Published online Dec 28, 2013. doi: 10.4329/wjr.v5.i12.491
Revised: October 26, 2013
Accepted: November 18, 2013
Published online: December 28, 2013
Processing time: 123 Days and 1.7 Hours
AIM: To study the prevalence and patterns of hepatic abnormal perfusion (HAP) visible by magnetic resonance imaging (MRI) in acute pancreatitis (AP).
METHODS: Enhanced abdominal MRI was performed on 51 patients with AP. These patients were divided into two groups according to the MRI results: those with signs of gallstones, cholecystitis, common bile duct (CBD) stones or dilatation of the CBD on MRI and those without. The prevalence, shape and distribution of HAP in the two groups were analyzed and compared. The severity of AP was graded using the MR severity index (MRSI). The correlation between the MRSI and HAP was then analyzed.
RESULTS: Of the 51 patients with AP, 32 (63%) showed at least one sign of gallbladder and CBD abnormalities on the MR images, while 19 (37%) showed no sign of gallbladder or CBD abnormalities. Nineteen patients (37%) had HAP visible in the enhanced images, including strip-, wedge- or patch-shaped HAP distributed in the hepatic tissue adjacent to the gallbladder and left and right liver lobes. There were no significant differences in the prevalence of HAP (χ2 = 0.305, P = 0.581 > 0.05) or HAP distribution in the liver (χ2 = 2.181, P = 0.536 > 0.05) between patients with and without gallbladder and CBD abnormalities. There were no significant differences in the MRSI score between patients with and without HAP (t = 0.559, P = 0.552 > 0.05). HAP was not correlated with the MRSI score.
CONCLUSION: HAP is common in patients with AP and appears strip-, patch- or wedge-shaped on MRI. HAP on MRI cannot be used to indicate the severity of AP.
Core tip: Hepatic abnormal perfusion (HAP) due to acute pancreatitis on enhanced magnetic resonance imaging (MRI) presents as a strip-shaped abnormality of the hepatic tissue adjacent to the gallbladder or a patch- or wedge-shaped abnormality with lobar distribution in the liver, which is most likely caused by both the inflamed gallbladder and acute pancreatitis. Indications of HAP resulting from acute pancreatitis should not be misinterpreted as primary liver abnormalities. The presence of HAP on MRI cannot be used to indicate the severity of acute pancreatitis.
- Citation: Tang W, Zhang XM, Zhai ZH, Zeng NL. Hepatic abnormal perfusion visible by magnetic resonance imaging in acute pancreatitis. World J Radiol 2013; 5(12): 491-497
- URL: https://www.wjgnet.com/1949-8470/full/v5/i12/491.htm
- DOI: https://dx.doi.org/10.4329/wjr.v5.i12.491
The pancreas is supplied by a rich plexus of arteries, derived from multiple branches of the coeliac trunk and superior mesenteric artery. Peripancreatic vessels are rich and complicated. Abnormal changes in major vessels and the microvasculature can be found in acute pancreatitis (AP), and the pathology of pancreatic and peripancreatic microcirculation has a significant impact on the early development and progression of the disease[1,2]. AP affects both the systematic and pancreatic vasculature[3-5]. The abnormal changes are not confined to the pancreas and may affect the gastrointestinal tract, liver, lung, kidney and skeletal muscle[6-11].
In AP, ex-pancreatic organ abnormalities are indicative of higher disease severity that can exacerbate pancreatic and systemic injury[1,10]. The identification of ex-pancreatic organ abnormalities is essential for predicting the severity of AP. Ex-pancreatic organ abnormalities have been found in the gallbladder and abdominal wall using magnetic resonance imaging (MRI); these abnormalities were correlated with the severity of AP[12,13].
