Published online Oct 28, 2011. doi: 10.4329/wjr.v3.i10.241
Revised: July 19, 2011
Accepted: July 26, 2011
Published online: October 28, 2011
AIM: To evaluate the dynamic contrast-enhanced magnetic resonance imaging (DCE-MRI) findings of bone metastasis in prostate cancer patients.
METHODS: Sixteen men with a diagnosis of metastatic prostate cancer to bones were examined with DCE-MRI at 1.5 Tesla. The mean contrast agent concentration vs time curves for bone metastasis and normal bone were calculated and Ktrans and ve values were estimated and compared.
RESULTS: An early significant enhancement (wash-out: n = 6, plateau: n = 8 and persistent: n = 2) was detected in all bone metastases (n = 16). Bone metastasis from prostate cancer showed significant enhancement and high Ktrans and ve values compared to normal bone which does not enhance in the elderly population. The mean Ktrans was 0.101/min and 0.0051/min (P < 0.001), the mean ve was 0.141 and 0.0038 (P < 0.001), for bone metastases and normal bone, respectively.
CONCLUSION: DCE-MRI and its quantitative perfusion parameters may have a role in improving the detection of skeletal metastasis in prostate cancer patients.
- Citation: Kayhan A, Yang C, Soylu FN, Lakadamyalı H, Sethi I, Karczmar G, Stadler W, Oto A. Dynamic contrast-enhanced MR imaging findings of bone metastasis in patients with prostate cancer. World J Radiol 2011; 3(10): 241-245
- URL: https://www.wjgnet.com/1949-8470/full/v3/i10/241.htm
- DOI: https://dx.doi.org/10.4329/wjr.v3.i10.241
Prostate cancer is the most commonly diagnosed non-skin cancer in men in the United States. As per the latest estimates by American Cancer Society in 2009 about 192 280 new cases of prostate cancer will be diagnosed and 27 360 men will die of the disease[1]. It is known that most patients with locally advanced prostate cancer will also have probable occult metastases at diagnosis. The most important determinant of potentially curative therapies and of appropriate palliative management for prostate cancer during early staging is accurate assessment of the extent of the metastatic process[2].
The most frequent sites of distant metastases of prostate cancer are bones and typically vertebra[3,4]. The diagnosis, location, burden and monitoring of metastatic bone involvement plays a crucial role in patient management and prognosis. Imaging bone disease in prostate carcinoma generally involves a cascade of studies starting with bone scintigraphy followed by magnetic resonance imaging (MRI), computed tomography (CT) or positron emission tomography/CT. Conventional MRI is sensitive to early changes in bone marrow that precede the osteoblastic response in the bone matrix. However, detection rates for bone metastases using MR range between 7% and 38% and its use is still limited[4,5]. Recently, newer MRI methods such as diffusion-weighted imaging and dynamic contrast-enhanced MRI (DCE-MRI) are also addressing the lack of quantitative assesment of skeletal metastases.
DCE-MRI has been increasingly used as an additional technique to characterize various bone lesions, grading disease, planning and guiding biopsy and monitoring response to radio- and/or chemotherapy and detecting early local recurrence[6,7]. It provides a powerful tool for assessing angiogenesis and measuring properties of tissue vasculature, including blood volume and vascular permeability in tumor tissues. In this study, we aimed to evaluate DCE-MRI findings of bone metastasis in patients with prostate cancer.
The study group consisted of 16 men (age range: 49-79 years;
median age: 65 years) with histologically proven adenocarcinoma of the prostate with skeletal metastasis. The study was approved by the institutional review board and informed consent was obtained. Each patient underwent clinical CT scan and bone scan prior to this study and the sites of bone metastasis was determined based on CT and bone scan findings. As part of the research protocol, bone metastases in regions with minimal motion artifact were scanned by research DCE-MRI protocol. In one patient, the scanned bone metastasis was in the shoulder and in 15 patients it was in the pelvic region. None of the metastatic lesions were treated before MRI.
