Published online Apr 28, 2016. doi: 10.4329/wjr.v8.i4.378
Peer-review started: September 12, 2015
First decision: October 8, 2015
Revised: December 24, 2015
Accepted: January 27, 2016
Article in press: January 29, 2016
Published online: April 28, 2016
Processing time: 222 Days and 19.1 Hours
AIM: To study the safety and effectiveness of preoperative embolization of primary bone tumors in relation to intraoperative blood loss, intraoperative blood transfusion volume and surgical time.
METHODS: Thirty-three patients underwent preoperative embolization of primary tumors of extremities, hip or vertebrae before resection and stabilization. The primary osseous tumors included giant cell tumors, aneurysmal bone cyst, osteoblastoma, chondroblastoma and chondrosarcoma. Twenty-six patients were included for the statistical analysis (embolization group) as they were operated within 0-48 h within preoperative embolization. A control group (non-embolization group, n = 28) with bone tumor having similar histological diagnosis and operated without embolization was retrieved from hospital record for statistical comparison.
RESULTS: The mean intraoperative blood loss was 1300 mL (250-2900 mL), the mean intraoperative blood transfusion was 700 mL (0-1400 mL) and the mean surgical time was 221 ± 76.7 min for embolization group (group I, n = 26). Non-embolization group (group II, n = 28), the mean intraoperative blood loss was 1800 mL (800-6000 mL), the mean intraoperative blood transfusion was 1400 mL (700-8400 mL) and the mean surgical time was 250 ± 69.7 min. On comparison, statistically significant (P < 0.001) difference was found between embolisation group and non-embolisation group for the amount of blood loss and requirement of blood transfusion. There was no statistical difference between the two groups for the surgical time. No patients developed any angiography or embolization related complications.
CONCLUSION: Preoperative embolization of bone tumors is a safe and effective adjunct to the surgical management of primary bone tumors that leads to reduction in intraoperative blood loss and blood transfusion volume.
Core tip: The current study shows that the preoperative embolisation of bone tumors performed 48 h prior to either limb salvage surgery or spinal stabilization leads to decrease in intraoperative blood loss and intraoperative blood transfusion volume. Therefore, would recommend that pre-operative embolisation is a safe procedure and should be used as part of the multi-disciplinary approach to the management of bone tumours in difficult anatomical locations especially those are known to be highly vascular.
- Citation: Jha R, Sharma R, Rastogi S, Khan SA, Jayaswal A, Gamanagatti S. Preoperative embolization of primary bone tumors: A case control study. World J Radiol 2016; 8(4): 378-389
- URL: https://www.wjgnet.com/1949-8470/full/v8/i4/378.htm
- DOI: https://dx.doi.org/10.4329/wjr.v8.i4.378
Bone tumors include primary and metastatic tumors[1]. They present in several ways, like bone destruction, expansion or, infiltration of periosteum. Bone tumors may be detected as an incidental finding or the patient may present with pain or loss of function, or with a pathological fracture[2]. Though there are many treatment modalities for bone tumors, surgical excision is the treatment of choice and may be coupled with other adjunctive therapies. However, several factors including tumor bulk, vascularity, vicinity to vital structures and potentially inaccessible location of the lesion may make the surgical treatment very difficult if not impossible[1].
Transarterial embolization (TAE) is an important adjuvant treatment modality and it may also be the primary and curative modality in some cases. It has been found that preoperative TAE is effective in both primary and metastatic bone tumors. Preoperative TAE reduces the tumor vascularity and intraoperative blood loss. It also reduces the need for blood transfusion and decreases the incidence of complications associated with surgery. TAE allows better definition of tissue planes at surgery leading to complete excision of the bone tumor, which ultimately leads to decrease tumor recurrence rates[2,3]. Refinement of angiographic techniques and advances in hardwares led to its use in preoperative embolization of both primary and secondary bone tumors[4,5]. The principle behind TAE of primary bone tumors is the precise targeting of the tumor feeding vessels and tumor capillary bed[6]. Non-target embolization and the occlusion of a non-target vessel leading to inadvertent necrosis of normal organ is the major concern during the procedure of trans arterial embolization of bone tumors, however, it can be prevented by careful review of the diagnostic angiograms obtained immediately before embolization and meticulous care during injection of the embolic agent into the selective vessels supplying the tumor.
The common types of bone tumor which usually require TAE are hypervascular in nature such as giant cell tumors (GCT), aneurysmal bone cysts (ABC), telengiactatic osteosarcomas, vertebral hemangiomas, arterio-venous malformations, osteoblastoma and vascular metastatic lesions especially from renal cell or thyroid[7-10].
Studies have documented the use of preoperative embolization as an effective intervention to reduce intraoperative blood loss, transfusion requirements and duration of surgery[11-13]. Many studies conducted worldwide have proved the efficacy of preoperative embolization but most of them were done on secondary bone tumors[14-22]. The aim of our study was to assess the effectiveness and safety of preoperative TAE of primary bone tumors in relation to the amount of intraoperative blood loss, requirement of amount of blood transfusion, surgical time and procedure related complications.
The prospective study was performed with the approval of the local ethical committee, 33 patients with primary bone tumors were included in this prospective study from January 2012 to October 2013. Patients with primary bone tumor of extremities amenable for limb salvage surgery and resectable primary vertebral/sacral tumor were included in this study. After obtaining informed consent, preoperative embolization was done in all these 33 patients. Twenty-six patients (15 men, 11 women, age range 14-82 years), who had surgical resection within 0-48 h of TAE, were included for statistical analysis and remaining 7 patients were excluded from the statistical analysis as they were operated after 48 h of embolization.
Among 26 patients who underwent TAE, most common histological type of tumor was GCT comprising of 20 patients (77%) predominantly located in lower limb and the second most common type was ABC and chondrosarcoma in two patients (7%) each, rest each had osteoblastoma and chondroblastoma. Axial skeletal skeleton was affected in 5 patients.