The liver is adjacent to the pancreas; abnormalities may be observed in the liver early in the course of AP because of abnormal peripancreatic microcirculation[8]. Liver abnormality has also been identified using computed tomography (CT) in patients with AP, while hepatic perfusion disorder was found in a different anatomic site of the liver[14]. Other studies have used various terms for hepatic perfusion disorder including transient hepatic signal intensity differences, transient hepatic attenuation differences, or transient hepatic parenchyma enhancement[15-17]. This study refers to these phenomena as hepatic abnormal perfusion (HAP). Causes of HAP include hepatic vein abnormality, hepatic tumor, liver cirrhosis, inflammatory changes of the liver or organs adjacent to the liver, and others[15,16].
Imaging tools including CT and MRI play an important role in predicting the severity of AP by revealing abnormalities of the pancreas, peri-pancreas and ex-pancreatic organs[12,13,18-20]. Arita et al[14] reported that HAP in AP is most likely caused by increased arterial blood flow, the result of an inflamed lobe of the liver or inflamed gallbladder; HAP disappeared when AP had subsided in 5 of 28 patients (according to CT imaging). Is HAP related to the severity of AP and inflamed gallbladder
To the best of our knowledge, this is the first report of HAP in AP on MRI. We present the spectrum of various HAP of AP visible by MRI, including the morphology, cause, prevalence, distribution, and pathogenesis of HAP as well as any association between HAP and the severity of AP.
This retrospective study was approved by our institutional review board. Patient informed consent was waived. Patients with AP admitted to our institution between February 2010 and February 2013 were considered for inclusion in this study. The diagnosis of AP was confirmed by the presence of acute typical abdominal pain and elevated serum levels of amylase or lipase, with levels three times normal indicating AP. The recruitment criteria for patients in this study were as follows: (1) AP at first onset; (2) enhanced abdominal MR examination; (3) upper abdominal MR examinations within 3 d of admission; (4) no related hepatic disease; (5) no hepatic vessel abnormality; and (6) the presence of a gallbladder.
Of the 51 patients enrolled in the study, there were 18 women and 33 men, with an average age of 49 ± 15 years (range 17 to 82 years).
MRI was performed on a 1.5-T MR scanner with 38 mT/m gradients and 120 mT/m per second slope (Signa Excite; GE Medical Systems, Milwaukee, Wis), using a phased-array torso-pelvis coil. The sequences included axial fast spoiled gradient echo (FSPGR) T1-weighted imaging with fat suppression, gradient-echo (GRE) T1-weighted in-phase and out-of-phase MRI, respiratory-triggered (R-T) axial fast recovery fast spin-echo (FRFSE) T2-weighted MRI with fat suppression, coronal and axial single shot fast spin-echo (SSFSE) T2-weighted MRI, SSFSE radial series slabs MR cholangiopancreatography (MRCP), and three-dimension (3D) FSPGR dynamic enhanced MRI.
FSPGR T1-weighted imaging with fat suppression was obtained in 1 or 2 breath holds, with the following parameters: repetition time (TR, ms)/echo time (TE, ms) = 150-170/1.6; flip angle = 80°; matrix = 512 × 160-192; field of view (FOV) = 26-32 cm; section thickness = 5 mm; number of excitation (NEX) = 1; and sampling bandwidth = 20.8 kHz.
GRE in-phase and out-of-phase MRI were acquired during breath hold, with the following parameters: TR (ms)/TE (ms) = 150/4.4, 2.2; flip angle = 90°; matrix = 256 × 192-224; FOV = 26-32 cm; section thickness = 5 mm; NEX = 1; and sampling bandwidth = 31 or 62 kHz.
R-T FRFSE T2-weighted sequences were obtained with the following parameters: TR (ms)/TE (ms) = 10000-12000/90-100, TR determined by the frequency of respiration; section thickness = 5 mm; intersection gap = 0.5 mm; matrix = 256 × 192; NEX = 3; and FOV = 34 cm × 34 cm. The acquisition took approximately 3-4 min to complete.
Coronal and axial SSFSE T2-weighted images were obtained during breath hold, with the following parameters: TE = 90-100 ms; 2 s between slice acquisitions; section thickness = 5 mm; intersection gap = 0.5 mm; matrix = 384 × 224; one-half signal acquired; and FOV = 33 cm × 33 cm.