MR images were acquired on a 1.5T GE MRI scanner (SIGNA™, GE Medical Systems, Waukesha, WI, USA). Following a scout scan to localize the lesions, T1-weighted (T1W) images were acquired at 2 s temporal resolution for 1 min before and 6 min after the injection of 0.1 mmoL/kg gadodiamide (Omniscan, GE Healthcare, Chalfont St. Giles, UK). The contrast agent and 20 mL saline flush was injected with an automated injector (Medrad, Indianola, PA, USA) at the rate of 2 mL/s in an antecubital vein. A 2D fast spoiled gradient-echo pulse sequence was used with TR/TE = 7.8/1.7 ms, flip angle 60°, matrix size 256 × 128, field of view 30-35 cm, 2 slices, slice thickness 8 mm, slice spacing 1 mm. The axial slices in which the lesion was in its largest dimension were selected.
For each subject, an experienced radiologist placed the region of interest (ROI) on the bone metastasis and normal bone in the DCE-MRI after reviewing the clinical CT and bone scan images. Any vessels at the lesion margin were carefully excluded from the bone metastasis ROI. For normal bone, muscle and bone metastasis the mean ROI size was 3.1 cm2 (median 3.2 cm2, range 1.1-5.7 cm2), 16.4 cm2 (median 13.9 cm2, range 6.6-33.8 cm2) and 14.3 cm2 (median 10.0 cm2, range 5.0-33.7 cm2), respectively.
The enhancement patterns of bone metastasis and normal bone were analyzed regarding presence of early enhancement, washout, plateau and persistence of enhancement. The contrast agent concentration was calculated as previously described[8]. Contrast agent arterial input function (AIF), which is the contrast agent concentration in the blood plasma, was estimated with a multiple reference tissue method using tumor voxels and muscle as described by Yang et al[8,9]. The mean contrast agent concentration vs time curve [Ct(t)] was calculated for each bone metastasis ROI and normal bone ROI. Using the estimated individual AIF, contrast agent transfer rate between blood and tissue (Ktrans) and the extra-vascular extra-cellular fractional volume (ve), were then estimated under the Tofts model[10].
Two-tailed paired Student’s t-test was used to test the difference in Ktrans and ve between bone metastasis and normal bone. Statistical analysis was performed using SPSS Software System version 15.0 (SPSS Inc., USA).
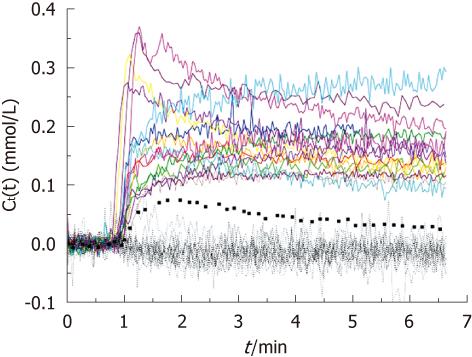
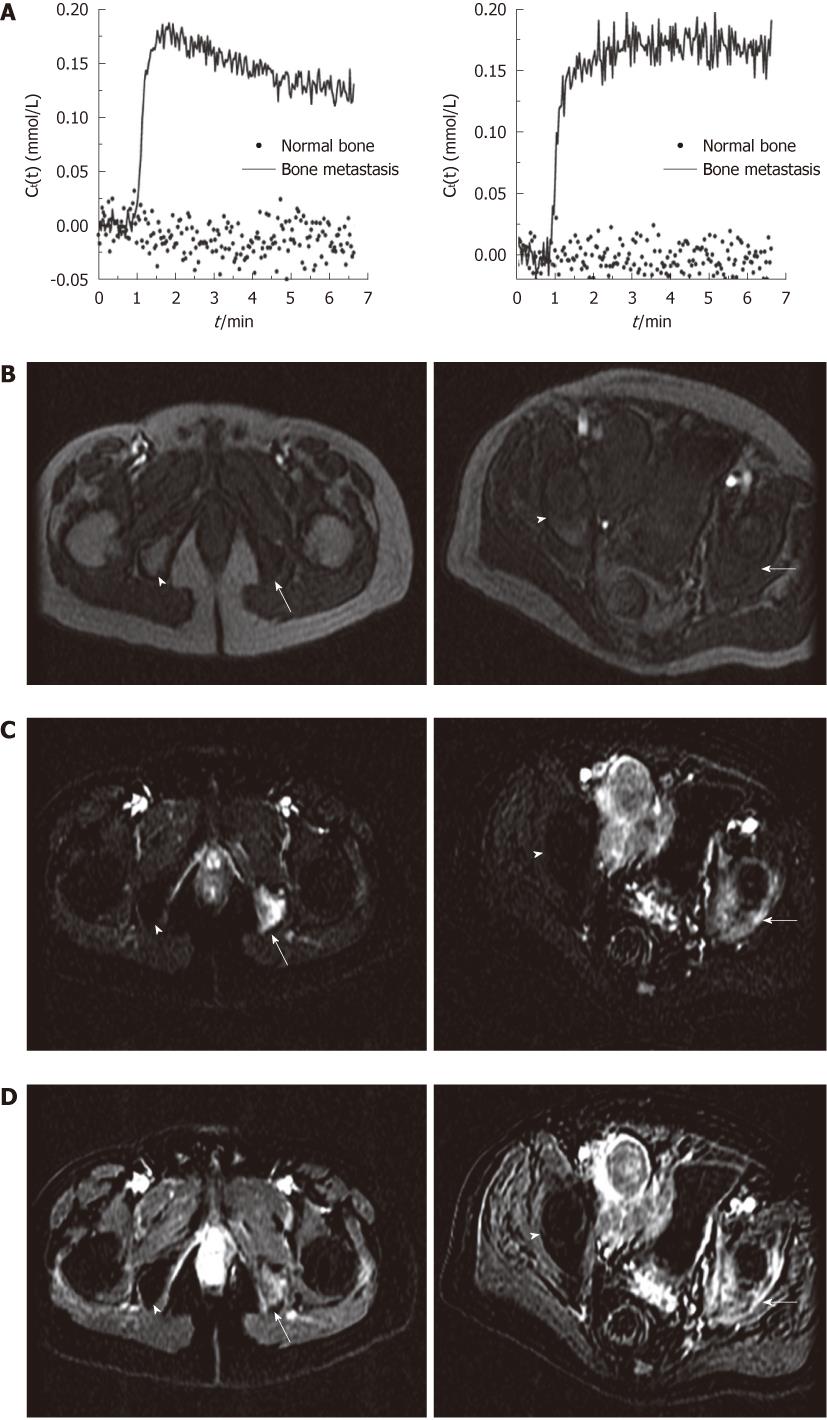
All of the bone metastases showed early significant enhancement (wash-out: 6, plateau: 8 and persistent: 2) (Figures 1 and 2). On the other hand, normal bone demonstrated negligible enhancement in 15 patients. There was minimal enhancement of normal bone in only one patient.
For the 16 bone metastases, the mean Ktrans was 0.101/min (range 0.034-0.290/min, median 0.071/min) and mean ve was 0.141/min (range 0.080-0.234/min, median 0.141/min). For the 16 normal bones, the mean Ktrans was 0.0051/min (range 0.0-0.080/min, median 0.0/min), (P < 0.001). The mean ve of normal bone was 0.0038 (range from 0.0-0.048, median 0.0), also significantly lower than that in bone metastases (P < 0.001). Based on quantitative analysis, normal bones showed slightly negative enhancement or very weak enhancement. In one 69-year-old patient, the normal bone showed a moderate enhancement with a Ktrans value of 0.080/min and a small ve value of 0.048. Figure 2 shows the Ct(t) curve of bone metastasis and normal bone, as well as the pre-contrast image, the average early subtraction image, and the average late subtraction image in two representative patients.
Approximately 70% of patients with advanced prostate cancer develop skeletal metastasis[11,12]. MRI appearance of normal bone marrow reflects variable amounts of its physiological components, primarily fat cells and hemopoietic cells. Although bone marrow that contains mostly fat cells can be depicted by conventional MRI techniques (including T1W, T2W and fat saturated imaging), these techniques are often not able to differentiate tumor infiltration, fibrosis and normal red bone marrow. Additionally, for a malignant marrow lesion to be visible on conventional MRI scan, it must replace sufficient normal marrow cells so that it can cause alterations in T1 and T2 relaxation values. However, as the perfusion of normal bone marrow is strongly influenced by the age of the patient and fat content of the marrow, the contrast enhancement of normal bone marrow decreases markedly with increasing age and conversion to fat, while the tumor cells demonstrate enhancement[7,13,14]. Our study group consisted of patients with an age range of 49-79 years and no enhancement was detected in normal bones in the vast majority (15/16) of the patients. As the metastatic tumor has increased enhancement levels, the tumor foci can be easily detected in the background of non-enhancing bone marrow on contrast-enhanced MR images. Therefore, contrast enhanced MRI may be an important tool for detection of bone metastasis for the elderly population of prostate cancer patients.
There have been many studies searching the microvascularization of bone marrow with different DCE-MRI techniques in which qualitative, semiquantitative and quantitative methods have been reported to depict tissue perfusion parameters[15-18]. Tokuda et al analyzed 34 patients with benign and malignant vertebral lesions in which peak enhancement, steepest slope and slope value were calculated from the time intensity curve (TIC)[19]. They showed that the steepest slopes of metastatic lesions were significantly higher than those of benign lesions and no characteristic distribution of the TIC pattern was found to help in differentiation of benign and metastatic lesions. Chen et al investigated the peak contrast enhancement percentage, enhancement slope and the TIC patterns of the first pass of contrast into vertebral lesions. They found that metastatic vertebral lesions had a higher peak enhancement percentage and steeper enhancement slope than lesions of benign etiology[6]. They also concluded that type D (rapid wash in and wash out) and E (rapid wash in followed by a second slow-rising phase) curves are valuable in differentiating benign and malignant vertebral lesions. Both of these studies evaluated angiogenesis and perfusion of bone metastasis using semi-quantitative parameters. Recently, a few studies have looked into more advanced quantitative analysis methods to potentially increase accuracy and reproducibility of DCE-MRI. Baurle et al evaluated the amplitude and exchange rate constant (Kep) of the enhancement of bone metastasis in an animal model of breast cancer[20]. They found that amplitude decreased significantly prior to changes in osteolytic lesion size following treatment of bone metastasis. On the other hand, there was no significant change in Kep between the treated group and control group.
In our study, by using quantitative parameters obtained from high temporal resolution DCE-MRI data, we demonstrated that in elderly prostate cancer patients, bone metastasis showed much faster and higher enhancement than normal appearing bones. The difference in their contrast concentration levels lasted for the entire 5.5 min of contrast enhancement duration. These results suggests that it may be possible to detect bone metastasis at a delayed contrast enhanced phase after 3 min of contrast administration instead of imaging the patients continuously at high temporal resolution for several minutes. However, quantitative analysis of DCE-MRI data can provide quantitative information about the bone metastasis which cannot be obtained by bone scan and CT. Further studies are needed to investigate whether DCE-MRI derived perfusion parameters may be used as biomarkers in evaluation of treatment response of bone metastasis in patients with prostate cancer.
The limitation of our study is that the quantitative parameters obtained from metastatic lesions were compared with the findings of normal bone in the same patient rather than benign bone lesions. The parameters of metastatic bone lesions other than prostate were also not compared. Moulopoulos et al[21] evaluated cancer patients with metastasis to bone marrow including lymphoma, chronic lymphocytic leukemia, carcinoma of the cervix, breast, lung and bladder. They compared the wash-in and wash-out rates, time to peak, and time to maximum slope values of control group with no history of malignancy and reported a significant difference for all values.
In conclusion, bone metastasis from prostate cancer demonstrates significant enhancement leading to high Ktrans and ve in contradiction to normal bone which does not enhance in the elderly population. DCE-MRI and its quantitative analysis may have a role in improving the detection of bone metastasis from prostate cancer.
Prostate cancer is a major health problem and a major cause of death in men. It is crucial to determine the assessment of the metastatic process of prostate cancer for designing a proper treatment. The most frequent sites of distant metastases of prostate cancer are bones. Dynamic contrast-enhanced magnetic resonance imaging (DCE-MRI) and its quantitative analysis may contribute to improve the detection of bone metastasis from prostate cancer.
DCE-MRI has been increasingly used as an additional technique to characterize various bone lesions. It provides a powerful tool for assessing the tissue vasculature in tumor tissues. In this study, the authors demonstrate the contribution of DCE-MRI for detection of bone metastasis in patients with prostate cancer.
In recent studies searching the microvascularization of bone marrow, mostly qualitative and semiquantitative DCE-MRI techniques were used. This study investigated quantitative parameters obtained from high temporal resolution DCE-MRI data of bone metastasis from prostate cancer. Furthermore, our study demonstrated that bone metastasis from prostate cancer shows significant enhancement leading to high Ktrans and ve in contradiction to normal bone which does not enhance in the elderly population.
Quantitative measurements of DCE-MRI data may improve the diagnosis of bone metastasis by providing quantitative analysis which cannot be obtained by bone scan and CT. Therefore, this study may represent a future perspective for DCE-MRI derived perfusion parameters, which may be used as biomarkers in evaluation of treatment response of bone metastasis in patients with prostate cancer.
DCE-MRI provides a powerful tool for measuring alterations in the microvascular environments of the tissue. Ktrans and ve, are quantitative parameters of DCE-MRI and they are expected to be increased in bone metastasis from prostate cancer, in contrast to normal bone in the elderly population.
Although this study did not perform the reproducibility of quantitative perfusion parameters in bone metastasis from prostate cancer, the topic of this article may draw the readers’ attention. This study may be an initial step to assess the roles of quantitative perfusion parameters in monitoring or predicting therapeutic responses for advanced prostate cancer patients in the future studies. Generally this article is well-written.
Peer reviewers: Liang Wang, MD, PhD, Professor, Department of Radiology, Tongji Medical College, Huazhong University of Science and Technology, Wuhan 430030, Hubei Province, China; Chan Kyo Kim, MD, Assistant Professor, Department of Radiology, Samsung Medical Center, Sungkyunkwan University School of Medicine, 50 Ilwon-dong, Kangnam-gu, Seoul 135-710, South Korea
S- Editor Cheng JX L- Editor O’Neill M E- Editor Zheng XM
| 1. | Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7953] [Cited by in RCA: 8103] [Article Influence: 506.4] [Reference Citation Analysis (2)] |
| 2. | Manyak MJ, Javitt MC. The role of computerized tomography, magnetic resonance imaging, bone scan, and monoclonal antibody nuclear scan for prognosis prediction in prostate cancer. Semin Urol Oncol. 1998;16:145-152. [PubMed] |
| 3. | Freedman GM, Negendank WG, Hudes GR, Shaer AH, Hanks GE. Preliminary results of a bone marrow magnetic resonance imaging protocol for patients with high-risk prostate cancer. Urology. 1999;54:118-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 22] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 4. | Venkitaraman R, Sohaib SA, Barbachano Y, Parker CC, Khoo V, Huddart RA, Horwich A, Dearnaley DP. Detection of occult spinal cord compression with magnetic resonance imaging of the spine. Clin Oncol (R Coll Radiol). 2007;19:528-531. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 26] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 5. | Lecouvet FE, Geukens D, Stainier A, Jamar F, Jamart J, d'Othée BJ, Therasse P, Vande Berg B, Tombal B. Magnetic resonance imaging of the axial skeleton for detecting bone metastases in patients with high-risk prostate cancer: diagnostic and cost-effectiveness and comparison with current detection strategies. J Clin Oncol. 2007;25:3281-3287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 152] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 6. | Chen WT, Shih TT, Chen RC, Lo HY, Chou CT, Lee JM, Tu HY. Blood perfusion of vertebral lesions evaluated with gadolinium-enhanced dynamic MRI: in comparison with compression fracture and metastasis. J Magn Reson Imaging. 2002;15:308-314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 85] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 7. | Chen WT, Shih TT, Chen RC, Lo SY, Chou CT, Lee JM, Tu HY. Vertebral bone marrow perfusion evaluated with dynamic contrast-enhanced MR imaging: significance of aging and sex. Radiology. 2001;220:213-218. [PubMed] |
| 8. | Yang C, Karczmar GS, Medved M, Oto A, Zamora M, Stadler WM. Reproducibility assessment of a multiple reference tissue method for quantitative dynamic contrast enhanced-MRI analysis. Magn Reson Med. 2009;61:851-859. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 40] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 9. | Yang C, Karczmar GS, Medved M, Stadler WM. Multiple reference tissue method for contrast agent arterial input function estimation. Magn Reson Med. 2007;58:1266-1275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 59] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 10. | Tofts PS, Brix G, Buckley DL, Evelhoch JL, Henderson E, Knopp MV, Larsson HB, Lee TY, Mayr NA, Parker GJ. Estimating kinetic parameters from dynamic contrast-enhanced T(1)-weighted MRI of a diffusable tracer: standardized quantities and symbols. J Magn Reson Imaging. 1999;10:223-232. [PubMed] |
| 11. | Coleman RE. Skeletal complications of malignancy. Cancer. 1997;80:1588-1594. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 12. | Mundy GR. Metastasis to bone: causes, consequences and therapeutic opportunities. Nat Rev Cancer. 2002;2:584-593. [PubMed] |
| 13. | Dunnill MS, Anderson JA, Whitehead R. Quantitative histological studies on age changes in bone. J Pathol Bacteriol. 1967;94:275-291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 201] [Cited by in RCA: 150] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 14. | Bluemke DA, Petri M, Zerhouni EA. Femoral head perfusion and composition: MR imaging and spectroscopic evaluation of patients with systemic lupus erythematosus and at risk for avascular necrosis. Radiology. 1995;197:433-438. [PubMed] |
| 15. | Bollow M, Knauf W, Korfel A, Taupitz M, Schilling A, Wolf KJ, Hamm B. Initial experience with dynamic MR imaging in evaluation of normal bone marrow versus malignant bone marrow infiltrations in humans. J Magn Reson Imaging. 1997;7:241-250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 46] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 16. | Erlemann R, Reiser MF, Peters PE, Vasallo P, Nommensen B, Kusnierz-Glaz CR, Ritter J, Roessner A. Musculoskeletal neoplasms: static and dynamic Gd-DTPA--enhanced MR imaging. Radiology. 1989;171:767-773. [PubMed] |
| 17. | Ma LD, Frassica FJ, McCarthy EF, Bluemke DA, Zerhouni EA. Benign and malignant musculoskeletal masses: MR imaging differentiation with rim-to-center differential enhancement ratios. Radiology. 1997;202:739-744. [PubMed] |
| 18. | van der Woude HJ, Verstraete KL, Hogendoorn PC, Taminiau AH, Hermans J, Bloem JL. Musculoskeletal tumors: does fast dynamic contrast-enhanced subtraction MR imaging contribute to the characterization? Radiology. 1998;208:821-828. [PubMed] |
| 19. | Tokuda O, Hayashi N, Taguchi K, Matsunaga N. Dynamic contrast-enhanced perfusion MR imaging of diseased vertebrae: analysis of three parameters and the distribution of the time-intensity curve patterns. Skeletal Radiol. 2005;34:632-638. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 45] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 20. | Bäuerle T, Bartling S, Berger M, Schmitt-Gräff A, Hilbig H, Kauczor HU, Delorme S, Kiessling F. Imaging anti-angiogenic treatment response with DCE-VCT, DCE-MRI and DWI in an animal model of breast cancer bone metastasis. Eur J Radiol. 2010;73:280-287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 42] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 21. | Moulopoulos LA, Maris TG, Papanikolaou N, Panagi G, Vlahos L, Dimopoulos MA. Detection of malignant bone marrow involvement with dynamic contrast-enhanced magnetic resonance imaging. Ann Oncol. 2003;14:152-158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 48] [Article Influence: 2.2] [Reference Citation Analysis (0)] |