Published online Jun 28, 2016. doi: 10.4329/wjr.v8.i6.610
Peer-review started: November 19, 2015
First decision: February 2, 2016
Revised: February 27, 2016
Accepted: April 21, 2016
Article in press: April 22, 2016
Published online: June 28, 2016
Processing time: 213 Days and 19.3 Hours
AIM: To determine whether contrast-enhanced ultrasound (CEUS) can improve the precision of breast imaging reporting and data system (BI-RADS) categorization.
METHODS: A total of 230 patients with 235 solid breast lesions classified as BI-RADS 4 on conventional ultrasound were evaluated. CEUS was performed within one week before core needle biopsy or surgical resection and a revised BI-RADS classification was assigned based on 10 CEUS imaging characteristics. Receiver operating characteristic curve analysis was then conducted to evaluate the diagnostic performance of CEUS-based BI-RADS assignment with pathological examination as reference criteria.
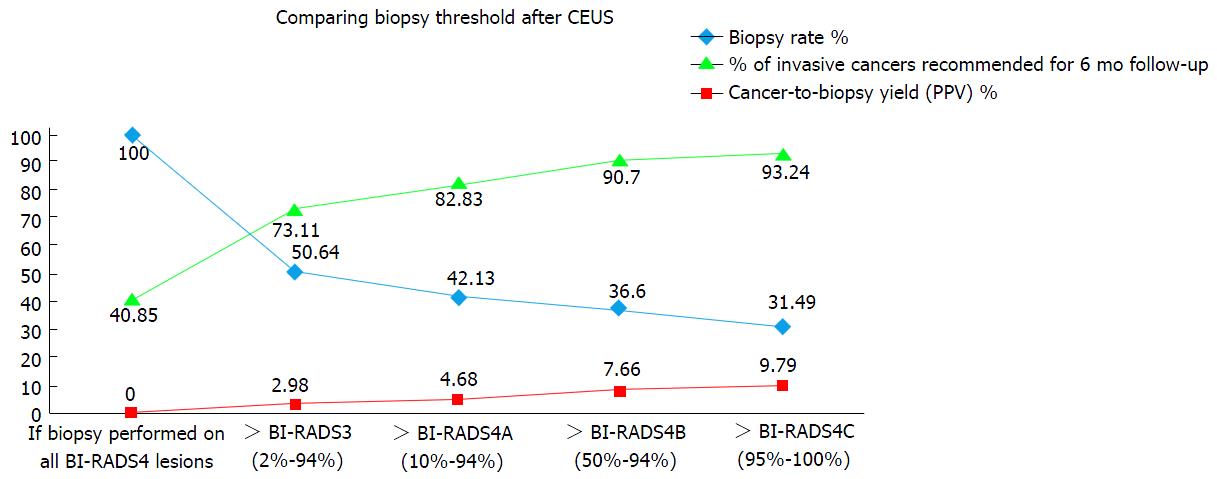
RESULTS: The CEUS-based BI-RADS evaluation classified 116/235 (49.36%) lesions into category 3, 20 (8.51%), 13 (5.53%) and 12 (5.11%) lesions into categories 4A, 4B and 4C, respectively, and 74 (31.49%) into category 5. Selecting CEUS-based BI-RADS category 4A as an appropriate cut-off gave sensitivity and specificity values of 85.4% and 87.8%, respectively, for the diagnosis of malignant disease. The cancer-to-biopsy yield was 73.11% with CEUS-based BI-RADS 4A selected as the biopsy threshold compared with 40.85% otherwise, while the biopsy rate was only 42.13% compared with 100% otherwise. Overall, only 4.68% of invasive cancers were misdiagnosed.
CONCLUSION: This pilot study suggests that evaluation of BI-RADS 4 breast lesions with CEUS results in reduced biopsy rates and increased cancer-to-biopsy yields.
Core tip: Many published studies show that overdiagnosis is now a problem faced if the breast imaging reporting and data system (BI-RADS) category is used in clinical practice. Many patients underwent unnecessary biopsies even if the final pathological results were benign lesions. It seems that BI-RADS is not good enough and one of the reasons may be that there is no microvascular information. Contrast-enhanced ultrasound (CEUS) can give us this information. We tried to determine whether CEUS can improve the precision of the BI-RADS categorization. Our results showed that in all BI-RADS 4 lesions which were suggested as needing a biopsy, CEUS-based BI-RADS can decrease false positive biopsies and increase cancer-to-biopsy yield and that only 4.68% invasive cancers were misdiagnosed.