Radial oblique slab SSFSE images were obtained for MRCP with the following parameters: TE = 1300 ms; 6 s between image acquisitions; section thickness = 40 mm; matrix = 384 × 224; one-half signal acquired; and FOV = 30 cm × 30 cm.
Dynamic enhanced imaging was performed with an axial fat saturated 3D FSPGR sequence. Gadolinium chelate was administered (0.2 mmol/L per kilogram of body weight) intravenously at approximately 3.5 mL/s by projector (Spectris MR Injection System, Medrad Inc., United States) injection, followed by a 20-mL saline solution flush. An additional delayed phase was acquired using 2D FSPGR fat suppression axial T1-weighted imaging.
The original MRI data were loaded onto a computer workstation (GE, AW4.1, Sun Microsystems, Palo Alto, CA, United States) for review. Two observers (with 5 and 8 years’ experience in interpreting abdominal MR images, respectively) independently reviewed MR images, and any discrepancies between the two reviewers were settled by consensus. The reviewed findings included: HAP was defined as an area of hepatic parenchyma enhancement that was different on the artery phase images, with a return to same signal intensity on venous phase images and delayed phase images compared with the surrounding normal hepatic tissues[15-17]. The presence or absence of HAP as well as the morphology and distribution of HAP in the liver were analyzed. Gallstones, cholecystitis, common bile duct (CBD) stones and dilatation of CBD: gallbladder and CBD stones were observed on T2 weight or MRCP images, which showed a hypointense area or a filling defect in the bright bile background[13]. Cholecystitis was diagnosed using MRI by identifying gallbladder wall thickening (more than 3 mm), gallbladder wall edema, gallbladder distention (diameter more than 40 mm), and pericholecystic fluid. The presence of one or more of the four criteria was indicative of cholecystitis[21,22]. Dilatation of the CBD was defined as the short diameter of the CBD over 7 mm on T2-weighted and MRCP images[23]. The severity of AP on the MRI was scored with the MR severity index (MRSI), which was derived from the CT severity index[18-20,24].
In China, the etiology of AP is closely related to the presence of gallbladder and common bile stones[13]. We therefore classified AP patients into two groups: those with gallbladder and CBD abnormalities (indicated by one or more signs of gallstones, cholecystitis, CBD stones and dilatation of CBD) and those without. The percentages of patients with HAP in all patients were calculated. The percentage of patients with HAP and the percentage of HAP distribution in the liver were calculated in each of the two groups. χ2 tests were used to analyze the difference between the two groups for the prevalence of HAP and HAP distribution in the liver.
