INTRODUCTION
Terahertz (THz, 1 THz = 1012 Hz) radiation, also known as THz waves, THz light, or T-rays, is situated in the frequency regime between optical and electronic techniques. This regime is typically defined as 0.1-10 THz and has become a new area for research in physics, chemistry, biology, materials science and medicine. Experiments with THz radiation date back to measurements of black body radiation using a bolometer in the 1890s[1,2]. However, for a long time, this region remained unexplored due to a lack of good sources and detectors, and it was commonly referred to as the “THz gap”. In 1975, David Auston at AT&T Bell Laboratories developed a photoconductive emitter gated with an optical pulse that led towards bridging this gap - the ‘Auston switch’ emitted broadband THz radiation up to 1 mW. A coherent method to detect THz pulses in the time domain was also proposed[3]. This became the foundation of THz time-domain spectroscopy (THz-TDS)[4], since then many improvements in the generation and detection of coherent THz radiation enabled THz-TDS and imaging techniques to be pioneered for applications in various fields such as biomedical engineering, physics, astronomy, security screening, communications, genetic engineering, pharmaceutical quality control and medical imaging[5]. In this paper, THz technology is introduced and some emerging applications in biology and medicine including molecular spectroscopy, tissue characterization and skin imaging are presented.
The aim of this article is to review the potential of THz pulsed imaging and spectroscopy as a promising diagnostic method. Several unique features make THz very suitable for medical applications. (1) THz radiation has very low photon energy, which is insufficient to cause chemical damage to molecules, or knock particles out of atoms. Thus, it will not cause harmful ionization in biological tissues; this makes it very attractive for medical applications; (2) THz radiation is very sensitive to polar substances, such as water and hydration state. For this reason, THz waves can provide a better contrast for soft tissues than X-rays; (3) THz-TDS techniques use coherent detection to record the THz wave’s temporal electric fields, which means both the amplitude and phase of the THz wave can be obtained simultaneously. The temporal waveforms can be further Fourier transformed to give the spectra. This allows precise measurements of the refractive index and absorption coefficient of samples without resorting to the Kramers-Kronig relations; and (4) The energy of rotational and vibrational transitions of molecules lies in the THz region and intermolecular vibrations such as hydrogen bonds exhibit different spectral characteristics in the THz range. These unique spectral features can be used to distinguish between different materials or even isomers.
PRINCIPLES OF THZ PULSED IMAGING AND SPECTROSCOPY
THz systems
Over the past two decades, technology for generating and detecting THz radiation has advanced considerably. Several commercialized systems are now available[6-10] and THz systems have been set up by many groups all over the world. According to the laser source used, THz systems can be divided into two general classes: continuous wave (CW) and pulsed.
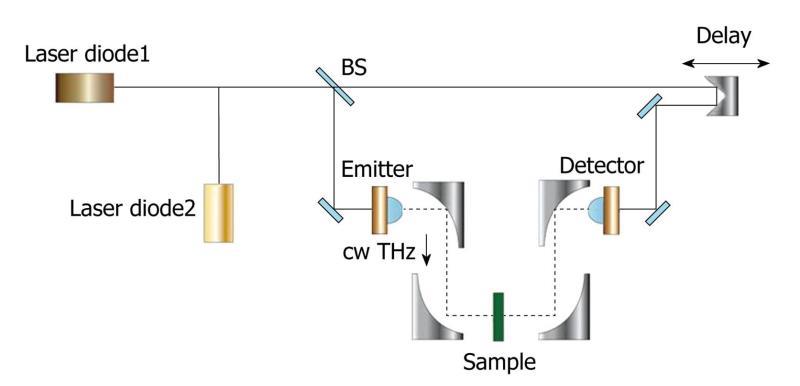
A typical CW system can produce a single fixed frequency or several discrete frequency outputs. Some of them can be tunable. Generation of CW THz radiation can be achieved by approaches such as photomixing[11], free-electron lasers[12] and quantum cascade lasers[13]. Figure 1 illustrates a CW THz system which photomixes two CW lasers in a photoconductor as an example[14]. The mixing of two above-bandgap (visible or near-infrared) wavelengths produces beating, which can modulate the conductance of a photoconductive switch at the THz difference frequency. The photomixing device is labeled “emitter” in Figure 1. Since the source spectrum of the CW system is narrow and sometimes only the intensity information is of interest, the data structures and post-processing are relatively simple. It is possible now to drive a whole CW system by laser diodes and thus it can be made compact and inexpensive. However, due to the limited information that CW systems provide, they are sometimes confined to those applications where only features at some specific frequencies are of interest.
Figure 1 Schematic illustration of a continuous wave THz imaging system in transmission geometry.
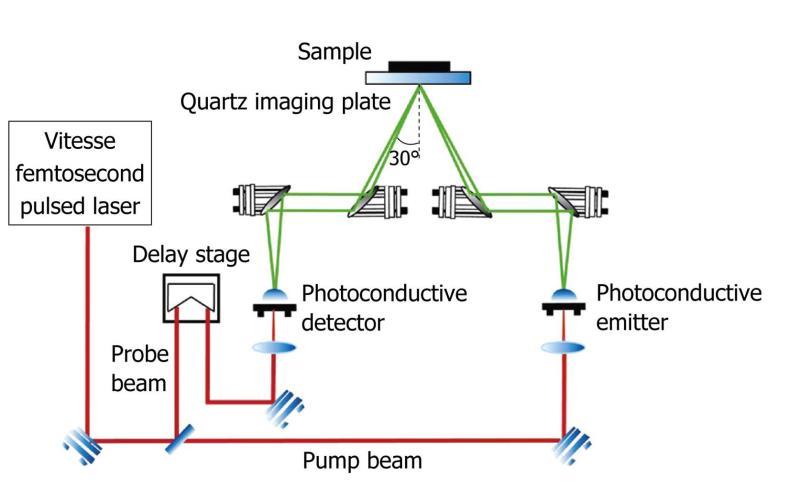
In pulsed systems, broadband emission up to several THz can be achieved. Currently, there are a number of ways to generate and detect pulsed THz radiation, such as ultrafast switching of photoconductive antennas[3], rectification of optical pulses in crystals[15], rapid screening of the surface field via photoexcitation of dense electron hole plasma in semiconductors[16] and carrier tunneling in coupled double quantum well structures[17]. Among them, the most established approaches based on photoconductive antennas, where an expensive femtosecond laser is required and configured as shown in Figure 2. Unlike CW THz imaging system, coherent detection in pulsed THz imaging techniques can record THz waves in the time domain, including both the intensity and phase information, which can be further used to obtain more details of the target such as spectral and depth information[18]. This key advantage lends coherent THz imaging to a wider range of applications.
Figure 2 Schematic illustration of a pulsed THz imaging system with reflection geometry.
Molecular interactions in the THz regime
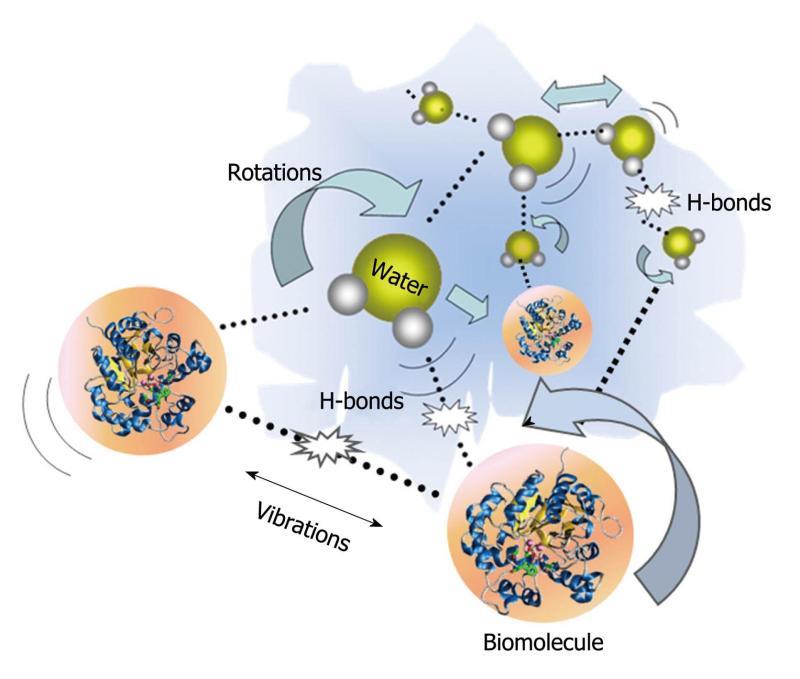
There has been an increased interest in understanding the interactions between molecules and THz radiation. Many of the intricate interactions on a molecular level rely on changes in biomolecular conformation of the basic units of proteins such as α helices and β sheets. In recent years, dynamic signatures of the THz frequency vibrations in RNA and DNA strands have been characterized[19,20]. Furthermore, studies of water molecule interactions with proteins have attracted significant research interest[21]. In a protein-water network, the protein’s structure and dynamics are affected by the surrounding water which is called biological water, or hydration water. As illustrated in Figure 3, hydrogen bonds, which are weak attractive forces, form between the hydrated water molecules and the side chains of protein. These affect the dynamic relaxation properties of protein and enable distinction between the hydration water layer and bulk water. The remarkable effects of the hydrogen bonds associated with the intermolecular information are able to be detected using THz spectroscopy. THz spectra contain information about intermolecular modes as well as intra-molecular bonds and thus usually carry more structural information than vibrations in the mid-infrared spectral region which tend to be dominated by intra-molecular vibrations.
Figure 3 Schematic representation of H-bond interactions between water and biomolecules.
Unique advantages and challenges for biomedical applications
The energy level of 1 THz is only about 4.14 meV (which is much less than the energy of X-rays 0.12 to 120 keV), it therefore does not pose an ionization hazard as in X-ray radiation. Research into safe levels of exposure has also been carried out through studies on keratinocytes[22] and blood leukocytes[23,24], neither of which has revealed any detectable alterations. This non-ionizing nature is a crucial property that lends THz techniques to medical applications.
The fundamental period of THz-frequency electromagnetic radiation is around 1ps, and so it is uniquely suited to investigate biological systems with mechanisms at picosecond timescales. The energy levels of THz light are very low, therefore damage to cells or tissue should be limited to generalized thermal effects, i.e. strong resonant absorption seems unlikely. From a spectroscopy standpoint, biologically important collective modes of proteins vibrate at THz frequencies, in addition, frustrated rotations and collective modes cause polar liquids (such as water) to absorb at THz frequencies. Many organic substances have characteristic absorption spectra in this frequency range[25,26] enabling research into THz spectroscopy for biomedical applications.
THz wavelengths have a diffraction limited spot size consistent with the resolution of a 1990’s vintage laser printer (1.22λ0 = 170 μm at 2.160 THz or 150 dots/in). At 1 THz, the resolution could be as good as a decent computer monitor (70 dots/in). Submillimeter-wavelength means that THz signals pass through tissue with only Mie or Tyndall scattering (proportional to f2) rather than much stronger Rayleigh scattering (proportional to f4) that dominates in the IR and optical since cell size is less than the wavelength.
Since most tissues are immersed in, dominated by, or preserved in polar liquids, the exceptionally high absorption losses at THz frequencies make penetration through biological materials of any substantial thickness infeasible. However, the same high absorption coefficient that limits penetration in tissue also promotes extreme contrast between substances with lesser or higher degrees of water content which can help to show distinctive contrast in medical imaging.
IMAGING VS SPECTROSCOPY
THz pulsed imaging
Early applications of THz technology were confined mostly to space science[27] and molecular spectroscopy[28,29], but interest in biomedical applications has been increasing since the first introduction of THz pulsed imaging (TPI) in 1995 by Hu and Nuss[30]. Their THz images of porcine tissue demonstrated a contrast between muscle and fats. This initial study promoted later research on the application of THz imaging to other biological samples. THz pulsed imaging actually can be viewed as an extension of the THz-TDS method. In addition to providing valuable spectral information, 2D images can be obtained with THz-TDS by spatial scanning of either the THz beam or the object itself. In this way, geometrical images of the sample can be produced to reveal its inner structures[31]. Thus, it is possible to obtain three-dimensional views of a layered structure.
When a THz pulse is incident on such a target, a train of pulses will be reflected back from the various interfaces. For each individual pulse in the detected signal, the amplitude and timing are different and can be measured precisely. The principle of time of flight technique is to estimate the depth information of the internal dielectric profiles of the target through the time that is required to travel over a certain distance. This permits looking into the inside of optically opaque material and it has been used for THz 3D imaging. Among the earliest demonstrations of THz 3D imaging, Mittleman et al[31] imaged a conventional floppy disk. In their work the various parts inside the disk were identified and the discontinuous refractive index profile was derived. This method was further extended into THz reflection computed tomography[32,33], where the target was rotated to provide back reflection from different angles. In a similar way to X-ray CT imaging, the filtered back projection algorithm was applied to reconstruct the edge map of the target’s cross-section. With advances in interactive publishing, Wallace et al[34] highlighted the ability of 3-D THz imaging in a number of niche applications. For example they were able to resolve two layers of drugs beneath the protective coating of a pharmaceutical tablet.
THz spectroscopy
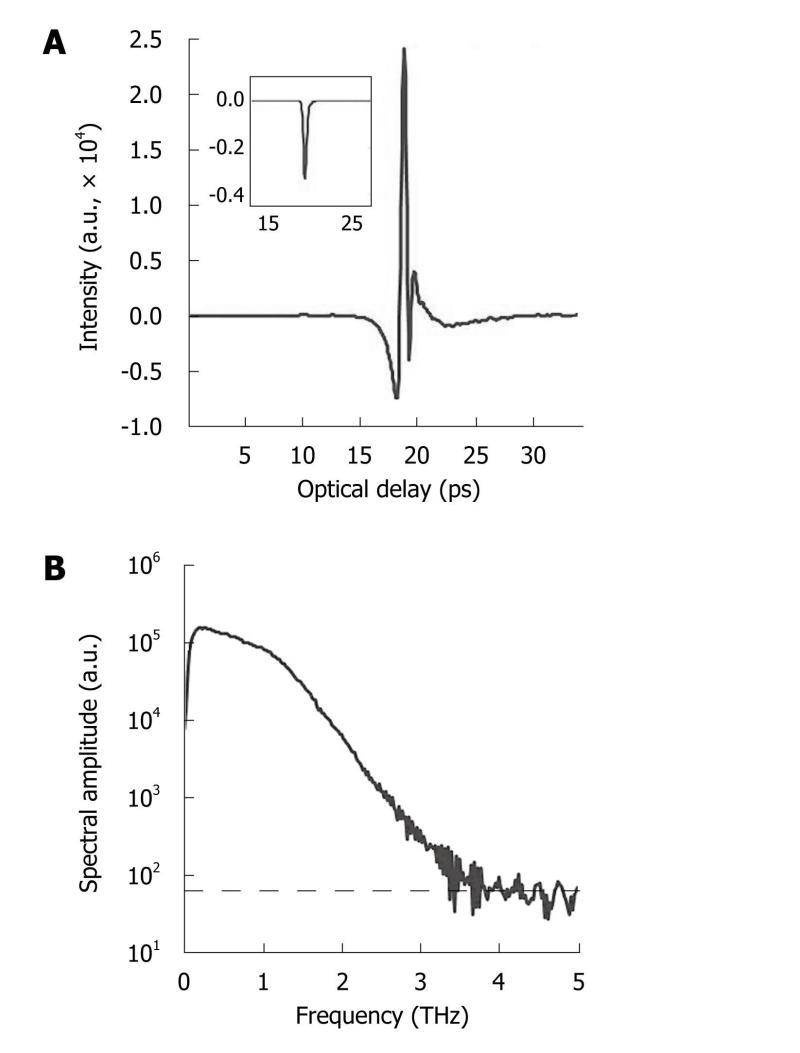
THz spectroscopy is typically done with a single point measurement (with transmission geometry in most cases) of a homogenous sample and the resulting THz electric field can be recorded as a function of time. Thus, it can be Fourier transformed to offer meaningful spectroscopic information due to the broadband nature of pulsed THz radiation, shown in Figure 4. Although the spectral resolution is not as good as that with narrowband techniques, coherent detection of THz-TDS can provide both high sensitivity and time-resolved phase information[35]. This spectroscopic technique is primarily used to probe material properties and it is helpful to see where it lies in the electromagnetic spectrum in relation to atomic and molecular transitions.
Figure 4 Typical terahertz pulse and corresponding spectrum.
A: Temporal waveform of a sample (drawn by raw data with the inset representing its corresponding processed data after deconvolution with a reference measurement); B: The corresponding spectrum of the raw data with the noise floor (indicated by dashed line).
THz spectroscopy is complementary to THz imaging and is primarily used to determine optical properties in the frequency domain. Since THz pulses are created and detected using short pulsed visible lasers with pulse widths varying from approximately 200 fs down to approximately 10 fs, it is now possible to make time resolved far-infrared studies with sub-picosecond temporal resolution[36]. This was not achievable with conventional far-infrared studies. An important aspect of THz time-domain spectroscopy is that both the phase and amplitude of the spectral components of the pulse are determined. The amplitude and phase are directly related to the absorption coefficient and refractive index of a sample and thus the complex permittivity is obtained without requiring Kramers-Kronig analysis. Furthermore, another advantage of THz spectroscopy is that it is able to non-destructively detect differences because it uses radiation of sufficiently long wavelength and low energy that does not induce any phase changes or photochemical reactions to living organisms.
BIOLOGICAL APPLICATIONS
Pharmaceuticals
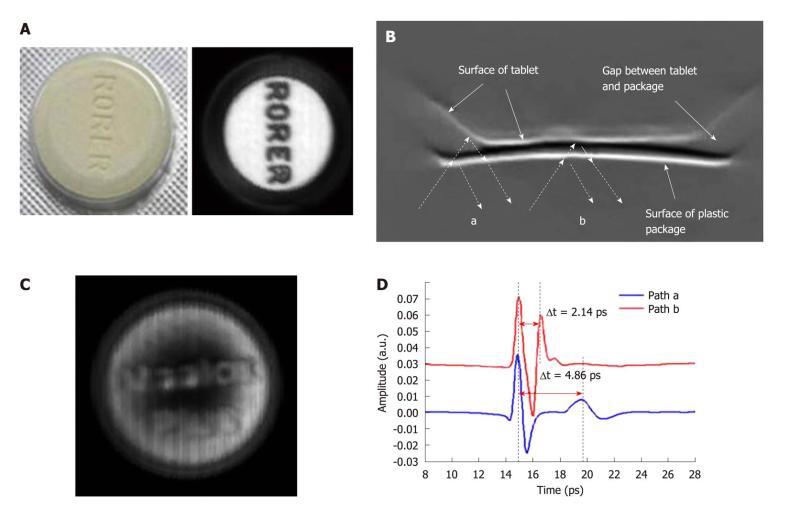
There has been a strong drive in the pharmaceutical industry for comprehensive quality assurance monitoring. This motivates development of new tools providing useful analysis of tablet formulations. The ability of THz technology to determine both spectral and structural information has fuelled interest in the pharmaceutical applications of this technique[37]. For example THz spectroscopy has been employed for polymorph identification and quantification[38], phase transition monitoring[39], and distinguishing between behaviors of hydrated forms[40]. THz radiation can penetrate through plastic packaging materials. To illustrate this we give an example using Maalox PlusTM - an over the counter medicine for stomach upsets. Each tablet has “Rorer” engraved on one side and “Maalox Plus” on the other side. Figure 5A contains a photo of a tablet in its packaging as well as a THz image taken after removing it from the packaging - the engraved lettering is clearly seen in the THz image. Figure 5C is a THz image of the tablet taken through the packaging - we can still see the lettering on the surface of the tablet. The cross-section of the tablet is better conveyed by an image of the depth profile. THz pulses are reflected first off the front surface of the package and then from any subsurface structure within it resulting in multiple pulses returning to the detector. The dashed arrows in Figure 5B mark out the THz light paths at the edge of the tablet (path A) and the center (path B). In path A the beveled edge means that there is an air gap between the packaging and the tablet and this corresponds to a greater optical delay between the reflected peaks in the waveforms illustrated in Figure 5D. Thus, THz imaging has non-destructively revealed the structure of the tablet through the packaging.
Figure 5 Terahertz imaging of the tablet.
A: Photograph of the tablet in the plastic package and THz C-Scan section imaging without the packaging; B: THz B-Scan image shows the structure of the cross-section of the tablet and the THz light paths at the edge of the tablet (path a) and the centre (path b); C: THz C-Scan image shows the tablet face inside the plastic package; D: THz deconvolved waveforms in the time-domain reflected from the paths a and b in Figure 5B.
Protein spectroscopy
Towards the higher frequency end of the THz range (from about 1 THz and above) there are vibrational modes corresponding to protein tertiary structural motion; such intermolecular interactions are present in many biomolecules. Other molecular properties that can be probed in the THz range include bulk dielectric relaxation modes[41] and phonon modes[42] these can be difficult to probe using other techniques. For instance nuclear magnetic resonance (NMR) spectroscopy can determine the presence of various carbon bonds, but it cannot be used to distinguish between molecules with the same molecular formula, but with different structural forms (isomers)[10]. THz spectroscopy is able to distinguish between isomers and polymorphs[43] and is therefore emerging as an important and highly sensitive tool to determine biomolecular structure and dynamics[44,45]. Indeed THz spectroscopy can distinguish between two types of artificial RNA strands when measured in dehydrated form[46]. Furthermore, Fischer et al[47] demonstrated that even when the molecular structure differs only in the orientation of a single hydroxyl group with respect to the ring plane, a pronounced difference in the THz spectra is observed. Intermolecular interactions are present in all biomolecules, and since biomolecules are the fundamental components of biological samples, they can be used to provide a natural source of image contrast in biomedical THz imaging[48].
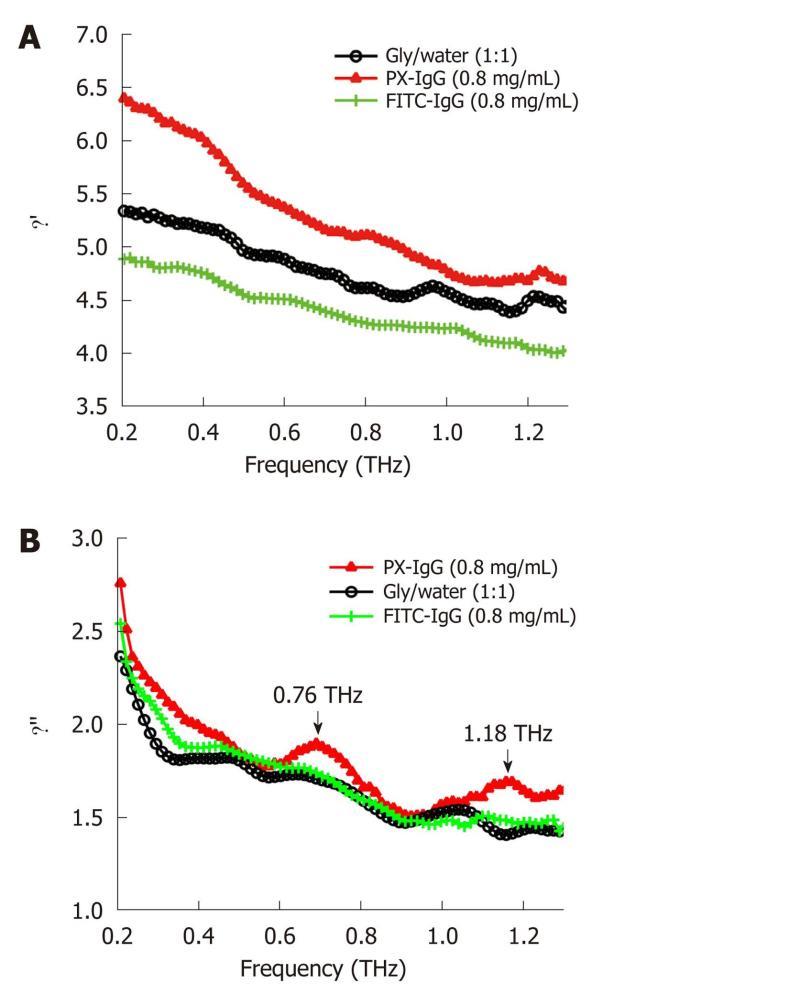
Biomolecules, especially proteins, which play an essential structural and catalytic role in cells and tissues, often require an aqueous phase in order to be transported to their target sites. In the protein-water system, the characteristic water structure induced near the surfaces of proteins arises not only through hydrogen bonding of the water molecules to available proton donor and proton acceptor sites, but also through electrostatic forces associated with the water molecule that arise from its large electric dipole moment. If an electric field is applied to such a system of protein-associated water, there will be a torque exerted on each water dipole moment inducing them to attempt to align along the direction of the field vector. The dielectric spectrum has been widely used to describe the interaction between protein and its solvent molecule in THz frequency[49]. A dielectric orientational relaxation time τ can then be defined as the time required for 1/e of the field-oriented water molecules to become randomly reoriented on removing the applied field. Measurements may be analyzed in terms of the complex dielectric constant ε(ω) (where ω denotes angular frequency) or the complex refractive index n(ω). The degree of orientational polarization and the rate of reorientational relaxation depend on how the water dipoles are influenced by local electrostatic forces and the extent to which the breaking and/or reforming of local hydrogen bonds is required to accommodate the changes in orientation. Relaxations of polar side-groups, vibrations of the polypeptide backbone, and fluctuating proton transfer between ionized side-groups of the protein also contribute to the overall polarizability of the protein-water system. If dielectric measurements are made on protein solutions, then orientational relaxations of the protein molecule itself will also be observed[50]. Figure 6 shows that the vibration mode of two types of labeled antibodies (peroxidase conjugated IgG and the fluorescein conjugated IgG) can be distinguished at 0.76 and 1.18 THz using the THz dielectric spectrum. By investigating the concentration dependence of the spectra it is also possible to obtain an estimate of the hydration shell thickness around the protein molecules[51].
Figure 6 Dielectric constant spectra (A) and dielectric loss spectra (B) of water/ glycerol mix (black line with circles), peroxidase conjugated IgG (red line with triangles) and the fluorescein conjugated IgG (green line with crosses) dissolved in the water/glycerol solution at the concentration of 0.
8 mg/mL in the frequency range of 0.1-1.3 THz. PX: Peroxidase conjugated; FITC: Fluorescein conjugated.
MEDICAL APPLICATIONS
Tissue characterization
There is also interest in tissue contrast for in vivo and ex vivo
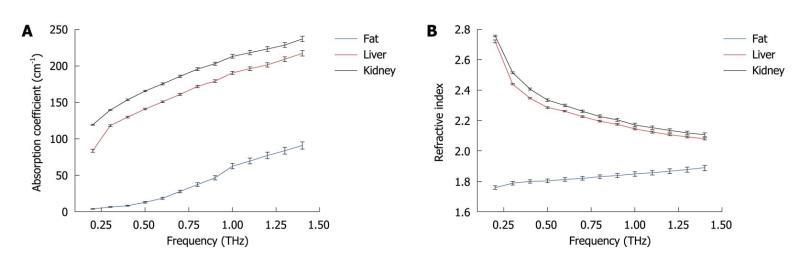
identification of abnormalities, hydration, and subdermal probing. Only a small number of measurements have been made to date, and systematic investigations to catalogue absorption coefficients, refractive indices and contrast mechanisms are just beginning to accumulate. Measurements on the absorption and refractive index of biological materials in the THz region go back at least to 1976[52]. Several research groups have investigated excised and fixed tissue samples, either alcohol perfused[8], formalin fixed[53-57], or freeze dried and wax mounted[58] looking for inherent contrast to define unique modalities. One of the first applications on human ex vivo wet tissue involved imaging of excised basal cell carcinoma[59,60]. In vivo work has focused on the skin[61] and accessible external surfaces of the body for measuring hydration[62] and tumor infiltration[60]. A catalogue of unfixed tissue properties (including blood constituents) was compiled by the University of Leeds, UK[54] for frequencies between 500-1500 GHz using a pulsed time-domain system. Difficulties in extrapolating measurements on excised tissue to in vivo results are numerous and include for example uptake of saline from the sample storage environment, changes in hydration level during measurement, temperature-dependent loss, measurement chamber interactions, and scattering effects. In our previous work[63], we performed reflection geometry spectroscopy to investigate the properties of several types of healthy organ tissues, including liver, kidney, heart muscle, leg muscle, pancreas and abdominal fat tissues using THz pulsed imaging. The frequency dependent refractive index and the absorption coefficient of the tissues are shown in Figure 7. All the results are the mean values of the ten samples and error bars represent the 95% confidence intervals. We found clear differences between the tissue properties, particularly the absorption coefficient. Since fatty tissue largely consists of hydrocarbon chains and relatively few polar molecules, the absorption coefficient and refractive index of the fatty tissue are much lower that those of the kidney and liver tissues.
Figure 7 Tissue characterization using THz spectroscopy.
A: Mean absorption coefficients of kidney, liver and abdominal fat; B: Mean refractive indices of all the tissue samples. Error bars represent 95% confidence intervals.
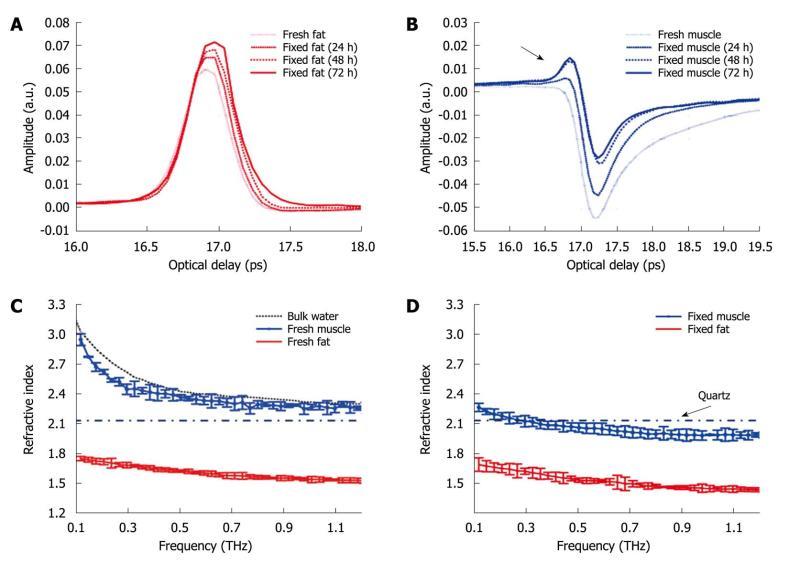
We have also investigated the optical properties of fresh and formalin fixed samples in the THz frequency range using THz reflection spectroscopy[64]. As seen in Figure 8A, when the fixing time increased the waveform amplitude of the adipose tissue also increased. This was primarily because the refractive index of the adipose tissue was decreasing over the majority of the bandwidth (due to the fixing) and this meant there was a greater difference between the refractive index of the quartz (n approximately 2.1) and that of the adipose tissue (e.g. 1.5 at 1 THz when fixed compared to 1.6 when it was fresh). From Fresnel theory, this increased difference in refractive indices resulted in a greater reflected amplitude. As the fixing time increased for the muscle, three main changes were apparent. A small peak started to appear preceding the trough and the width and magnitude of the trough decreased (Figure 8B). These changes can also be explained by considering the effects of the formalin on the refractive index and absorption coefficient of the muscle. For the muscle, the formalin significantly reduced both the refractive index and the absorption coefficient. Before fixing, the refractive index of the fresh muscle was greater than that of quartz over the whole of the frequency range measured (Figure 8C). The fixing reduced the refractive index so much that the refractive index became lower than that of quartz at higher frequencies (Figure 8D) and this was the cause for the small peak which appeared (arrow in Figure 8B) and increased as the fixing time was increased.
Figure 8 The deconvolved mean waveforms for adipose tissue (A) and skeletal muscle (B) as the fixing time progressed and the mean refractive index for fresh (C) and fixed samples (D) of adipose tissue and skeletal muscle.
The dot-dash line indicates the refractive index of the quartz window on which the sample was measured. Error bars represent 95% CI. The water data were acquired in transmission and the error bars are too small to be seen on this graph.
Skin cancer, breast tumors and dental caries
Due to the low penetration depth of THz in biological tissues, THz biomedical applications investigated to date have been limited to easily accessible parts of the body, such as skin and teeth, or those that would benefit from intra-operative imaging such as breast cancer. Figure 9 is a photograph of the reflection geometry THz probe from TeraView Ltd. which we use in Hong Kong.
Figure 9 Photograph of the THz hand-held probe.
One potential application of THz imaging is the diagnosis of skin cancer. Work by Woodward et al[60] has demonstrated the potential to use THz imaging to determine regions of skin cancer non-invasively using a reflection geometry imaging system. The first ex vivo measurements on skin cancer revealing the ability of TPI to differentiate basal cell carcinoma (BCC) from normal skin were produced by Woodward et al[59].
Breast-conserving surgery is also an area of medicine which may benefit from THz imaging. Fitzgerald et al[65] conducted ex vivo studies of breast cancer to investigate the potential of THz imaging to aid the removal of breast cancer intra-operatively. In particular, they studied the feasibility of THz pulsed imaging to map the tumor margins on freshly excised human breast tissue. Good correlation was found for the area and shape of tumor in the THz images compared with that of histology. They also performed a spectroscopy study comparing the THz optical properties (absorption coefficient and refractive index) of the excised normal breast tissue and breast tumor. Both the absorption coefficient and refractive index were higher for tissue that contained tumor and this is a very positive indication that THz imaging could be used to detect margins of tumor and provide complementary information to techniques such as infrared and optical imaging, thermography, electrical impedance, and magnetic resonance imaging[66,67].
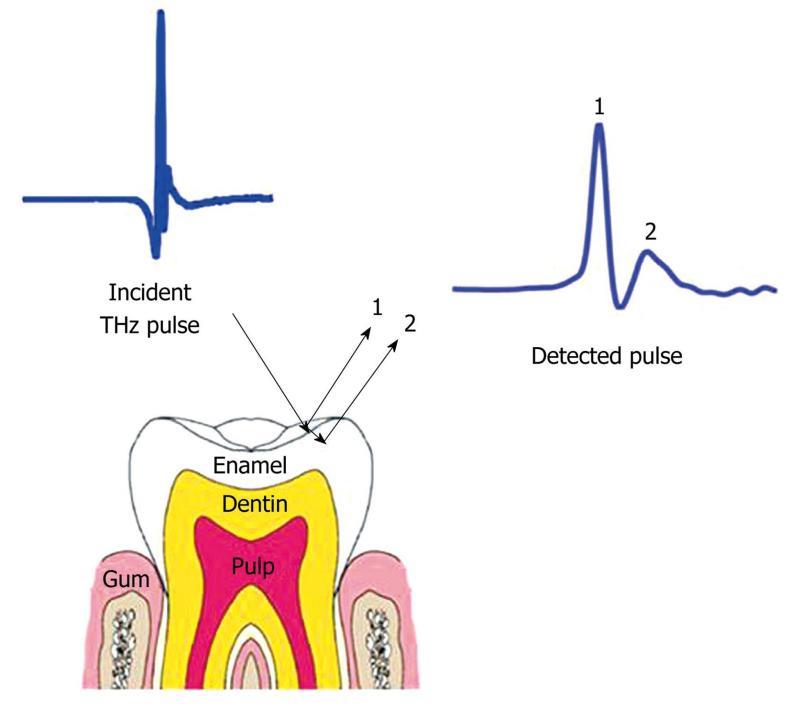
Since in vivo THz imaging is currently limited to surface features, another potential application of THz imaging is the diagnosis of dental caries[68,69]. Figure 10 is a schematic diagram of a THz reflection from the outer layer of enamel. Caries are a result of mineral loss from enamel, and this causes a change in refractive index within the enamel. The change in refractive index means that small lesions, smaller than those detected by the naked eye, can be detected[70]. However, in practice THz imaging systems are large and cumbersome - even structures as obvious as teeth can make a challenging target. In this respect THz imaging is still some way off offering a non-ionizing alternative to X-rays in dentistry.
Figure 10 Schematic representation of the THz reflections from enamel.
Reflection 1 is the reflection from the surface of the enamel and reflection 2 is the reflection from within the enamel due to tooth decay causing mineral loss.
Burn depth diagnosis
Since THz waves can penetrate several hundreds of microns into the skin and most burn injuries are superficial, this opens up the possibility of employing THz techniques for burn assessment[71]. The waveforms and optical parameters of the burn wounds were investigated and THz images have shown contrast between burn-damaged tissue and healthy tissue. Their results indicate that THz imaging may be promising in evaluating skin burn severity, especially for characterizing burn areas. Moreover, the time of flight technique is able to reveal the depth profile thus could be used to evaluate burn depth.
The potential of THz imaging as a burn diagnostic has been demonstrated using chicken breast[6]. It is also conceivable that THz imaging could be of use in monitoring treatment of skin conditions (like psoriasis), since THz imaging is cheaper than MRI and does not require a coupling gel like ultrasound[72].
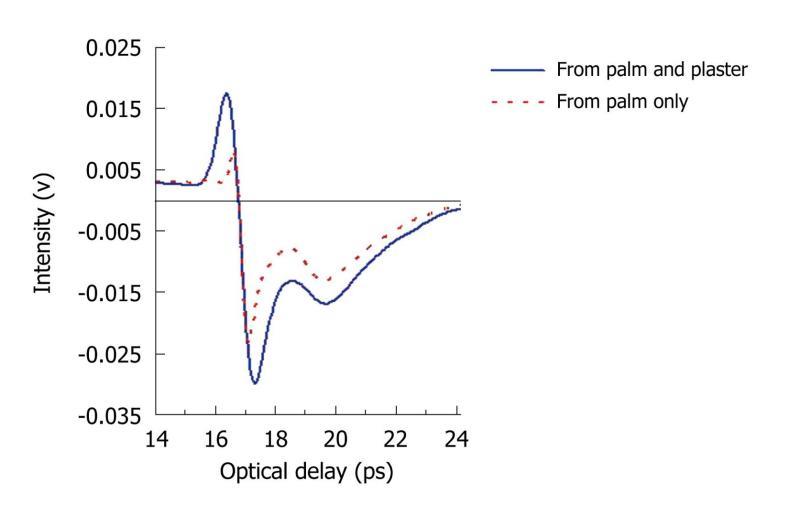
THz light can penetrate many materials: we have investigated whether it can resolve skin layers beneath a Tegaderm® plaster as this would also be of benefit in monitoring burn wounds. Normal skin comprises three different layers: the stratum corneum, epidermis, and dermis. The stratum corneum on the palm of the hand is 100-200 μm thick and thus has been resolved in previous in vivo skin studies. We placed the plaster on the palm of the hand and the resulting THz reflected waveform is shown in Figure 11. For comparison the measurement of the palm alone is also plotted. In the figure, there are 2 troughs, which correspond to the top and bottom surface of the stratum corneum. The optical delay between them can be used to calculate the thickness of this layer. These results show that THz light is able to penetrate through wound dressings with only slight attenuation to reveal the skin depth information. This therefore indicates that THz imaging is likely to be able to detect and measure changes to the stratum corneum and epidermis which could for example, be caused by burns. To illustrate how sensitive THz imaging is to the stratum corneum, we have also imaged the side of a healthy hand. As the position of the measurement changes, as indicated by the arrow in
Figure 11 Measured pulses from the palm.
The blue line is the measurement of the palm through a tegaderm plaster and the red dotted line is the measurement of the palm alone. In both cases, two troughs are seen - they are the reflections from the top and bottom surfaces of the stratum corneum.
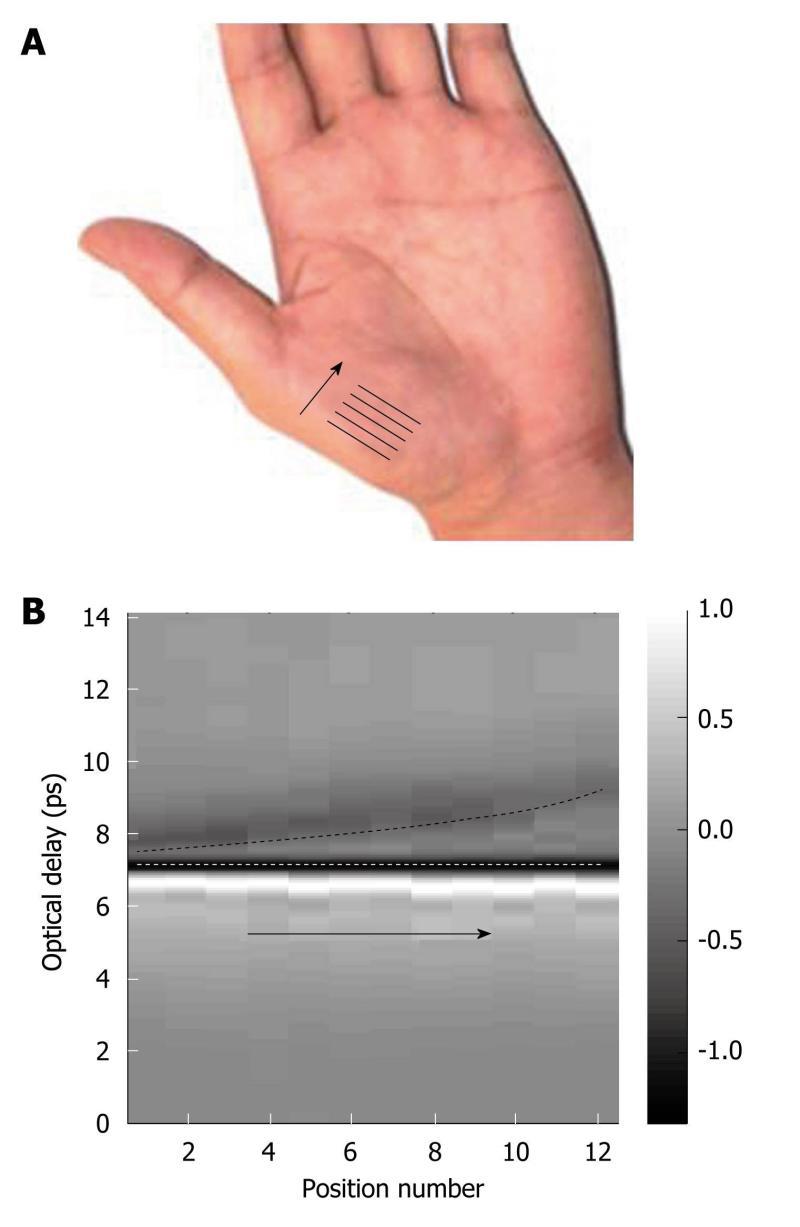
Figure 12A, the optical delay between the reflections from the top and bottom of the stratum corneum increases. This is because the stratum corneum is thicker at the tip of the arrow than at the arrow foot. By plotting the reflected amplitude intensity against position we obtain the depth profile image of the palm in Figure 12B. By using frequency and wavelet domain deconvolution we are able to resolve thinner layers of skin than if basic deconvolution is used[73].
Figure 12 In vivo measurement of the variation in stratum corneum thickness of the palm.
A: Photograph of the palm, indicating where the THz measurements were taken; B: A b-scan image showing the variation in stratum corneum thickness with palm position. The white dashed line indicates the interface between the quartz window and the stratum corneum; and the black dashed line indicates the interface between the stratum corneum and the epidermis. The grey bar represents the ratio of the electric field at a given optical delay, t, over the maximum electric field [E(t)/Emax].
COMPARISON WITH OTHER TECHNOLOGIES
Numerous groups have investigated direct transmission or reflection THz imaging as a means of distinguishing tissue types[30,55,74] and recognizing diseases including tumors penetrating below the surface layers of skin or into organs[56,58,75]. Although progress is being made, the competition from other more developed imaging modalities is fierce. Optical coherence tomography, ultrasound, near-IR, and Raman spectroscopy, MRI, positron emission tomography, in situ confocal microscopy, and X-ray techniques have all received much more attention and currently offer enhanced resolution, greater penetration, higher acquisition speeds, and specifically targeted contrast mechanisms. This does not preclude THz imaging from finding a niche in this barrage of already favorable modalities. There is still no technique that can readily distinguish benign from malignant lesions macroscopically at the surface or subdermally. The sensitivity of THz signals to skin moisture, which is often a key indictor, is very high, and competing techniques such as high-resolution MRI are less convenient and more costly.
The resolution of ordinary THz imaging is diffraction limited, however, its high sensitivity to water content and great surface imaging capability provide motivation for further development and sub-wavelength resolution has been achieved in near-field studies[76]. Indeed shallow subsurface images can be very revealing and the first few hundred micrometers are hard to image with other modalities. The high sensitivity of THz radiation to fluid composition and the variable conductivity in tissue[77] is likely to lead to statistically significant differences between nominally identical samples taken at different locations in the body at different times or from different subjects. This may ultimately prove advantageous; however, in the short term, it will tend to mask sought for differences that are indicative of diseases.
CONCLUSION
THz imaging is still in its early stage of development, but as this paper has shown, has great potential to be a valuable imaging technique in the future. In the past decade, THz imaging applications in biomedical fields have drawn extensive interest and advancements in imaging methods and theoretical analysis continue to enable further applications to be investigated.