For statistical analysis, 28 patients (16 men, 12 women, age range 10-60 years) with similar histological diagnosis were retrieved from hospital record who were operated in past without preoperative embolization (control group, n = 28). Among these 28 patients, the most common histological type was GCT comprising of 23 patients (82%) followed by ABC and osteoblastoma comprising of 2 patients (7%) each. There was no case of chondrosarcoma in the control group.
Patient demographics and histological diagnosis of tumors of both study group and control group were shown in Table 1.
| Study group (embolisation group, n = 26) | Control group (non-embolisation group, n = 28) | ||
| Characteristics | Number (%) | Characteristics | Number (%) |
| Age Group | Age Group | ||
| 0-40 yr | 18 (69%) | 0-40 yr | 18 (64%) |
| 40-80 yr | 7 (27%) | 40-80 yr | 10 (36%) |
| > 80 yr | 1 (4%) | ||
| Sex | Sex | ||
| Male | 15 (58%) | Male | 16 (57%) |
| Female | 11 (42%) | Female | 12(43%) |
| Tumor histology | Tumor histology | ||
| Giant cell tumor | 20 (78%) | Giant cell tumor | 23 (82%) |
| Aneurysmal bone cyst | 2 (7%) | Aneurysmal bone cyst | 2 (7%) |
| Chondrosarcoma | 2 (7%) | Chondrosarcoma | 0 (0%) |
| Osteoblastoma | 1 (4%) | Osteoblastoma | 2 (7%) |
| Chondroblastoma | 1 (4%) | Chondroblastoma | 1 (4%) |
Angiography and embolization was done using state of art, digital substraction angiography unit (Allura Xper FD 20, Philips Medical Systems, Best, The Netherlands), under local anesthesia by a single interventional radiologist with experience of more than 10 years. All the angiographies were performed through transfemoral route, most vessels could be catheterized selectively either with 5 French diagnostic catheter (Picard, Cook, Bloomington, IN, United States) or using coaxial microcatheter system 2.7 F (Progreat, Terumo, Tokyo, Japan) with 0.018 inch wire. Coaxial microcatheter system was used in 20 patients (77%) for superselective cannulation of smaller feeder arteries.
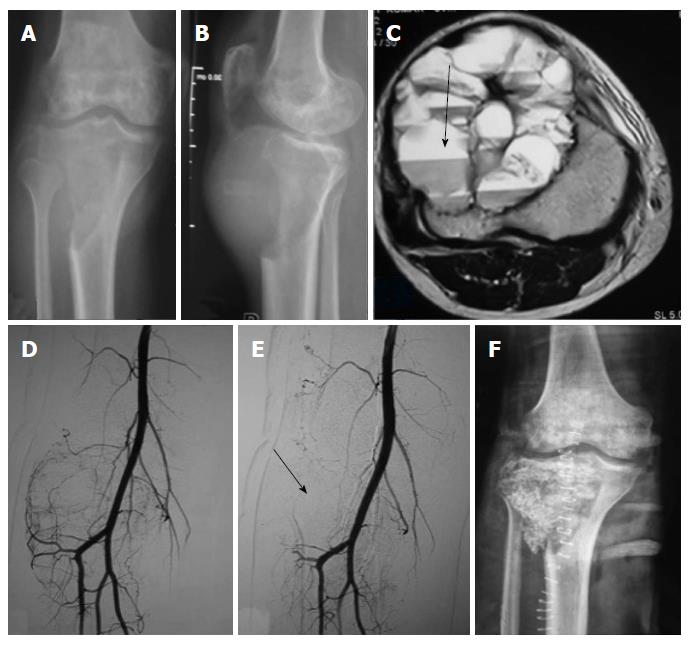
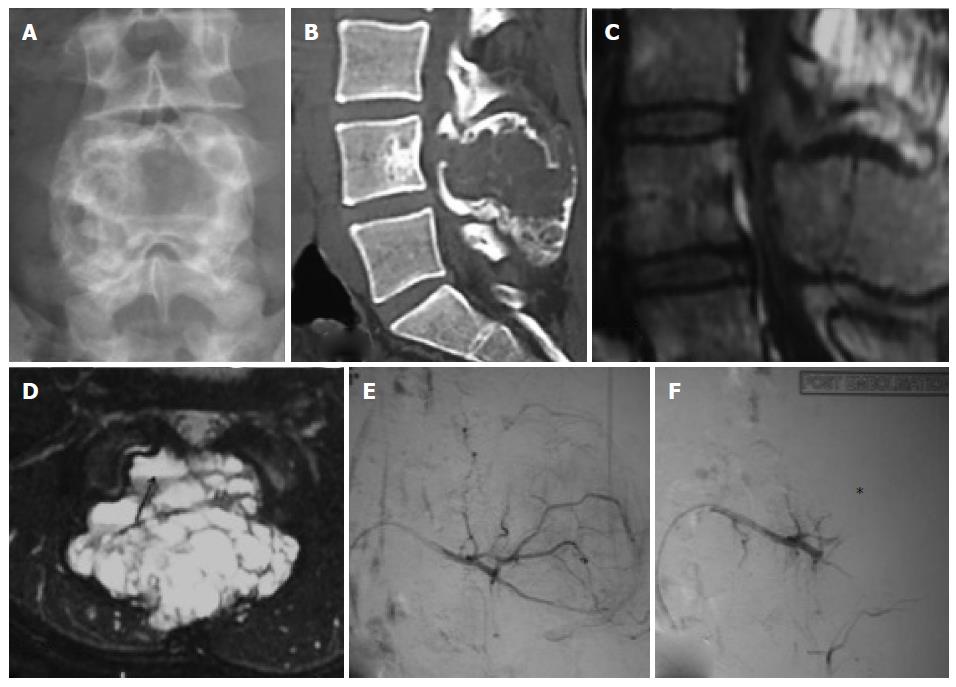
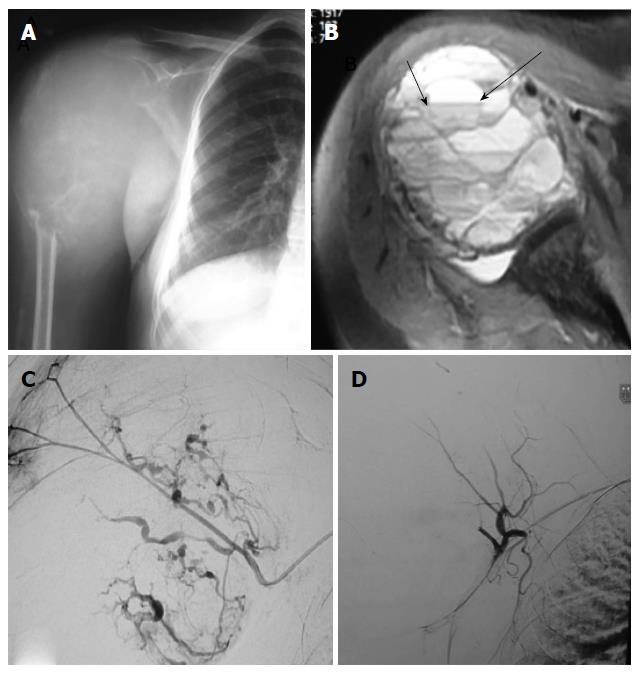
Thirteen embolisations were performed using combination of gelfoam (Gelfoam; Pfizer, New York, NY, United States) and polyvinyl alcohol (PVA, Cook, Bloomington, IN, United States) particles of size 300-500 and 550-700 microns[23], 12 embolisations were performed using only PVA particles and remaining one embolisation was using only gelfoam, following selective catheterisation of individual vessels supplying the tumor as ascertained on angiograms. The choice of embolising material was done on the basis of personal preference, catheter size, position of catheter tip and diameter of the feeding vessel. The embolisation endpoint was decided to be when all major vessels supplying the tumor were embolised and there was essentially near complete obliteration of tumor blush (Figure 1). Percentage of reduction of tumor blush was categorized into three groups as mentioned in previous studies, < 50% reduction, 50%-75% reduction and > 75% reduction[16,24]. More than > 75% reduction of tumor blush was achieved in 24 patients (92%), and 50%-75% in 2 patients (8%).
All the patients underwent tumor resection and stabilization within 0-48 h of embolization. Three orthopedic surgeons having experience of more than 15 years performed all surgical procedures. Intraoperative blood loss was derived from operative a note which was calculated by the surgical team with use of standard surgical assessment like weighing of blood soaked gauze pieces and measuring the collected blood in the drain (Figure 2). Similarly intraoperative blood transfusion volume and surgical time taken were also noted from the surgical note. Any adverse events related to angiography or embolization and related to surgery were noted up to 48 h of postoperative period. We also recorded the same parameters from the surgical notes of the 28 patients of the control group operated in past without undergoing any preoperative embolization.