The MRSI is given as the range and the mean ± SD, and independent-sample t tests were used to test the difference between the patients with and without HAP.
Data analysis was performed using the Statistical Package for Social Sciences for Windows (Version 13.0, Chicago, IL, United States). P≤ 0.05 was considered significant.
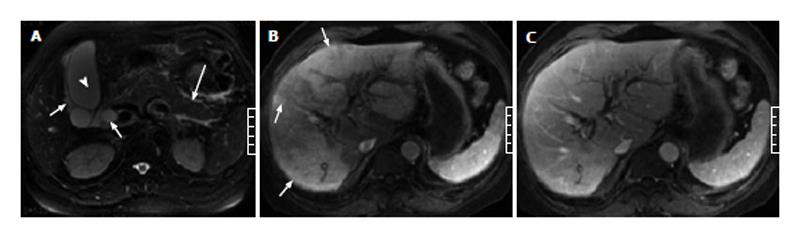
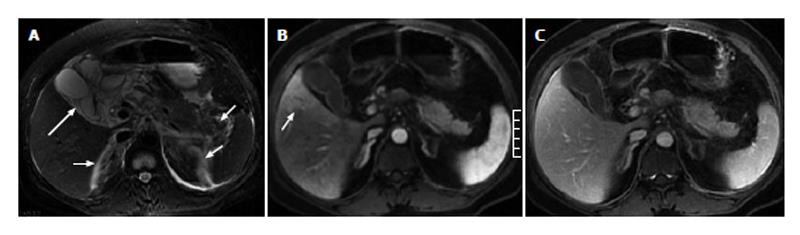
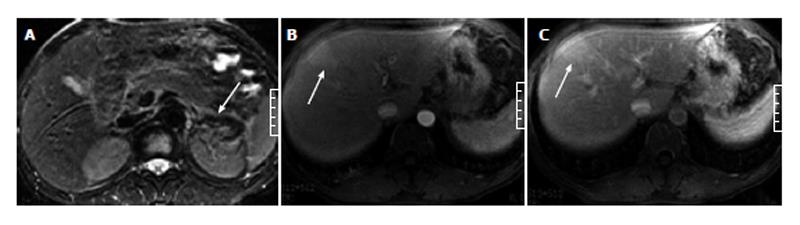
Of the 51 patients with AP, 32 (63%) had at least one sign of gallbladder and CBD abnormalities on the MR images, and 19 (37%) showed no signs of gallbladder or CBD abnormalities. In the 32 patients with gallbladder and CBD abnormalities, gallbladder distention was found in 3 patients (Figure 1), pericholecystic fluid in 15 patients (Figures 1 and 2), gallbladder wall thickening in 19 patients, gallbladder wall edema in 4 patients, gallstones in 21 patients (Figure 3), CBD stones in 2 patients, and CBD dilation in 8 patients (Figures 1 and 3).
In the 51 patients with AP, 19 (37%) showed indications of HAP on enhanced images. Of the 19 patients with HAP, 15 (80%) had HAP on the arterial phase images and returned to normal on the venous phase images (Figures 1-4), while 4 (20%) had HAP on both the arterial and venous phase images with a return to normal on the delayed phase images (Figure 5).
In the 19 patients with HAP, there were 11 patients (58%) with gallbladder and CBD abnormalities and 8 patients (42%) without. The prevalence of HAP in patients with AP in combination with gallbladder or CBD abnormalities was greater than that in patients with AP without gallbladder or CBD abnormalities, but the difference between the two groups was not significant (χ2 = 0.305, P = 0.581 > 0.05) (Table 1).
| Acute pancreatitis (n = 51) | Visible hepatic abnormal perfusion | No visible hepatic abnormal perfusion |
| Acute pancreatitis combined with gallbladder and common bile duct abnormalities (group 1) (n = 32) | 11 | 21 |
| Acute pancreatitis without gallbladder and common bile duct abnormalities (group 2) (n = 19) | 8 | 11 |
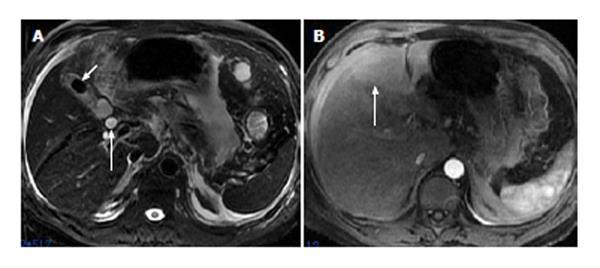
Of the 11 patients with AP in combination with gallbladder and CBD abnormalities, 7 (64%) showed strip-shaped HAP in the hepatic tissues adjacent to the gallbladder (Figure 2), 2 (18%) showed wedge- or patch-shaped HAP in both the left and right hepatic lobes (Figure 1), 1 (9%) showed wedge- or patch-shaped HAP only in the right hepatic lobe, and 1 (9%) showed wedge- or patch-shaped HAP only in the left hepatic lobe (Figure 3).
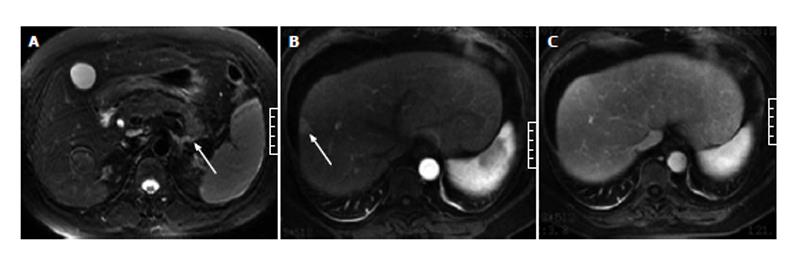
In the 8 AP patients without gallbladder and CBD abnormalities, 3 (38%) showed strip-shaped HAP in hepatic tissues adjacent to the gallbladder, 1 (12%) showed wedge- or patch-shaped HAP in both the left and right hepatic lobes, 2 (25%) showed wedge- or patch-shaped HAP only in the right hepatic lobe (Figure 4), and 2 (25%) showed wedge- or patch-shaped HAP only in the left hepatic lobe (Figure 5).
There were no significant differences in the HAP distribution in the liver (χ2 = 2.181, P = 0.536 > 0.05) between the patients with and without gallbladder and CBD abnormalities (Table 2).
| Patients with hepatic abnormal perfusion (n = 19) | Hepatic abnormal perfusion distribution in the liver | |||
| Adjacent to the gallbladder | Both left and right | Right | Left | |
| Acute pancreatitis combined with gallbladder and common bile duct abnormalities (group 1) (n = 11) | 7 | 2 | 1 | 1 |
| Acute pancreatitis without gallbladder and common bile duct abnormalities (group 2) (n = 8) | 3 | 1 | 2 | 2 |
In all 51 patients with AP, the mean MRSI was 2.7 ± 1.3, with scores ranging from 1 to 4. In the 19 patients with HAP, the mean MRSI was 3.2 ± 1.0, with scores ranging from 1 to 4. In the 32 patients without HAP, the mean MRSI was 3.0 ± 1.0, with scores ranging from 2 to 4. There were no significant differences in the MRSI scores between patients with and without HAP (t = 0.559, P = 0.552 > 0.05).
Pancreatic disease is related to gallbladder disorders because the gallbladder is located close to the pancreas[25,26]. Gallbladder abnormalities in carcinoma of the pancreatic head and AP have been reported[13,27]. In our study, 63% of patients with AP also showed signs of gallbladder and CBD abnormalities, which were discovered mainly by identifying cholecystitis on MRI images. The results demonstrate that gallbladder abnormalities are correlated with inflammation due to AP. Similarity, the inflammatory process of AP often spreads to the liver because the body of the pancreas is located close to the left lobe of the liver[14,28-30].
Inflammatory changes in the organs adjacent to liver can lead to HAP in the liver, as indicated by a previous study that reported HAP was found on CT images of patients with AP[14]. In our study, we used MRI images to categorize the patients with HAP into two groups: one group of patients with AP in combination with gallbladder and CBD abnormalities and another group of patients with AP and no gallbladder or CBD abnormality. Although the prevalence of HAP in patients with AP in combination with gallbladder and CBD abnormalities (58%) is higher than that in patients with AP but no gallbladder or CBD abnormalities (42%), there is no significant difference between the two groups. We speculate that the first cause of HAP in AP is the inflammatory process of AP spreading to the liver, while the second cause is the inflammatory process of cholecystitis spreading to the liver.
In the patients with AP and no gallbladder or CBD abnormalities, HAP was identified in hepatic tissue adjacent to the gallbladder (38%) and in the left and right lobes of the liver (62%). In patients with AP in combination with gallbladder and CBD abnormalities, HAP was identified in hepatic tissue adjacent to the gallbladder (64%) and in the left and right lobes of the liver (36%). There were no significant differences in the HAP distribution between the two groups. The distribution of HAP in AP is different from that of HAP caused only by cholecystitis. HAP caused by cholecystitis is mainly distributed in hepatic tissue adjacent to the gallbladder, with no distribution on the left or right lobes of the liver[31,32]. Our results of the HAP distribution indicate that the biliary and pancreatic diseases influence each other because of their close anatomic site and related functions[25].
We found that HAP was strip, patch or wedge-shaped on enhanced MR images of patients with AP. Our results are similar to those obtained by Arita et al[14] by CT. The shape of HAP can elucidate the pathogenic mechanisms by which AP causes HAP. Strip-shaped HAP adjacent to the gallbladder is attributable to hepatic arterial hyperemia and increased venous drainage caused by the inflammatory process in the gallbladder[14,31]. Patch- and wedge-shaped HAP in the left and right lobes of the liver are caused by the inflammatory process of AP, which may spread to the liver through the hepatoduodenal ligament or gastrohepatic ligament toward the porta hepatis, finally spreading along the Glisson sheath; another pathway is through the lesser sac to the left lobe of the liver[28-30]. Understanding the pathway of AP spread can help predict the severity of AP.
MRSI is used for staging AP by grading both the degree of pancreatic and peripancreatic fluid and the extent of pancreatic necrosis. The sum of these parameters resulted in the development of an MRSI[18-20]. The MRSI has more advantages for predicting local complications and has a limited role in predicting systemic complications[19]. Ex-pancreatic organ abnormalities in patients with AP reflect systemic complications, as abdominal wall edema and gallbladder abnormalities were reported on MRI images. The presence of these abnormalities had a positive correlation with the severity of AP and may be a supplementary factor for grading the severity of AP[12,13]. In our study, HAP in AP had no correlation with the severity of AP identified using MRI. We speculate that HAP in AP is caused by both cholecystitis and AP, which is the reason for the correlation between HAP and the severity of AP.
In addition, in our study, 4 patients (20%) had HAP on the both arterial phase and venous phase images that returned to normal on the delayed phase images (Figure 1). This finding differs from another study on HAP. Another study reported that HAP was found on arterial phase images and returned to normal on venous phase images[15-17]. These special findings warrant further study.
In conclusion, the presence of HAP on enhanced MRI is common in patients with AP, which presents as strip-shaped in the hepatic tissue adjacent to the gallbladder and with a patch- or wedge-shaped lobar distribution in the liver. HAP is most likely caused by increased arterial blood flow attributable to both inflamed gallbladder and AP, and should not be misinterpreted as other primary liver abnormalities. The presence of HAP on MRI images cannot be used to determine the severity of AP.
In acute pancreatitis (AP), ex-pancreatic organ abnormality is a feature of disease severity that has the potential to exacerbate pancreatic and systemic injury. The identification of ex-pancreatic organ abnormality is essential in predicting the severity of AP. Hepatic abnormal perfusion (HAP) in AP is most likely caused by increased arterial blood flow due to the inflamed lobe of the liver or the inflamed gallbladder. Is HAP related to the severity of AP and the inflamed gallbladder
The abnormal changes in AP are not confined to the pancreas and may affect the gastrointestinal tract, liver, lung, kidney and skeletal muscle. In the area of AP, current research has focused on predicting the relationship between the severity of AP and ex-pancreatic organ abnormality using imaging tools.
In a previous study, HAP due to AP was found using computed tomography. However, there are no previous reports on the use of magnetic resonance imaging (MRI) to evaluate HAP in AP. the authors study presents information on HAP in patients with AP by using MRI, and discusses the morphology, cause, prevalence, distribution, and pathogenesis of HAP and any association with the severity of AP.
The study results suggest that HAP on enhanced MRI presents as strip-shaped in the hepatic tissue adjacent to the gallbladder, and as patch-shaped or wedge-shaped with lobar distribution in the liver. HAP on MRI cannot be used to determine the severity of AP.
HAP is defined as an area with a hepatic parenchymal enhancement difference on arterial phase images that returns to the same signal intensity on venous phase images and delayed phase images compared with the surrounding normal hepatic tissues.
This is a good descriptive study in which the authors analyze HAP due to AP visible by MRI. The results are interesting and suggest that HAP is common in AP and cannot be used to determine the severity of AP.
P- Reviewers: Pan CX, Sun ZH, S- Editor: Ma YJ L- Editor: Wang TQ E- Editor: Liu SQ
| 1. | Cuthbertson CM, Christophi C. Disturbances of the microcirculation in acute pancreatitis. Br J Surg. 2006;93:518-530. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 211] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 2. | Zhou ZG, Chen YD, Sun W, Chen Z. Pancreatic microcirculatory impairment in experimental acute pancreatitis in rats. World J Gastroenterol. 2002;8:933-936. [PubMed] |
| 3. | Beattie GC, Hardman JG, Redhead D, Siriwardena AK. Evidence for a central role for selective mesenteric angiography in the management of the major vascular complications of pancreatitis. Am J Surg. 2003;185:96-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 49] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 4. | Bergert H, Hinterseher I, Kersting S, Leonhardt J, Bloomenthal A, Saeger HD. Management and outcome of hemorrhage due to arterial pseudoaneurysms in pancreatitis. Surgery. 2005;137:323-328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 145] [Article Influence: 7.3] [Reference Citation Analysis (1)] |
| 5. | Inoue K, Hirota M, Beppu T, Ishiko T, Kimura Y, Maeda K, Ogawa M. Angiographic features in acute pancreatitis: the severity of abdominal vessel ischemic change reflects the severity of acute pancreatitis. JOP. 2003;4:207-213. [PubMed] |
| 6. | Schiller WR, Anderson MC. Microcirculation of the normal and inflamed canine pancreas. Ann Surg. 1975;181:466-470. [PubMed] |
| 7. | Chen HM, Sunamura M, Shibuya K, Yamauchi JI, Sakai Y, Fukuyama S, Mikami Y, Takeda K, Matsuno S. Early microcirculatory derangement in mild and severe pancreatitis models in mice. Surg Today. 2001;31:634-642. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 28] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 8. | Kelly DM, McEntee GP, McGeeney KF, Fitzpatrick JM. Microvasculature of the pancreas, liver, and kidney in cerulein-induced pancreatitis. Arch Surg. 1993;128:293-295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 9. | Dobosz M, Mionskowska L, Hac S, Dobrowolski S, Dymecki D, Wajda Z. Heparin improves organ microcirculatory disturbances in caerulein-induced acute pancreatitis in rats. World J Gastroenterol. 2004;10:2553-2556. [PubMed] |
| 10. | Foitzik T, Eibl G, Hotz B, Hotz H, Kahrau S, Kasten C, Schneider P, Buhr HJ. Persistent multiple organ microcirculatory disorders in severe acute pancreatitis: experimental findings and clinical implications. Dig Dis Sci. 2002;47:130-138. [PubMed] |
| 11. | Nishiwaki H, Ko I, Hiura A, Ha SS, Satake K, Sowa M. Renal microcirculation in experimental acute pancreatitis of dogs. Ren Fail. 1993;15:27-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 12. | Yang R, Jing ZL, Zhang XM, Tang W, Xiao B, Huang XH, Yang L, Feng ZS. MR imaging of acute pancreatitis: correlation of abdominal wall edema with severity scores. Eur J Radiol. 2012;81:3041-3047. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 13. | Ji YF, Zhang XM, Li XH, Jing ZL, Huang XH, Yang L, Zhai ZH. Gallbladder patterns in acute pancreatitis: an MRI study. Acad Radiol. 2012;19:571-578. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 14. | Arita T, Matsunaga N, Takano K, Hara A, Fujita T, Honjo K. Hepatic perfusion abnormalities in acute pancreatitis: CT appearance and clinical importance. Abdom Imaging. 1999;24:157-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 17] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 15. | Colagrande S, Centi N, Galdiero R, Ragozzino A. Transient hepatic intensity differences: part 1, Those associated with focal lesions. AJR Am J Roentgenol. 2007;188:154-159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 43] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 16. | Colagrande S, Centi N, Galdiero R, Ragozzino A. Transient hepatic intensity differences: part 2, Those not associated with focal lesions. AJR Am J Roentgenol. 2007;188:160-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 43] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 17. | Lupescu IG, Grasu M, Capsa R, Pitrop A, Georgescu SA. Hepatic perfusion disorders: Computer-tomographic and magnetic resonance imaging. J Gastrointestin Liver Dis. 2006;15:273-279. [PubMed] |
| 18. | Arvanitakis M, Koustiani G, Gantzarou A, Grollios G, Tsitouridis I, Haritandi-Kouridou A, Dimitriadis A, Arvanitakis C. Staging of severity and prognosis of acute pancreatitis by computed tomography and magnetic resonance imaging-a comparative study. Dig Liver Dis. 2007;39:473-482. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 39] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 19. | Tang W, Zhang XM, Xiao B, Zeng NL, Pan HS, Feng ZS, Xu XX. Magnetic resonance imaging versus Acute Physiology And Chronic Healthy Evaluation II score in predicting the severity of acute pancreatitis. Eur J Radiol. 2011;80:637-642. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 28] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 20. | Arvanitakis M, Delhaye M, De Maertelaere V, Bali M, Winant C, Coppens E, Jeanmart J, Zalcman M, Van Gansbeke D, Devière J. Computed tomography and magnetic resonance imaging in the assessment of acute pancreatitis. Gastroenterology. 2004;126:715-723. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 232] [Cited by in RCA: 197] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 21. | Smith EA, Dillman JR, Elsayes KM, Menias CO, Bude RO. Cross-sectional imaging of acute and chronic gallbladder inflammatory disease. AJR Am J Roentgenol. 2009;192:188-196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 82] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 22. | Altun E, Semelka RC, Elias J, Braga L, Voultsinos V, Patel J, Balci NC, Woosley JT. Acute cholecystitis: MR findings and differentiation from chronic cholecystitis. Radiology. 2007;244:174-183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 51] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 23. | Songür Y, Temuçin G, Sahin B. Endoscopic ultrasonography in the evaluation of dilated common bile duct. J Clin Gastroenterol. 2001;33:302-305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 34] [Article Influence: 1.4] [Reference Citation Analysis (1)] |
| 24. | Lecesne R, Taourel P, Bret PM, Atri M, Reinhold C. Acute pancreatitis: interobserver agreement and correlation of CT and MR cholangiopancreatography with outcome. Radiology. 1999;211:727-735. [PubMed] |
| 25. | Kimura W. Surgical anatomy of the pancreas for limited resection. J Hepatobiliary Pancreat Surg. 2000;7:473-479. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 55] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 26. | Jaffray C, Yang J, Norman J. Elastase mimics pancreatitis-induced hepatic injury via inflammatory mediators. J Surg Res. 2000;90:95-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 35] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 27. | Zhang XM, Mitchell DG, Byun JH, Verma SK, Bergin D, Witkiewicz A. Gallbladder abnormalities in carcinoma of pancreatic head: findings on MR imaging. Abdom Imaging. 2009;34:507-513. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 28. | Meyers MA, Oliphant M, Berne AS, Feldberg MA. The peritoneal ligaments and mesenteries: pathways of intraabdominal spread of disease. Radiology. 1987;163:593-604. [PubMed] |
| 29. | Fujiwara T, Takehara Y, Ichijo K, Tooyama N, Kodaira N, Yamamoto H, Watahiki H. Anterior extension of acute pancreatitis: CT findings. J Comput Assist Tomogr. 1995;19:963-966. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 30. | Siegelman SS, Copeland BE, Saba GP, Cameron JL, Sanders RC, Zerhouni EA. CT of fluid collections associated with pancreatitis. AJR Am J Roentgenol. 1980;134:1121-1132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 158] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 31. | Yamashita K, Jin MJ, Hirose Y, Morikawa M, Sumioka H, Itoh K, Konish J. CT finding of transient focal increased attenuation of the liver adjacent to the gallbladder in acute cholecystitis. AJR Am J Roentgenol. 1995;164:343-346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 74] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 32. | Matsui O, Takashima T, Kadoya M, Konishi H, Kawamura I, Hirose J, Kameyama T, Chohtou S. Staining in the liver surrounding gallbladder fossa on hepatic arteriography caused by increased cystic venous drainage. Gastrointest Radiol. 1987;12:307-312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 32] [Article Influence: 0.8] [Reference Citation Analysis (0)] |