcinoma. J Nucl Med. 2003;44:1905-1910. [PubMed] |
| 108. | Chen L, Luo Q, Shen Y, Yu Y, Yuan Z, Lu H, Zhu R. Incremental value of 131I SPECT/CT in the management of patients with differentiated thyroid carcinoma. J Nucl Med. 2008;49:1952-1957. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 96] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 109. | Grewal RK, Tuttle RM, Fox J, Borkar S, Chou JF, Gonen M, Strauss HW, Larson SM, Schöder H. The effect of posttherapy 131I SPECT/CT on risk classification and management of patients with differentiated thyroid cancer. J Nucl Med. 2010;51:1361-1367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 64] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 110. | Barwick T, Murray I, Megadmi H, Drake WM, Plowman PN, Akker SA, Chew SL, Grossman AB, Avril N. Single photon emission computed tomography (SPECT)/computed tomography using Iodine-123 in patients with differentiated thyroid cancer: additional value over whole body planar imaging and SPECT. Eur J Endocrinol. 2010;162:1131-1139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 46] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 111. | Blum M, Tiu S, Chu M, Goel S, Friedman K. I-131 SPECT/CT elucidates cryptic findings on planar whole-body scans and can reduce needless therapy with I-131 in post-thyroidectomy thyroid cancer patients. Thyroid. 2011;21:1235-1247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 35] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 112. | Oh JR, Byun BH, Hong SP, Chong A, Kim J, Yoo SW, Kang SR, Kim DY, Song HC, Bom HS. Comparison of ¹³¹I whole-body imaging, ¹³¹I SPECT/CT, and ¹8F-FDG PET/CT in the detection of metastatic thyroid cancer. Eur J Nucl Med Mol Imaging. 2011;38:1459-1468. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 46] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 113. | Qiu ZL, Xue YL, Song HJ, Luo QY. Comparison of the diagnostic and prognostic values of 99mTc-MDP-planar bone scintigraphy, 131I-SPECT/CT and 18F-FDG-PET/CT for the detection of bone metastases from differentiated thyroid cancer. Nucl Med Commun. 2012;33:1232-1242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 17] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 114. | Weber K, Berger F, Mustafa M, Reiser MF, Bartenstein P, Haug A. [SPECT/CT for staging and treatment monitoring in oncology. Applications in differentiated thyroid cancer and liver tumors]. Radiologe. 2012;52:646-652. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 115. | Qiu ZL, Xu YH, Song HJ, Luo QY. Localization and identification of parapharyngeal metastases from differentiated thyroid carcinoma by 131I-SPECT/CT. Head Neck. 2011;33:171-177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 116. | Agriantonis DJ, Hall L, Wilson MA. Utility of SPECT/CT as an adjunct to planar whole body I-131 imaging: liver metastasis from papillary thyroid cancer. Clin Nucl Med. 2009;34:247-248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 21] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 117. | Aide N, Lehembre E, Gervais R, Bardet S. Unusual intratracheal metastasis of differentiated thyroid cancer accurately depicted by SPECT/CT acquisition after radioiodine ablation. Thyroid. 2007;17:1305-1306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 118. | Dümcke CW, Madsen JL. Usefulness of SPECT/CT in the diagnosis of intrathoracic goiter versus metastases from cancer of the breast. Clin Nucl Med. 2007;32:156-159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 8] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 119. | Macdonald W, Armstrong J. Benign struma ovarii in a patient with invasive papillary thyroid cancer: detection with I-131 SPECT-CT. Clin Nucl Med. 2007;32:380-382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 29] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 120. | Qiu ZL, Luo QY. Erector spinae metastases from differentiated thyroid cancer identified by I-131 SPECT/CT. Clin Nucl Med. 2009;34:137-140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 31] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 121. | Rachinsky I, Driedger A. Iodine-131 uptake in a menstruating uterus: value of SPECT/CT in distinguishing benign and metastatic iodine-positive lesions. Thyroid. 