Revised: January 18, 2011
Accepted: January 25, 2011
Published online: January 28, 2011
AIM: To highlight various patterns of nodal involvement and post treatment changes in pediatric chest tuberculosis based on contrast enhanced computed tomography (CECT) scans of chest.
METHODS: This was a retrospective study consisting of 91 patients aged less than 17 years, who attended pediatric OPD of All India Institute of Medical Sciences with clinically diagnosed tuberculosis or with chest radiographs suggestive of chest tuberculosis. These patients had an initial chest radiograph as well as CECT of the chest and follow up imaging after 6 mo, and in some cases 9 mo, of completion of anti-tubercular treatment (ATT). CECT of these patients was reviewed for the location and extent of nodal involvement along with determination of site, size, enhancement pattern and calcification.
RESULTS: Enlargement of mediastinal or hilar lymph nodes was found in 88/91 patients (96.7%), with the most common locations being paratracheal (84.1%), and subcarinal (76.1%). The most common pattern of enhancement was found to be inhomogenous. The nodes were conglomerate in 56.8% and discrete in 43.2%. In addition, perinodal fat was obscured in 84.1% of patients. In the post-treatment scan, there was 87.4% reduction in the size of the nodes. All nodes post-treatment were discrete and homogenous with perinodal fat present. Calcification was found both pre- and post-treatment, but there was an increase in incidence after treatment (41.7%). There was hence a reduction in size, change in enhancement pattern, and appearance of perinodal fat with treatment.
CONCLUSION: Tubercular nodes have varied appearance and enhancement pattern. Conglomeration and obscuration of perinodal fat suggest activity. In residual nodes decision to continue ATT requires clinical correlation.
- Citation: Mukund A, Khurana R, Bhalla AS, Gupta AK, Kabra SK. CT patterns of nodal disease in pediatric chest tuberculosis. World J Radiol 2011; 3(1): 17-23
- URL: https://www.wjgnet.com/1949-8470/full/v3/i1/17.htm
- DOI: https://dx.doi.org/10.4329/wjr.v3.i1.17
Tuberculosis is one of the major causes of morbidity and mortality worldwide. Children represent one of the high risk groups in the resurgence of the disease. Pediatric patients generally have primary tuberculosis, presenting as Ghon’s complex, consisting of small parenchymal infiltrates with lymphatic spread leading to mediastinal and hilar lymphadenopathy. This generally heals but may progress to progressive pulmonary tuberculosis in 5%-10% of patients.
Chest radiography may not be sensitive in detecting lymphadenopathy, which is considered to be the fingerprint of primary pulmonary tuberculosis. Moreover, computed tomography (CT) is considered the imaging modality of choice to diagnose the presence, location and characteristics of mediastinal adenopathy. The advantages of CT are the characterization of lesions by showing low attenuation nodes with rim enhancement, calcification, nodules of bronchogenic spread, or miliary disease, in defining the extent of tuberculous disease and its complications and in differentiating active from inactive phases[1-5]. This study describes the various CT patterns of nodal involvement in pediatric chest tuberculosis in active as well as in post-treatment phases.
In this retrospective study (from December 2006 to December 2009) we analyzed the CT scans of 91 patients less than 17 years of age who were diagnosed with tuberculosis on the basis of clinical and/or radiographic and/or pathological data. CT was performed after administration of non ionic contrast [Iomeron 400 (Iomeprol, Bracco, Milano, Italy), Iohexol 300 (Omnipaque, GE Health care, Ireland)] which was injected by hand leading to an average delay of 50 to 70 s and thus providing venous phase images. The dose of contrast was calculated based on the body weight of the child, not exceeding 2 mL/kg body weight. CT scans of these patients were reviewed by two radiologists with 20 years and 5 years experience, respectively, in thoracic imaging.
Medical records of these patients were thoroughly studied by a clinical investigator who looked for presenting clinical symptoms, history of Mycobacterium tuberculosis contact, previous diagnoses of tuberculosis, demographic information, and status of BCG vaccination, a Mantoux test result and isolation of acid fast bacilli (AFB).
Patients who presented with clinical symptoms suggestive of active tuberculosis like cough for more than 2 wk, fever, weight loss, hemoptysis, anorexia, dyspnea and weakness were included. Apart from these clinical features at least 2 of the following 4 criteria had to be fulfilled: (1) A tuberculin skin test with 5 tuberculin units PPD, showing area of induration more than 10 mm; (2) Radiographic findings compatible with Mycobacterium tuberculosis like miliary disease, cavitary lesions, hilar lymphadenopathy or primary complex; (3) History of adult source patient with contagious disease, caused by Mycobacterium tuberculosis; and (4) Isolation of AFB from sputum, FNAC from cervical nodes, bronchioalveolar lavage, or other sites like empyema, etc.
Informed consent and clearance from the local ethical committee was not required due to the retrospective nature of the study.
An initial CT scan done within 30 d of commencement of anti-tubercular treatment (ATT) was available in all these patients. Amongst these, 45 patients had a follow up CT performed following 6 mo of treatment. Some patients with persistent nodes underwent a 3rd scan after 9 mo of ATT.
Contrast enhanced CT (CECT) was assessed for the location and extent of nodal, parenchymal, airway, and pleural involvement. Particular attention was given to the site, size, enhancement pattern and calcification of the lymph nodes. Mediastinal nodes having a size > 1 cm in the short axis were considered abnormal. In addition sites of nodal involvement were also analyzed. The enhancement pattern was categorized into three groups consisting of homogeneously enhancing nodes, nodes with peripheral enhancement and central necrosis and inhomogeneous nodes. The nodes were also classified as being discrete or conglomerate and we looked for presence or obscuration of perinodal fat. Discrete nodes were defined as having smooth, well defined margins with preserved adjacent fat; whereas clubbed or clusters of nodes with ill defined margins and adjacent fat streakiness were called conglomerate. Nodes with ill defined fuzzy margins with loss of adjacent fat or fat stranding were said to have obscuration of perinodal fat.
Further size as well as the enhancement pattern of the nodes was compared with the follow up CT done after 6 and 9 mo.
The clinical profile of the study population is depicted in Table 1. The 91 patients (47 males, 44 females) aged 3 mo to 17 years had a majority of patients (42.9%) in the age group of 10 to 15 years, with a median age of 11 years. Most of the patients presented with a plethora of symptoms with the commonest being fever (96.7%), weight loss (85.7%) and loss of appetite (98.9%). Of these, Mantoux positivity was seen in all patients (100%) whereas elevated ESR in 61 (71.4%) cases and AFB could be isolated in only 20 (22%) cases.
| Variables | Frequency (n = 91) |
| Age group (yr) | |
| 0-5 | 19 (20.9) |
| 5-10 | 26 (28.6) |
| 10-15 | 39 (42.9) |
| 15-17 | 7 (7.7) |
| Sex | |
| Male | 47 (51.6) |
| Female | 44 (48.4) |
| Presenting symptoms | |
| Fever | 88 (96.7) |
| Cough | 56 (61.5) |
| Weight loss | 78 (85.7) |
| Loss of appetite | 90 (98.9) |
| Lymphadenopathy | 38 (41.7) |
| History of contact | 41 (45.0) |
| Laboratory investigations | |
| Mantoux positivity | 91 (100) |
| Elevated ESR | 65 (71.4) |
| AFB detection | 20 (22.0) |
An initial CT scan performed within 30 d of commencement of ATT was available for all 91 patients. It was reviewed for nodal, parenchymal, airway, and pleural lesions (Table 2). Enlargement of mediastinal or hilar lymph nodes was found in 88 patients (96.7%). The nodal distribution and lymph node size is described in Table 3. The most common site for lymphadenopathy was the paratracheal location, and the largest nodes were seen in prevascular and subcarinal locations. The incidence of lymphadenopathy in children < 5 years was 19/19 (100%), while that in children > 5 years was 69/72 (96%).
| Findings | Frequency (n = 91) |
| Lymph nodal involvement | 88 (96.7) |
| Parenchymal lesion | 64 (70.3) |
| Airway disease | 32 (35.2) |
| Pleural lesion | 18 (19.8) |
| Frequency (n = 88) | % | |
| Nodal distribution | Frequency and mean size | |
| Paratracheal | 74 (2.21 cm) | 84.1 |
| Pretracheal | 50 (1.62 cm) | 56.8 |
| Precarinal | 48 (1.72 cm) | 54.4 |
| Subcarinal | 67 (2.03 cm) | 76.1 |
| Right hilar | 46 (1.87 cm) | 52.3 |
| Left hilar | 32 (1.68 cm) | 36.4 |
| Prevascular | 22 (2.82 cm) | 25.0 |
| Azygoesophageal | 30 (1.9 cm) | 34.1 |
| Aorto-pulmonary window | 10 (2.0 cm) | 11.4 |
| Nodal characteristics | ||
| Enhancement pattern | ||
| Peripheral rim | 12 | 13.6 |
| Homogeneous | 30 | 34.1 |
| Inhomogeneous | 46 | 52.3 |
| Calcification | 25 | 28.4 |
| Conglomerate | 50 | 56.8 |
| Discrete | 38 | 43.2 |
| Obscured perinodal fat | 74 | 84.1 |
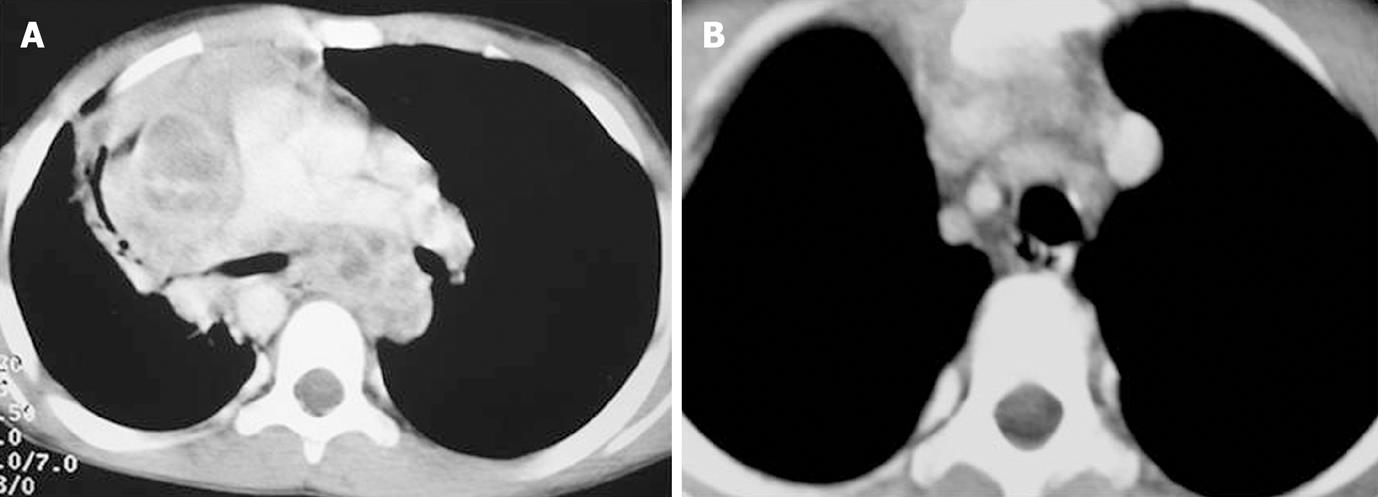
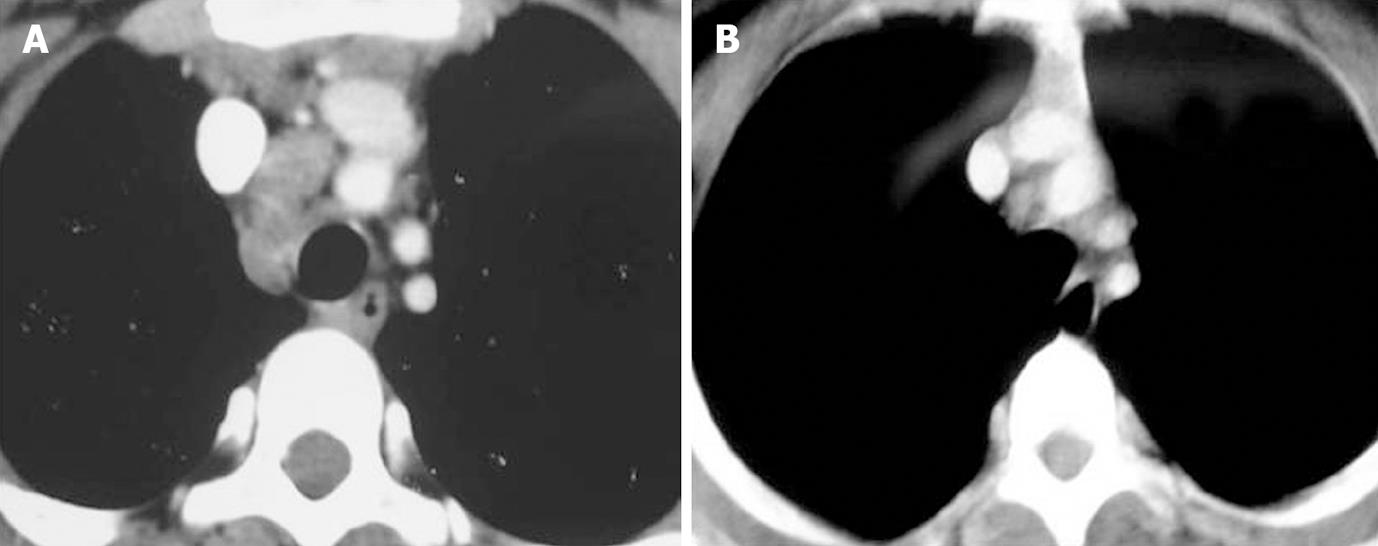
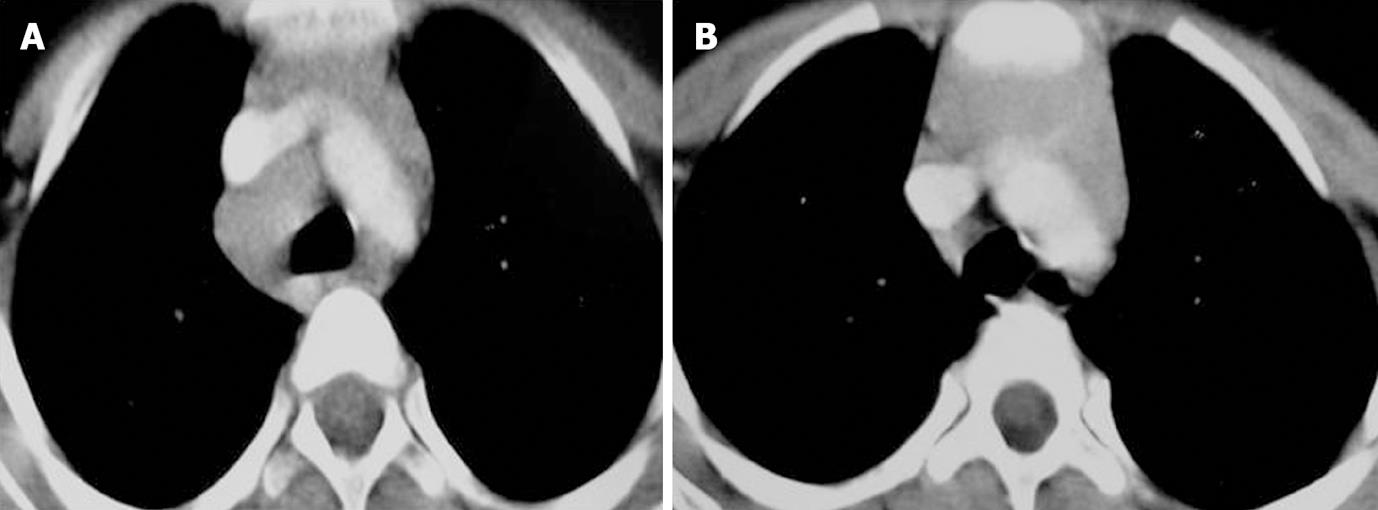
Enlarged nodes displayed 3 patterns of enhancement consisting of homogeneous, inhomogeneous and peripherally enhancing nodes with central necrosis (Figures 1, 2 and 3). A homogeneous and inhomogenous pattern of enhancement was more common than peripheral enhancement. Nodal calcification was seen even in the pre-treatment scan of 25 patients (28.4%). The majority of patients had conglomerate nodes in the initial scan and most of the patients (84.1%) showed obscuration of perinodal fat.