- Citation: Luo J, Chen JD, Chen Q, Yue LX, Zhou G, Lan C, Li Y, Wu CH, Lu JQ. Contrast-enhanced ultrasound improved performance of breast imaging reporting and data system evaluation of critical breast lesions. World J Radiol 2016; 8(6): 610-617
- URL: https://www.wjgnet.com/1949-8470/full/v8/i6/610.htm
- DOI: https://dx.doi.org/10.4329/wjr.v8.i6.610
The breast imaging reporting and data system (BI-RADS)[1] is the most commonly used classification system for breast lesions. It is used primarily to assess the risk of breast lesion malignancy and can facilitate treatment selection. However, because most Chinese women have relatively small and dense breasts which can complicate interpretation of traditional mammography images[2], sonography is usually considered the primary clinical work-up tool in China. Unfortunately, the BI-RADS system for ultrasound (US) which was updated in 2013 still only addresses two-dimensional (2D) gray-scale and color Doppler US[1]. Although the BI-RADS-US makes breast US diagnosis more standardized and objective, poor interobserver agreement and high false positive biopsy rates are still frequent problems in clinical diagnosis[3-5]. This is particularly the case for the BI-RADS 4 category in which the risk of malignancy ranges from 2%-95%. In the United States, most (69%-95%) patients with BI-RADS 4 lesions undergo biopsy[6,7], even although the cancer-to-biopsy yield rates are only 22%-33%[8-10]. This compares with 50%-64% in the United Kingdom[11,12].
The aim of our study was to prospectively evaluate contrast-enhanced ultrasound (CEUS) for the determination of the malignant risk of BI-RADS 4 lesions in order to increase diagnostic accuracy and reduce the number of unnecessary biopsies. A secondary aim was to explore the role of CEUS as a possible adjunct to the BI-RADS-US classification scheme.
From January 2013 to July 2014, all patients referred to our institution with solid breast lesions classified as BI-RADS 4 on conventional US were considered for inclusion in the study. Patients were ineligible for inclusion if they were pregnant or breastfeeding, had lesions that were reclassified as BI-RADS 3 after reassessment, or had undergone any previous treatment or interventional diagnosis for confirmed malignant breast lesions. The study was approved by the institutional ethics committee of the Sichuan Provincial People’s Hospital and written informed consent was obtained from all patients.
US examinations were performed within one week before surgery or core needle biopsy. All examinations were performed by the same sonographer who had 10 years of experience in breast US and 2 years of experience in CEUS. Conventional US imaging was performed with Mylab90 and Twice (Esaote, Genoa, Italy) with an 8-13 MHz linear transducer (LA532). Color and power Doppler US were performed to evaluate intralesional vascularity and to compare images obtained in different planes; the plane with the most extensive vascularity or most irregular shape was selected for CEUS. Conversely, planes with macrocalcifications and shadowing were avoided. The selected plane had to include the lesion and its surrounding normal tissue whenever possible. When the lesion was too big to be scanned in one plane, a part of the lesion with adjacent normal tissue was chosen.
CEUS was performed with a 4.5-7.5 MHz linear transducer (LA522) using the same equipment as described above. The machine parameters were adjusted to give a mechanical index of < 0.1 and a gain of 100-120 dB. No parameters were changed during the examination.
CEUS was performed with 4.8 mL of SonoVue (Bracco, Milan, Italy) administered as a bolus via a peripheral vein, followed by a 5-10 mL saline flush. Continuous imaging was performed for 2 min beginning immediately after the contrast agent injection. US images and video clips were stored electronically for subsequent analysis. The dual image mode was applied to locate breast lesions, particularly small lesions, accurately during the procedure. The selected plane remained unchanged during the examination. The probe was placed gently on the skin to avoid exerting pressure on the lesion, particularly when the lesion was superficial. The patients were told to remain still and to attempt to maintain eupnea during the examination to minimize motion artifacts.
All images were read by two sonographers who each had at least 10 years of experience with breast US and 2 years of experience with breast CEUS. Both sonographers were blinded to patients’ individual clinical data and the final pathological diagnoses. Image assessment was performed by each sonographer separately. A consensus decision was reached through discussion if differences in opinion occurred during independent image assessment. Each reader initially evaluated all conventional US images and classified all lesions detected using established BI-RADS-US criteria. Thereafter, each reader evaluated all CEUS data and assigned new BI-RADS categories to all lesions based on information from relevant published literature and the sonographer’s specific personal clinical experience with CEUS. A BI-RADS 3 diagnosis was given to lesions that demonstrated one of the following 3 enhancement patterns: (1) rapid wash-in with homogeneous hyperenhancement, equal size after enhancement compared with the size demonstrated on routine 2D gray-scale images, with clear margins and regular shape, and without evidence of penetrating vessels or perfusion defect; (2) synchronous or slow wash-in with isonenhancement, indistinguishable shape and margins after enhancement, and without evidence of penetrating vessels or perfusion defect; and (3) synchronous or slow wash-in with hypoenhancement, equal or smaller size after enhancement compared with the size demonstrated on 2D gray-scale images and without perfusion defect. A BI-RADS 5 diagnosis was given to lesions that demonstrated one of the following 3 enhancement patterns: (1) hyperenhancement with larger size compared with the size demonstrated on 2D gray-scale images, irregular shape; (2) hyperenhancement with centripetal perfusion, clear evidence of perfusion defect, with or without an enlarged size; and (3) rapid or synchronous wash-in with hyper or isoenhancement, presence of penetrating vessels or a crab claw-like pattern, with or without evidence of perfusion defect. All remaining lesions were classified as BI-RADS 4.