2007;17:901-902. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 17] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 122. | Thust S, Fernando R, Barwick T, Mohan H, Clarke SE. SPECT/CT identification of post-radioactive iodine treatment false-positive uptake in a simple renal cyst. Thyroid. 2009;19:75-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 22] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 123. | von Falck C, Beer G, Gratz KF, Galanski M. Renal metastases from follicular thyroid cancer on SPECT/CT. Clin Nucl Med. 2007;32:751-752. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 25] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 124. | Wong KK, Avram AM. Posttherapy I-131 thymic uptake demonstrated with SPECT/CT in a young girl with papillary thyroid carcinoma. Thyroid. 2008;18:919-920. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 125. | Zhao LX, Li L, Li FL, Zhao Z. Rectus abdominis muscle metastasis from papillary thyroid cancer identified by I-131 SPECT/CT. Clin Nucl Med. 2010;35:360-361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 23] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 126. | Carlisle MR, Lu C, McDougall IR. The interpretation of 131I scans in the evaluation of thyroid cancer, with an emphasis on false positive findings. Nucl Med Commun. 2003;24:715-735. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 68] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 127. | Mitchell G, Pratt BE, Vini L, McCready VR, Harmer CL. False positive 131I whole body scans in thyroid cancer. Br J Radiol. 2000;73:627-635. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 53] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 128. | Shapiro B, Rufini V, Jarwan A, Geatti O, Kearfott KJ, Fig LM, Kirkwood ID, Gross MD. Artifacts, anatomical and physiological variants, and unrelated diseases that might cause false-positive whole-body 131-I scans in patients with thyroid cancer. Semin Nucl Med. 2000;30:115-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 102] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 129. | Bizhanova A, Kopp P. Minireview: The sodium-iodide symporter NIS and pendrin in iodide homeostasis of the thyroid. Endocrinology. 2009;150:1084-1090. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 135] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 130. | Carvalho DP, Ferreira AC. The importance of sodium/iodide symporter (NIS) for thyroid cancer management. Arq Bras Endocrinol Metabol. 2007;51:672-682. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 40] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 131. | Filetti S, Bidart JM, Arturi F, Caillou B, Russo D, Schlumberger M. Sodium/iodide symporter: a key transport system in thyroid cancer cell metabolism. Eur J Endocrinol. 1999;141:443-457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 177] [Cited by in RCA: 164] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 132. | Shen DH, Kloos RT, Mazzaferri EL, Jhian SM. Sodium iodide symporter in health and disease. Thyroid. 2001;11:415-425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 48] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 133. | Dohán O, De la Vieja A, Paroder V, Riedel C, Artani M, Reed M, Ginter CS, Carrasco N. The sodium/iodide Symporter (NIS): characterization, regulation, and medical significance. Endocr Rev. 2003;24:48-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 580] [Cited by in RCA: 548] [Article Influence: 24.9] [Reference Citation Analysis (0)] |
| 134. | Glazer DI, Brown RK, Wong KK, Savas H, Gross MD, Avram AM. SPECT/CT evaluation of unusual physiologic radioiodine biodistributions: pearls and pitfalls in image interpretation. Radiographics. 2013;33:397-418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 32] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 135. | Oh JR, Ahn BC. False-positive uptake on radioiodine whole-body scintigraphy: physiologic and pathologic variants unrelated to thyroid cancer. Am J Nucl Med Mol Imaging. 2012;2:362-385. [PubMed] |
| 136. | Jammah AA, Driedger A, Rachinsky I. Incidental finding of ovarian teratoma on post-therapy scan for papillary thyroid cancer and impact of SPECT/CT imaging. Arq Bras Endocrinol Metabol. 2011;55:490-493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 14] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 137. | Yuoness S, Rachinsky I, Driedger AA, Belhocine TZ. Differentiated thyroid cancer with epiphora: detection of nasolacrimal duct obstruction on I-131 SPECT/CT. Clin Nucl Med. 2011;36:1149-1152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 138. | Mebarki M, Menemani A, Medjahedi A, Boualou F, Slama A, Ouguirti S, Kherbouche FZ, Berber N. Radioiodine accumulation in a giant ovarian cystadenofibroma detected incidentally by 131-I whole body scans. Case Rep Radiol. 2012;2012:295617. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 139. | Sakahara H, Yamashita S, Suzuki K, Imai M, Kosugi T. Visualization of nasolacrimal drainage system after radioiodine therapy in patients with thyroid cancer. Ann Nucl Med. 2007;21:525-527. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 140. | Savas H, Wong KK, Saglik B, Hubers D, Ackermann RJ, Avram AM. SPECT/CT characterization of oral activity on radioiodine scintigraphy. J Clin Endocrinol Metab. 2013;98:4410-4416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 141. | Burlison JS, Hartshorne MF, Voda AM, Cocks FH, Fair JR. SPECT/CT localization of oral radioiodine activity: a retrospective study and in-vitro assessment. Nucl Med Commun. 2013;34:1216-1222. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 142. | Ciappuccini R, Hardouin J, Heutte N, Vaur D, Quak E, Rame JP, Blanchard D, de Raucourt D, Bardet S. Stimulated thyroglobulin level at ablation in differentiated thyroid cancer: the impact of treatment preparation modalities and tumor burden. Eur J Endocrinol. 2014;171:247-252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 30] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 143. | Jeong SY, Lee SW, Kim HW, Song BI, Ahn BC, Lee J. Clinical applications of SPECT/CT after first I-131 ablation in patients with differentiated thyroid cancer. Clin Endocrinol (Oxf). 2014;81:445-451. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 20] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 144. | Kim HY, Gelfand MJ, Sharp SE. SPECT/CT imaging in children with papillary thyroid carcinoma. Pediatr Radiol. 2011;41:1008-1012. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 18] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 145. | Tuttle RM, Leboeuf R, Robbins RJ, Qualey R, Pentlow K, Larson SM, Chan CY. Empiric radioactive iodine dosing regimens frequently exceed maximum tolerated activity levels in elderly patients with thyroid cancer. J Nucl Med. 2006;47:1587-1591. [PubMed] |
| 146. | Kulkarni K, Van Nostrand D, Atkins F, Aiken M, Burman K, Wartofsky L. The relative frequency in which empiric dosages of radioiodine would potentially overtreat or undertreat patients who have metastatic well-differentiated thyroid cancer. Thyroid. 2006;16:1019-1023. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 63] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 147. | Sisson JC, Dewaraja YK, Wizauer EJ, Giordano TJ, Avram AM. Thyroid carcinoma metastasis to skull with infringement of brain: treatment with radioiodine. Thyroid. 2009;19:297-303. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 20] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 148. | Song H, He B, Prideaux A, Du Y, Frey E, Kasecamp W, Ladenson PW, Wahl RL, Sgouros G. Lung dosimetry for radioiodine treatment planning in the case of diffuse lung metastases. J Nucl Med. 2006;47:1985-1994. [PubMed] |
| 149. | Klimstra DS, Modlin IR, Coppola D, Lloyd RV, Suster S. The pathologic classification of neuroendocrine tumors: a review of nomenclature, grading, and staging systems. Pancreas. 2010;39:707-712. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 776] [Cited by in RCA: 751] [Article Influence: 50.1] [Reference Citation Analysis (2)] |
| 150. | Vinik AI, Woltering EA, Warner RR, Caplin M, O’Dorisio TM, Wiseman GA, Coppola D, Go VL. NANETS consensus guidelines for the diagnosis of neuroendocrine tumor. Pancreas. 2010;39:713-734. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 188] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 151. | Mottaghy FM, Reske SN. Functional imaging of neuroendocrine tumours with PET. Pituitary. 2006;9:237-242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 18] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 152. | Bombardieri E, Seregni E, Villano C, Chiti A, Bajetta E. Position of nuclear medicine techniques in the diagnostic work-up of neuroendocrine tumors. Q J Nucl Med Mol Imaging. 2004;48:150-163. [PubMed] |
| 153. | Amthauer H, Denecke T, Rohlfing T, Ruf J, Böhmig M, Gutberlet M, Plöckinger U, Felix R, Lemke AJ. Value of image fusion using single photon emission computed tomography with integrated low dose computed tomography in comparison with a retrospective voxel-based method in neuroendocrine tumours. Eur Radiol. 2005;15:1456-1462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 38] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 154. | Apostolova I, Riethdorf S, Buchert R, Derlin T, Brenner W, Mester J, Klutmann S. SPECT/CT stabilizes the interpretation of somatostatin receptor scintigraphy findings: a retrospective analysis of inter-rater agreement. Ann Nucl Med. 2010;24:477-483. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 155. | Castaldi P, Rufini V, Treglia G, Bruno I, Perotti G, Stifano G, Barbaro B, Giordano A. Impact of 111In-DTPA-octreotide SPECT/CT fusion images in the management of neuroendocrine tumours. Radiol Med. 2008;113:1056-1067. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 42] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 156. | Gabriel M, Hausler F, Bale R, Moncayo R, Decristoforo C, Kovacs P, Virgolini I. Image fusion analysis of (99m)Tc-HYNIC-Tyr(3)-octreotide SPECT and diagnostic CT using an immobilisation device with external markers in patients with endocrine tumours. Eur J Nucl Med Mol Imaging. 2005;32:1440-1451. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 38] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 157. | Hillel PG, van Beek EJ, Taylor C, Lorenz E, Bax ND, Prakash V, Tindale WB. The clinical impact of a combined gamma camera/CT imaging system on somatostatin receptor imaging of neuroendocrine tumours. Clin Radiol. 2006;61:579-587. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 43] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 158. | Ingui CJ, Shah NP, Oates ME. Endocrine neoplasm scintigraphy: added value of fusing SPECT/CT images compared with traditional side-by-side analysis. Clin Nucl Med. 2006;31:665-672. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 35] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 159. | Krausz Y, Keidar Z, Kogan I, Even-Sapir E, Bar-Shalom R, Engel A, Rubinstein R, Sachs J, Bocher M, Agranovicz S. SPECT/CT hybrid imaging with 111In-pentetreotide in assessment of neuroendocrine tumours. Clin Endocrinol (Oxford). 2003;59:565-573. [RCA] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 126] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 160. | Perri M, Erba P, Volterrani D, Lazzeri E, Boni G, Grosso M, Mariani G. Octreo-SPECT/CT imaging for accurate detection and localization of suspected neuroendocrine tumors. Q J Nucl Med Mol Imaging. 2008;52:323-333. [PubMed] |