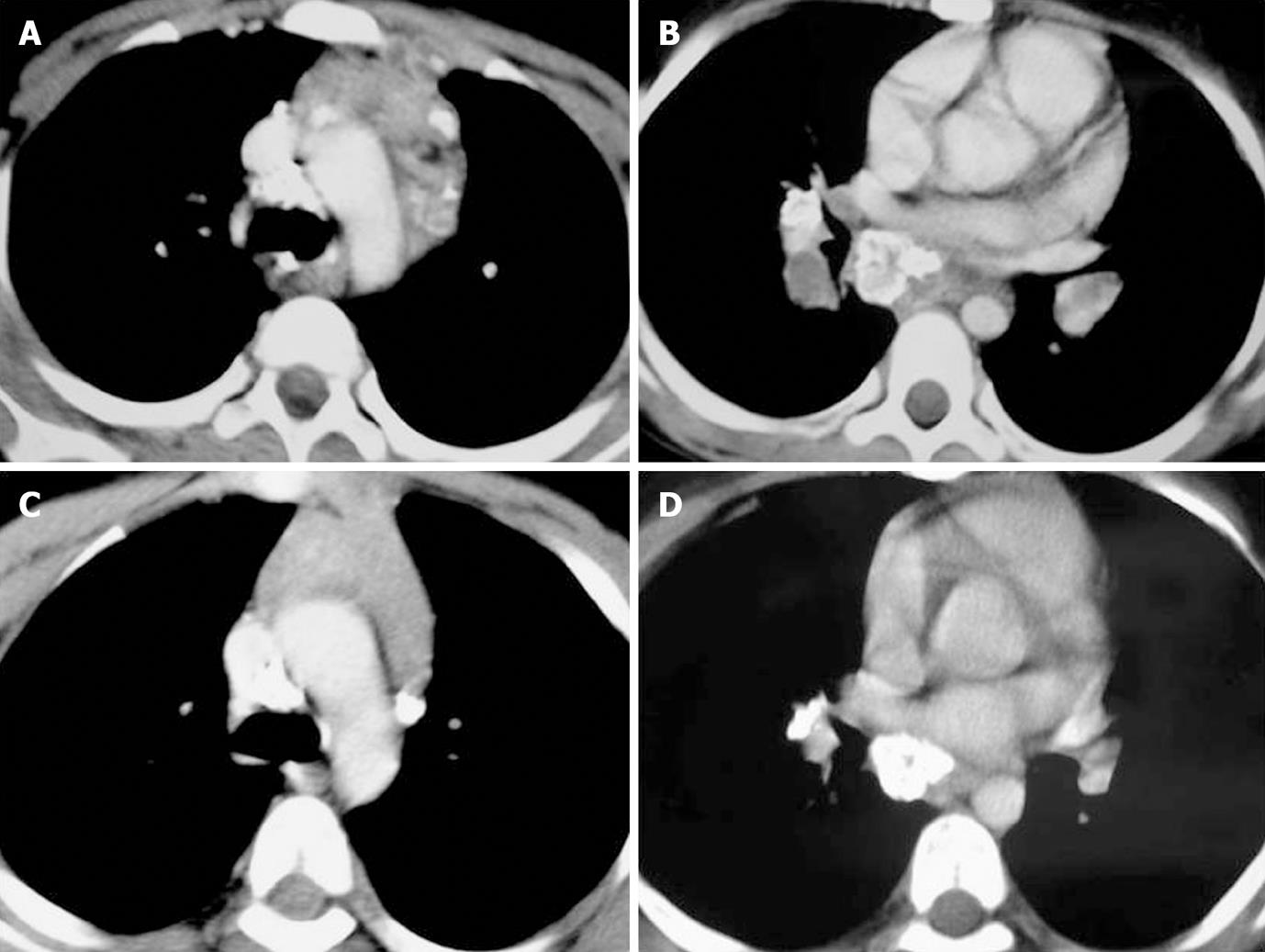
In 45 of these 91 patients a follow up scan (performed 6-7 mo after ATT) was available. Comparison of pre- and post-treatment scans of these 45 patients was made. Regression of nodes was seen in 30 patients (Figures 1-3). Out of 30 patients (Table 4), 6 patients showed complete resolution of nodes whereas 24 had nodes < 1 cm in the short axis. These nodes were insignificant according to the size criteria; we, however, studied these small nodes for morphological characteristics. In the post-treatment CT, these nodes were discrete. Perinodal fat was obscured in 17 patients in pre-treatment scans and it reappeared in all patients in post-treatment scans (Figures 1-3). A peripheral and inhomogenous pattern of enhancement changed to a homogenous pattern of enhancement (Figure 2). Calcification was found in 8 patients in the pre-treatment scan and 10 patients after treatment (41.7%) (Figure 4 and Table 4).
| Lymph nodal characteristics | Frequency (n = 30) | P value | |
| Pre-treatment | Post-treatment | ||
| Lymph nodes seen with mean size | 30 (1.83 cm) | 24 (0.23 cm) | < 0.05 |
| Enhancement pattern | |||
| Peripheral rim | 3 | 0 | < 0.05 |
| Homogeneous | 7 | 24 | > 0.05 |
| Inhomogeneous | 20 | 0 | < 0.05 |
| Calcification | 8 | 10 | > 0.05 |
| Conglomerate | 11 | 0 | < 0.05 |
| Discrete | 19 | 24 | > 0.05 |
| Obscured perinodal fat | 17 | 0 | < 0.05 |
The comparison between active and inactive disease (n = 30) was made using McNemar test and a value of P < 0.05 was regarded as significant. For comparison between size of nodes, Wilcoxon signed ranks test was used. There was a statistically significant reduction in the size of nodes. The difference in the incidence of peripheral, inhomogenous, and mixed patterns of enhancement, conglomerate nodes and obscuration of perinodal fat was statistically significant.
Fifteen patients had nodes > 1 cm, which were hence within the significant range using the size criteria. The decision for continuing or stopping ATT in these patients was based on correlation of radiological and clinical findings. A further follow up scan after 3 mo was available in 9 patients divided into 2 groups - one in whom ATT was continued (n = 7) and the other in whom ATT was discontinued (n = 2) (Table 5). Though a constant reduction in the mean and maximum size of the nodes was noted as the duration of treatment progressed, at the end of 9 mo of ATT, significant nodes (> 1 cm) persisted in 3 cases, with few showing rim enhancement and conglomeration, suggesting activity.
| Nodes | Pre-treatment | 6 mo | 9 mo |
| Mean size (cm) | 2.4 | 1.8 | 1.4 |
| Maximum size (cm) | 5 | 4.5 | 3.5 |
| Enhancement | |||
| Peripheral rim | 2 | 2 | 1 |
| Homogeneous | 0 | 1 | 2 |
| Inhomogeneous | 1 | 0 | 0 |
| Calcification | 1 | 1 | 1 |
| Conglomerate | 1 | 1 | 1 |
| Discrete | 2 | 2 | 2 |
| Obscured perinodal fat | 2 | 1 | 0 |
Tuberculosis continues to be a major health problem all over the world, especially in developing countries. Pediatric cases are particularly important epidemiologically because each usually represents a new infection rather than reactivation of prior disease. Indeed it is said that, “Children with primary tuberculosis are the reservoir from which future cases will emerge”[6].
Lymphadenopathy is the most common finding in primary TB. In a study by Lamont et al[7], it was concluded that lymphadenopathy was the most common single manifestation of primary tuberculosis in children, occurring in two-thirds of patients in a series of 154 patients, 90% of whom had associated parenchymal abnormalities. Weber et al[8] reported lymphadenopathy in 96% of cases. Leung et al[9] found lymphadenopathy was present in 92% cases on chest radiography, and it was the most common abnormality detected. We found enlargement of mediastinal or hilar lymph nodes in 88/91 patients (96.7%), which is comparable to other reports.
The common sites of lymphadenopathy reported in primary tuberculosis are right paratracheal, hilar and subcarinal. Leung et al[9] noted that the most common sites were right paratracheal and hilar. Andronikou et al[10] observed that the most frequently involved location was subcarinal (90%), followed by the hilar (85%), anterior mediastinum (79%), precarinal (64%) and right paratracheal (63%). Multiple sites were involved in 88% of patients and a single site (subcarinal) was present in only 4% of patients. Nodes at all the sites simultaneously were present in 35% of patients. Kim et al[11] found that right paratracheal nodes (73%) were the most common, followed by right hilar (34.1%). Our study was in concordance with the above studies, with the most common locations being paratracheal (84.1%), subcarinal (76.1%), pretracheal (56.8%), precarinal (54.5%), and right hilar (52.3%). Prevascular nodes were enlarged in 25% of patients. Lymph node involvement was found in a single site in only 1 (1.1%) patient, involving the right paratracheal node, while in the rest of the patients multiple sites were involved. Lymph nodes were seen in all the sites in 4 (4.4%) patients.
In a study by Andronikou et al[10], subcarinal was the most commonly affected site and also the site with the largest nodes. Other sites with the largest lymph nodes were prevascular and paratracheal. We also observed that the largest nodes were found in the prevascular (6.5 cm), subcarinal (5.5 cm), and paratracheal (5 cm) nodes.
Though the typical enhancement pattern reported in tuberculosis is peripheral rim enhancement with central necrosis, several studies have described other patterns, like homogenous and inhomogenous enhancement. Kim et al[11] found that 70.7% of patients had nodes with a low attenuation centre and enhancing rim. In the study by Andronikou et al[10], enhancement was present in 67% and was invariably ghost like, rather than ring enhancing. In our study, we found the most common pattern enhancement to be inhomogeneous (52.3%) followed by homogeneous (34.1%) and rim enhancing (13.6%). The major difference observed in our study was the presence of homogeneous nodes which has not been reported in pediatric studies; however in a study in adults, the incidence was reported to be 9%[12].
We also observed calcification in 28.4% of patients, as compared to the reported incidence of 9% by Andronikou et al[10], and 12.2% by Kim et al[11]. In the present study, we observed conglomeration of nodes and obliteration of perinodal fat as an important indicator of active disease. The nodes were conglomerate in 56.8% and discrete in 43.2%. In addition, perinodal fat was obscured in 84.1% patients. The pediatric studies did not make a mention of these characteristics. In a study in adults, by Pombo et al[12], obliteration of perinodal fat was found in 39.5% of patients.
To the best of our knowledge, there is no study in a pediatric population which has compared the nodal characteristics on imaging before and after treatment in children. In a study in adults, by Moon et al[13], 49 patients were studied, of which 37 were classified as active and 12 as inactive on the basis of lymph nodal biopsy. They reported that in 100% of patients with active disease, the nodes had peripheral rim enhancement with central low attenuation on CT. Calcification was observed in 19%. On the other hand, in inactive disease the nodes were homogenous, without central low attenuation areas, and calcification was found in 83% of patients. The central hypodense areas pathologically corresponded to areas of caseation necrosis. In the present study, we found small nodes in 24/30 patients (80%), which were insignificant as they were < 1 cm in size. The mean size of the nodes was 0.23 cm, with 87.4% reduction in size of the nodes, as compared to the initial scan. All nodes in the post-treatment scan were discrete and homogenous with visible surrounding perinodal fat. Calcification was found in both pre- and post-treatment scans, but there was an increase in its incidence after treatment (41.7%). Hence, although nodes were seen on follow up, these were insignificant as per size criteria.
Thus, there was a reduction in size, change in the enhancement pattern, and reappearance of perinodal fat with treatment. Our study correlated with the study by Moon et al[13] in adults, in terms of homogenous nodes without necrosis in the post-treatment CT. However, the incidence of calcification in inactive disease was lower in our study.
In our study, there were 15 patients who had residual disease on imaging after 6 mo of ATT. Even though there was a 52% reduction in the size of nodes the mean size was still significant. There was an increase in the number of patients showing homogenous nodes, but still 33.3% patients showed peripheral and inhomogenous enhancement. Obscuration of perinodal fat was found in 13.3% of patients. Thus, though there was a response to treatment, significant nodes persisted after 6 mo. Further follow-up CT at 9 mo was available in 9 patients which showed regression of nodes, either with ATT (n = 4), or without ATT (n = 2). Hence, in all patients, nodes became insignificant, except in 3 patients in whom the clinical signs of activity still persisted. The decision for continuation or discontinuation of ATT was based on clinical signs of activity.
Hence, residual sizable nodes at the end of 6 mo of ATT did not necessarily indicate an active disease on the basis of radiological finding alone. Rather the evaluation of activity required a clinical correlation. Hence, nodes of size > 1 cm represented an active disease only in symptomatic patients. We have reached this conclusion based on the fact that our patients who became clinically inactive at 6 mo (n = 2) showed regression of nodes at 9 mo to insignificant size despite stopping the ATT at 6 mo. The rate of regression, however, was variable in children, ranging from 6 to 9 mo. We do not have a pathological correlation to say that the residual nodes were active or inactive, and the decision to treat these nodes was based on clinical correlation. Also to ascertain the significance of residual nodes, particularly in asymptomatic children, and to determine whether nodes should determine the end point of treatment, further studies with histopathological correlation can only conclusively prove activity vs inactivity in these patients. Although the numbers in our study are small for making any definite conclusions we recommend that in patients having > 1 cm size nodes on completion of 6 mo of treatment the decision to continue or stop treatment should be based on clinical and radiological correlation.
To conclude, tubercular nodes may have varied appearances, the most common being inhomogeneous and only a small number of patients having typical rim enhancement with central necrosis. However, homogeneous enlargement does not exclude tubercular etiology. Major determinants of disease activity are nodal enlargement, conglomeration and obscuration of perinodal fat. The decision to continue or withdraw ATT should always be taken after correlating clinical and imaging findings as persistent homogeneous and discrete but enlarged/equivocal nodes may not always suggest active disease.
This study aims to highlight various patterns of nodal involvement and post-treatment changes in pediatric chest tuberculosis based on contrast enhanced computed tomography (CECT) scans of the chest.
We inferred that tubercular nodes which are < 1 cm, homogenous, discrete and with surrounding perinodal fat are inactive, while > 1 cm nodes with any pattern of enhancement (homogenous, rim like, or inhomogenous) should be considered active. Calcification can be seen in both active and inactive nodes.
Residual mediastinal/hilar lymph nodes do not necessarily indicate disease activity; rather it is the clinical scenario along with the trend of regression of nodes which suggests if the disease is active or not.
This is a very interesting and well conducted study. However there are some points to be clarified.
Peer reviewer: Francesco Lassandro, MD, Department of Radiology, Monaldi Hospital, via Leonardo Bianchi, Napoli, 80129, Italy
S- Editor Cheng JX L- Editor O’Neill M E- Editor Zheng XM
| 1. | Im JG, Itoh H, Shim YS, Lee JH, Ahn J, Han MC, Noma S. Pulmonary tuberculosis: CT findings--early active disease and sequential change with antituberculous therapy. Radiology. 1993;186:653-660. |
| 2. | Moon WK, Im JG, Yeon KM, Han MC. Mediastinal tuberculous lymphadenitis: CT findings of active and inactive disease. AJR Am J Roentgenol. 1998;170:715-718. |
| 3. | Lee KS, Hwang JW, Chung MP, Kim H, Kwon OJ. Utility of CT in the evaluation of pulmonary tuberculosis in patients without AIDS. Chest. 1996;110:977-984. |
| 4. | Hatipoğlu ON, Osma E, Manisali M, Uçan ES, Balci P, Akkoçlu A, Akpinar O, Karlikaya C, Yüksel C. High resolution computed tomographic findings in pulmonary tuberculosis. Thorax. 1996;51:397-402. |
| 5. | Poey C, Verhaegen F, Giron J, Lavayssiere J, Fajadet P, Duparc B. High resolution chest CT in tuberculosis: evolutive patterns and signs of activity. J Comput Assist Tomogr. 1997;21:601-607. |
| 6. | Agrons GA, Markowitz RI, Kramer SS. Pulmonary tuberculosis in children. Semin Roentgenol. 1993;28:158-172. |
| 7. | Lamont AC, Cremin BJ, Pelteret RM. Radiological patterns of pulmonary tuberculosis in the paediatric age group. Pediatr Radiol. 1986;16:2-7. |
| 8. | Weber AL, Bird KT, Janower ML. Primary tuberculosis in childhood with particular emphasis o hanges affecting the tracheobronchial tree. Am J Roentgenol Radium Ther Nucl Med. 1968;103:123-132. |
| 9. | Leung AN, Müller NL, Pineda PR, FitzGerald JM. Primary tuberculosis in childhood: radiographic manifestations. Radiology. 1992;182:87-91. |
| 10. | Andronikou S, Joseph E, Lucas S, Brachmeyer S, Du Toit G, Zar H, Swingler G. CT scanning for the detection of tuberculous mediastinal and hilar lymphadenopathy in children. Pediatr Radiol. 2004;34:232-236. |
| 11. | Kim WS, Moon WK, Kim IO, Lee HJ, Im JG, Yeon KM, Han MC. Pulmonary tuberculosis in children: evaluation with CT. AJR Am J Roentgenol. 1997;168:1005-1009. |
| 12. | Pombo F, Rodríguez E, Mato J, Pérez-Fontán J, Rivera E, Valvuena L. Patterns of contrast enhancement of tuberculous lymph nodes demonstrated by computed tomography. Clin Radiol. 1992;46:13-17. |
| 13. | Moon WK, Im JG, Yeon KM, Han MC. Tuberculosis of the central airways: CT findings of active and fibrotic disease. AJR Am J Roentgenol. 1997;169:649-653. |