All patients underwent surgery or core biopsy 1-2 d after the CEUS examination. The pathology findings were used as the final diagnostic standard.
Continuous data were described as mean ± SD. Dichotomous data were summarized by calculating proportions in each category. The performance of the BI-RADS classification system in distinguishing benign from malignant lesions was determined using the receiver operating curve (ROC) method. Data analysis was performed with routine statistical software (SPSS for Windows, version 13.0; SPSS, Chicago, Ill).
A total of 230 patients (mean age 44 years, range: 11-84 years) with 235 solid breast lesions met the inclusion criteria and were enrolled in the study. The mean diameter of the lesions was 18.1 mm ± 9.3 mm (range: 10.3 mm to 50.9 mm). After histological assessment of pathology specimens, 96 (41%) lesions were confirmed as benign and 139 (59%) as malignant (Table 1).
| Histopathological diagnosis | BI-RADS after CEUS | Total | ||||
| 3 | 4A | 4B | 4C | 5 | ||
| Benign lesions | 139 | |||||
| Fibroadenoma | 47 | 3 | 1 | 2 | 53 | |
| Fibrocystic mastopathy | 34 | 1 | 35 | |||
| Complex sclerosing adenosis | 2 | 2 | 1 | 5 | ||
| Hyperplasia | 2 | 1 | 1 | 4 | ||
| Chronic mastitis | 12 | 5 | 3 | 1 | 21 | |
| Granulomatous mastitis | 2 | 1 | 1 | 4 | ||
| Intraductal papilloma | 4 | 2 | 3 | 9 | ||
| Benign phyllodes tumor | 2 | 1 | 3 | |||
| Hamartoma | 1 | 1 | 2 | |||
| Radial scar | 2 | 2 | ||||
| Bolus material after operation | 1 | 1 | ||||
| Malignant lesions | 96 | |||||
| IDC | 7 | 1 | 3 | 5 | 62 | 78 |
| DCIS | 2 | 1 | 3 | 3 | 9 | |
| Mucinous carcinoma | 2 | 1 | 3 | |||
| Infiltrating lobular carcinoma | 2 | 2 | ||||
| Diffused large B-cell lymphoma | 1 | 1 | 2 | |||
| Malignant phyllodes tumor | 1 | 1 | ||||
| Solid neuroendocrine carcinoma | 1 | 1 | ||||
| Total | 235 | |||||
All 235 (100%) breast nodules were diagnosed as BI-RADS 4 on conventional US before CEUS. After CEUS, 116 (49.4%) lesions were diagnosed as BI-RADS 3, 45 (19.2%) were diagnosed as BI-RADS 4 and 74 (31.5%) were diagnosed as BI-RADS 5. The diagnostic sensitivity, specificity, accuracy, positive predictive value, negative predictive value, positive likelihood ratio, negative likelihood ratio, diagnostic odds ratio and Youden index were 90.7%, 79.0%, 83.8%, 75.2%, 92.4%, 4.33, 0.11, 36.67 and 0.697, respectively. In the BI-RADS 4 category, 20 (8.5%) lesions were diagnosed as BI-RADS 4A, 13 (5.5%) as BI-RADS 4B and 12 (5.1%) as BI -RADS 4C (Table 1).
The maximum area under the curve from the ROC analysis was 0.914, which occurred for a benign/malignant threshold set at BI-RADS 4A. If BI-RADS 3 and 4A were judged as benign after CEUS, the diagnostic sensitivity and specificity were 85.4% and 87.8%, respectively. A total of 14 false negative and 17 false positive lesions were recorded when the benign/malignant threshold was set at BI-RADS 4A (Table 1). The 14 false negative lesions comprised 8 invasive ductal carcinoma (IDC) (7 classified as BI-RADS 3 and 1 as BI-RADS 4A), 3 ductal carcinoma in suit (DCIS) (2 classified as BI-RADS 3 and 1 as BI-RADS 4A), 2 mucinous carcinomas and 1 diffuse large B-cell lymphoma. The 7 IDC classified as BI-RADS 3 included 4 triple negative IDC (1 accompanied with DCIS), 1 Luminal A type, 1 Luminal B type and 1 HER2 (Table 2).
| No. | Size (mm) | LN | Histology | Mammography |
| 1 | 18 × 15 | Negative | IDC (triple negative) | Hyperplasia fibrocystic |
| 2 | 14.6 × 10.7 | Negative | IDC (triple negative) | Mastopathy |
| 3 | 12 × 8 | Negative | IDC (HER2) | Solid lesion |
| 4 | 19 × 15 | Negative | Mixed mucinous carcinoma(Luminal A) | Hyperplasia |
| 5 | 25 × 17 | Negative | IDC (triple negative) | Phyllodes tumor |
| 6 | 30 × 15 | Positive | IDC (triple negative) | Solid lesion |
| 7 | 30 × 25 | Negative | IDC (Luminal B) | Without |
| 8 | 18.8 × 15.7 | Negative | IDC (triple negative) | Solid lesion |
| 9 | 38 × 37 | Negative | Diffused B-cell lymphoma | Without |
| 10 | 11 × 10 | Negative | Mucinous carcinoma | Without |
| 11 | 17 × 14 | Negative | Mucinous carcinoma | Adenoma |
The 17 false positive lesions comprised 3 fibroadenomas, 3 complex sclerosing adenosis lesions, 1 hyperplasia, 5 mastitis lesions (4 chronic, 1 granulomatous), 3 intraductal papillomas, 1 benign phyllodes tumor and 1 hamartoma (Tables 1 and 3).
| No. | Enhancement patterns | ||||||
| Time | Intensity | Scope after enhancement | Direction | Craw-like pattern | Nourishing vessel | Shape after enhancement | |
| 1 | Rapid | Hyper | Equal | Complex | Absent | Absent | Regular |
| 2 | Synchronous | Iso | Equal | Complex | Absent | Absent | Irregular |
| 3 | Rapid | Hyper | Smaller | Centripetal | Absent | Absent | Regular |
| 4 | Rapid | Hyper | Equal | Complex | Absent | Absent | Regular |
| 5 | Rapid | Hyper | Equal | Centripetal | Absent | Absent | Regular |
| 6 | Rapid | Hyper | Larger | Complex | Absent | Present | Regular |
| 7 | Rapid | Hyper | Larger | Complex | Absent | Absent | Irregular |
| 8 | Synchronous | Hyper | Equal | Complex | Absent | Absent | Regular |
| 9 | Slow | Hypo | Equal | Centripetal | Absent | Absent | Regular |
| 10 | Rapid | Hyper | Smaller | Complex | Absent | Present | Irregular |
| 11 | Slow | Hypo | Smaller | Centripetal | Absent | Absent | Regular |
Hypothetical biopsy thresholds based on BI-RADS after CEUS were malignant risk assessment, resulting biopsy rates and cancer-to-biopsy yields. Based on the BI-RADS classifications assigned before CEUS (i.e., based on conventional US), all 235 (100%) lesions were classified as BI-RADS 4 and would have been referred for biopsy, but the cancer-to-biopsy rate was only 40.85% (Figure 1).
If BI-RADS 3 after CEUS was regarded as having a lesion malignancy risk of not more than 2%, permitting follow-up instead of immediate biopsy, the cancer-to-biopsy rate would rise to 73.11%, with a biopsy rate of only 50.64%. In this case, only 3.83% of malignant lesions would be missed. If BI-RADS 4A is set as the cut-off point for biopsy, the cancer-to-biopsy rate rises to 82.83% for a biopsy rate of only 42.13%. In this case, 5.96% of malignant lesions would be missed (Table 4). Finally, if BI-RADS 4B is set as the cut-off point for biopsy, the cancer-to-biopsy rate, biopsy rate and missed malignancy rate would be 90.7%, 36.6% and 7.66%, respectively, while if BI-RADS 4C is set as the cut-off point for biopsy, the corresponding rates would be 93.24%, 31.49% and 11.49%, respectively.
| BI-RADS category | Biopsy rate (%) | Invasive cancers recommended for 6 mo follow-up (%) | Cancer-to-biopsy yield (%) |
| If biopsy performed on all BI-RADS4 lesions | 235 (100) | 0 (0) | 96/235 (40.85) |
| > BI-RADS 3 (2%-94%) | 119 (50.64) | 7 (2.98) | 87/119 (73.11) |
| > BI-RADS 4A (10%-94%) | 99 (42.13) | 11 (4.68) | 82/99 (82.83) |
| > BI-RADS 4B (50%-94%) | 86 (36.60) | 15 (7.66) | 78/86 (90.70) |
| > BI-RADS 4C (95%-100%) | 74 (31.49) | 21 (9.79) | 69/74 (93.24) |
If invasive malignancy is the principal lesion of interest, then classification of lesions as BI-RADS 3, 4A, 4B, 4C diagnosis after CEUS would bring missed malignancy rates of 2.98%, 4.68%, 7.66 and 9.79%, respectively (Table 4).
Currently, the application of BI-RADS-US is based on diagnostic information from 2D gray-scale and color Doppler. According to published research literature and our own clinical experience, there are two main problems: (1) inter-observer agreement is relatively poor; and (2) there is an overlap of US imaging features between benign and malignant lesions. These limitations result in the overdiagnosis of a considerable number of benign lesions as BI-RADS 4, the generation of false positives and an increased number of unnecessary biopsies[3,4,13].
It is well-known that benign and malignant breast tumors differ in terms of microvasculature and microcirculation (references needed). Unfortunately, the currently used BI-RADS classification system does not incorporate this information into the assessment of malignancy risk. CEUS can provide this information, allowing us to better optimize the assigned BI-RADS. In our study, although we initially reassessed all lesions prior to CEUS and excluded those lesions reclassified as BI-RADS 3, there were still 116/235 (49.4%) lesions reclassified in BI-RADS 3 after CEUS.
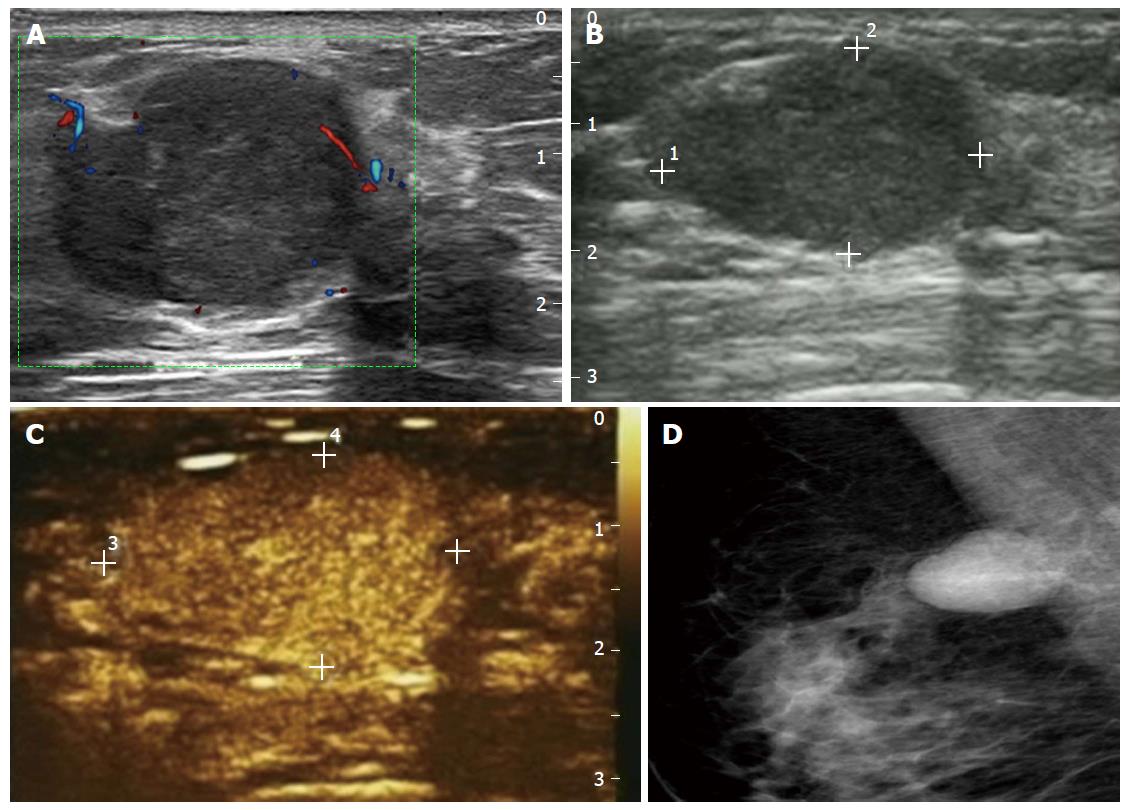
When the cut-off point for biopsy was increased to BI-RADS 4A, 57.9% (136/235) of biopsies would have been avoided for a missed malignancy rate of less than 5% (11/235). This suggests that increasing the cut-off point for recommending biopsy after CEUS and substituting a short-term follow-up protocol for biopsy may safely reduce the number of false positive biopsies. BI-RADS with CEUS can assess the risk of malignant lesions more accurately. The 14 false negative malignant lesions included 11 cases of invasive malignant tumors and 3 DCIS. CEUS showed slow or synchronous wash-in with hypo or isoenhancement, accompanying clear margins and a regular shape after enhancement, without enlarged size, penetrating vessels or a crab claw-like pattern (Figure 2) in 4 triple negative breast cancers. These findings are similar to the results of Uematsu et al[14] who demonstrated a correlation between triple negative breast cancer and MRI, showing rim enhancement with smooth mass margins. This phenomenon might be associated with the histopathology of triple negative cancer, which typically shows characteristics of a benign tumor with pushing margins and a “scar-like fibrous area” or necrosis in the center[15,16]. Another 2 pure mucinous carcinomas and 1 mixed mucinous carcinoma showed a similar appearance to that of a benign lesion (slow wash-in with hypo or rapid wash-in with hyper, heterogeneous enhancement, equal or smaller size after contrast and almost regular shape). This may reflect the fact that mucinous cancer contains an extensive mucus component in which the tumor cell nests float and that there is a lack of microvasculature. Furthermore, 13 of the 14 missed malignant tumors had negative axillary lymph nodes. Notably, 8 of these false negative tumors were also false negative at mammography. This may indicate that these malignant lesions were early grade tumors; in this regard, it is known that the diagnostic performance of CEUS is relatively poor for DCIS, early stage IDC and rare or special types of malignant tumors. On the other hand, there is some controversy about the overdiagnosis and possible overtreatment of DCIS, especially for low-to-intermediate grade DCIS. The question is whether only high-grade DCIS should be a focus for early detection. DCIS now accounts for 20%-30% of all ‘‘malignant’’ diagnoses of breast cancer, which derive almost entirely from screening. Yet, after removal of approximately 60000 DCIS cases annually for over 10 years, there has not been a concomitant drop in invasive cancer, suggesting that many of these lesions would not necessarily progress to invasive cancer if left undetected[17]. In our study, 2 DCIS classified as BI-RADS 3 and 1 DCIS classified as BI-RADS 4A showed slow wash-in with hypoenhancement or synchronous wash-in with isoenhancement, with equal, smaller or indistinguishable size after contrast enhancement. This kind of microcirculation may imply low-to-intermediate grade DCIS and a reduced risk of progression to invasive cancer. However, further studies are needed to confirm this.
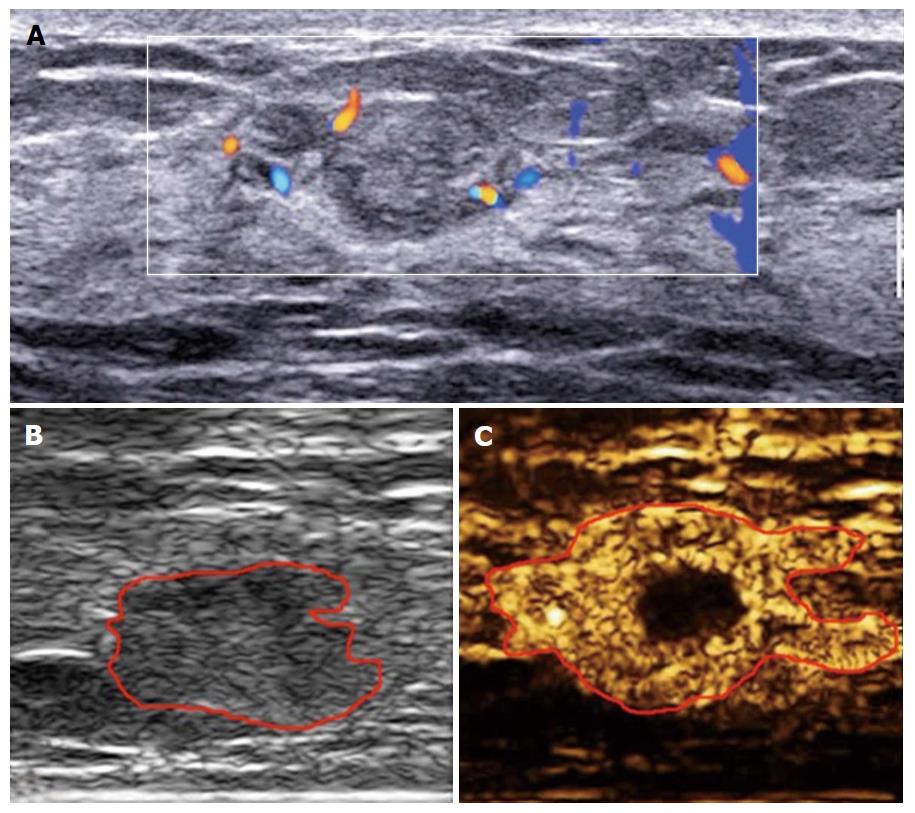
In addition to the false negative malignant lesions, we also recorded 17 benign lesions as BI-RADS 4B, 4C or 5 (i.e., false positive diagnoses). One possible explanation is that some benign lesions, such as intraductal papilloma, hypervascular inflammatory lesions, adenosis and hyperplasia, demonstrate active cell proliferation or infantile features[18,19] which may result in the overlapping enhancement behavior with that of malignant lesions on CEUS. In common with the findings of others[20,21], 9 of 15 benign lesions with enlarged size after enhancement were inflammatory lesions in our study. Inflammatory lesions are always hypervascular with inflammatory cells infiltrating into the surrounding tissue irregularly. This is similar to the histological features of invasive cancers (Figure 3).
The development of breast cancer is a complex and gradual process and different types of benign lesions, including different grades of DCIS, have different degrees of risk of progressing to IDC. The challenge we now face is how to safely reduce the false positive biopsy rate that accompanies high sensitivity. Our study showed that CEUS may have predictive value. An experienced sonographer can assess the malignant risk of lesions more accurately based on different CEUS appearances, resulting in higher positive predictive values while avoiding the possibility of a false positive biopsy in nearly 50% of patients.
At the same time, although the risk of delay in treating malignant lesions is relatively very low at < 5%, the fear of missing cancers is a potent driver of excess biopsies. However, there is increasing support for the view that some screen-detected cancers are slow-growing low-risk tumors with indolent behavior[22-24]. The challenge for doctors is to distinguish between benign and slow-growing lesions and those in which there is an urgent need for resolution, not missing invasive cancer but avoiding a false positive biopsy as much as possible, which is why we need BI-RADS. Malignant lesions classified as BI-RADS 3 and 4A in this study seem to be low grade and demonstrate indolent behavior. If this judgment can be confirmed by a prospective multi-center study in a larger patient population, a new and optimized BI-RADS category may be born.
This study had limitations that should be noted: (1) the number of patients enrolled in this study was small and further multi-center prospective studies with a larger sample size are needed to confirm our findings; (2) although CEUS seems to markedly reduce the false positive biopsy rate, there are still a small number of patients that will face delays in the diagnosis of malignancy if immediate biopsy is replaced by follow-up at 6 mo. Even although these lesions may be low risk, meaning that a 6 mo delay in diagnosis is unlikely to cause real harm, patients may not fully understand the risk and importance of follow-up, leading to anxiety on the part of both the patient and the physician regarding the risk of misdiagnosis; (3) BI-RADS categories with CEUS are still subject to interobserver variation with regards to the selection of the region of interest and classification of the enhancement patterns. This reflects the fact that there is no consensus as yet regarding contrast patterns for the differential diagnosis of benign and malignant breast lesions; and (4) in this study, we usually chose the plane with a rich blood supply or irregular shape for CEUS. A single plane may not represent the entire lesion and may result in the loss of important information.
In conclusion, this pilot study found that CEUS can optimize the BI-RADS classification of breast lesions. Using risk-based biopsy thresholds for BI-RADS 4 lesions by recommending a 6 mo follow-up for the lowest risk lesions after CEUS may safely reduce biopsy rates and increase cancer-to-biopsy yields. These thresholds are not meant to be the definitive standards for biopsy but rather a starting point to move forward to determine what thresholds best improve cancer-to-biopsy yields while avoiding a delay in diagnosis for consequential invasive lesions. If it can be proven by further studies, a new BI-RADS category with CEUS may give radiologists and clinicians the justification and support to allow disease dynamics to determine what is consequential and worthy of bringing to clinical attention and finally to avoid overdiagnosis and overtreatment.
Overdiagnosis and a high rate of false positive biopsies is a worldwide problem that most doctors meet in clinical practice when using the breast imaging reporting and data system (BI-RADS) because the BI-RADS ultrasound the authors use now has no microvascular information, which is very important in the differential diagnosis between benign and malignant breast lesions. Contrast-enhanced ultrasound (CEUS) can give the authors this information.
How to reduce false positive biopsies and improve the BI-RADS the authors use now are current hot spots in the research field, which the authors’ study tried to address.
The authors’ study is the first to claim that CEUS can optimize the BI-RADS classification of breast lesions and finally avoid overdiagnosis and overtreatment.
It shows that CEUS is useful in breast lesions and that BI-RADS can be improved by CEUS. Further study to improve the results can still be done.
False positive biopsy means those biopsied breast nodules that were positive but not confirmed in the final pathological results. Cancer-to-biopsy yield means the percentage of malignant nodules of all nodules which were biopsied.
The manuscript is well written.
P- Reviewer: Casciaro S, Jales RM, Razek AAKA S- Editor: Qiu S L- Editor: Roemmele A E- Editor: Li D
| 1. | Breast Imaging Reporting and Data System. BI-RADS: Ultrasound. Reston, VA: American College of Radiology 2014; . |
| 2. | del Carmen MG, Hughes KS, Halpern E, Rafferty E, Kopans D, Parisky YR, Sardi A, Esserman L, Rust S, Michaelson J. Racial differences in mammographic breast density. Cancer. 2003;98:590-596. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 43] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 3. | Elverici E, Zengin B, Nurdan Barca A, Didem Yilmaz P, Alimli A, Araz L. Interobserver and Intraobserver Agreement of Sonographic BIRADS Lexicon in the Assessment of Breast Masses. Iran J Radiol. 2013;10:122-127. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 4. | Calas MJ, Almeida RM, Gutfilen B, Pereira WC. Interobserver concordance in the BI-RADS classification of breast ultrasound exams. Clinics (Sao Paulo). 2012;67:185-189. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 5. | Berg WA, Zhang Z, Lehrer D, Jong RA, Pisano ED, Barr RG, Böhm-Vélez M, Mahoney MC, Evans WP, Larsen LH. Detection of breast cancer with addition of annual screening ultrasound or a single screening MRI to mammography in women with elevated breast cancer risk. JAMA. 2012;307:1394-1404. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 857] [Cited by in RCA: 801] [Article Influence: 61.6] [Reference Citation Analysis (0)] |
| 6. | Geller BM, Ichikawa LE, Buist DS, Sickles EA, Carney PA, Yankaskas BC, Dignan M, Kerlikowske K, Yabroff KR, Barlow W. Improving the concordance of mammography assessment and management recommendations. Radiology. 2006;241:67-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 7. | Poplack SP, Tosteson AN, Grove MR, Wells WA, Carney PA. Mammography in 53,803 women from the New Hampshire mammography network. Radiology. 2000;217:832-840. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 100] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 8. | Kerlikowske K, Hubbard RA, Miglioretti DL, Geller BM, Yankaskas BC, Lehman CD, Taplin SH, Sickles EA. Comparative effectiveness of digital versus film-screen mammography in community practice in the United States: a cohort study. Ann Intern Med. 2011;155:493-502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 201] [Cited by in RCA: 206] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 9. | Bent CK, Bassett LW, D’Orsi CJ, Sayre JW. The positive predictive value of BI-RADS microcalcification descriptors and final assessment categories. AJR Am J Roentgenol. 2010;194:1378-1383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 140] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 10. | Weaver DL, Rosenberg RD, Barlow WE, Ichikawa L, Carney PA, Kerlikowske K, Buist DS, Geller BM, Key CR, Maygarden SJ. Pathologic findings from the Breast Cancer Surveillance Consortium: population-based outcomes in women undergoing biopsy after screening mammography. Cancer. 2006;106:732-742. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 95] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 11. | Smith-Bindman R, Chu PW, Miglioretti DL, Sickles EA, Blanks R, Ballard-Barbash R, Bobo JK, Lee NC, Wallis MG, Patnick J. Comparison of screening mammography in the United States and the United kingdom. JAMA. 2003;290:2129-2137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 239] [Cited by in RCA: 230] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 12. | Consolidated Guidance on Standards for the NHS Breast Screening Programme. NHSBSP Publication No 60 (Version 2). NHS Cancer Screening Programmes: Sheffield 2005; . |
| 13. | Breast cancer screening - An overview from the US National Cancer Institute (NCI) - Patient version. PDQ Cancer Information Summaries, 2014-12-25. . |
| 14. | Uematsu T, Kasami M, Yuen S. Triple-negative breast cancer: correlation between MR imaging and pathologic findings. Radiology. 2009;250:638-647. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 247] [Cited by in RCA: 252] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 15. | Schrading S, Kuhl CK. Mammographic, US, and MR imaging phenotypes of familial breast cancer. Radiology. 2008;246:58-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 155] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 16. | Rakha EA, El-Sayed ME, Green AR, Lee AH, Robertson JF, Ellis IO. Prognostic markers in triple-negative breast cancer. Cancer. 2007;109:25-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 874] [Cited by in RCA: 935] [Article Influence: 51.9] [Reference Citation Analysis (0)] |
| 17. | Esserman L, Shieh Y, Thompson I. Rethinking screening for breast cancer and prostate cancer. JAMA. 2009;302:1685-1692. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 367] [Cited by in RCA: 368] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 18. | Weind KL, Maier CF, Rutt BK, Moussa M. Invasive carcinomas and fibroadenomas of the breast: comparison of microvessel distributions--implications for imaging modalities. Radiology. 1998;208:477-483. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 50] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 19. | Ellis RL. Differentiation of benign versus malignant breast disease. Radiology. 1999;210:878-880. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 20. | Wang L, Du J, Li FH, Fang H, Hua J, Wan CF. Diagnostic efficacy of contrast-enhanced sonography by combined qualitative and quantitative analysis in breast lesions: a comparative study with magnetic resonance imaging. J Ultrasound Med. 2013;32:1805-1814. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 21. | Xiao X, Ou B, Yang H, Wu H, Luo B. Breast contrast-enhanced ultrasound: is a scoring system feasible? A preliminary study in China. PLoS One. 2014;9:e105517. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 49] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 22. | Kalager M, Adami HO, Bretthauer M, Tamimi RM. Overdiagnosis of invasive breast cancer due to mammography screening: results from the Norwegian screening program. Ann Intern Med. 2012;156:491-499. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 170] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 23. | Welch HG, Black WC. Overdiagnosis in cancer. J Natl Cancer Inst. 2010;102:605-613. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1042] [Cited by in RCA: 1145] [Article Influence: 76.3] [Reference Citation Analysis (0)] |
| 24. | Nelson HD, Tyne K, Naik A, Bougatsos C, Chan BK, Humphrey L. Screening for breast cancer: an update for the U.S. Preventive Services Task Force. Ann Intern Med. 2009;151:727-737, W237- W242. [PubMed] |