| 161. | Pfannenberg AC, Eschmann SM, Horger M, Lamberts R, Vonthein R, Claussen CD, Bares R. Benefit of anatomical-functional image fusion in the diagnostic work-up of neuroendocrine neoplasms. Eur J Nucl Med Mol Imaging. 2003;30:835-843. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 74] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 162. | Roach PJ, Schembri GP, Ho Shon IA, Bailey EA, Bailey DL. SPECT/CT imaging using a spiral CT scanner for anatomical localization: Impact on diagnostic accuracy and reporter confidence in clinical practice. Nucl Med Commun. 2006;27:977-987. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 65] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 163. | Ruf J, Steffen I, Mehl S, Rosner C, Denecke T, Pape UF, Plotkin M, Amthauer H. Influence of attenuation correction by integrated low-dose CT on somatostatin receptor SPECT. Nucl Med Commun. 2007;28:782-788. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 20] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 164. | Sepúlveda-Méndez J, de Murphy CA, Pedraza-López M, Murphy-Stack E, Rojas-Bautista JC, González-Treviño O. Specificity and sensitivity of 99mTc-EDDA/HYNIC-Tyr³-octreotide (99mTc-TOC) for imaging neuroendocrine tumors. Nucl Med Commun. 2012;33:69-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 165. | Wong KK, Cahill JM, Frey KA, Avram AM. Incremental value of 111-in pentetreotide SPECT/CT fusion imaging of neuroendocrine tumors. Acad Radiol. 2010;17:291-297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 35] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 166. | Sainz-Esteban A, Olmos R, González-Sagrado M, González ML, Ruiz MÁ, García-Talavera P, Gamazo C, Villanueva JG, Cobo A, de Luis D. Contribution of ¹¹¹In-pentetreotide SPECT/CT imaging to conventional somatostatin receptor scintigraphy in the detection of neuroendocrine tumours. Nucl Med Commun. 2015;36:251-259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 167. | Wong KK, Wynn EA, Myles J, Ackermann RJ, Frey KA, Avram AM. Comparison of single time-point [111-In] pentetreotide SPECT/CT with dual time-point imaging of neuroendocrine tumors. Clin Nucl Med. 2011;36:25-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 18] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 168. | Koçyiğit Deveci E, Ocak M, Bozkurt MF, Türker S, Kabasakal L, Uğur O. The Diagnostic Efficiency of 99mTc-EDDA/HYNIC-Octreotate SPECT-CT in Comparison with 111In-Pentetrotide in the Detection of Neuroendocrine Tumours. Mol Imaging Radionucl Ther. 2013;22:76-84. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 169. | Schreiter NF, Bartels AM, Froeling V, Steffen I, Pape UF, Beck A, Hamm B, Brenner W, Röttgen R. Searching for primaries in patients with neuroendocrine tumors (NET) of unknown primary and clinically suspected NET: Evaluation of Ga-68 DOTATOC PET/CT and In-111 DTPA octreotide SPECT/CT. Radiol Oncol. 2014;48:339-347. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 29] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 170. | Etchebehere EC, de Oliveira Santos A, Gumz B, Vicente A, Hoff PG, Corradi G, Ichiki WA, de Almeida Filho JG, Cantoni S, Camargo EE. 68Ga-DOTATATE PET/CT, 99mTc-HYNIC-octreotide SPECT/CT, and whole-body MR imaging in detection of neuroendocrine tumors: a prospective trial. J Nucl Med. 2014;55:1598-1604. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 81] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 171. | Kubota K, Okasaki M, Minamimoto R, Miyata Y, Morooka M, Nakajima K, Sato T. Lesion-based analysis of (18)F-FDG uptake and (111)In-Pentetreotide uptake by neuroendocrine tumors. Ann Nucl Med. 2014;28:1004-1010. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 34] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 172. | Grimes J, Celler A, Birkenfeld B, Shcherbinin S, Listewnik MH, Piwowarska-Bilska H, Mikolajczak R, Zorga P. Patient-specific radiation dosimetry of 99mTc-HYNIC-Tyr3-octreotide in neuroendocrine tumors. J Nucl Med. 2011;52:1474-1481. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 36] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 173. | Sandström M, Garske-Román U, Granberg D, Johansson S, Widström C, Eriksson B, Sundin A, Lundqvist H, Lubberink M. Individualized dosimetry of kidney and bone marrow in patients undergoing 177Lu-DOTA-octreotate treatment. J Nucl Med. 2013;54:33-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 194] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 174. | Jackson PA, Beauregard JM, Hofman MS, Kron T, Hogg A, Hicks RJ. An automated voxelized dosimetry tool for radionuclide therapy based on serial quantitative SPECT/CT imaging. Med Phys. 2013;40:112503. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 63] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 175. | Kashyap R, Jackson P, Hofman MS, Eu P, Beauregard JM, Zannino D, Hicks RJ. Rapid blood clearance and lack of long-term renal toxicity of 177Lu-DOTATATE enables shortening of renoprotective amino acid infusion. Eur J Nucl Med Mol Imaging. 2013;40:1853-1860. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 32] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 176. | Sarkar SD, Beierwaltes H, Ice RD, Basmadjian GP, Hetzel KR, Kennedy WP, Mason MM. A new and superior adrenal scanning agent, NP-59. J Nucl Med. 1975;16:1038-1042. [PubMed] |
| 177. | Kloos RT, Gross MD, Francis IR, Korobkin M, Shapiro B. Incidentally discovered adrenal masses. Endocr Rev. 1995;16:460-484. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 21] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 178. | White ML, Gauger PG, Doherty GM, Cho KJ, Thompson NW, Hammer GD, Miller BS. The role of radiologic studies in the evaluation and management of primary hyperaldosteronism. Surgery. 2008;144:926-333; discussion 933. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 46] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 179. | Volpe C, Enberg U, Sjögren A, Wahrenberg H, Jacobsson H, Törring O, Hamberger B, Thorén M. The role of adrenal scintigraphy in the preoperative management of primary aldosteronism. Scand J Surg. 2008;97:248-253. [PubMed] |
| 180. | Kazerooni EA, Sisson JC, Shapiro B, Gross MD, Driedger A, Hurwitz GA, Mattar AG, Petry NA. Diagnostic accuracy and pitfalls of [iodine-131]6-beta-iodomethyl-19-norcholesterol (NP-59) imaging. J Nucl Med. 1990;31:526-534. [PubMed] |
| 181. | Wong KK, Komissarova M, Avram AM, Fig LM, Gross MD. Adrenal cortical imaging with I-131 NP-59 SPECT-CT. Clin Nucl Med. 2010;35:865-869. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 25] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 182. | Lu CC, Wu VC, Wu KD, Liu KL, Lin WC, Cheng MF, Tzen KY, Yen RF. Prognostic value of semiquantification NP-59 SPECT/CT in primary aldosteronism patients after adrenalectomy. Eur J Nucl Med Mol Imaging. 2014;41:1375-1384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 20] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 183. | Chen YC, Chiu JS, Wang YF. NP-59 SPECT/CT imaging in stage 1 hypertensive and atypical primary aldosteronism: a 5-year retrospective analysis of clinicolaboratory and imaging features. ScientificWorldJournal. 2013;2013:317934. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 184. | Hahner S, Stuermer A, Kreissl M, Reiners C, Fassnacht M, Haenscheid H, Beuschlein F, Zink M, Lang K, Allolio B. [123 I]Iodometomidate for molecular imaging of adrenocortical cytochrome P450 family 11B enzymes. J Clin Endocrinol Metab. 2008;93:2358-2365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 59] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 185. | Hahner S, Sundin A. Metomidate-based imaging of adrenal masses. Horm Cancer. 2011;2:348-353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 26] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 186. | Hahner S, Kreissl MC, Fassnacht M, Haenscheid H, Knoedler P, Lang K, Buck AK, Reiners C, Allolio B, Schirbel A. [131I]iodometomidate for targeted radionuclide therapy of advanced adrenocortical carcinoma. J Clin Endocrinol Metab. 2012;97:914-922. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 58] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 187. | Ilias I, Pacak K. A clinical overview of pheochromocytomas/paragangliomas and carcinoid tumors. Nucl Med Biol. 2008;35 Suppl 1:S27-S34. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 43] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 188. | Pacak K, Eisenhofer G, Goldstein DS. Functional imaging of endocrine tumors: role of positron emission tomography. Endocr Rev. 2004;25:568-580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 105] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 189. | Jacobson AF, Deng H, Lombard J, Lessig HJ, Black RR. 123I-meta-iodobenzylguanidine scintigraphy for the detection of neuroblastoma and pheochromocytoma: results of a meta-analysis. J Clin Endocrinol Metab. 2010;95:2596-2606. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 105] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 190. | Harisankar CN, Mittal BR, Bhattacharya A, Kashyap R, Bhansali A. Iodine-131 meta-iodobezylguanidine single photon emission computed tomography/computerized tomography in diagnosis of neuro-endocrine tumors. Indian J Nucl Med. 2012;27:55-58. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 191. | Fukuoka M, Taki J, Mochizuki T, Kinuya S. Comparison of diagnostic value of I-123 MIBG and high-dose I-131 MIBG scintigraphy including incremental value of SPECT/CT over planar image in patients with malignant pheochromocytoma/paraganglioma and neuroblastoma. Clin Nucl Med. 2011;36:1-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 57] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 192. | Rozovsky K, Koplewitz BZ, Krausz Y, Revel-Vilk S, Weintraub M, Chisin R, Klein M. Added value of SPECT/CT for correlation of MIBG scintigraphy and diagnostic CT in neuroblastoma and pheochromocytoma. AJR Am J Roentgenol. 2008;190:1085-1090. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 89] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 193. | Meyer-Rochow GY, Schembri GP, Benn DE, Sywak MS, Delbridge LW, Robinson BG, Roach PJ, Sidhu SB. The utility of metaiodobenzylguanidine single photon emission computed tomography/computed tomography (MIBG SPECT/CT) for the diagnosis of pheochromocytoma. Ann Surg Oncol. 2010;17:392-400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 35] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 194. | Sharma P, Dhull VS, Jeph S, Reddy RM, Singh H, Naswa N, Bal C, Kumar R. Can hybrid SPECT-CT overcome the limitations associated with poor imaging properties of 131I-MIBG?: Comparison with planar scintigraphy and SPECT in pheochromocytoma. Clin Nucl Med. 2013;38:e346-e353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 15] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 195. | Derlin T, Busch JD, Wisotzki C, Schoennagel BP, Bannas P, Papp L, Klutmann S, Habermann CR. Intraindividual comparison of 123I-mIBG SPECT/MRI, 123I-mIBG SPECT/CT, and MRI for the detection of adrenal pheochromocytoma in patients with elevated urine or plasma catecholamines. Clin Nucl Med. 2013;38:e1-e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 17] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 196. | Kroiss A, Putzer D, Uprimny C, Decristoforo C, Gabriel M, Santner W, Kranewitter C, Warwitz B, Waitz D, Kendler D. Functional imaging in phaeochromocytoma and neuroblastoma with 68Ga-DOTA-Tyr 3-octreotide positron emission tomography and 123I-metaiodobenzylguanidine. Eur J Nucl Med Mol Imaging. 2011;38:865-873. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 92] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 197. | Rufini V, Treglia G, Castaldi P, Perotti G, Calcagni ML, Corsello SM, Galli G, Fanti S, Giordano A. Comparison of 123I-MIBG SPECT-CT and 18F-DOPA PET-CT in the evaluation of patients with known or suspected recurrent paraganglioma. Nucl Med Commun. 2011;32:575-582. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 43] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 198. | Kroiss A, Shulkin BL, Uprimny C, Frech A, Gasser RW, Url C, Gautsch K, Madleitner R, Nilica B, Sprinzl GM. (68)Ga-DOTATOC PET/CT provides accurate tumour extent in patients with extraadrenal paraganglioma compared to (123)I-MIBG SPECT/CT. Eur J Nucl Med Mol Imaging. 2015;42:33-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 40] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 199. | Andersen JB, Mortensen J, Bech BH, Højgaard L, Borgwardt L. First experiences from Copenhagen with paediatric single photon emission computed tomography/computed tomography. Nucl Med Commun. 2011;32:356-362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 200. | Biermann M, Schwarzlmüller T, Fasmer KE, Reitan BC, Johnsen B, Rosendahl K. Is there a role for PET-CT and SPECT-CT in pediatric oncology? Acta Radiol. 2013;54:1037-1045. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 201. | Tang HR, Da Silva AJ, Matthay KK, Price DC, Huberty JP, Hawkins RA, Hasegawa BH. Neuroblastoma imaging using a combined CT scanner-scintillation camera and 131I-MIBG. J Nucl Med. 2001;42:237-247. [PubMed] |
| 202. | Rufini V, Castaldi P, Treglia G, Perotti G, Gross MD, Al-Nahhas A, Rubello D. Nuclear medicine procedures in the diagnosis and therapy of medullary thyroid carcinoma. Biomed Pharmacother. 2008;62:139-146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 32] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 203. | Bozkurt MF, Uğur O, Banti E, Grassetto G, Rubello D. Functional nuclear medicine imaging of medullary thyroid cancer. Nucl Med Commun. 2008;29:934-942. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 12] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 204. | Czepczyński R, Parisella MG, Kosowicz J, Mikołajczak R, Ziemnicka K, Gryczyńska M, Sowiński J, Signore A. Somatostatin receptor scintigraphy using 99mTc-EDDA/HYNIC-TOC in patients with medullary thyroid carcinoma. Eur J Nucl Med Mol Imaging. 2007;34:1635-1645. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 25] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 205. | Chowdhury FU, Scarsbrook AF. The role of hybrid SPECT-CT in oncology: current and emerging clinical applications. Clin Radiol. 2008;63:241-251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 56] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 206. | Fiore D, Rubello D, Casara D, Pelizzo MR, Franchi A, Muzzio PC. [Role of CT/111In-octreotide SPECT digital fusion imaging in the localization of loco-regional recurrence of medullary thyroid carcinoma]. Minerva Chir. 2004;59:295-299. [PubMed] |
| 207. | De Bonilla-Damiá A, Calvo-Morón C, De La Riva-Pérez PA, Iglesias-Jerez R, Molina-Mora M, Castro-Montaño J. [Detection by SPECT-CT scan with (99m)Tc-(V) DMSA of bone metastases in patient with medullary thyroid cancer]. Rev Esp Med Nucl. 2011;30:365-367. [PubMed] |
| 208. | Giovanella L, Suriano S, Lunghi L, Bongiovanni M, Ceriani L. Radioguided surgery of thyroid carcinoma recurrences: the role of preoperative (99m)Tc-labeled human serum albumin macroaggregates-SPECT/CT mapping. Clin Nucl Med. 2013;38:e207-e209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 209. | Ahn BC. Radioiodine SPET/CT guided needle aspiration as a useful technique for recurrence surveillance in a thyroidectomized differentiated thyroid cancer patient with negative US and serum Tg and positive Tg of the lymph node aspirate. Hell J Nucl Med. 2013;16:142-143. [PubMed] |
| 210. | Tangjaturonrasme N, Vasavid P, Sombuntham P, Keelawat S. Pre-operative prediction of cervical nodal metastasis in papillary thyroid cancer by 99mTc-MIBI SPECT/CT; a pilot study. J Med Assoc Thai. 2013;96:696-702. [PubMed] |
| 211. | Zhao R, Wang J, Deng J, Yang W, Wang J. Efficacy of (99m)Tc-EDDA/HYNIC-TOC SPECT/CT scintigraphy in Graves’ ophthalmopathy. Am J Nucl Med Mol Imaging. 2012;2:242-247. [PubMed] |
| 212. | Galt JR, Halkar RK, Evans CO, Osman NA, LaBorde D, Fox TH, Faraj BA, Kumar K, Wang H, Oyesiku NM. In vivo assay of folate receptors in nonfunctional pituitary adenomas with 99mTc-folate SPECT/CT. J Nucl Med. 2010;51:1716-1723. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |