Published online May 28, 2013. doi: 10.4329/wjr.v5.i5.193
Revised: April 18, 2013
Accepted: May 17, 2013
Published online: May 28, 2013
Processing time: 150 Days and 3.8 Hours
Crohn’s disease, a transmural inflammatory bowel disease, remains a difficult entity to diagnose clinically. Over the last decade, multidetector computed tomography (CT) has become the method of choice for non-invasive evaluation of the small bowel, and has proved to be of significant value in the diagnosis of Crohn’s disease. Advancements in CT enterography protocol design, three dimensional (3-D) post-processing software, and CT scanner technology have allowed increasing accuracy in diagnosis, and the acquisition of studies at a much lower radiation dose. The cases in this review will illustrate that the use of 3-D technique, proper enterography protocol design, and a detailed understanding of the different manifestations of Crohn’s disease are all critical in properly diagnosing the full range of possible complications in Crohn’s patients. In particular, CT enterography has proven to be effective in identifying involvement of the small and large bowel (including active inflammation, stigmata of chronic inflammation, and Crohn’s-related bowel neoplasia) by Crohn’s disease, as well as the extra-enteric manifestations of the disease, including fistulae, sinus tracts, abscesses, and urologic/hepatobiliary/osseous complications. Moreover, the proper use of 3-D technique (including volume rendering and maximum intensity projection) as a routine component of enterography interpretation can play a vital role in improving diagnostic accuracy.
Core tip: Advancements in computed tomography (CT) enterography protocol design, three dimensional (3-D) post-processing software, and CT scanner technology have allowed increasing accuracy in diagnosis, and the acquisition of studies at a much lower radiation dose. The cases in this review will illustrate that the use of 3-D technique, proper enterography protocol design, and a detailed understanding of the different manifestations of Crohn’s disease are all critical in properly diagnosing the full range of possible complications in Crohn’s patients.
- Citation: Raman SP, Horton KM, Fishman EK. Computed tomography of Crohn’s disease: The role of three dimensional technique. World J Radiol 2013; 5(5): 193-201
- URL: https://www.wjgnet.com/1949-8470/full/v5/i5/193.htm
- DOI: https://dx.doi.org/10.4329/wjr.v5.i5.193
Crohn’s disease, a form of transmural inflammatory bowel disease affecting over 1.5 million Americans and Europeans, remains a difficult entity to diagnose clinically: While involvement of any segment of the gastrointestinal tract is possible, the disease most often affects the mesenteric small bowel, making direct endoscopic evaluation and biopsy difficult. Moreover, symptoms tend to be nonspecific, and there are no clinical symptoms or laboratory markers which allow a specific diagnosis[1]. With the development of the newest generation of drugs aimed at the treatment of Crohn’s disease (including tumor necrosis factor-α inhibitors, steroids, and salicylic acid), some of which have proven efficacious even in moderate to severe cases, the accurate, timely diagnosis of Crohn’s has become increasingly important[1,2].
Over the last decade, multidetector computed tomography (MDCT) has become the method of choice for non-invasive evaluation of the small bowel, and has proved to be of significant value in the diagnosis of Crohn’s disease[3]. Computed tomography (CT) enterography has proven to be quite effective not only in identifying involvement of the small and large bowel by Crohn’s, but also in the diagnosis of the extra-enteric manifestations of the disease, including fistulae, sinus tracts, and abscesses[4,5]. Improvements in enterography protocols, MDCT scanner technology, and image post-processing software have further improved the utility of MDCT in Crohn’s, allowing increasingly subtle diagnoses, while at the same time, allowing acquisition of studies with markedly reduced radiation doses. This review will focus on the enteric and extra-enteric manifestations of Crohn’s disease on MDCT, the importance of proper MDCT enterography protocols, the use of low-radiation techniques on modern MDCT scanners, and the utility of three dimensional (3-D) technique in improving diagnostic accuracy.
At our institution, all patients undergoing CT enterography are told to avoid any oral intake for at least 4-6 h prior to the study. Positive oral contrast is never used, as beam-hardening artifact from such contrast agents can obscure subtle bowel wall thickening, and make it difficult to appreciate changes in bowel wall and mucosal enhancement. Moreover, positive oral contrast agents can interfere with 3-D post-processing of MDCT data sets, an increasingly important component of enterography interpretation.
Instead, neutral contrast agents are preferred, typically 0.1% wt/vol barium sulfate suspension (VoLumen; Braco Diagnostics, Princeton, NJ, United States), although a few other products are also commercially available[6,7]. Notably, the literature suggests that VoLumen (compared to other neutral and positive contrast media) provides the best distension of the small bowel. Neutral contrast agents, which are near water density (but are not absorbed as rapidly as ingested water), are effective in distending the small bowel, but at the same time, allow detailed evaluation of small bowel wall thickness, density, and enhancement, without any negative impact upon 3-D post-processing[1,8].
Several different protocols have been described in the literature regarding the administration of oral contrast media for CT enterography, including protocols solely comprised of VoLumen, protocols with a combination of water and VoLumen, and protocols composed almost entirely of water[9]. Our institution’s enterography protocol involves the administration of a total of 1350 cc of VoLumen (450 cc at 60 min prior to scanning, 450 cc at 40 min prior to scanning, and 450 cc at 20 min prior to scanning), followed by 500 cc of water 10 min before scanning. While this administration schedule represents the ideal, it is important to note that many patients may be unable to tolerate the ingestion of this large a volume of contrast media[1]. Even when patients are unable to drink the entire volume of oral contrast, adequate distension is still often possible.
Subsequently, a rapid injection of 100 cc of intravenous (IV) contrast is performed (3-5 cc/s), with the acquisition of both arterial and venous phase images at 30 s and 60 s respectively. The arterial phase images are critical for appreciating subtle bowel wall or mucosal hyperenhacement, as well as engorgement of the adjacent vasa recta, all of which are important signs of bowel inflammation. The venous phase images are important not only for evaluating the bowel, but also the other parenchymal organs of the abdomen (i.e., liver, spleen, etc.), the extra-enteric manifestations of Crohn’s disease, the venous mesenteric vasculature, and hypovascular bowel tumors.
Images are acquired with thin collimation, with acquisition of 0.625-0.75 mm slices, which are then reconstructed into 3-5 mm axial slices for routine interpretation. Coronal and sagittal multiplanar reconstructions are directly created at the CT scanner following the acquisition of the axial source images. At the same time, isotropic 0.5-0.75 mm images are used for 3-D post-processing.
At our institution, two separate sets of 3-D reconstructions are interactively created by the interpreting radiologist at an independent workstation: (1) Maximum intensity projection (MIP) imaging is based upon a computer algorithm which extracts the highest attenuation voxels in a data set, and projects these voxels into a 3-D display which can be manipulated and rotated by the radiologist into the desired plane. These images have proven the most effective for evaluation of the mesenteric vasculature, and are useful not only for visualizing the main aortic branch vessels, but also tiny mesenteric branches which are typically not readily visualized on the axial source images. Areas of bowel hyperemia and mesenteric vascular engorgement (i.e., “comb sign”, opacification of the vasa recta) are also easily identified using this technique; and (2) Volume rendering (VR) is based upon a more complex computer algorithm which assigns a specific color and transparency to each voxel in a data set based on its underlying attenuation (and relationship to other adjacent voxels), before projecting this data into an interactive 3-D display. We have found this technique to be most useful in displaying the entirety of the small bowel, and illustrating the relationship of adjacent small bowel loops, subtle areas of bowel wall thickening, abnormal mucosal enhancement, and extra-enteric manifestations of Crohn’s disease[10-12].
It is important to be cognizant that (1) the peak incidence of Crohn’s disease is in patients between the ages of 20-40 years; (2) a sizeable percentage of cases are diagnosed in children (15%); and (3) the disease has a mild female predominance[13,14]. In other words, Crohn’s disease is most often diagnosed in a particularly radiation-sensitive population, and the waxing and waning course of the disease (with multiple relapses over the patient’s lifetime) places the patient at risk for a significant cumulative lifetime radiation dose[13-16]. However, several dose-reduction techniques are now available on the latest generation of CT scanners, all of which should be used for Crohn’s patients (when available). These include (1) automated tube current modulation, which alters the tube current (mAs) based on the patient’s size and density; (2) automated tube potential modulation, which alters the scanner’s tube potential (kVp) based on the patient’s size and density; and (3) iterative reconstruction, an alternative to traditional filtered back projection reconstruction techniques, which allows the acquisition and reconstruction of diagnostic quality images at far lower radiation doses[17]. Notably, while the details of each of these dose-reduction techniques is beyond the scope of this article, several studies have illustrated that enterography studies in patients with Crohn’s disease can be performed at substantially lower radiation doses using these techniques, and can still be interpreted with a high degree of diagnostic confidence by the radiologist[13-17].
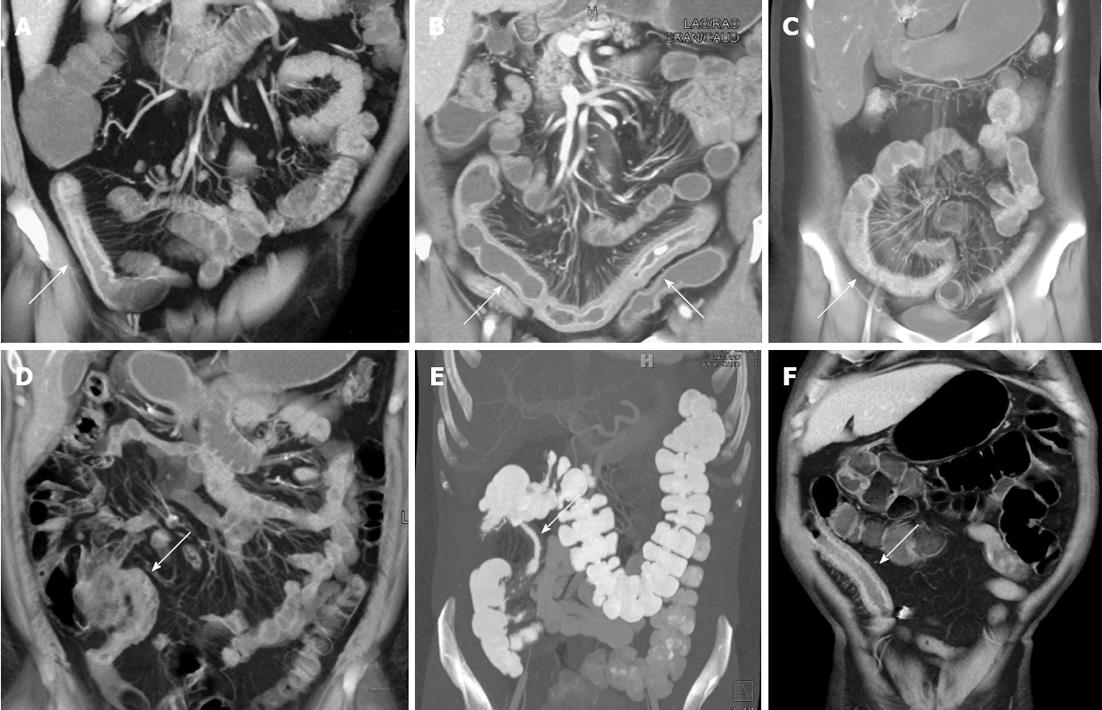
Crohn’s disease can involve any portion of the gastrointestinal tract from the mouth to the anus, although the small bowel is the most commonly affected portion of the bowel, particularly the distal and terminal ileum (Figure 1)[18]. The earliest phases of small bowel inflammation may be characterized only by subtle mucosal hyperenhancement on the arterial phase images, with little or no wall thickening or venous phase enhancement abnormalities[19,20]. However, as the degree of inflammation progresses, thickening of the bowel wall is typically visualized (in addition to frank mucosal hyperemia on the venous phase images), with evidence of mural stratification (“target” or “double-halo appearance”)[19]. This mural stratification most often represents the juxtaposition of avidly enhancing mucosa with hypodense submucosal edema in the bowel wall itself, and in some cases, hyperemia of the serosal surface of the bowel[21].
