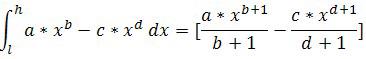Published online Nov 28, 2013. doi: 10.4329/wjr.v5.i11.421
Revised: September 24, 2013
Accepted: November 2, 2013
Published online: November 28, 2013
Processing time: 133 Days and 19.6 Hours
AIM: To compare the computed tomography (CT) dose and image quality with the filtered back projection against the iterative reconstruction and CT with a minimal electronic noise detector.
METHODS: A lung phantom (Chest Phantom N1 by Kyoto Kagaku) was scanned with 3 different CT scanners: the Somatom Sensation, the Definition Flash and the Definition Edge (all from Siemens, Erlangen, Germany). The scan parameters were identical to the Siemens presetting for THORAX ROUTINE (scan length 35 cm and FOV 33 cm). Nine different exposition levels were examined (reference mAs/peek voltage): 100/120, 100/100, 100/80, 50/120, 50/100, 50/80, 25/120, 25/100 and 25 mAs/80 kVp. Images from the SOMATOM Sensation were reconstructed using classic filtered back projection. Iterative reconstruction (SAFIRE, level 3) was performed for the two other scanners. A Stellar detector was used with the Somatom Definition Edge. The CT doses were represented by the dose length products (DLPs) (mGycm) provided by the scanners. Signal, contrast, noise and subjective image quality were recorded by two different radiologists with 10 and 3 years of experience in chest CT radiology. To determine the average dose reduction between two scanners, the integral of the dose difference was calculated from the lowest to the highest noise level.
RESULTS: When using iterative reconstruction (IR) instead of filtered back projection (FBP), the average dose reduction was 30%, 52% and 80% for bone, soft tissue and air, respectively, for the same image quality (P < 0.0001). The recently introduced Stellar detector (Sd) lowered the radiation dose by an additional 27%, 54% and 70% for bone, soft tissue and air, respectively (P < 0.0001). The benefit of dose reduction was larger at lower dose levels. With the same radiation dose, an average of 34% (22%-37%) and 25% (13%-46%) more contrast to noise was achieved by changing from FBP to IR and from IR to Sd, respectively. For the same contrast to noise level, an average of 59% (46%-71%) and 51% (38%-68%) dose reduction was produced for IR and Sd, respectively. For the same subjective image quality, the dose could be reduced by 25% (2%-42%) and 44% (33%-54%) using IR and Sd, respectively.
CONCLUSION: This study showed an average dose reduction between 27% and 70% for the new Stellar detector, which is equivalent to using IR instead of FBP.
Core tip: A computed tomography dose reduction between 30% and 80% can be expected when using iterative reconstruction instead of filtered back projection. The benefit of dose reduction is larger at lower dose levels. An additional dose reduction between 27% and 70% can be obtained by applying the new Stellar detector.
- Citation: Christe A, Heverhagen J, Ozdoba C, Weisstanner C, Ulzheimer S, Ebner L. CT dose and image quality in the last three scanner generations. World J Radiol 2013; 5(11): 421-429
- URL: https://www.wjgnet.com/1949-8470/full/v5/i11/421.htm
- DOI: https://dx.doi.org/10.4329/wjr.v5.i11.421
Engineering progress has greatly reduced the radiation dose required for computer tomography. Dose modulation along the x-, y- and z-axes[1-6] and shielding[7-12] represent major manufacturing steps involved in radiation protection. Increasing computing capacity has allowed iterative reconstruction to be introduced in the clinical routine, leading to dose reductions between 27% and 65%[13-18]. Problems with electronic noise during image acquisition can be overcome by integrating analog-digital-converters with the photodiodes on the CT-detectors contained on the same silicon chip (Stellar detector, Siemens Healthcare, Erlangen, Germany) with the potential to further reduce radiation dose[19].
Image quality can be characterized by calculating the noise, signal to noise ratio (SNR) and contrast to noise ratio (CNR)[20-24]. The signal at a region of interest (ROI) corresponds to the attenuation in Hounsfield units (HU). The noise corresponds to the standard deviation of the pixel attenuation within the ROI[20]. The signal will remain the same with lower tube current and unchanged tube voltage, but the noise will increase[21]. Several studies have demonstrated the feasibility of low dose imaging using a lowest acceptable tube current below 50 mAs for lung nodule detection[25-28].
Changing tube voltage changes the signal and the noise, depending on the absorption spectrum of the scanned object[21]. The dose (D) is coupled with many variables: D~(signal to noise)2/(pixel-size*image-thickness), D~mAs (Miliamperesecond) and D~kVp2 (Kilovolt peak)[20,21,25]. Noise reduction can either be used for dose reduction or to increase the image quality.
It is not known if the same CT dose is required to generate the same CNR with the new generation of CT scanners. Therefore, we investigated how the lowest acceptable signal to noise acquired at 50 mAs/80 kVp and used for filtered back projection can be transferred to iteratively reconstructed images using a new detector. We compared the dependency of dose and noise (SNR and CNR) for the last three generations of CT scanners.
A lung phantom (Chest Phantom N1 by Kyoto Kagaku) was used in this study (Figure 1). This phantom is an accurate life-size anatomical model of a male human torso with a synthetic heart, trachea, pulmonary vessels (right and left) and abdomen (diaphragm) block. The thickness of the chest wall is based on measurement of clinical data. The soft tissue substitute material (polyurethane, gravity 1.06) and synthetic bones (epoxy resin) have X-ray absorption rates very close to those of human tissues. The abducted arm positions of the torso are appropriate for CT scanning. The pulmonary vessels are also spatially traceable.
The phantom size was 43 cm × 40 cm × 48 cm with a chest girth of 94 cm and a weight of 18 kg. The pleural cavity measured 268 mm (craniocaudal). The phantom was measured at the level of the lung apices using axial slices (140 mm × 400 mm) and at the level of the diaphragm (208 mm × 279 mm).
The phantom was scanned using 3 different CT scanners (Siemens SOMATOM Sensation, SOMATOM Definition Flash and SOMATOM Definition Edge, all from Siemens Healthcare, Erlangen, Germany). The scan parameters were identical to the manufacturer’s standard presetting for THORAX ROUTINE: 24 mm × 1.2 mm, pitch 0.8 mm and slice thickness 1.5 mm for the SOMATOM Sensation 64; 128 mm × 0.6 mm, pitch 0.6 and slice thickness 1 mm for the SOMATOM Definition Flash; and 128 mm × 0.6 mm, pitch 0.6 and slice thickness 1 mm for the SOMATOM Definition Edge. The scan length and field of view (FOV) were 35 cm and 33 cm, respectively. Nine different exposition levels were used (reference mAs/tube voltage): 100/120, 100/100, 100/80, 50/120, 50/100, 50/80, 25/120, 25/100 and 25 mAs/80 kV. The option CARE kV setting that automatically adjusts tube voltage to an optimal level was disabled so that we could set the voltage to the predefined values. Reference mAs were used to keep the study parameters as close as possible to those used for routine scans. Images from the SOMATOM Sensation 64 were reconstructed using the classic filtered back projection method with a soft tissue kernel of B20 and a lung kernel of B60. Iterative reconstruction (SAFIRE, level 3) was performed for the two other scanners using the I26f and I70f Kernels. The dose was represented by the dose length product DLP (mGycm) provided by the scanners for a 32 cm diameter phantom for each scan with a constant scan length of 35 cm.
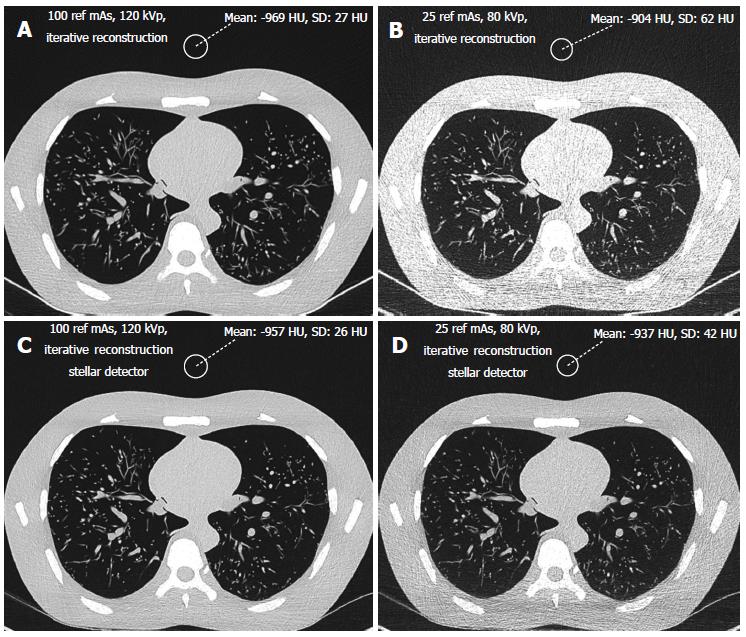
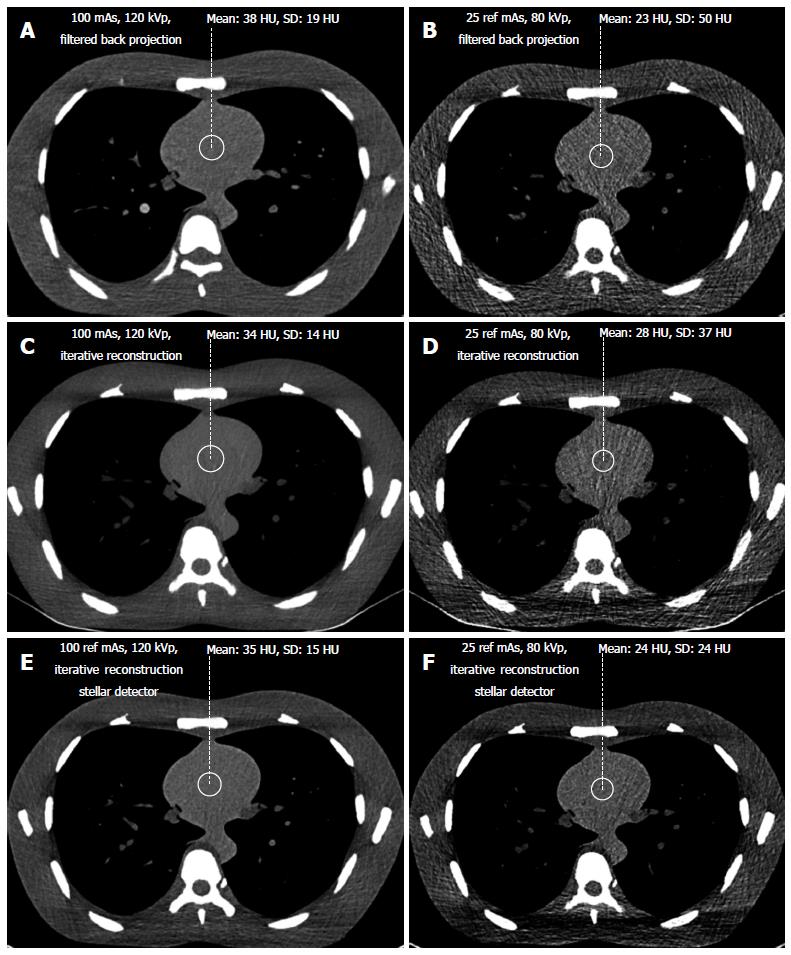
Signal and noise were recorded by two different radiologists with 10 and 3 years of experience, respectively, in chest CT radiology using a Picture Archiving and Communication System (PACS Philips Netherlands/Sectra Sweden). They measured the density in HU (signal) and the standard deviation of the CT values (noise) from regions of interest (ROIs) of 2 cm (3.14 cm2) in diameter. The ROIs were placed in air outside the phantom, anterior to the sternum (Figure 1) in bone (middle of the vertebral body) and soft tissue (heart, Figure 2). Each radiologist chose 5 different levels at which to place the ROIs in the phantom scans. Signal and noise were measured from the same ROIs. Measurements were recorded for air and bone using a hard Kernel and for soft tissue using a soft Kernel. The image quality (SNR) was calculated for soft tissue. Only the noise was recorded for air because the signal in air was negligible. Dose was represented by the dose length product DLP (mGycm) calculated automatically by the scanner for a 32 cm diameter phantom for each scan with a constant scan length of 35 cm. CNR was defined as the difference between the signal from the bone and the soft tissue divided by the noise: HUbone-HUsoft tissue/noise[29].
In addition, both radiologists scored the subjective image quality from 1 to 5 in the lung window (level -500 HU, width 1500 HU, Figure 1) using a hard Kernel on a Picture Archiving and Communication System (PACS Philips Netherlands/Sectra Sweden). The subjective image quality scale was as follows: (1) non-diagnostic; (2) poor, diagnostic confidence significantly reduced; (3) moderate, but sufficient for diagnosis; (4) good; and (5) excellent. Curves were fitted for the noise vs dose and for the SNR vs dose. The highest R-square correlation coefficients were observed for power curves. For the same noise or same SNR, this allows the corresponding CT acquisition dose for the different scanners to be exactly determined. The lowest acceptable doses of 50 mAs and 80 kVp determined from the filtered back projection method were transferred to image acquisition with iterative reconstruction with Stellar detectors.
To compare the different scans, it was necessary to transform the noise and the dose to obtain the same acquisition parameters as the SOMATOM Sensation. The dose and noise were therefore calculated for a standard slice thickness of 1.5 mm (SOMATOM Sensation). Because the dose remains constant when slice thickness is increased from 1 to 1.5 mm for the new generation Siemens scanners (SOMATOM Definition Flash and Edge), only the noise was corrected by a factor of 1/(1.5)(1/2)[22,23].
Graphs for image noise vs radiation dose and SNR vs dose were evaluated for air, soft tissue and bone. Trend lines were determined for the data points using regression to produce power curves in MedCalc® Version 7.6.0.0 and Microsoft Excel 2007. Noise reduction between two scanners was determined by subtracting the power curves. The integral of this subtraction equals the area between the curves. This area was divided by the dose to obtain the average noise reduction. The integral for the subtraction of the trend lines was calculated from the lowest (l) to the highest (h) joint radiation exposure according to
Math 1
The dose reduction for a constant noise value was integrated along the noise scale to determine the average dose reduction. The smallest and highest reductions were determined to indicate the range. The Wilcoxon test for non-normally distributed paired samples was used to calculate the significance levels for noise reduction among the different scanners[30,31]. Inter-observer comparisons of the image quality score were performed, calculating the agreement levels with the Fleiss’ K statistic[32,33]. The K strengths were categorized as follows: < 0.20 poor, 0.21-0.40 fair, 0.41-0.60 moderate, 0.61-0.80 good, and 0.81-1.00 very good[34]. The Wilcoxon test and the Fleiss’ K statistic were analyzed in MedCalc® Version 7.6.0.0.
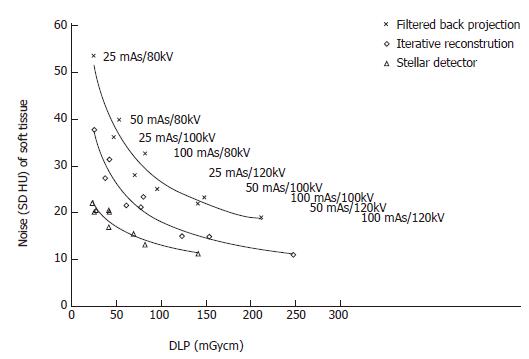
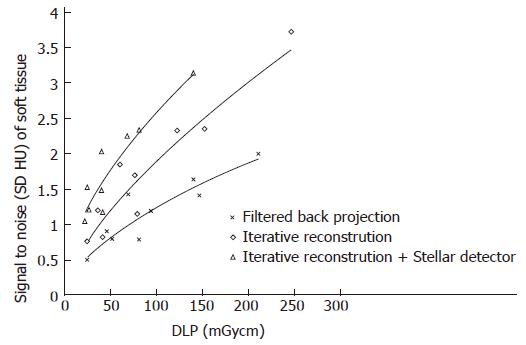
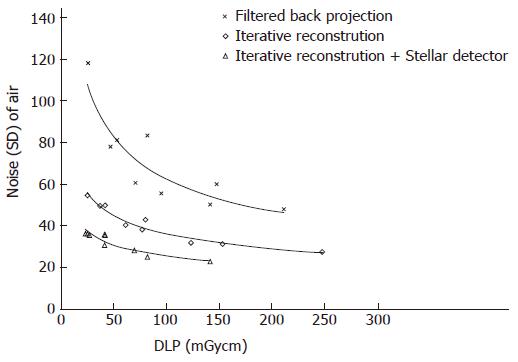
The noise levels measured in air, soft tissue and bone were significantly lower for iterative reconstruction compared to filtered back projection (P < 0.0001) and for using Stellar detectors in combination with IR compared to IR alone (P < 0.0001). The inter-observer agreement on image quality was moderate (kappa = 0.508). Noise/dose signature graphs for each scanner were plotted for air and soft tissue (Figures 3-5).
Noise reduction: The average noise reduction from filtered back projection (FBP, SOMATOM Sensation) and iterative reconstruction (IR, Somatom Definition Flash) was 10 HU (7-15 HU) for the same radiation dose, generating 31% (30%-31%) less noise. Changing from IR to the Stellar detector (Sd, SOMATOM edge) allowed another 7 HU (4-16 HU) of noise to be removed. Noise was reduced on average by (32%); 24% for the highest dose and 42% for the lowest dose (Figure 3 and Table 1).
| Soft tissue | Air | Bone | ||||||||||
| Lowest dose | Highest dose | Lowest dose | Highest dose | Lowest dose | Highest dose | |||||||
| Δ HUsd | Range | Δ HUsd | Range | Δ HUsd | Range | |||||||
| Noise reduction from FBP to IR | 10 (31%) | 15 (30%) | to | 7 (31%) | 31 (44%) | 53 (49%) | to | 22 (41%) | 64 (30%) | 128 (37%) | to | 28 (21%) |
| Noise reduction from IR to IR with Sd | 7 (32%) | 16 (42%) | to | 4 (24%) | 12 (31%) | 19 (34%) | to | 9 (28%) | 24 (15%) | 51 (22%) | to | 9 (7%) |
| Δ DLP | Range | Δ DLP | Range | Δ DLP | Range | |||||||
| Dose reduction from FBP to IR for the same noise level | 53 (52%) | 116 (55%) | to | 25 (50%) | 133 (80%) | 165 (78%) | to | 106 (81%) | 48 (30%) | 24 (12%) | to | 40 (55%) |
| Dose reduction from IR to IR with Sd for the same noise level | 69 (54%) | 96 (39%) | to | 46 (65%) | 106 (70%) | 166 (67%) | to | 65 (72%) | 35 (27%) | 33 (21%) | to | 25 (43%) |
Dose reduction: For a constant noise level, the dose could be reduced by an average of 53 (25-116) mGycm by applying IR instead of FBP, corresponding to an average dose reduction of 52%. At the lowest dose (high noise level), the dose reduction was 50%. At the highest dose, the dose reduction was 55%. An additional 69 (46-96) mGycm reduction was possible using the Stellar detector (54%). More dose reduction was achieved for the highest noise level (65%) compared to the lowest noise level (39%, Table 1).
Signal to noise (SNR ranged from 0.5 to 3.7, Figure 4): When dose was held constant, the SNR could be increased on average to 0.7 (36%, ranging from 0.2 to 1) and 0.6 (38%, ranging from 0.5 to 0.8) using IR and IR/Stellar detectors, respectively. Using a constant SNR, the dose could be reduced on average by 59 mGycm (45%, ranging from 20 to 107 mGycm) and 52 mGycm (41%, ranging from 23 to 72 mGycm) for IR and IR/Sd, respectively (Figure 4 and Table 2).
| Soft tissue | lung | ||||||||
| △HUsd | Range | △Points | Range | ||||||
| Lowest dose | Highest dose | Lowest dose | Highest dose | ||||||
| Signal to noise reduction from FBP to IR | 0.7 (36%) | 0.2 (31%) | to | 1 (39%) | Improving subjective image quality from FBP to IR | 0.2 (7%) | 0.4 (13%) | to | 0.1 (3%) |
| Signal to noise reduction from IR to IR with Sd | 0.6 (38%) | 0.5 (41%) | to | 0.8 (32%) | Improving subjective image quality from IR to IR with Sd | 0.5 (13%) | 0.2 (8%) | to | 0.9 (17%) |
| △DLP | Range | △DLP | Range | ||||||
| Lowest SNR | Highest SNR | lowest SNR | highest SNR | ||||||
| Dose reduction from FBP to IR for the same SNR | 59 (45%) | 20 (40%) | to | 107 (47%) | Dose reduction from FBP to IR for same subjective quality | 19 (25%) | 22 (42%) | to | 5 (2%) |
| Dose reduction from IR to IR with Sd for the same SNR | 52 (41%) | 23 (49%) | to | 72 (36%) | Dose reduction from IR to IR with Sd for same subjective quality | 57 (44%) | 10 (33%) | to | 130 (54%) |
Noise reduction: A noise range of 23 to 118 HU was
measured for all scans. Noise levels for Sd were always lower than for FBP, even when comparing the lowest dose of Sd against the highest dose of FBP (Figure 5). Noise at the same dose level could be lowered by 31 HU (44%) and 12 HU (31%) on average by changing from FBP to IR and from IR to Sd, respectively. The largest noise reductions were possible at lower dose levels (Figure 5, Table 1).
Dose reduction: To maintain a constant noise level, the dose was reduced by 133 (106-165) mGycm and 106 (65-166) mGycm when using IR instead of FBP and Sd instead of IR only, averaging dose reductions of 80% and 70%, respectively. The relative dose reductions were higher at higher noise levels (Figure 5, Table 1).
Bone: The noise ranged from 80 to 382 HU. The average noise reductions at constant radiation levels were 64 HU (30%) and 24 HU (15%) using IR and Sd, respectively. At higher dose levels, there was less noise reduction (Table 1). For a constant noise level, the doses could be lowered by 48 mGycm (30%) and 35 mGycm (27%) on average for IR and Sd, respectively. The dose reduction was higher at higher noise levels (Table 1).
Contrast to noise: With the same radiation dose, an average of 34% (22%-37%) and 25% (13%-46%) greater CNR was achieved by changing from FBP to IR and from IR to Sd, respectively. For the same CNR, the dose could be reduced on average by 59% (46%-71%) and 51% (38%-68%) for IR and Sd, respectively.
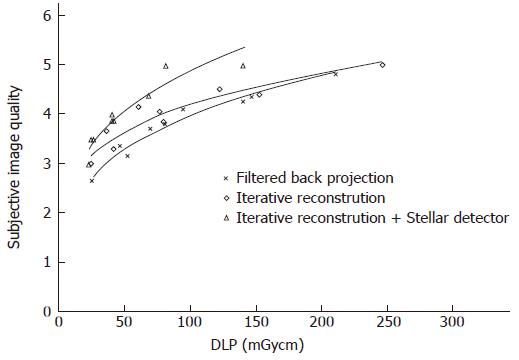
Subjective image quality (5 points maximal, Table 2): Image quality rose by 0.2 (0.1-0.4) points and 0.5 (0.2-0.9) points on average by changing from FBP to IR and from IR to Sd, respectively (Figure 6). For the same image quality, dose could be reduced by 25% (2%-42%) and 44% (33%-54%) using IR and Sd, respectively (Table 2).
By setting the image quality along with the noise, SNR and CNR, our study showed that iterative reconstruction and Stellar detectors produce better quality images than scans reconstructed with filtered back projection using lower CT doses. Using the SOMATOM Definition Flash instead of the Sensation enabled either a dose reduction of 80% or a noise reduction of 44% when imaging air. It is not possible to achieve simultaneous dose and noise reduction. Additionally, at the level of maximal dose reduction, it is not possible to further increase image quality and vice versa. It is known that iterative reconstruction approaches (Adaptive Statistical Iterative Reconstruction or Sinogram Affirmed Iterative Reconstruction) can in some cases reduce radiation dose by 27% to 65%[13-18]. Our phantom study results confirm these findings, showing dose reductions of 30%, 52% and 80% for imaging bone, soft tissue and air, respectively.
Using the recently introduced Stellar detector, signal-transformation was implemented into the detector itself to reduce electronic noise. With this technique, it was possible to further reduce radiation dose by an average 27%, 54% and 70% for bone, soft tissue and air, respectively.
With lower tube voltage, not only did the image noise increase, but the density of the bone also increased, which is well known for dual energy scans. When the signal increased, the SNR also increased, altering the bone diagram. This is most likely the main reason why the Stellar detectors did not have the same effect on dose reduction when imaging bone. The benefit of dose reduction is greater for low doses, especially for bone and air. The absolute dose reduction, measured in mGycm, was larger at high doses, but the relative reduction was always larger at lower doses. This was true with the exceptions of subjective image quality and SNR, which are influenced by increased signal at lower doses (such as the case of bone). The manufacturer claims that Stellar detectors reduce noise by 20%[19]. However, because dose increases by a power of 2 as noise decreases, a potential dose reduction of 36% should be possible. We produced dose reductions of 27%-70% and noise reductions of 15%-32%. As mentioned above, the lower the dose levels, the bigger the relative benefit. Our scan range covered only low dose levels, with a high dose of 250 mGycm (100 reference mAs/120 kVp), equivalent to approximately 3 mSv. At high dose levels, the noise reduction approached 20% (24%, 28% and 7% for air, soft tissue and bone, respectively). The noise level was strongly influenced by the chosen kernel. For FBP reconstruction, there was a tradeoff between spatial resolution and noise. This tradeoff could be adjusted by selecting different kernels. Soft tissue kernels had much lower standard deviations in the signal than hard kernels for air and bone. Therefore, it was necessary to use the same kernels for comparisons. Some radiation protectors believe that the risk from high radiation (atomic bombs) can be extrapolated to much lower radiation exposures (CT) and that the risk of death due to radiation-induced cancer for the general population is 0.005%/mSv[35]. The conversion coefficient from DLP to mSv is 0.014 for lung[36,37], equivalent to a reduction of radiation induced cancer death per chest CT from 1 in 13000 for iterative reconstruction to 1 in 32000 when using Stellar detectors. These estimates neglect the potential bio-positive effects of low radiation exposure[38-42]. Nevertheless, radiation is a cumulative physical quantity that may lead to stochastic processes such as carcinogenesis. Therefore, radiation protection is mandatory even at lower dose levels.
In a previous phantom study using the SOMATOM Sensation, it was shown that the sensitivity for lung nodule detection was not significantly altered for images acquired with 50 mAs and 80 kVp compared to 100 mAs and 120 kVp (article submitted). With the findings from our present study, it is possible to transfer these parameters to new scanners that use iterative reconstruction and better detectors. Our images had noise levels of 81 and 40 HU for air and mediastinum, respectively. For similar noise levels, parameters as low as 25 mAs and 80 kVp for the SOMATOM Definition Flash and Edge are theoretically sufficient for detection without loss of sensitivity (Figures 3-5).
Obviously, image quality is not only defined by signal but also by contrast and noise because a loss of subjective image quality is less impressive than an increase in noise level. In other words, radiologists rate image quality higher than the measured noise would suggest. Perception of image quality is influenced by spatial resolution, individual vision and pattern recognition, all of which cannot be precisely measured. Fortunately, subjective image quality was not rated below what the noise level would predict.
Noise is one parameter of image quality, but we did not investigate scan plane and z-axis spatial resolution (Z-sensitivity) or high-contrast resolution[22]. Further studies are needed to investigate these parameters to understand how the type of scanner can influence image quality. The pre-settings for THORAX ROUTINE were not the same for the three scanner types. For example, the pitch for the Sensation was 0.8, but this value was altered to 0.6 for the other scanners. Increasing the pitch from 0.6 to 0.8 did not change the noise; however, the dose decreased corresponding to a Siemens specific unchanged tube current time product/pitch[43]. Therefore, it is possible that the images acquired by the Somatom Sensation have slightly different dose levels at lower pitches. The resolution was slightly different for the B- and I-Kernels for the different reconstruction approaches, which might have negatively influenced the noise of the I-Kernel. Ongoing studies will address this topic.
As varying kVp lead to CT number changes, it can be difficult to interpret results, especially for SNR and CNR. Possible differences in gantry geometry, detector efficiency and tube spectrum for the three investigated scanners were also neglected because our intent was to give an approximation of dose and noise reduction among the last three generations of CT for given clinical presettings. Varying the kVp also provoked questions about how beam-hardening effects impact the images, which will require further investigation.
To evaluate the dose reduction provided by the new Stellar detector, it would be desirable to compare images reconstructed using the FBP algorithm, which is a linear algorithm with predictable performance. We only compared SAFIRE reconstructed images with and without the Stellar detector. Due to the nonlinearity of iterative reconstruction methods, reconstruction may be object and dose dependent. Thus, it is difficult to characterize the improvements provided by the Stellar detector alone from the results in our study. Further investigations need to address this topic.
In conclusion, this study demonstrated an average dose reduction of 27% to 70% by applying the new Stellar detector. This dose reduction was equivalent to using IR instead of FBP.
Due to increasing computing capacity, iterative image reconstruction can be introduced into clinics, leading to the potential for dose reduction by replacing older filtered back projection methods. The problem of electronic noise during image acquisition was overcome by integrating the analog-digital-converter with the photodiode of the computed tomography (CT)-detectors on the same silicon chip. This noise reduction can either be used for dose reduction or to increase the image quality. The three latest CT-scanner generations were examined to compare their potential for dose reduction.
Several studies demonstrated the feasibility of low dose imaging without loss of sensitivity for pulmonary diseases. Radiologists are able to lower the CT tube current and/or the tube voltage, using the lowest acceptable published dose levels for older CT scanners. Manufacturer dependent progress in CT-technology was only partly investigated. Dose or noise reduction using iterative reconstruction has been published, but the potential for new detectors in the clinic is not yet known.
This study demonstrates the dependency of CT radiation dose, image quality and CT-generation. It is possible to obtain the same image quality with a dose reduction of 30% to 80% by substituting filtered back projection with iterative image reconstruction. Using the new CT-detectors, radiation dose can be reduced by an additional 27% to 70%, depending on the scanned tissue.
With the results, the lowest acceptable tube currents and voltages for the older CT-generations can be transferred to the newest scanners.
CT-exams produce cross sectional images of the body, based on the radiation absorption of the body tissues. Radiation absorption is measured at every angle circularly around the body and is back-projected on a virtual pixel field in the scanned plane, delivering a filtered back projection image. New iterative reconstruction methods distribute the absorption of one angle to all of the pixels in a direction, adjusting the pixel values based on the effective absorption for each angle position. For clinical routines, three iterations of 360° rotation are used, which is very time-consuming. Only recently was it possible to deliver sufficient computing power for these clinical scanners.
This manuscript described an interest work that the average dose could be reduced 30%, 52% and 80% for imaging bone, soft tissue and air for the same image quality by using iterative reconstruction instead of filtered back projection. In addition, employing the new Stellar detector could further lower radiation dose additionally by 27%, 54% and 70% for bone, soft tissue and air, respectively. The manuscript can be accepted for publication as it is.
P- Reviewer: Meng LJ S- Editor: Wen LL L- Editor: A E- Editor: Liu XM
| 1. | Kalender WA, Wolf H, Suess C. Dose reduction in CT by anatomically adapted tube current modulation. II. Phantom measurements. Med Phys. 1999;26:2248-2253. |
| 2. | Kalra MK, Maher MM, Toth TL, Schmidt B, Westerman BL, Morgan HT, Saini S. Techniques and applications of automatic tube current modulation for CT. Radiology. 2004;233:649-657. |
| 3. | Graser A, Wintersperger BJ, Suess C, Reiser MF, Becker CR. Dose reduction and image quality in MDCT colonography using tube current modulation. AJR Am J Roentgenol. 2006;187:695-701. |
| 4. | Greess H, Wolf H, Baum U, Kalender WA, Bautz W. [Dosage reduction in computed tomography by anatomy-oriented attenuation-based tube-current modulation: the first clinical results]. Rofo. 1999;170:246-250. |
| 5. | Mulkens TH, Bellinck P, Baeyaert M, Ghysen D, Van Dijck X, Mussen E, Venstermans C, Termote JL. Use of an automatic exposure control mechanism for dose optimization in multi-detector row CT examinations: clinical evaluation. Radiology. 2005;237:213-223. |
| 6. | Gies M, Kalender WA, Wolf H, Suess C. Dose reduction in CT by anatomically adapted tube current modulation. I. Simulation studies. Med Phys. 1999;26:2235-2247. |
| 7. | Kojima H, Tsujimura A, Yabe H. [Usefulness of the adaptive dose shield for the infant CT]. Nihon Hoshasen Gijutsu Gakkai Zasshi. 2011;67:57-61. |
| 8. | Hein E, Rogalla P, Klingebiel R, Hamm B. Low-dose CT of the paranasal sinuses with eye lens protection: effect on image quality and radiation dose. Eur Radiol. 2002;12:1693-1696. |
| 9. | Hopper KD, King SH, Lobell ME, TenHave TR, Weaver JS. The breast: in-plane x-ray protection during diagnostic thoracic CT--shielding with bismuth radioprotective garments. Radiology. 1997;205:853-858. |
| 10. | Hopper KD. Orbital, thyroid, and breast superficial radiation shielding for patients undergoing diagnostic CT. Semin Ultrasound CT MR. 2002;23:423-427. |
| 11. | Hopper KD, Neuman JD, King SH, Kunselman AR. Radioprotection to the eye during CT scanning. AJNR Am J Neuroradiol. 2001;22:1194-1198. |
| 12. | McLaughlin DJ, Mooney RB. Dose reduction to radiosensitive tissues in CT. Do commercially available shields meet the users’ needs? Clin Radiol. 2004;59:446-450. |
| 13. | Pontana F, Duhamel A, Pagniez J, Flohr T, Faivre JB, Hachulla AL, Remy J, Remy-Jardin M. Chest computed tomography using iterative reconstruction vs filtered back projection (Part 2): image quality of low-dose CT examinations in 80 patients. Eur Radiol. 2011;21:636-643. |
| 14. | Leipsic J, Labounty TM, Heilbron B, Min JK, Mancini GB, Lin FY, Taylor C, Dunning A, Earls JP. Estimated radiation dose reduction using adaptive statistical iterative reconstruction in coronary CT angiography: the ERASIR study. AJR Am J Roentgenol. 2010;195:655-660. |
| 15. | Singh S, Kalra MK, Shenoy-Bhangle AS, Saini A, Gervais DA, Westra SJ, Thrall JH. Radiation dose reduction with hybrid iterative reconstruction for pediatric CT. Radiology. 2012;263:537-546. |
| 16. | Hara AK, Paden RG, Silva AC, Kujak JL, Lawder HJ, Pavlicek W. Iterative reconstruction technique for reducing body radiation dose at CT: feasibility study. AJR Am J Roentgenol. 2009;193:764-771. |
| 17. | Kalra MK, Woisetschläger M, Dahlström N, Singh S, Lindblom M, Choy G, Quick P, Schmidt B, Sedlmair M, Blake MA. Radiation dose reduction with Sinogram Affirmed Iterative Reconstruction technique for abdominal computed tomography. J Comput Assist Tomogr. 2012;36:339-346. |
| 18. | Winklehner A, Karlo C, Puippe G, Schmidt B, Flohr T, Goetti R, Pfammatter T, Frauenfelder T, Alkadhi H. Raw data-based iterative reconstruction in body CTA: evaluation of radiation dose saving potential. Eur Radiol. 2011;21:2521-2526. |
| 19. | Ulzheimer S. Stellar Detector Performance in Computed Tomography: The first fully-integrated detector in the CT industry sets a new reference in image quality with HiDynamics, TrueSignal and Ultra Fast Ceramics. Somatom Sessions. 2011;29:64-66. |
| 20. | Romans L. Introduction to Computed Tomography. Baltimore, MD: Williams & Wilkins 1995; . |
| 21. | Seeram E. Computed Tomography: Physical Principles, Clinical Applications & Quality Control. Philadelphia, Penn: WB Saunders Co 1994; . |
| 22. | Goldman LW. Principles of CT: radiation dose and image quality. J Nucl Med Technol. 2007;35:213-225; quiz 226-228. |
| 23. | Stoyanov D, Vassileva J. Influence of exposure parameters on patient dose and image noise in computed tomography. Pol J Med Phys Eng. 2009;15:215-226. |
| 24. | Shuman WP, Branch KR, May JM, Mitsumori LM, Lockhart DW, Dubinsky TJ, Warren BH, Caldwell JH. Prospective versus retrospective ECG gating for 64-detector CT of the coronary arteries: comparison of image quality and patient radiation dose. Radiology. 2008;248:431-437. |
| 25. | Diederich S, Lenzen H, Windmann R, Puskas Z, Yelbuz TM, Henneken S, Klaiber T, Eameri M, Roos N, Peters PE. Pulmonary nodules: experimental and clinical studies at low-dose CT. Radiology. 1999;213:289-298. |
| 26. | Christe A, Lin MC, Yen AC, Hallett RL, Roychoudhury K, Schmitzberger F, Fleischmann D, Leung AN, Rubin GD, Vock P. CT patterns of fungal pulmonary infections of the lung: comparison of standard-dose and simulated low-dose CT. Eur J Radiol. 2012;81:2860-2866. |
| 27. | Weng MJ, Wu MT, Pan HB, Kan YY, Yang CF. The feasibility of low-dose CT for pulmonary metastasis in patients with primary gynecologic malignancy. Clin Imaging. 2004;28:408-414. |
| 28. | Gergely I, Neumann C, Reiger F, Dorffner R. [Lung nodule detection with ultra-low-dose CT in routine follow-up of cancer patients]. Rofo. 2005;177:1077-1083. |
| 29. | Schindera ST, Graca P, Patak MA, Abderhalden S, von Allmen G, Vock P, Szucs-Farkas Z. Thoracoabdominal-aortoiliac multidetector-row CT angiography at 80 and 100 kVp: assessment of image quality and radiation dose. Invest Radiol. 2009;44:650-655. |
| 30. | Zwillinger D. Standard mathematical Tables and Formula. A Chapman & Hall Book. Boca Raton, Florida: CRC Press LLC 2003; e. 31st e. |
| 31. | Altman DG. Practical statistics for medical research. London: Chapman and Hall 1991; . |
| 32. | Fleiss JL. Measuring nominal scale agreement among many raters. Psychol Bull. 1971;76:378-383. |
| 33. | Fleiss JL. Statistical methods for rates and proportions. 2nd edition. New York: John Wiley 1981; 38-46. |
| 34. | Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33:159-174. |
| 35. | National Council on Radiation Protection and Measurements. Risk Estimates for Radiation Protection. NCRP Report No. 115, Bethesda, Maryland, December 31, 1993. ISBN 0-929600-34-7. |
| 36. | Shrimpton P. Assessment of patient dose in CT: Appendix C. In: European guidelines for multislice computed tomography funded by the European Commission 2004; contract number FIGMCT2000-20078-CT-TIP. Luxembourg: European Commission, 2004. |
| 37. | Shrimpton PC, Hillier MC, Lewis MA, Dunn M. Doses from computed tomography (CT) examinations in the UK: 2003 review. Chilton, UK: National Radiological Protection Board 2005; report NRPB-W67. |
| 38. | Yang J, Yu Y, Hamrick HE, Duerksen-Hughes PJ. ATM, ATR and DNA-PK: initiators of the cellular genotoxic stress responses. Carcinogenesis. 2003;24:1571-1580. |
| 39. | Ye N, Bianchi MS, Bianchi NO, Holmquist GP. Adaptive enhancement and kinetics of nucleotide excision repair in humans. Mutat Res. 1999;435:43-61. |
| 40. | Yu Y, Okayasu R, Weil MM, Silver A, McCarthy M, Zabriskie R, Long S, Cox R, Ullrich RL. Elevated breast cancer risk in irradiated BALB/c mice associates with unique functional polymorphism of the Prkdc (DNA-dependent protein kinase catalytic subunit) gene. Cancer Res. 2001;61:1820-1824. |
| 41. | Yukawa O, Nakajima T, Yukawa M, Ozawa T, Yamada T. Induction of radical scavenging ability and protection against radiation-induced damage to microsomal membranes following low-dose irradiation. Int J Radiat Biol. 1999;75:1189-1199. |
| 42. | Zeeb H, Blettner M, Langner I, Hammer GP, Ballard TJ, Santaquilani M, Gundestrup M, Storm H, Haldorsen T, Tveten U. Mortality from cancer and other causes among airline cabin attendants in Europe: a collaborative cohort study in eight countries. Am J Epidemiol. 2003;158:35-46. |