It was performed using Stata 11.2 software. Statistical comparison was done between the study population - embolisation group and the control group - non-embolisation group (data retrieved from the hospital medical record). To compare the difference between the two groups (embolization and non embolization group) and to calculate the P-value, Student’s t test was applied.
All 26 embolisation procedures were technically and clinically successful. All the tumors were hypervascular on angiograms (Figures 3 and 4). Following embolisation, more than > 75% reduction of tumor blush was achieved in 24 patients (92%) and reduction was in the range of 50%-75% in 2 patients (8%).
None of the patients developed any major complications related to either angiography or embolization during the procedure or during observation period. Minor complications like local site pain, shivering were noted in 4 patients which did not warrant any medical treatment. None of the patients had postembolization syndrome.
The type of surgical procedures performed in study group included, tumor resection in 7 patients, curettage and bone grafting in 8 patients, tumor resection, bone grafting and fixation using compression plate in 7 patients and tumor resection with posterior element fixation and cage placement in 4 patients.
The mean intraoperative blood loss was 1300 mL (range 250-2900 mL), with mean requirement of intraoperative blood transfusion of 700 mL (range 0-1400 mL). Five patients did not require any blood transfusion. The mean surgical time was 221 min (range 115-450 min).
The mean intraoperative blood loss was 1800 mL (range 800-6000 mL), with mean requirement of intraoperative blood transfusion of 1400 mL (range 700-8400 mL). The mean surgical time was 250 min (range 145-450 min).
On comparison, statistically significant (P < 0.001) difference was found between embolisation group and non-embolisation group for the amount of blood loss and requirement of blood transfusion. There was no statistical difference between the two groups for the surgical time.
The comparison of intraoperative blood loss, intraoperative blood transfusion and surgical time is depicted in Table 2. In order to extract safer conclusions; we have made two homogenous groups, i.e., GCT in extremities of same grade and underwent same type of operation.
| Parameters | Study group, n = 26 | Control group, n = 28 | P value |
| Intraoperative blood loss (mL) | |||
| Minimum | 250 | 800 | |
| Maximum | 2900 | 6000 | < 0.0015 |
| Median | 1300 | 1800 | |
| Intraoperative blood transfusion (mL) | |||
| Minimum | 0 | 700 | |
| Maximum | 1400 | 8400 | < 0.0001 |
| Median | 700 | 1400 | |
| Surgical time (min) | |||
| Minimum | 115 | 145 | > 0.14 |
| Maximum | 450 | 450 | |
The comparison of intraoperative blood loss, intraoperative blood transfusion and surgical time for the GCT in extremities between embolisation (study group) and non-embolisation group (control group) is depicted in Table 3.
| Parameters | Study group, n = 18 | Control group, n = 22 | P value |
| Intraoperative blood loss (mL) | |||
| Minimum | 250 | 800 | |
| Maximum | 2900 | 6000 | < 0.0012 |
| Median | 1300 | 1800 | |
| Intraoperative blood transfusion (mL) | |||
| Minimum | 0 | 700 | |
| Maximum | 1400 | 8400 | < 0.0007 |
| Median | 525 | 1400 | |
| Surgical time (min) | |||
| Minimum | 115 | 145 | |
| Maximum | 340 | 450 | > 0.0079 |
| Median | 210 | 252.5 | |
Embolisation group (n = 18): The mean intraoperative blood loss was 1300 mL (range 250-2900 mL), with mean requirement of intraoperative blood transfusion of 525 mL (range 0-1400 mL). Four patients did not require any blood transfusion. The mean surgical time was 210 min (range 115-340 min).
Non-embolisation group (n = 22): The mean intraoperative blood loss was 1800 mL (range 800-6000 mL), with mean requirement of intraoperative blood transfusion of 1400 mL (range 700-8400 mL). The mean surgical time was 252.5 min (range 145-450 min).
On comparison, statistically significant (P < 0.001) difference was found between embolisation group and non-embolisation group for the GCT in extremities for the amount of blood loss, requirement of blood transfusion and also for surgical time.
Patient demographics, histological diagnosis, location of tumor, blood loss, transfusion requirement and surgical time of study group and control group were shown in Table 4 for entire study population and in Table 5 for GCT in extremities.
| S.No | Study group (embolisation group), n = 26 | Control group (non-embolisation group), n = 28 | |||||||||||
| Age/sex | Diagnosis | Site | Embolising agent | Blood loss (mL) | Transfusion (mL) | Surgical time (min) | Age/sex | Diagnosis | Site | Blood loss (mL) | Transfusion (mL) | Surgical time (min) | |
| 1 | 52 M | GCT | LL | PVA + GEL FOAM | 2900 | 1400 | 230 | 10 F | ABC | LL | 1450 | 750 | 170 |
| 2 | 30 M | GCT | LL | PVA + GEL FOAM | 1740 | 700 | 231 | 46 F | GCT | LL | 1300 | 1400 | 240 |
| 3 | 18 M | OB | SPINE | PVA + GEL FOAM | 1650 | 700 | 240 | 58 M | GCT | LL | 1600 | 1450 | 230 |
| 4 | 17 M | ABC | SPINE | PVA + GEL FOAM | 1600 | 700 | 355 | 20 F | ABC | UL | 1800 | 1400 | 195 |
| 5 | 42 M | GCT | LL | PVA + GEL FOAM | 1500 | 700 | 340 | 28 F | OB | SPINE | 2500 | 1750 | 195 |
| 6 | 14 F | ABC | SPINE | PVA + GEL FOAM | 1200 | 700 | 230 | 31 M | GCT | LL | 1250 | 1050 | 220 |
| 7 | 34 M | CS | ILIUM | GEL FOAM | 1900 | 1400 | 450 | 21 M | GCT | LL | 2500 | 1050 | 145 |
| 8 | 39 F | GCT | LL | PVA + GEL FOAM | 1600 | 700 | 240 | 34 M | GCT | SPINE | 2500 | 1400 | 295 |
| 9 | 45 F | GCT | LL | PVA + GEL FOAM | 1300 | 350 | 180 | 34 F | GCT | LL | 1800 | 1400 | 150 |
| 10 | 31 M | GCT | LL | PVA | 250 | 0 | 115 | 60 F | GCT | LL | 1200 | 700 | 150 |
| 11 | 20 F | GCT | UL | PVA + GEL FOAM | 1300 | 700 | 250 | 20 M | GCT | LL | 6000 | 8400 | 450 |
| 12 | 25 F | GCT | SPINE | PVA | 1300 | 700 | 230 | 28 M | OB | SPINE | 1300 | 700 | 230 |
| 13 | 48 M | GCT | SACRUM | PVA | 1400 | 700 | 240 | 30 M | GCT | LL | 2000 | 2100 | 350 |
| 14 | 15 M | GCT | UL | PVA + GEL FOAM | 500 | 350 | 170 | 45 F | GCT | LL | 1500 | 1400 | 225 |
| 15 | 32 F | GCT | LL | PVA + GEL FOAM | 1600 | 700 | 260 | 22 M | GCT | LL | 4300 | 2100 | 315 |
| 16 | 30 F | GCT | UL | PVA | 350 | 0 | 135 | 40 F | GCT | LL | 1800 | 1400 | 245 |
| 17 | 28 F | GCT | LL | PVA + GEL FOAM | 1400 | 700 | 240 | 24 F | GCT | LL | 800 | 700 | 290 |
| 18 | 32 M | GCT | LL | PVA | 400 | 350 | 190 | 16 M | CB | LL | 1400 | 1050 | 185 |
| 19 | 45 F | GCT | UL | PVA | 2100 | 1050 | 245 | 17 M | GCT | UL | 1800 | 700 | 180 |
| 20 | 20 M | GCT | LL | PVA | 600 | 0 | 125 | 22 F | GCT | LL | 1500 | 1050 | 260 |
| 21 | 20 F | GCT | LL | PVA | 800 | 350 | 145 | 34 M | GCT | UL | 900 | 700 | 245 |
| 22 | 82 M | GCT | LL | PVA | 900 | 350 | 155 | 29 F | GCT | LL | 1500 | 1400 | 270 |
| 23 | 15 M | CB | LL | PVA | 750 | 350 | 135 | 26 M | GCT | LL | 1600 | 1050 | 290 |
| 24 | 22 M | GCT | UL | PVA+GEL FOAM | 1800 | 1400 | 230 | 27 F | GCT | LL | 2600 | 2100 | 335 |
| 25 | 52 M | CS | UL | PVA | 500 | 0 | 250 | 18 M | GCT | LL | 3450 | 2450 | 330 |
| 26 | 42 F | GCT | UL | PVA | 300 | 0 | 140 | 23 F | GCT | UL | 2600 | 2050 | 285 |
| 27 | 21 F | GCT | LL | 2100 | 1400 | 245 | |||||||
| 28 | 24 M | GCT | LL | 1900 | 1400 | 290 | |||||||
| S.No | Study group (embolisation group), n = 18 | Control group (non-embolisation group), n = 22 | |||||||||||
| Age/sex | Diagnosis | Site | Embolising agent | Blood loss (mL) | Transfusion (mL) | Surgical time (min) | Age /sex | Diagnosis | Site | Blood loss (mL) | Transfusion (mL) | Surgical time (min) | |
| 1 | 52 M | GCT | LL | PVA + GEL FOAM | 2900 | 1400 | 230 | 46 F | GCT | LL | 1300 | 1400 | 240 |
| 2 | 30 M | GCT | LL | PVA + GEL FOAM | 1740 | 700 | 231 | 58 M | GCT | LL | 1600 | 1450 | 230 |
| 3 | 42 M | GCT | LL | PVA + GEL FOAM | 1500 | 700 | 340 | 31 M | GCT | LL | 1250 | 1050 | 220 |
| 4 | 39 F | GCT | LL | PVA + GEL FOAM | 1600 | 700 | 240 | 21 M | GCT | LL | 2500 | 1050 | 145 |
| 5 | 45 F | GCT | LL | PVA + GEL FOAM | 1300 | 350 | 180 | 34 F | GCT | LL | 1800 | 1400 | 150 |
| 6 | 31 M | GCT | LL | PVA | 250 | 0 | 115 | 60 F | GCT | LL | 1200 | 700 | 150 |
| 7 | 20 F | GCT | UL | PVA + GEL FOAM | 1300 | 700 | 250 | 20 M | GCT | LL | 6000 | 8400 | 450 |
| 8 | 15 M | GCT | UL | PVA + GEL FOAM | 500 | 350 | 170 | 30 M | GCT | LL | 2000 | 2100 | 350 |
| 9 | 32F | GCT | LL | PVA + GEL FOAM | 1600 | 700 | 260 | 45F | GCT | LL | 1500 | 1400 | 225 |