Clearly, interpretation of wall thickening must take into account the degree of luminal distention, but a wall thickness of > 3 mm in well distended small bowel loops has traditionally been considered as abnormal[22]. Although sometimes difficult to appreciate even on the highest quality studies, this wall thickening usually begins on the mesenteric side of the bowel, before progressing towards the antimesenteric side[19]. Notably, more than the wall thickening itself, the degree of mucosal enhancement most highly correlates with disease activity, although one must be careful not to confuse pathologic hyperenhancement with the normal greater enhancement of the jejunum relative to the ileum on arterial phase images. Similarly, collapsed bowel loops often appear to have higher attenuation walls, a finding which should not be confused with pathologic hyperenhancement[18,23].
Coronal multiplanar reformats, volume rendered images, and MIP images can be very helpful in properly evaluating abnormal small bowel loops. The coronal reformats are often most useful to visualize the small bowel as a whole, and better gauge which small bowel loops are truly abnormal, rather than simply collapsed. In a study by Liu et al[19], several instances of abnormal small bowel loops were not perceptible on the standard axial images, but were clearly present on coronal multiplanar reformats. The coronal reformations can also be helpful in cases of small bowel obstruction as a result of active inflammation, particularly in identifying the site of transition. Moreover, subtle mucosal hyperemia and thickening is often best appreciated on the volume rendered and MIP images, which accentuate these abnormalities. The use of clip planes is helpful to ensure visualization of the entire mesenteric small bowel, by removing overlapping loops, and following the entire bowel from duodenum to the terminal ileum.
While involvement of the small bowel is more common, Crohn’s disease can also involve the large bowel, and in some cases, affect only the large bowel without small bowel involvement. While findings similar to those previously described in the small bowel should be sought, it should be noted that CT enterography studies are not designed to optimally distend the colon. As a result, the determination of bowel wall thickening and mucosal hyperenhancement should be made carefully, particularly when the colon is largely decompressed.
In the chronic phases of the disease, intramural deposition of fat is a common finding, and hypodense/soft tissue attenuation wall thickening and mucosal hyperemia should not be present in the absence of active inflammation (Figure 2). Notably, however, intramural fat deposition is a nonspecific finding that can be seen not only in other causes of chronic bowel inflammation, but also in the setting of obesity, steroid use, and diabetes[8]. As a result of the disease’s preferential involvement of the mesenteric side of the bowel, asymmetric fibrosis and pseudosacculations along the mesenteric border are also common in the chronic setting[6].
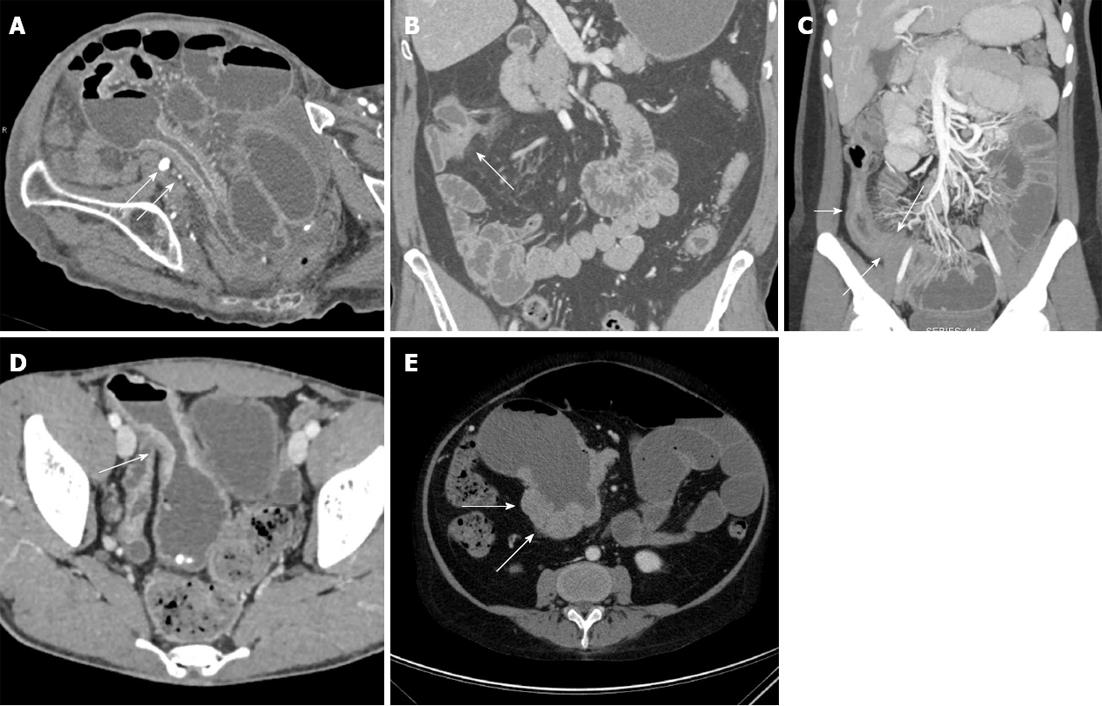
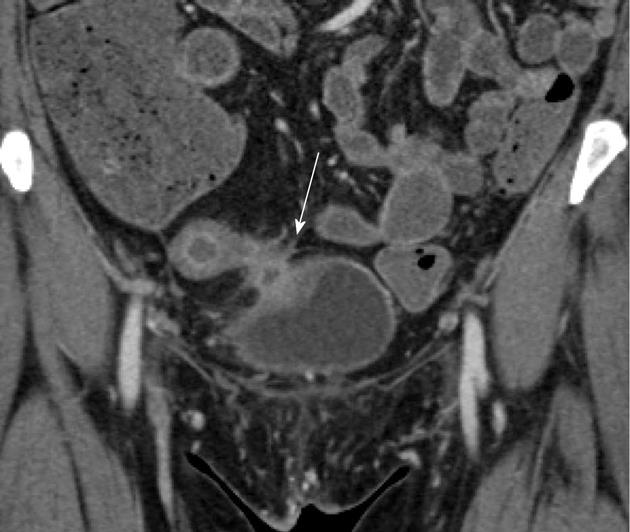
CT enterography can also be helpful in identifying sites of strictures and narrowing, representing sites of fibrosis as a result of prior bouts of active inflammation. However, while sites of narrowing and thickening can be identified on CT, it is not always easy to distinguish a true stricture from peristalsis. Signs of true bowel obstruction should be sought, including proximal bowel dilatation with a discrete caliber transition at the stricture, distal decompression of small bowel loops, and fecal material in the proximal small bowel as a result of delayed bowel transit and stasis (Figure 3A-C). In some cases, it can be difficult to determine if a site of luminal narrowing is secondary to active inflammation or chronic fibrosis, particularly in the absence of adjacent inflammatory change and mesenteric hyperemia[24]. Regardless of whether luminal narrowing is acute or chronic, the presence of a stricture is a critical finding to communicate to gastroenterologists, as small bowel endoscopy in this setting can result in capsule retention and small bowel obstruction[6].
Patients with Crohn’s disease are at increased risk for both small bowel and colonic adenocarcinoma and lymphoma (Figure 3D and E). Corresponding to the most common sites of inflammation in Crohn’s disease patients, the most common sites of small bowel adenocarcinoma are in the distal and terminal ileum, as opposed to the general population, where small bowel adenocarcinomas are most common in the duodenum. The overall risk of small bowel adenocarcinoma may be 15-50 times greater than in the general population, and are most commonly seen at the sites of greatest inflammation in each specific patient[25].
As a result, given the absence of any other clear means by which to screen the small bowel for tumors, the possibility of a tumor must be considered when evaluating any CT enterography study. In addition to the classic appearances of a tumor (i.e., focal soft tissue mass, ulcerated nodule, annular constricting mass or “apple-core” lesion), any abnormal bowel loop must be evaluated critically: Any fixed site of narrowing (whether inflammatory or fibrotic) should be treated as a site of suspicion until proven otherwise, even if a discrete soft tissue mass is not identified. Moreover, asymmetric wall thickening and irregularity should not automatically be assumed to simply represent a site of active inflammation, particular if mural stratification of the thickened wall is not seen.
In a series by Soyer et al[25], four different patterns were seen with Crohn’s related small bowel adenocarcinomas: (1) focal soft tissue mass; (2) short severe stenosis; (3) long stenosis with wall irregularity; and (4) irregular circumferential wall thickening of a bowel loop. The use of VR techniques in the coronal plane can be particularly useful in some of these cases, nicely illustrating the irregularity and mass-like nature of some areas of wall thickening, and suggesting the presence of a neoplasm. It is also critical to assess local adenopathy. Although reactive nodes are commonly noted in patients with active Crohn’s disease, large nodes (> 2 cm) should raise the possibility of an underlying malignancy.
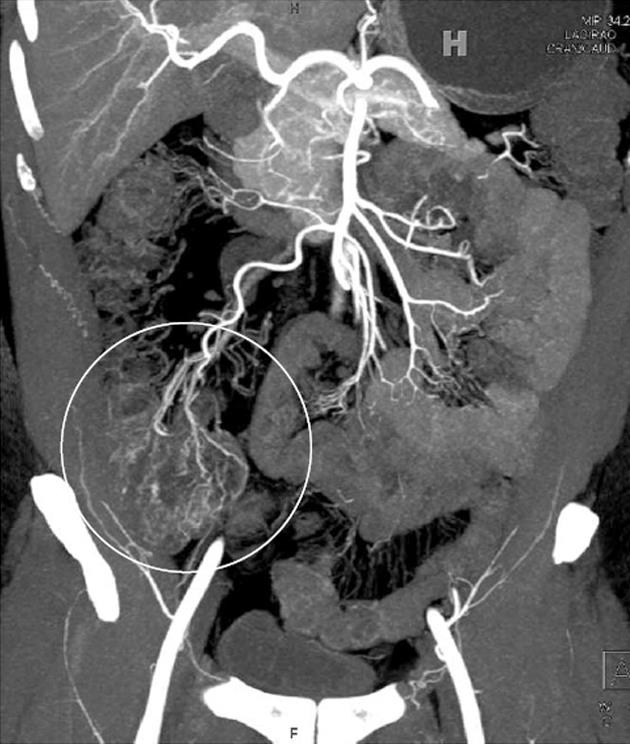
In the acute inflammatory setting, engorgement of the vasa recta, mesenteric hyperemia, fat stranding, and increased attenuation of the mesenteric fat are all common imaging findings, and are typically localized adjacent to the sites of greatest bowel inflammation (Figures 1A-C and 4). These signs are particularly important in those cases where bowel wall thickening and mucosal hyperemia are equivocal, as well as those cases where collapsed loops of bowel limit subtle evaluation of the bowel wall and mucosa (Figure 4). All of these mesenteric findings have been associated with active bowel inflammation, elevated C-reactive protein levels, and severity of disease, and are important findings to note in every examination[22]. In particular, engorgement of the vasa recta (sometimes termed the “comb” sign) is often best appreciated on coronal MIP images, which accentuate those areas of greatest vascular engorgement.
Over time, as a result of multiple bouts of active inflammation, fibrofatty proliferation (often termed as “creeping fat”) can develop along the mesenteric border of the involved bowel segments (Figure 2). This fatty proliferation is associated with chronic disease, although it is unclear whether this fat is merely reactive to the patient’s chronic inflammation, or alternatively, is hormonally active and may potentially drive the patient’s inflammation[8,22].
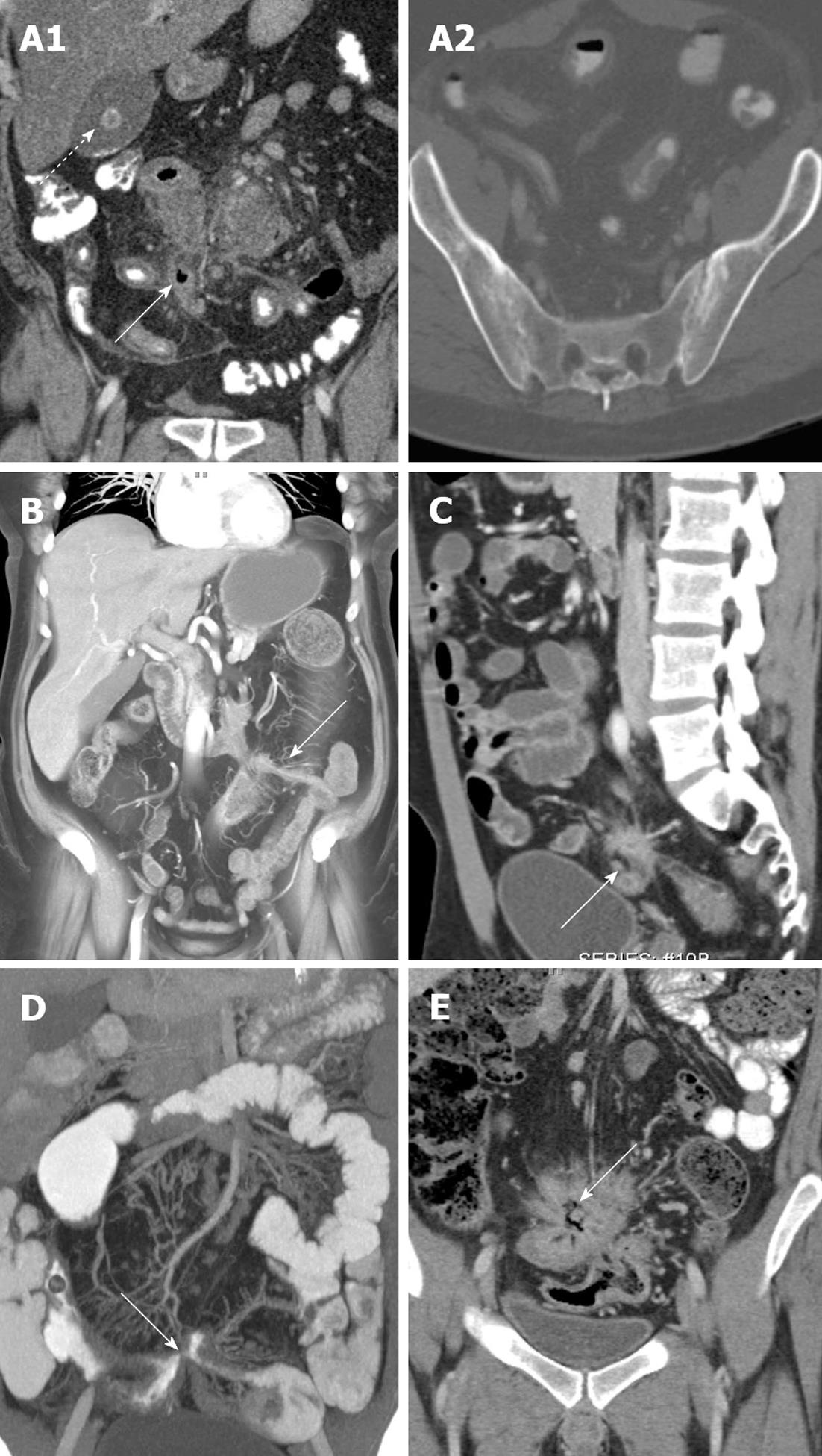
Up to 1/3 of Crohn’s patients develop a fistula within the first ten years after exhibiting symptoms of Crohn’s disease (Figures 5 and 6A). While the perianal region is the most common site of fistula formation, fistulas can develop anywhere in the abdomen, including enteroenteric, coloenteric, colocolic, rectovaginal, enterocutaneous, and enterovesicular fistulas. The sensitivity of CT for fistulas may be as high as 94%, although the appearance can be subtle in some cases. In the most obvious cases, an enhancing tract can be traced, clearly identifying the presence of a fistula[8].
However, in many cases a discrete tract will not be identified, and the presence of a fistula must be surmised by secondary signs. In particular, the presence of ectopic gas in the midst of bowel loops, tethering and spiculation of adjacent bowel loops, and soft tissue stranding and density in the midst of tethered bowel loops can be seen in the presence “complex fistulizing” Crohn’s disease. In such cases, these imaging features are suggestive of the presence of fistulous tracts connecting these abnormally oriented loops of bowel (Figure 5A, C and E). Ectopic gas in other locations, including the bladder and subcutaneous soft tissues, should also raise concern for a fistula, and should not automatically be assumed to be secondary to a foley catheter or soft tissue injections[22]. Notably, CT is much less sensitive to the presence of a perianal fistula compared to magnetic resonance imaging, and a discrete tract or hyperenhancement is very rarely visualized on CT[26]. Nevertheless, the presence of any soft tissue stranding, induration, or fluid in this location should raise concern, and at the very least, should precipitate clinical examination of this area (Figure 6A)[8].
From a protocol perspective, while the use of a neutral oral contrast agent is the norm in CT enterography studies, better delineation of a fistula is one of the few indications where a positive oral contrast agent may be helpful. The use of volume rendered images can also be very useful in delineating the full extent of a patient’s fistulous disease, particularly in cases of complex fistulizing Crohn’s disease with multiple involved bowel loops. Coronal VR images can improve visualization of sites of involvement, delineate the size and extent of fistulous tracts, and in some cases, can facilitate visualization of fistulous tracts which are difficult to appreciate on routine axial images. Finally, patients with Crohn’s disease are at high risk of developing abscesses in the leaves of the mesentery, some of which can fistulize with the adjacent bowel (Figure 6B). These abscesses can be difficult to visualize, and can blend in with adjacent bowel loops given the routine use of VoLumen in CT enterography studies.
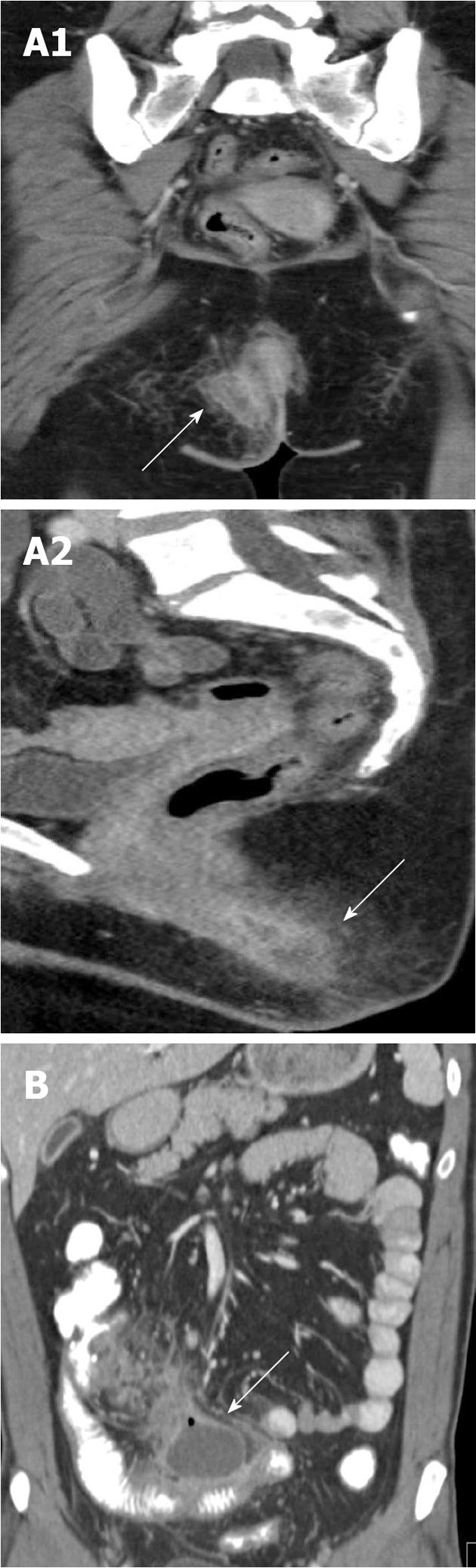
In the setting of acute Crohn’s-related inflammatory disease in the abdomen, the two most common severe urological complications are the development of (1) obstructive uropathy; and (2) enterovesicular fistulas: The development of obstructive uropathy is relatively common in Crohn’s, and may be present in up to 6% of patients with acute inflammatory disease. Most common on the right side, hydronephrosis and hydroureter are typically the result of either acute inflammatory change enveloping a portion of the ureter, or alternatively, fibrotic narrowing of the ureter as a result of a prior inflammatory episode[27].
Enterovesicular fistulas are a rare, but serious, complication, present in up to 3.5% of patients. Typically the result of an adjacent inflamed loop of bowel, women are less likely to develop this complication because of the protective presence of the uterus and adnexa. In the most obvious cases, a direct enhancing tract can be identified extending from an adjacent bowel loop (usually ileum) to the bladder. However, in the absence of directly visualizing a tract, the presence of ectopic gas in the bladder, focal bladder wall thickening adjacent to an inflamed loop of bowel, or the tethering of a bowel loop towards the bladder should all raise concern for the presence of a fistula. Evaluation of the bladder in the coronal plane using multiplanar reformations and VR is a necessity, especially when abnormal bowel loops are identified in close proximity to the bladder (Figure 7)[27]. Notably, patients with Crohn’s disease are also at increased risk of developing both renal stones and urinary infections, even in the absence of an active inflammatory episode[27].
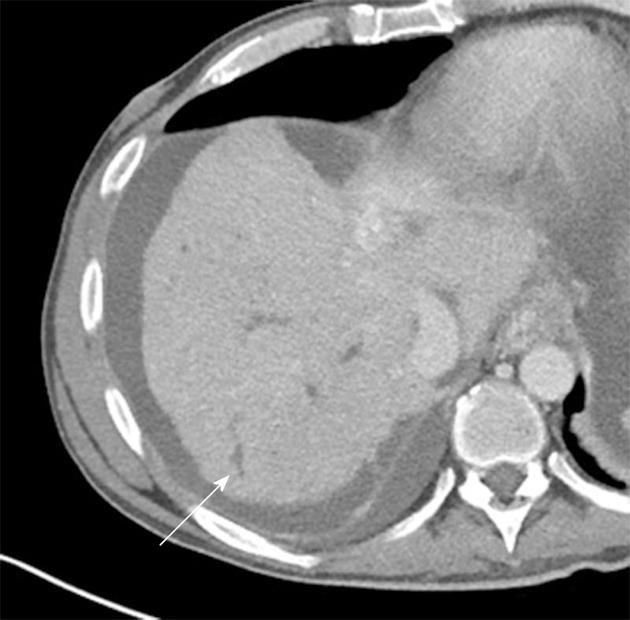
In addition to the previously mentioned predilection for renal stones, patients with Crohn’s disease also demonstrate an increased incidence of gallstones (perhaps up to 9.3% in one series)[28] (Figure 5A). Moreover, although rare, there is a known association between Crohn’s disease and primary sclerosing cholangitis (PSC). Although this entity may sometimes be difficult to appreciate on CT, the presence of ductal beading and irregularity, cirrhosis, and significant enlargement of the caudate lobe are all signs which should be suggestive of PSC in the setting of known Crohn’s disease (Figure 8).
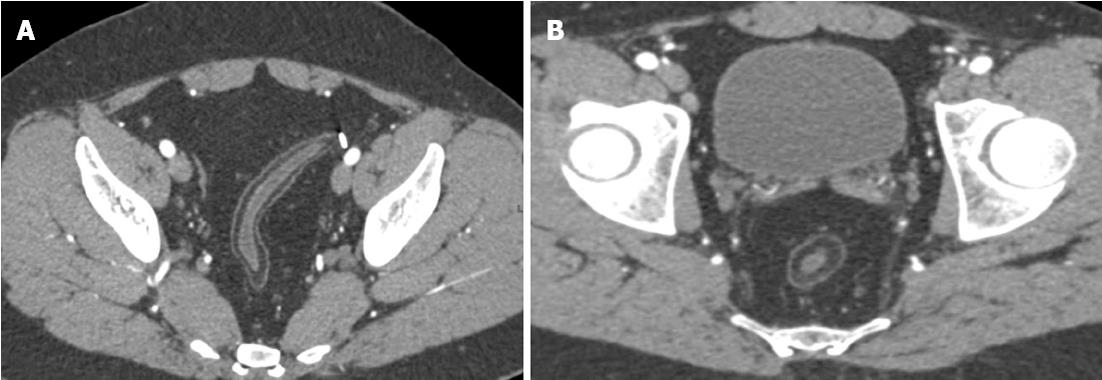
The association between sacroiliitis and Crohn’s disease has been well described in the literature, with between 11%-35% of patients with Crohn’s disease demonstrating evidence of bilateral, symmetric sacroiliitis on either CT or nuclear medicine studies[29]. Careful attention should be paid to the sacroiliac joints on every study in a Crohn’s patient, searching for evidence of joint space narrowing, erosions, sclerosis, and fusion[5] (Figure 5A). Moreover, given the relatively common use of steroids and other immunomodulators in the treatment of Crohn’s, the radiologist must carefully note any evidence of new sclerosis, deformity, or irregularity of either the femoral or humeral heads, as the use of these medications places this patient population at increased risk of avascular necrosis[5].
There is a roughly four-fold increased risk of non-Hodgkins lymphoma in patients with Crohn’s disease, although it is unclear whether this increased risk is secondary to these patients’ underlying Crohn’s disease (and disease severity), or the use of immunomodulating drugs (such as azathioprine and 6-mercaptopurine)[30] (Figure 3E).
The utility of MDCT in the diagnosis of Crohn’s disease and its complications is undeniable, with a proven efficacy in identifying the enteric and extra-enteric manifestations of the disease. However, advancements in CT enterography protocol design, 3-D post-processing software, and CT scanner technology have allowed increasing accuracy in diagnosis, and the acquisition of studies at a much lower radiation dose. As the cases in this review illustrate, the use of 3-D technique, proper protocol design, and a detailed understanding of the different manifestations of Crohn’s disease are all critical in properly diagnosing the full range of possible complications in Crohn’s patients.
P- Reviewer Plataniotis G S- Editor Huang XZ L- Editor A E- Editor Ma S
| 1. | Huprich JE, Fletcher JG. CT enterography: principles, technique and utility in Crohn’s disease. Eur J Radiol. 2009;69:393-397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 61] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 2. | Wu YW, Tang YH, Hao NX, Tang CY, Miao F. Crohn’s disease: CT enterography manifestations before and after treatment. Eur J Radiol. 2012;81:52-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 31] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 3. | Raptopoulos V, Schwartz RK, McNicholas MM, Movson J, Pearlman J, Joffe N. Multiplanar helical CT enterography in patients with Crohn’s disease. AJR Am J Roentgenol. 1997;169:1545-1550. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 105] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 4. | Hara AK, Leighton JA, Heigh RI, Sharma VK, Silva AC, De Petris G, Hentz JG, Fleischer DE. Crohn disease of the small bowel: preliminary comparison among CT enterography, capsule endoscopy, small-bowel follow-through, and ileoscopy. Radiology. 2006;238:128-134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 226] [Cited by in RCA: 189] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 5. | Kerner C, Carey K, Mills AM, Yang W, Synnestvedt MB, Hilton S, Weiner MG, Lewis JD. Use of abdominopelvic computed tomography in emergency departments and rates of urgent diagnoses in Crohn’s disease. Clin Gastroenterol Hepatol. 2012;10:52-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 38] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 6. | Elsayes KM, Al-Hawary MM, Jagdish J, Ganesh HS, Platt JF. CT enterography: principles, trends, and interpretation of findings. Radiographics. 2010;30:1955-1970. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 92] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 7. | Al-Hawary M, Zimmermann EM. A new look at Crohn’s disease: novel imaging techniques. Curr Opin Gastroenterol. 2012;28:334-340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 8. | Paulsen SR, Huprich JE, Fletcher JG, Booya F, Young BM, Fidler JL, Johnson CD, Barlow JM, Earnest F. CT enterography as a diagnostic tool in evaluating small bowel disorders: review of clinical experience with over 700 cases. Radiographics. 2006;26:641-657; discussion 657-662. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 326] [Cited by in RCA: 282] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 9. | Furukawa A, Saotome T, Yamasaki M, Maeda K, Nitta N, Takahashi M, Tsujikawa T, Fujiyama Y, Murata K, Sakamoto T. Cross-sectional imaging in Crohn disease. Radiographics. 2004;24:689-702. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 194] [Cited by in RCA: 136] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 10. | Johnson PT, Horton KM, Fishman EK. Nonvascular mesenteric disease: utility of multidetector CT with 3D volume rendering. Radiographics. 2009;29:721-740. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 11. | Raman SP, Horton KM, Fishman EK. Transitional cell carcinoma of the upper urinary tract: optimizing image interpretation with 3D reconstructions. Abdom Imaging. 2012;37:1129-1140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 15] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 12. | Raman SP, Horton KM, Fishman EK. Multimodality imaging of pancreatic cancer-computed tomography, magnetic resonance imaging, and positron emission tomography. Cancer J. 2012;18:511-522. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 80] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 13. | Kambadakone AR, Chaudhary NA, Desai GS, Nguyen DD, Kulkarni NM, Sahani DV. Low-dose MDCT and CT enterography of patients with Crohn disease: feasibility of adaptive statistical iterative reconstruction. AJR Am J Roentgenol. 2011;196:W743-W752. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 92] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 14. | Kambadakone AR, Prakash P, Hahn PF, Sahani DV. Low-dose CT examinations in Crohn’s disease: Impact on image quality, diagnostic performance, and radiation dose. AJR Am J Roentgenol. 2010;195:78-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 67] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 15. | Kielar AZ, Tao H, McKeever C, El-Maraghi RH. Low-Radiation-Dose Modified Small Bowel CT for Evaluation of Recurrent Crohn’s Disease. Gastroenterol Res Pract. 2012;2012:598418. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 16. | Lee SJ, Park SH, Kim AY, Yang SK, Yun SC, Lee SS, Jung GS, Ha HK. A prospective comparison of standard-dose CT enterography and 50% reduced-dose CT enterography with and without noise reduction for evaluating Crohn disease. AJR Am J Roentgenol. 2011;197:50-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 89] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 17. | Raman SP, Johnson PT, Deshmukh S, Mahesh M, Grant KL, Fishman EK. CT dose reduction applications: available tools on the latest generation of CT scanners. J Am Coll Radiol. 2013;10:37-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 102] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 18. | Zalis M, Singh AK. Imaging of inflammatory bowel disease: CT and MR. Dig Dis. 2004;22:56-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 23] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 19. | Liu YB, Liang CH, Zhang ZL, Huang B, Lin HB, Yu YX, Xie SF, Wang QS, Zheng JH. Crohn disease of small bowel: multidetector row CT with CT enteroclysis, dynamic contrast enhancement, CT angiography, and 3D imaging. Abdom Imaging. 2006;31:668-674. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 23] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 20. | Gatta G, Di Grezia G, Di Mizio V, Landolfi C, Mansi L, De Sio I, Rotondo A, Grassi R. Crohn’s disease imaging: a review. Gastroenterol Res Pract. 2012;2012:816920. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 29] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 21. | Lalitha P, Reddy MCh, Reddy KJ, Kumari MV. Computed tomography enteroclysis: a review. Jpn J Radiol. 2011;29:673-681. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 22. | Hara AK, Swartz PG. CT enterography of Crohn’s disease. Abdom Imaging. 2009;34:289-295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 42] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 23. | Booya F, Fletcher JG, Huprich JE, Barlow JM, Johnson CD, Fidler JL, Solem CA, Sandborn WJ, Loftus EV, Harmsen WS. Active Crohn disease: CT findings and interobserver agreement for enteric phase CT enterography. Radiology. 2006;241:787-795. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 139] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 24. | Dillman JR, Adler J, Zimmermann EM, Strouse PJ. CT enterography of pediatric Crohn disease. Pediatr Radiol. 2010;40:97-105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 33] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 25. | Soyer P, Hristova L, Boudghène F, Hoeffel C, Dray X, Laurent V, Fishman EK, Boudiaf M. Small bowel adenocarcinoma in Crohn disease: CT-enterography features with pathological correlation. Abdom Imaging. 2012;37:338-349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 24] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 26. | Pariente B, Peyrin-Biroulet L, Cohen L, Zagdanski AM, Colombel JF. Gastroenterology review and perspective: the role of cross-sectional imaging in evaluating bowel damage in Crohn disease. AJR Am J Roentgenol. 2011;197:42-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 40] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 27. | Tonolini M, Villa C, Campari A, Ravelli A, Bianco R, Cornalba G. Common and unusual urogenital Crohn’s disease complications: spectrum of cross-sectional imaging findings. Abdom Imaging. 2013;38:32-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 28. | Bruining DH, Siddiki HA, Fletcher JG, Tremaine WJ, Sandborn WJ, Loftus EV. Prevalence of penetrating disease and extraintestinal manifestations of Crohn’s disease detected with CT enterography. Inflamm Bowel Dis. 2008;14:1701-1706. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 99] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 29. | Steer S, Jones H, Hibbert J, Kondeatis E, Vaughan R, Sanderson J, Gibson T. Low back pain, sacroiliitis, and the relationship with HLA-B27 in Crohn’s disease. J Rheumatol. 2003;30:518-522. [PubMed] |
| 30. | Koronakis N, Lagoudianakis E, Keramidaris D, Pappas A, Gemenetzis G, Seretis C, Chrysikos J, Manouras A. Mesentery lymphoma in a patient with Crohn’s disease: An extremely rare entity. Int J Surg Case Rep. 2012;3:343-345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |