Published online Oct 28, 2014. doi: 10.4329/wjr.v6.i10.846
Revised: April 8, 2014
Accepted: August 27, 2014
Published online: October 28, 2014
Peripheral primitive neuroectodermal tumor (PNET) of the kidney is a rare, aggressive tumor known for its recurrence and metastatic potential. Despite the frequency of venous extension to the renal veins and inferior vena cava, pulmonary tumor embolism at the initial presentation is not common. We report a case of 22-year-old female with PNET of the kidney who presented with tumor embolism in the inferior vena cava (IVC) and bilateral pulmonary artery. The patient underwent surgical resection and histopathological analysis confirmed the presence of tumor within the IVC and pulmonary arteries. The patient received adjuvant chemotherapy and is currently doing well on follow-up.
Core tip: Traditionally, an aggressive renal mass with pulmonary tumor embolism is an entity well described in renal cell carcinomas. However, renal primitive neuroectodermal tumor (PNET), a rare and aggressive tumor, can have a similar presentation. The severity of the clinical manifestations of pulmonary tumor embolism is highly variable and less predictable. Bland thrombus often mimics tumor emboli in imaging and histopathology is often confirmatory. Tumoral extension into the pulmonary artery is not necessarily associated with pulmonary metastasis. Surgical removal of the thrombus along with the primary tumor followed by adjuvant chemotherapy may prolong the survival in these patients.
- Citation: Chinnaa S, Das CJ, Sharma S, Singh P, Seth A, Purkait S, Mathur SR. Peripheral primitive neuroectodermal tumor of the kidney presenting with pulmonary tumor embolism: A case report. World J Radiol 2014; 6(10): 846-849
- URL: https://www.wjgnet.com/1949-8470/full/v6/i10/846.htm
- DOI: https://dx.doi.org/10.4329/wjr.v6.i10.846
Peripheral primitive neuroectodermal tumor (PNET)/Ewing’s sarcoma belongs to the small round cell tumor family and most commonly occurs in the central nervous system. The rarity of these tumors in the kidneys and their aggressive nature precludes the necessity for complete imaging work up for metastasis preoperatively. Although the radiological features of PNETs are non-specific, multiphasic computed tomography (CT) with 3D reconstruction can assess for vascular invasion and is essential prior to embolectomy. Histopathologically, the differential diagnosis for PNETs is wide since they share immunophenotypical and genetic similarities with other small round cell tumors.
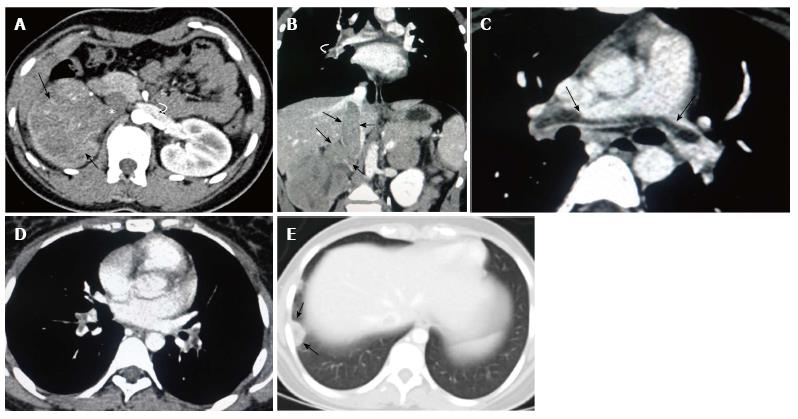
A 22-year-old female presented with complaints of right flank pain and hematuria for six weeks. The patient described the pain as mild, dull, aching and non-radiating. There were six to seven episodes of gross hematuria and each episode subsided spontaneously. There was no history of fever or burning micturition. She was a non smoker and non alcoholic with no significant family history. Blood hemoglobin levels, renal and liver function tests were normal. Abdominal ultrasound showed a heterogeneous right renal mass 7.2 cm × 6.3 cm × 3.1 cm with right renal vein thrombus extending into the inferior vena cava (IVC). There was no hydronephrosis or dilated ureter. Contrast enhanced CT of the chest and abdomen was planned in a helical 256 slice CT scanner (Definition flash, Siemens). After obtaining a non-contrast scan of the renal mass, a bolus of 80-100 mL of IV contrast material (iopromide, ultravist) was administered at a rate of 2-3 mL/s. Non-contrast CT scan revealed an iso-hypodense mass arising from the right kidney without evidence of calcification or hemorrhage. A contrast enhanced CT scan showed a 7.1 cm × 8.2 cm × 4.0 cm inhomogeneous mass with a predominantly non-enhancing center and peripheral rim enhancement suggestive of necrosis (Figure 1A). It was a mass with extension into the renal pelvis. There was no infiltration into the perinephric fat or adjacent organs. Coronal contrast-enhanced CT (CECT) images demonstrated the vascular extension of the tumor mass expanding the lumen of right renal vein and suprarenal part of inferior vena cava (Figure 1B). The presence of contour abnormality of the vessel with area of contact of the tumor exceeding more than 50% of the circumference of the vessel raised suspicion of vascular invasion. The left kidney and left renal vein were normal. Contrast enhanced CT of the chest showed a saddle type non occlusive filling defect in the main pulmonary artery with extension noted into the segmental branches bilaterally (Figure 1C and D). Pleural based patchy consolidation with central lucency was noted in the posterior segment of the right lower lobe (Figure 1E). Considering the shape, location and nature of consolidation, wedge shaped pulmonary infarct from the saddle pulmonary embolism was our first differential compared to cavitating pulmonary metastasis. No evidence of liver or bone metastasis was noted in the CT and the bone scan was normal.
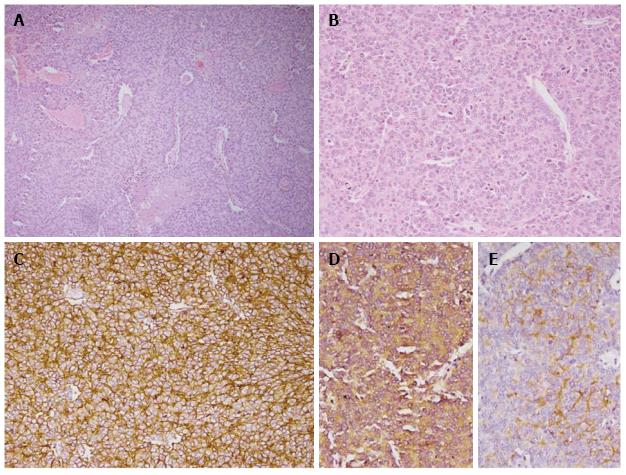
The patient underwent a right radical nephrectomy with IVC thrombectomy and cardiopulmonary bypass with pulmonary embolectomy and right atrial thrombectomy under deep hypothermic cardiac arrest (cardioplegia). The tumor was seen infiltrating into the renal sinus without perinephric extension. Hilar vessels showed tumor emboli in the lumen. Microscopically, the tumor cells were arranged in nests and small sheets with focal rosette formation (Figure 2). The cells demonstrated round nuclei with granular chromatin and scant to moderate cytoplasm. Immunohistochemical analysis revealed diffuse positivity for MIC-2 and focal positivity for neuron specific enolase, CD 56 and synaptophysin, while negative for pan CK, chromogranin. MIB-1 labelling index was 20%-25% at the highest proliferating area. Thrombi within the atrium, IVC and pulmonary artery revealed identical histology of the primary tumor. The patient had an uneventful postoperative period and was started on adjuvant chemotherapy.
Peripheral primitive neuroectodermal tumor/Ewing’s sarcoma (PNET/ES) belongs to “Ewing family of tumors” and comprises 1% of all sarcomas[1]. They have a predilection for the adolescent age group. More commonly, peripheral PNETs are found to arise in the chest wall and paraspinal regions[2]. Skin, soft tissue and viscera (kidney, lungs, adrenal) and the retroperitoneum are less commonly affected[3]. ES/PNET of the kidney with pulmonary tumor embolism is very unusual, with only two cases reported to date[4,5]. A similar presentation of renal PNET with pulmonary embolism was reported in a 30-year-old male and 21-year-old female patient, with tumor resection and thrombectomy done in both cases and later confirmed on histopathology. A good chemotherapeutic response with over a 1 year disease free post operative period was mentioned in the female patient[4].
Renal PNET mostly presents with nonspecific symptoms such as abdominal pain, palpable masses or hematuria. Imaging features of renal PNET are indistinguishable from renal cell carcinoma. Although abdominal ultrasound can visualize the thrombus in the renal vein and suprarenal IVC, contrast enhanced CT is mandatory to identify the cranial extent of tumor into supradiaphragmatic IVC, atrial chambers and pulmonary artery because of the surgical implications. Imaging differentiation of a bland (benign) thrombus and tumor thrombus is necessary because of the high rate of recurrence and dismal prognosis associated with the latter. Tumor thrombus causes expansion of the vascular lumen with thread and streak arterial enhancement and shows continuity with the primary tumor. Restricted diffusion may also be noted in the tumor thrombus. Infiltration of the IVC wall is considered the most specific sign for tumor embolism; however, it is less sensitive and if present, surgical resection of the involved segment of IVC is necessary[6]. CECT is also useful in providing information about the loco regional invasion of the tumor and to identify the presence of metastatic deposits in liver, bone, lungs and lymph nodes.
Histopathologically, several diagnostic techniques need to be adopted to differentiate PNET/ES from other small round cell tumors. Peripheral PNET typically expresses high amounts of the MIC2 antigen (CD99) and exhibits highly characteristic chromosomal translocation [t (11, 22) (q24; q12)][7]. The most important identifying histological feature to diagnose renal PNET is the presence of pseudorosettes[8]. Neuron-specific enolase has been shown to stain positive in 95% of renal PNET cases[8]. EWS gene arrangement, as seen in renal PNET, may also be observed in a desmoplastic small round cell tumor, clear cell sarcoma and neuroblastoma[9].
A case of spontaneous regression of metastatic lung nodules from renal PNET postnephrectomy has been documented[10]. Pulmonary embolism secondary to vascular invasion does not always cause pulmonary metastasis as establishment of pulmonary metastasis requires both mechanical trapping and invasive potential of the tumor cells[11]. The most common sites of metastasis reported in the literature are distant lymph nodes, lungs and liver[8]. Despite the combination of surgery, radiotherapy and chemotherapy, overall 5-year disease-free survival rate for peripheral PNETs is between 45% and 55%[8].
PNET of the kidney is an uncommon tumor and its presentation as a pulmonary tumor embolism is extremely rare. Pulmonary infarct needs to be differentiated from metastasis in such conditions and adequate surgical resection of both primary tumor and thrombus can result in a better outcome. We suggest that in a young patient presenting with a renal mass invading the renal vein, a differential diagnosis of PNET needs to be considered and adequate preoperative imaging to include the chest is mandatory to rule out pulmonary tumor embolism.
Non-radiating flank pain and gross hematuria for six weeks.
Renal malignancy.
Renal calculi.
Anemia.
Renal cell carcinoma.
Primitive neuroectodermal tumor.
Right radical nephrectomy with inferior vena cava (IVC) thrombectomy and cardiopulmonary bypass with pulmonary embolectomy and adjuvant chemotherapy.
Pulmonary tumor embolism is different from thromboembolism.
Not all heterogeneous renal masses with IVC extension are renal cell carcinomas and a preoperative biopsy should be done.
The manuscript describes a very interesting presentation of a rare tumor. The images provided also nicely correlate with the clinical presentation.
P- Reviewer: Reilly C, Triantopoulou C S- Editor: Ji FF L- Editor: Roemmele A E- Editor: Lu YJ
| 1. | Funahashi Y, Hattori R, Yamamoto T, Mizutani K, Yoshino Y, Matsukawa Y, Sassa N, Okumura K, Gotoh M. Ewing’s sarcoma / primitive neuroectodermal tumor of the kidney. Aktuelle Urol. 2009;40:247-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 2. | Gonlusen G, Ergin M, Paydas S, Bolat FA. Primitive neuroectodermal tumor of the kidney: a rare entity. Int Urol Nephrol. 2001;33:449-451. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 3. | Wedde TB, Lobmaier IV, Brennhovd B, Lohne F, Hall KS. Primary Ewing‘s Sarcoma of the Kidney in a 73-Year-Old Man. Sarcoma. 2011;2011:978319. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 4. | Kim MS, Kim B, Park CS, Song SY, Lee EJ, Park NH, Kim HS, Kim SH, Cho KS. Radiologic findings of peripheral primitive neuroectodermal tumor arising in the retroperitoneum. AJR Am J Roentgenol. 2006;186:1125-1132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 41] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 5. | Castro EC, Parwani AV. Ewing sarcoma/primitive neuroectodermal tumor of the kidney: two unusual presentations of a rare tumor. Case Rep Med. 2012;2012:190581. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 6. | Didier D, Racle A, Etievent JP, Weill F. Tumor thrombus of the inferior vena cava secondary to malignant abdominal neoplasms: US and CT evaluation. Radiology. 1987;162:83-89. [PubMed] |
| 7. | Dedeurwaerdere F, Giannini C, Sciot R, Rubin BP, Perilongo G, Borghi L, Ballotta ML, Cornips E, Demunter A, Maes B. Primary peripheral PNET/Ewing’s sarcoma of the dura: a clinicopathologic entity distinct from central PNET. Mod Pathol. 2002;15:673-678. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 74] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 8. | Ellinger J, Bastian PJ, Hauser S, Biermann K, Müller SC. Primitive neuroectodermal tumor: rare, highly aggressive differential diagnosis in urologic malignancies. Urology. 2006;68:257-262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 90] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 9. | Gardner LJ, Ayala AG, Monforte HL, Dunphy CH. Ewing sarcoma/peripheral primitive neuroectodermal tumor: adult abdominal tumors with an Ewing sarcoma gene rearrangement demonstrated by fluorescence in situ hybridization in paraffin sections. Appl Immunohistochem Mol Morphol. 2004;12:160-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 24] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 10. | Bostedt H. [Studying the immobility of sheep in the perinatal period]. Berl Munch Tierarztl Wochenschr. 1976;89:156-161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 11. | Masumori N, Kumamoto Y, Tsukamoto T, Otani N, Miyao N, Yanase M, Takahashi A, Iwabe H. [Studies on pulmonary metastasis of renal cell carcinoma--pulmonary embolism revealed by lung-perfusion imaging and metastasis]. Nihon Hinyokika Gakkai Zasshi. 1991;82:769-775. [PubMed] |