| 10 | 30 F | GCT | UL | PVA | 350 | 0 | 135 | 22 M | GCT | LL | 4300 | 2100 | 315 |
| 11 | 28 F | GCT | LL | PVA + GEL FOAM | 1400 | 700 | 240 | 40 F | GCT | LL | 1800 | 1400 | 245 |
| 12 | 32 M | GCT | LL | PVA | 400 | 350 | 190 | 24 F | GCT | LL | 800 | 700 | 290 |
| 13 | 45 F | GCT | UL | PVA | 2100 | 1050 | 245 | 17 M | GCT | UL | 1800 | 700 | 180 |
| 14 | 20 M | GCT | LL | PVA | 600 | 0 | 125 | 22 F | GCT | LL | 1500 | 1050 | 260 |
| 15 | 20 F | GCT | LL | PVA | 800 | 350 | 145 | 34 M | GCT | UL | 900 | 700 | 245 |
| 16 | 82 M | GCT | LL | PVA | 900 | 350 | 155 | 29 F | GCT | LL | 1500 | 1400 | 270 |
| 17 | 22 M | GCT | UL | PVA + GEL FOAM | 1800 | 1400 | 230 | 26 M | GCT | LL | 1600 | 1050 | 290 |
| 18 | 42 F | GCT | UL | PVA | 300 | 0 | 140 | 27 F | GCT | LL | 2600 | 2100 | 335 |
| 19 | 18 M | GCT | LL | 3450 | 2450 | 330 | |||||||
| 20 | 23 F | GCT | UL | 2600 | 2050 | 285 | |||||||
| 21 | 21 F | GCT | LL | 2100 | 1400 | 245 | |||||||
| 22 | 24M | GCT | LL | 1900 | 1400 | 290 | |||||||
The management of primary bone tumors, either benign or malignant, varies with the individual lesions; however, surgical resection remains the main treatment modality. However, heavy, uncontrollable blood loss during surgery has been common problem, leading to massive transfusion requirements. Preoperative embolization has significantly reduced peri-operative blood loss and better delineation of the tumor margins from the surrounding, facilitating the tumor excision with reduced blood transfusion requirement.
Barton et al[24] reported a blood loss of 2000-18500 mL (mean 6800 mL) in 20 patients operated without preoperative embolization, whereas only 500-1500 mL blood loss occurred when PAE was performed and cases were operated within 72 h. For better result, close cooperation between the orthopedic surgeon and interventional radiologist is essential. In our study, we studied the role of PAE in bone tumors where limb salvage surgery was possible.
Majority of the patients in our study had GCT (n = 20, 77%) followed by ABC and chondrosarcoma that comprised of two patients (7%) each. Similarly 23 patients (82%) of our control group had GCT. None of the previous studies from literature comprised of this number of GCT and the most common histology in previous studies was metastatic tumors[16,19,21,22]. In a prospective study done by Lee et al[13] total 6 patients were studied. Out of 6 patients they studied, three patients had GCT and 3 patients had ABC. There are scattered case reports, describing the role of TAE in limb-girdle location where GCT and ABCs were the common histology[25,26,27].
Barton et al[24] also reported that, surgery occurred within 3 d of embolization, blood loss was 500-1500 mL, comparing to 1500-2800 mL at 4-14 d post embolisation, due to re-canalization and angiogenesis. In our study, all the cases were operated within 48 h of embolisation.
In our study, significant number of bone tumors where TAE was performed, were located in appendicular skeleton (n = 24, 76%) than axial skeleton (n = 6, 24%). In contrast, as reported by the previous studies where TAE was performed, spinal column was the most common sites and most of these tumors were metastatic in nature than primary bone tumor[15,16,19,22,28,29]. Retrospective study done by Radeleff et al[17] studied four metastatic lower limb tumors and five metastatic upper limb tumors out of 31 patients.
Majority of the studies from literature suggests that TAE was performed under local anaesthesia as in our study. However, in a study done by Thiex et al[29] all angiograms were done under general anesthesia, since all the angiograms were done for spinal column metastatic lesions.
PVA particles, as an embolising agent is considered as a workhorse in pre-operative TAE[15,23,30]. It was used as sole embolising agent in 12 (46%) patients and both PVA and gelfoam were used in 13 (50%) patients in our study. In a study done by Radeleff et al[17] apart from gelfoam and PVA particles, they have also used embosphere and liquid embolising material like cyanoacrylate-lipoidal mixture and ethibloc. They have also advocated the use of embosphere as a particulate embolising material due to its uniform size. Another study reported by Kobayashi et al[27] used PVA and embosphere as embolising material. In study reported by Lee et al[13] only gelfoam was used for embolization. In few studies coils were also used for embolization[3,18]. We compared the various perioperative findings in the PVA only and PVA with gelfoam groups. There was statistically significant difference (P < 0.005) when only PVA was used as embolizing material as there was lesser intraoperative blood loss, lesser intraoperative and post operative blood transfusion volume.
Final angiogram was taken after the completion of TAE and degree of tumor devascularity was assessed subjectively. We achieved reduction of tumor blush by more than > 75% in 24 (92%) patients and in rest, the reduction of tumor blush was in range of 50%-75%. In a study done by Börüban et al[12] they had achieved 80%-100% devascularisation in 5 of 11 patients. In another study done by Sun et al[15], more than 70% reduction in tumor blush (stain) was noted in 12 of 16 patients, while in 2 patients reduction of tumor blush was less than 50%. In comparison, none of our patients had reduction of tumor blush less than 50%. This suggests that our study was comparable with many of the studies from the literature. In our study, statistical analysis between the amount of blood loss and amount of devascularisation could not be done since majority of our patients (n = 24) had > 75% devascularisation and the other group which had 50%-75% devascularisation was very small (n = 2). However, we did not find any significant difference during the surgery.
A known complication of embolisation is the possibility of wound healing problems. Wirbel et al[14] noted that 2 patients in their embolisation group (spinal tumours) had wound problems due to psoas muscle necrosis and subsequent skin necrosis. None of the patients from our study population developed any such complication or any other complications related to angiography and embolization. This is in concordance with previous studies where no major complications were reported[11,30-32].
Our results showed significantly less blood in a variety of surgically resected bone tumours as compared with the pre-embolisation era, and comparable blood loss to other studies[12,27,29].
In a study done by Thiex et al[29] on 104 spinal tumors, the blood loss was found to be in the range of 200-15000 mL. In another study done by Kobayashi et al[27], the blood loss was found to be in the range of 250-11000 mL with a mean of 2554 mL. In a study done by Zhang et al[32], the blood loss was in the range of 60-580 mL with a mean blood loss of 290 mL. The lower blood loss in this study can be attributed to the fact that this study was done on osteosarcomas which is not very vascular tumor except for the telengiactatic form. In other study done by Börüban et al[12] on bone and soft tissue tumors of extremities, the blood loss was in the range of 100-2500 mL with a mean blood loss of 1360 mL. Our study had blood loss in comparison to this study as mean blood loss in the range of 1300 mL.
In a study done by Thiex et al[29], the requirement of perioperative blood transfusion requirement was found to be 0-99 units. In another study done by Kobayashi et al[27] on spinal tumors; the requirement of intraoperative blood transfusion volume was noted in the range of 0-28 units with mean of 7 units. In study done by Zhang et al[32], the volume of blood infusion was noted to be 689 ± 133.89 mL. The intraoperative blood transfusion in our study group was comparable to study done by Zhang et al[32], while lower than the study done by Thiex et al[29] and Kobayashi et al[27]. In our study, mean requirement of intraoperative blood transfusion was 700 mL (range 0-1400 mL).
Many retrospective studies from the literature have shown that intraoperative blood loss is significantly lower in embolization group than non-embolisation group (Table 6). In non-embolisation group, the intraoperative blood loss ranged from 4350-8750 mL vs 300-4300 mL as compared to embolisation group[14,30-36]. In our study, the mean intraoperative blood loss was 1300 mL (range 250-2900 mL), with mean requirement of intraoperative blood transfusion of 700 mL (range 0-1400 mL) in embolisation group as compared to mean intraoperative blood loss was 1800 mL (range 800-6000 mL), with mean requirement of intraoperative blood transfusion of 1400 mL (range 700-8400 mL) in non-embolisation group.
| Ref. | Year | No. of patients | Type of study | Result |
| Thiex et al[29] | 2013 | 104 | Retrospective | EBL = 100-15000 mL |
| BT = 0-99 units | ||||
| Ibrahim et al[36] | 2013 | 18 | Retrospective | EBL = 1100-2600 mL |
| MBL = 1400 mL | ||||
| BT = 0-8 units | ||||
| Kobayashi et al[27] | 2012 | 62 | Retrospective | EBL = 250-11000 mL |
| MBL = 2554 mL | ||||
| BT = 0-28 units | ||||
| Al-Hadithy et al[20] | 2011 | 26 | Retrospective | EBL = 100-1800 mL |
| BT = 0-3 units | ||||
| Zhang et al[32] | 2009 | 47 | Retrospective | EBL = 705±120 mL |
| BT = 689±133 mL | ||||
| Lee et al[13] | 2008 | 6 | Prospective | EBL = 200-830 mL |
| BT = Nil | ||||
| Our study | 2012-2013 | 26 (study group) | Case control study | For study group |
| EBL = 250-2900 mL | ||||
| BT = 0-1400 mL | ||||
| 28 (control group) | For control group | |||
| EBL = 800-6000 mL | ||||
| BT = 700-8400 mL | ||||
| P < 0.05 |
We also compared surgical time in our study group and control group. We found that mean surgical time was less in study group (221.19 min) in comparison to the control group (250.35 min), however, we did not found significant correlation (P > 0.14) between preoperative embolization and surgical time.
The important limitations of our study were, first, the sample size of our study was small and study was not randomized according to the tumor size and volume and its effect on blood loss. Second, we did not study osseous metastatic tumors. Third, variables like patient characteristics, tumor characteristics, surgical skill and techniques that can influence perioperative blood loss and BT were not evaluated, as it was not our aim of study. Fourth, no long-term clinical follow up of the patients were available to evaluate the tumor recurrence and survival rates in these patients.
The type, grade and staging of the tumor are important characteristics that affect its “bleeding potential” and type of operation. In our study, we have examined the effectiveness of preoperative embolization in 2 groups with at least 4 different types of tumors or tumor like lesions, unknown staging and grading and different locations, thus these two are heterogeneous group of patients. Comparison of same type of tumor and same location between two groups was not attempted. Since in our study predominant histological pattern was GCT in both study group and control group. Rest of histological pattern was small number and was in different location, hence could not be compared. However in order to extract safer conclusion we have compared the effect of TAE in case of GCT in extremities, who underwent same type of operation. On comparison, we found statistically significant (P < 0.001) difference between embolisation group and non-embolisation group for the GCT in extremities for the amount of blood loss, requirement of blood transfusion and also for surgical time.
In conclusion, the results of the current study show that the preoperative embolisation of bone tumor performed 0-48 prior to either limb salvage surgery or stabilization in vertebral and sacral tumor leads to decrease in intraoperative blood loss and intraoperative blood transfusion volume. We experienced no procedure related complications. Therefore, would recommend that pre-operative embolisation is a safe procedure and should be used as part of the multi-disciplinary approach to the management of bone tumours in difficult anatomical locations and those are known to be highly vascular. Further larger prospective randomized studies are needed to address these issues and generalize it for primary bone tumor resection.
The authors thank Professor Sreenivas V for helping in statistical analysis of the study.
Preoperative transarterial embolisation (TAE) reduces the tumor vascularity and intraoperative blood loss. It also reduces the need for blood transfusion and decreases the incidence of complications associated with surgery. Many studies conducted worldwide have proved the efficacy of preoperative embolization but most of them were done on secondary bone tumors. The aim of this study was to assess the effectiveness and safety of preoperative TAE of primary bone tumors in relation to the amount of intraoperative blood loss, requirement of amount of blood transfusion, surgical time and procedure related complications.
Selective TAE was first described by Feldman et al[3] in 1975, since then embolization of tumors has been a widely practiced interventional radiology technique. Refinement of techniques and advances in hardwares led to its use in preoperative embolization of both primary and secondary bone tumors. The principle behind TAE of primary bone tumors is the precise targeting of the tumor feeding vessels and tumor capillary bed, while simultaneously reducing non-target embolisation effects.
The common types of bone tumor which usually require TAE are hypervascular in nature such as giant cell tumors, aneurysmal bone cysts, telengiactatic osteosarcomas, vertebral hemangiomas, arterio-venous malformations, osteoblastoma and vascular metastatic lesions especially from renal cell or thyroid. The management of primary bone tumors, either benign or malignant, varies with the individual lesions; however, surgical resection remains the main treatment modality. However, heavy, uncontrollable blood loss during surgery has been common problem, leading to massive transfusion requirements. Preoperative embolization has significantly reduced peri-operative blood loss and better delineation of the tumor margins from the surrounding, facilitating the tumor excision with reduced blood transfusion requirement. The results of the current study show that the preoperative TAE performed 0-48 h before limb salvage surgery in extremities tumor and tumor resection and stabilization in vertebral and sacral tumor leads to decrease in intraoperative blood loss and intraoperative blood transfusion volume.
The results of this study suggest that pre-operative embolisation is a safe and effective procedure, could be used as part of the multi-disciplinary approach to the management of bone tumors in difficult anatomical locations and those that are known to be highly vascular.
Preoperative TAE reduces the tumor vascularity, intraoperative blood loss, the need for blood transfusion and decreases the incidence of complications associated with surgery.
This is really well designed and written paper regarding the importance of preoperative embolization of primary bone tumors.
P- Reviewer: Bicanic G, Drampalos E, Fernandez-Fairen M S- Editor: Gong XM L- Editor: A E- Editor: Jiao XK
| 1. | Gupta P, Gamanagatti S. Preoperative transarterial Embolisation in bone tumors. World J Radiol. 2012;4:186-192. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 12] [Cited by in RCA: 14] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 2. | Owen RJ. Embolization of musculoskeletal bone tumors. Semin Intervent Radiol. 2010;27:111-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 49] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 3. | Feldman F, Casarella WJ, Dick HM, Hollander BA. Selective intra-arterial embolization of bone tumors. A useful adjunct in the management of selected lesions. Am J Roentgenol Radium Ther Nucl Med. 1975;123:130-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 74] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 4. | Bücheler E, Hupe W, Hertel EU, Klosterhalfen H. [Catheter embolisation of renal tumours (author’s transl)]. Rofo. 1976;124:134-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 5. | Carpenter PR, Ewing JW, Cook AJ, Kuster AH. Angiographic assessment and control of potential operative hemorrhage with pathologic fractures secondary to metastasis. Clin Orthop Relat Res. 1977;6-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 6. | Kauffmann G, Wimmer B, Bischoff W, Adler C, Strecker EP. [Fundamental experiments for therapeutic artery occlusion by angiography catheters (author’s transl)]. Radiologe. 1977;17:489-491. [PubMed] |
| 7. | Turcotte RE, Sim FH, Unni KK. Giant cell tumor of the sacrum. Clin Orthop Relat Res. 1993;215-221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 27] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 8. | Simpson AH, Porter A, Davis A, Griffin A, McLeod RS, Bell RS. Cephalad sacral resection with a combined extended ilioinguinal and posterior approach. J Bone Joint Surg Am. 1995;77:405-411. [PubMed] |
| 9. | Fraser RK, Coates CJ, Cole WG. An angiostatic agent in treatment of a recurrent aneurysmal bone cyst. J Pediatr Orthop. 1993;13:668-671. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 10. | Chu JP, Chen W, Li JP, Zhuang WQ, Huang YH, Huang ZM, Yang JY. Clinicopathologic features and results of transcatheter arterial chemoembolization for osteosarcoma. Cardiovasc Intervent Radiol. 2007;30:201-206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 22] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 11. | Breslau J, Eskridge JM. Preoperative embolization of spinal tumors. J Vasc Interv Radiol. 1995;6:871-875. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 51] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 12. | Börüban S, Sancak T, Yildiz Y, Sağlik Y. Embolization of benign and malignant bone and soft tissue tumors of the extremities. Diagn Interv Radiol. 2007;13:164-171. [PubMed] |
| 13. | Lee VN, Nithyananth M, Cherian VM, Amritanand R, Venkatesh K, Sundararaj GD, Raghuram LN. Preoperative embolisation in benign bone tumour excision. J Orthop Surg (Hong Kong). 2008;16:80-83. [PubMed] |
| 14. | Wirbel RJ, Roth R, Schulte M, Kramann B, Mutschler W. Preoperative embolization in spinal and pelvic metastases. J Orthop Sci. 2005;10:253-257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 75] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 15. | Sun S, Lang EV. Bone metastases from renal cell carcinoma: preoperative embolization. J Vasc Interv Radiol. 1998;9:263-269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 71] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 16. | Chatziioannou AN, Johnson ME, Pneumaticos SG, Lawrence DD, Carrasco CH. Preoperative embolization of bone metastases from renal cell carcinoma. Eur Radiol. 2000;10:593-596. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 76] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 17. | Radeleff B, Eiers M, Lopez-Benitez R, Noeldge G, Hallscheidt P, Grenacher L, Libicher M, Zeifang F, Meeder PJ, Kauffmann GW. Transarterial embolization of primary and secondary tumors of the skeletal system. Eur J Radiol. 2006;58:68-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 26] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 18. | Kickuth R, Waldherr C, Hoppe H, Bonel HM, Ludwig K, Beck M, Triller J. Interventional management of hypervascular osseous metastasis: role of embolotherapy before orthopedic tumor resection and bone stabilization. AJR Am J Roentgenol. 2008;191:W240-W247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 39] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 19. | Koike Y, Takizawa K, Ogawa Y, Muto A, Yoshimatsu M, Yagihashi K, Nakajima Y. Transcatheter arterial chemoembolization (TACE) or embolization (TAE) for symptomatic bone metastases as a palliative treatment. Cardiovasc Intervent Radiol. 2011;34:793-801. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 30] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 20. | Al-Hadithy N, Gikas P, Perera J, Aston W, Pollock R, Skinner J, Lotzof K, Cannon S, Briggs T. Pre-operative Embolization of Primary and Secondary Bone Tumours is safe and Effective: a Retrospective Study. World J Oncol. 2011;2:319-322. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 21. | Robial N, Charles YP, Bogorin I, Godet J, Beaujeux R, Boujan F, Steib JP. Is preoperative embolization a prerequisite for spinal metastases surgical management? Orthop Traumatol Surg Res. 2012;98:536-542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 43] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 22. | Kato S, Murakami H, Minami T, Demura S, Yoshioka K, Matsui O, Tsuchiya H. Preoperative embolization significantly decreases intraoperative blood loss during palliative surgery for spinal metastasis. Orthopedics. 2012;35:e1389-e1395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 53] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 23. | Coldwell DM, Stokes KR, Yakes WF. Embolotherapy: agents, clinical applications, and techniques. Radiographics. 1994;14:623-643; quiz 645-646. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 114] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 24. | Barton PP, Waneck RE, Karnel FJ, Ritschl P, Kramer J, Lechner GL. Embolization of bone metastases. J Vasc Interv Radiol. 1996;7:81-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 98] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 25. | Pogoda P, Linhart W, Priemel M, Rueger JM, Amling M. Aneurysmal bone cysts of the sacrum. Clinical report and review of the literature. Arch Orthop Trauma Surg. 2003;123:247-251. [PubMed] |
| 26. | Emori M, Kaya M, Sasaki M, Wada T, Yamaguchi T, Yamashita T. Pre-operative selective arterial embolization as a neoadjuvant therapy for proximal humerus giant cell tumor of bone: radiological and histological evaluation. Jpn J Clin Oncol. 2012;42:851-855. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 13] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 27. | Kobayashi K, Ozkan E, Tam A, Ensor J, Wallace MJ, Gupta S. Preoperative embolization of spinal tumors: variables affecting intraoperative blood loss after embolization. Acta Radiol. 2012;53:935-942. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 39] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 28. | Dahlin DC. Caldwell Lecture. Giant cell tumor of bone: highlights of 407 cases. AJR Am J Roentgenol. 1985;144:955-960. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 135] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 29. | Thiex R, Harris MB, Sides C, Bono CM, Frerichs KU. The role of preoperative transarterial embolization in spinal tumors. A large single-center experience. Spine J. 2013;13:141-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 44] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 30. | Manke C, Bretschneider T, Lenhart M, Strotzer M, Neumann C, Gmeinwieser J, Feuerbach S. Spinal metastases from renal cell carcinoma: effect of preoperative particle embolization on intraoperative blood loss. AJNR Am J Neuroradiol. 2001;22:997-1003. [PubMed] |
| 31. | Olerud C, Jónsson H, Löfberg AM, Lörelius LE, Sjöström L. Embolization of spinal metastases reduces peroperative blood loss. 21 patients operated on for renal cell carcinoma. Acta Orthop Scand. 1993;64:9-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 88] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 32. | Zhang HJ, Yang JJ, Lu JP, Lai CJ, Sheng J, Li YX, Hao Q, Zhang SM, Gupta S. Use of intra-arterial chemotherapy and embolization before limb salvage surgery for osteosarcoma of the lower extremity. Cardiovasc Intervent Radiol. 2009;32:672-678. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 12] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 33. | Berkefeld J, Scale D, Kirchner J, Heinrich T, Kollath J. Hypervascular spinal tumors: influence of the embolization technique on perioperative hemorrhage. AJNR Am J Neuroradiol. 1999;20:757-763. [PubMed] |
| 34. | Hess T, Kramann B, Schmidt E, Rupp S. Use of preoperative vascular embolisation in spinal metastasis resection. Arch Orthop Trauma Surg. 1997;116:279-282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 52] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 35. | Guzman R, Dubach-Schwizer S, Heini P, Lovblad KO, Kalbermatten D, Schroth G, Remonda L. Preoperative transarterial embolization of vertebral metastases. Eur Spine J. 2005;14:263-268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 104] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 36. | Ibrahim WH, Safran ZA, Hasan H, Zeid WA. Preoperative and therapeutic embolization of extremities of bone and soft tissue tumors. Angiology. 2013;64:151-156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |