Published online Sep 28, 2010. doi: 10.4329/wjr.v2.i9.358
Revised: August 20, 2010
Accepted: August 27, 2010
Published online: September 28, 2010
AIM: To evaluate the efficacy of percutaneous imaging-guided biliary interventions in the management of acute biliary disorders in high surgical risk patients.
METHODS: One hundred and twenty two patients underwent 139 percutaneous imaging-guided biliary interventions during the period between January 2007 to December 2009. The patients included 73 women and 49 men with a mean age of 61 years (range 35-90 years).
Fifty nine patients had acute biliary obstruction, 26 patients had acute biliary infection and 37 patients had abnormal collections. The procedures were performed under computed tomography (CT)- (73 patients), sonographic- (41 patients), and fluoroscopic-guidance (25 patients). Success rates and complications were determined. The χ2 test with Yates’ correction for continuity was applied to compare between these procedures. A P value < 0.05 was considered significant.
RESULTS: The success rates for draining acute biliary obstruction under CT- , fluoroscopy- or ultrasound-guidance were 93.3%, 62.5% and 46.1%, respectively with significant P values (P = 0.026 and 0.002, respectively). In acute biliary infection, successful drainage was achieved in 22 patients (84.6%). The success rates in patients drained under ultrasound- and CT-guidance were 46.1% and 88.8%, respectively and drainage under CT-guidance was significantly higher (P = 0.0293). In 13 patients with bilomas, percutaneous drainage was successful in 11 patients (84.6%). Ten out of 12 cases with hepatic abscesses were drained with a success rate of 83.3%. In addition, the success rate of drainage in 12 cases with pancreatic pseudocysts was 83.3%. The reported complications were two deaths, four major and seven minor complications.
CONCLUSION: Percutaneous imaging-guided biliary interventions help to promptly diagnose and effectively treat acute biliary disorders. They either cure the disorders or relieve sepsis and jaundice before operations.
- Citation: Donkol RH, Latif NA, Moghazy K. Percutaneous imaging-guided interventions for acute biliary disorders in high surgical risk patients. World J Radiol 2010; 2(9): 358-367
- URL: https://www.wjgnet.com/1949-8470/full/v2/i9/358.htm
- DOI: https://dx.doi.org/10.4329/wjr.v2.i9.358
Acute disorders of the biliary tract affect a significant portion of the population. These conditions include biliary obstruction, biliary sepsis, hepato-biliary trauma, and their complications. Over the past few decades, biliary interventions have evolved a great deal in diagnosing and managing patients with different biliary diseases, especially in critically ill patients who are unfit for any surgical intervention or induction of general anesthesia[1].
In most cases of acute biliary disorders, common cross-sectional imaging techniques such as ultrasonography (US), computed tomography (CT) or magnetic resonance cholangio-pancreatography are highly capable of depicting the diagnosis[2]. The two common procedures used to evaluate biliary anatomy are endoscopic retrograde cholangiopancreatography (ERCP) and percutaneous transhepatic cholangiography (PTC). A number of acute biliary disorders remain unexplained in critically ill patients as the clinical, radiologic, and biochemical features of the disorders are nonspecific in these patients. Currently, no single test is reliable for diagnosing acute biliary disorders in these patients. Usually those patients are surgically unfit and only interventional biliary procedures should be performed for diagnosis and management of such diseases.
Over the past three decades, endoscopic and percutaneous biliary interventions have become readily available in most hospitals, and these minimally invasive techniques have revolutionized the treatment of patients with acute biliary disorders. Today, imaging-guided percutaneous biliary interventions are safe and effective means for non-operative biliary decompression of biliary obstruction, sepsis and their complications as they can be performed with relative ease and carry a lower morbidity than surgical decompression. Percutaneous biliary drainage procedures can be lifesaving in many acute biliary disorders. These current percutaneous biliary interventions include PTC, external and internal biliary drainage (PTD) and percutaneous cholecystostomy. In addition, percutaneous treatment of biliary stone disease with or without choledochoscopy is still performed in selected cases. Other applications include cholangioplasty for biliary strictures, biopsy of biliary duct tumors, and management of complications from laparoscopic cholecystectomy, abdominal trauma, and liver transplantation[3-9].
Acute biliary disorders in critically ill patients are common in our tertiary care hospital. The clinical presentations of these conditions are often nonspecific and management by open surgery carries a high risk. Consequently, imaging-guided interventions are usually pivotal in the management of these patients. In this study, we illustrate the spectrum of acute biliary disorders in patients with high surgical risk and evaluate the success rate and complications of percutaneous imaging-guided interventions in their diagnosis and management.
This was a retrospective study from a tertiary care hospital. The study was approved by the institutional review board. The records in our imaging database and medical files from Jan 2007 to Dec 2009 were reviewed for critically ill patients who underwent imaging-guided interventions for variable acute biliary disorders. The patient population included 122 patients; 73 women (60%) and 49 men (40%) with a mean age of 61 years (range 35-90 years). The patients had various clinical symptoms of acute biliary disorders including abdominal discomfort or pain, abdominal tenderness, fever, chills, and jaundice. These patients were of high surgical risk for different reasons (Table 1).
| Condition | Patients |
| Advanced cardiovascular disease | 24 (19.68) |
| Advanced multisystem disease | 21 (17.22) |
| Advanced malignancy | 19 (15.57) |
| Uncontrolled diabetes | 14 (11.48) |
| Severe pancreatitis | 12 (9.83) |
| Advanced respiratory disease | 11 (9.01) |
| Advanced neurologic disease | 9 (7.38) |
| Advanced liver disease | 7 (5.74) |
| Morbid obesity | 5 (4.09) |
| Total number of patients | 122 (100) |
The patients were initially scanned by US using a 3.5 MHz convex probe (Voluson 730 Expert, GE, Austria) and CT scan was performed using a double and a 64-detector helical CT scanner (GE Healthcare). After written informed consent, different imaging-guided percutaneous biliary procedures were performed under CT guidance in 73 patients, sonographic guidance in 41 patients, and fluoroscopic guidance in 25 patients. Sonographic guidance was used whenever the dilated gallbladder, dilated biliary ducts or abdominal collections were obvious on sonography and a safe pathway could be documented for passage of the needle or catheter. CT-guidance was used for ductal puncture and immediate external drainage. After relief of obstruction and if internal drainage was needed, the procedure was performed within 3 d under fluoroscopic-guidance. We did not use CT fluoroscopy to guide the entire procedure. We prefer the use of percutaneous biliary interventions under CT-guidance to ensure immediate relief of obstruction and sepsis. In addition, a CT scan was carried out, whenever the clinical status did not improve after sonographic- and/or fluoroscopic-guided interventional procedures or when the draining catheter was not draining adequately.
PTC and PTD were performed in the standard fashion described elsewhere[3-5,10,11]. Under local anesthesia and intravenous sedation, a 22-gauge Chiba needle (Cook, Bloomington, MD, USA) was inserted. If drainage was required, a Jeifries set (Cook) was used to gain access to an appropriate duct. We use a small amount of dilute contrast media (5%) to prevent CT streaky artefacts and to prevent post-procedure sepsis.
Attempts to cross obstructions or strictures were made at initial drainage and subsequent sessions. The initial catheter guide-wire combination used for these attempts was a 5-F JB1 catheter (Cook) combined with a 0.035-inch angled guide-wire (Meditech/Boston Scientific, Watertown, Mass, USA), manipulated through the sheath of the Jeifries set. If this combination failed, other guide-wire and catheter combinations were tried. An 8-F Mueller drainage catheter (Cook) was placed in cases in which external drainage was required and an 8.3-F Ring catheter (Cook) in cases in which internal drainage was required. If a larger catheter was used at subsequent changes, a 10-F Percuflex VTCB catheter (Medi-tech/Boston Scientific) was inserted in place of the smaller catheter.
The technique of percutaneous cholecystostomy was used for high surgical risk patients with acute cholecystitis according to the technique first described by Radder[12] in 1980 and then modified by others in the following years[13-16]. In brief, the gallbladder is punctured using the Seldinger technique. Then, tract dilatation and catheter placement were performed using a guide-wire. The tract chosen depended on the anatomy and whether stone extraction was planned. The trans-hepatic route was preferred to the sub-hepatic route, as it carries less risk of bile leakage[17].
Drainage of intra-abdominal fluid collections was performed using US- or CT-guidance under local anesthesia, and intravenous sedation was used in some irritable patients. The procedure was performed as described elsewhere[18]. Briefly, after choosing a suitable needle track, an 18-gauge needle (Cook) was used to enter the collection and an 8-10-F APD drainage catheter (Meditech/Boston Scientific) was inserted into the collection with use of the Seldinger technique. Gravity drainage was used until the collection had resolved and drainage had ceased, at that point, the catheter was removed.
Success of the biliary intervention was achieved when there was clinical improvement, relief of obstruction or resolution of the collection on follow-up imaging. Failure was considered if biliary obstruction or collection did not improve with the intervention trial over at least 1 wk, or when the patient’s clinical condition worsened despite optimal percutaneous management. The χ2 test with Yates’ correction for continuity was used to compare the differences in the success rate between the different procedures. A P value of less than 0.05 was considered significant.
The 122 patients were classified into three groups according to their clinical presentation (Table 2). One hundred and thirty nine different percutaneous imaging-guided biliary interventions were performed on these patients under CT- , US- and fluoroscopy-guidance (Table 3).
| Group | Clinical presentation | Patients |
| Group 1 | Acute biliary obstruction | 59 (48.36) |
| Group 2 | Acute biliary infection | 26 (21.31) |
| Group 3 | Abnormal intra-abdominal collections related to acute biliary tract disorders | 37 (30.33) |
| Total | 122 |
| Type of procedure | Patients (n) | |
| 1 | Computed tomography-guided biliary interventions | 73 |
| 2 | Ultrasonography-guided biliary interventions | 41 |
| 3 | Fluoroscopy-guided interventions | 25 |
| Total number of procedures | 139 | |
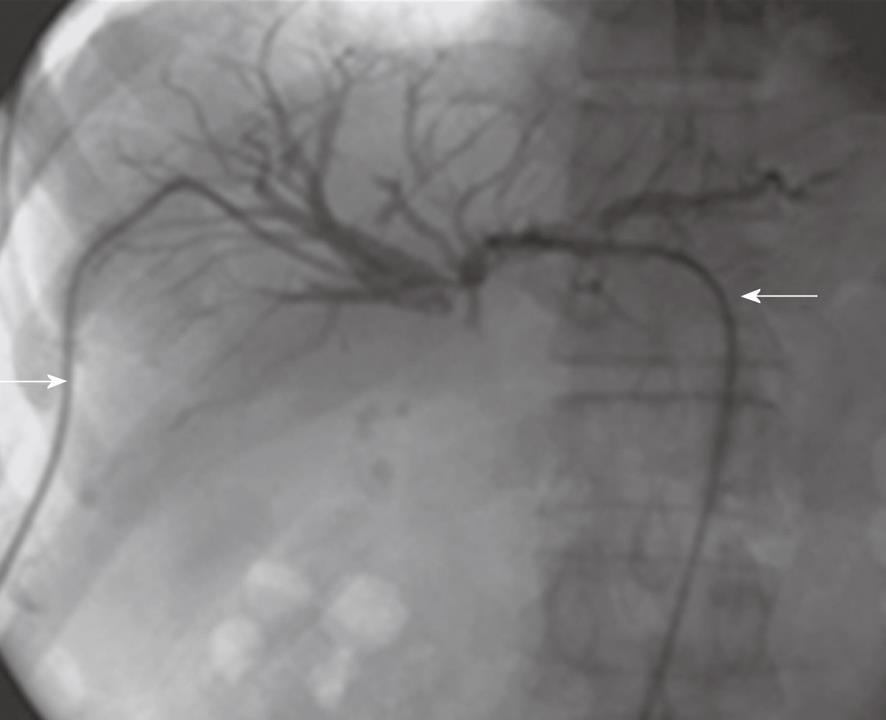
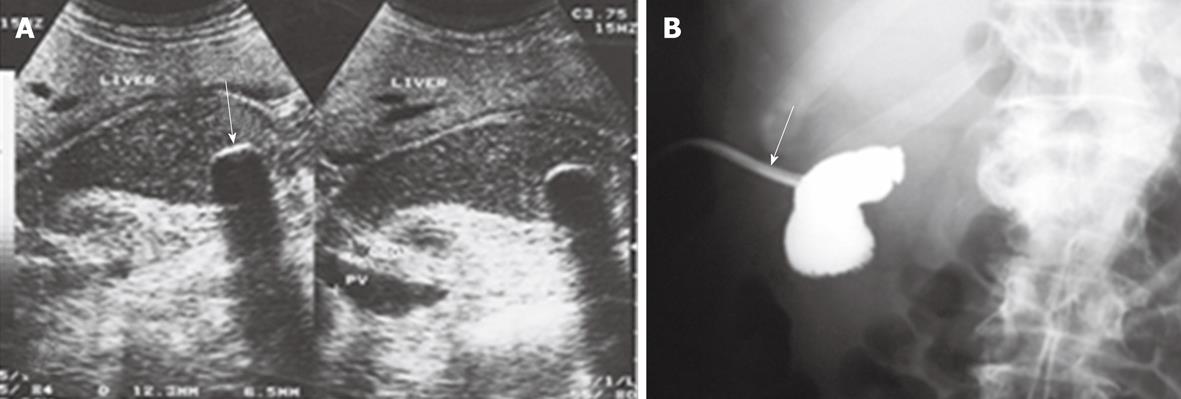
Of the patients in group 1, acute biliary obstruction was diagnosed in 59 cases. In this group, ERCP was able to demonstrate cause and level of obstruction in 21 patients (35.5%). ERCP failed to opacify the biliary tree in the remaining 38 patients (64.5%). In addition, the endoscopic intervention failed in 12 patients and the obstruction recurred in nine cases after endoscopic interventions. PTC was performed in these patients and biliary obstructions were located at the biliary bifurcation in 16 cases (27%), in the common extrahepatic bile duct in 29 cases (49%), the right hepatic duct in 8 cases (14%) and the left hepatic duct in 6 cases (10%). In this group, percutaneous imaging-guided drainage procedures were carried out under fluoroscopy- (Figure 1), US- or CT-guidance (Figure 2) with variable success rates as shown in Table 4. Additional catheters were used in 30 patients when the biliary dilatation was not communicating or the collections were either multiloculated or the initial catheter failed. Catheter upsizing was performed in 22 patients when the initial catheter was well positioned in the collection and still had low drainage rates of less than 10 mL/d.
| Type of procedure | No. of procedures | Successful procedures (n) | Success rate (%) |
| Fluoroscopy-guided drainage | 16 | 10 | 62.5 |
| US-guided drainage | 13 | 7 | 46.15 |
| CT-guided drainage | 30 | 28 | 93.331 |
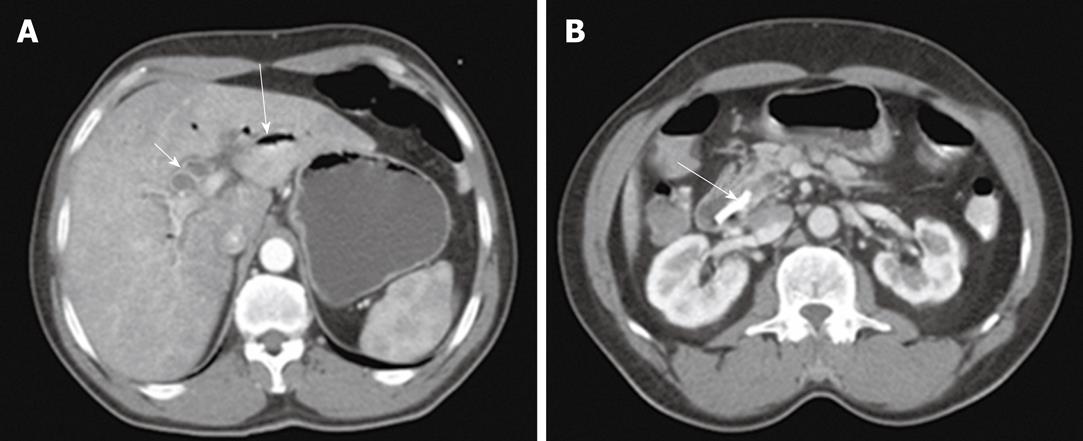
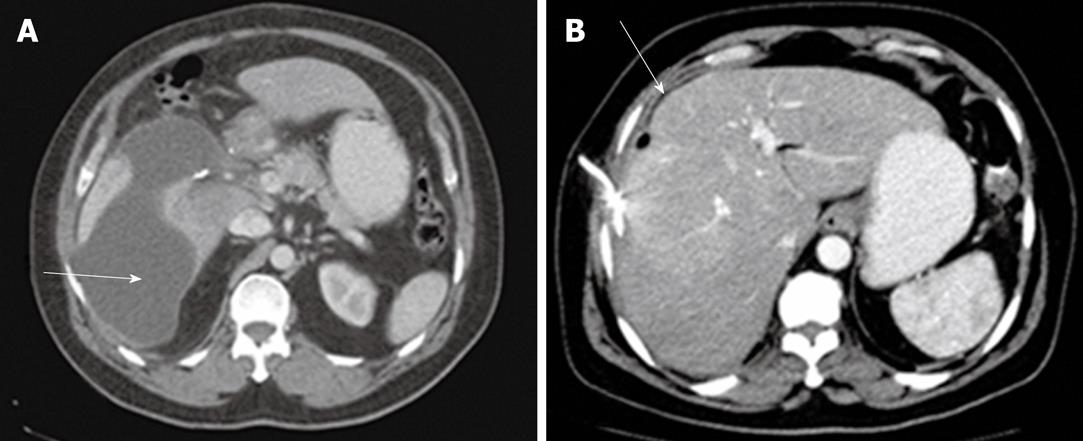
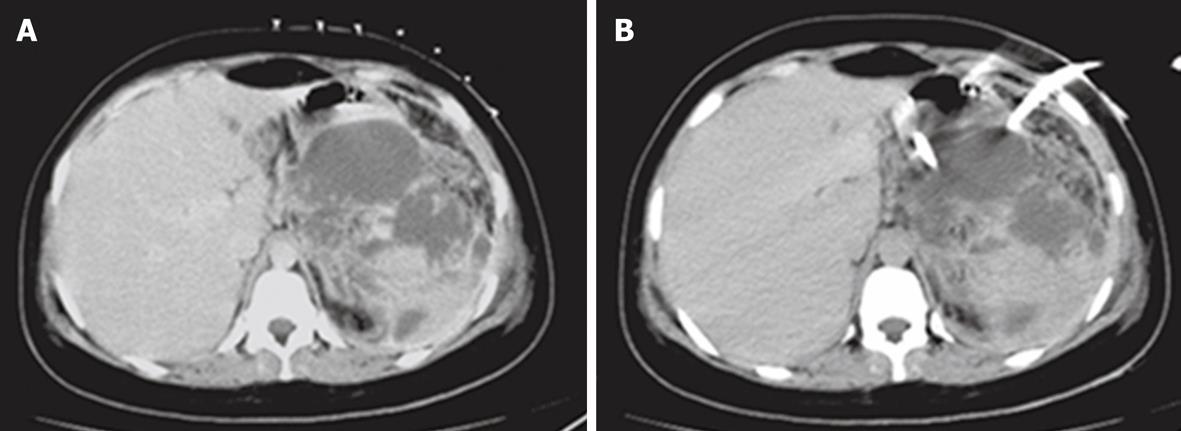
Group 2 consisted of 26 patients with acute biliary sepsis as follow: acute cholangitis (6 cases), gallbladder empyema (3 cases), emphysematous cholecystitis (2 cases), acute cholecystitis (5 cases), and pericholecystic abscess (10 cases). Examples of these conditions are seen in Figures 3, 4, 5, 6 and 7. Successful drainage in patients with biliary infection was achieved in 22 patients (84.6%). These patients were managed by either US +/- fluoroscopy- or CT-guided percutaneous drainage in conjunction with systemic antibiotics with different success rates as shown in Table 5.
| Procedure | US-guided procedures | CT-guided procedures | ||
| No. of procedures | Successful procedures | No. of procedures | Successful procedures | |
| Pericholecystic abscess drainage | 5 | 2 (40) | 7 | 6 (85.7) |
| Percutaneous cholecystostomy | 5 | 3 (60) | 5 | 5 (100) |
| Drainage for cholangitis | 3 | 1 (33.3) | 6 | 5 (83.3) |
| Total | 13 | 6 (46.15) | 18 | 16 (88.8)1 |
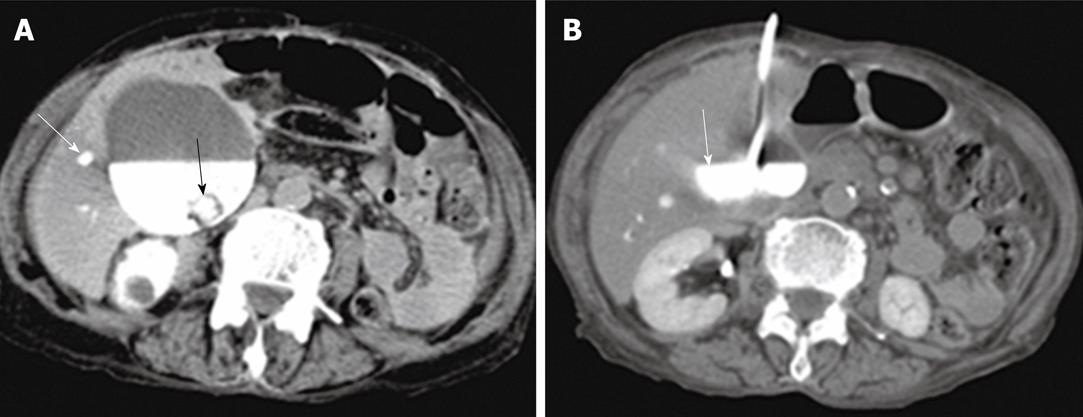
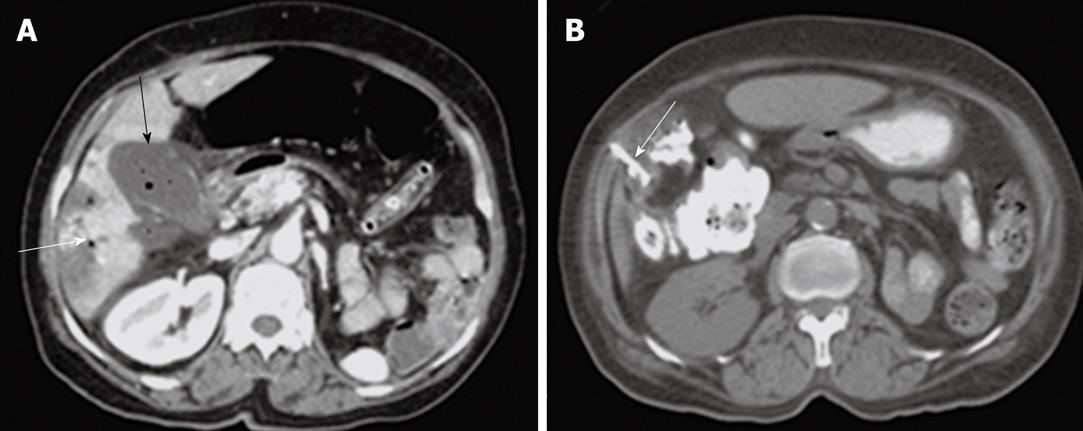
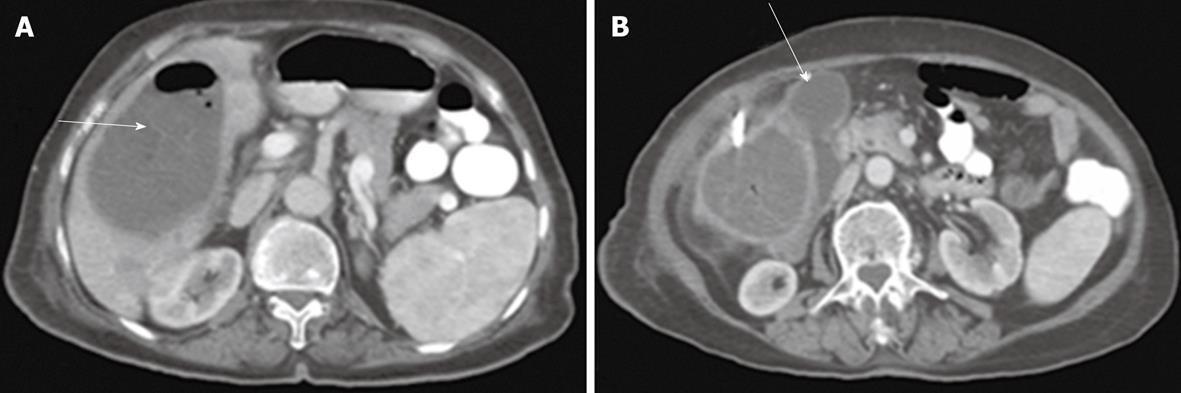
Percutaneous drainage of intra-abdominal pathological fluid collections was performed in 37 cases (group 3) under US- or CT-guidance. In 13 patients with bilomas (Figure 8), percutaneous drainage was successful in 11 patients with a total success rate of 84.6%. In cases of hepatic abscess (Figure 9) 10 out of 12 cases were drained successfully (83.3%). In 12 cases of pancreatic pseudocysts (Figure 10), successful drainage was achieved in 10 cases (83.3%). The details of the success rates of biliary interventions for drainage of abnormal fluid collections are shown in Table 6.
| Type of collection | US-guided procedures | CT-guided procedures | ||
| No. of procedures | Successful procedures | No. of procedures | Successful procedures | |
| Bilomas | 4 | 2 (50) | 10 | 9 (90) |
| Abscess | 6 | 3 (50) | 8 | 7 (87.5) |
| Pancreatic pseudocysts | 5 | 3 (60) | 7 | 7 (100) |
| Total | 15 | 8 (53.33) | 25 | 23 (92)1 |
Two deaths occurred within 30 d among the patients in this study resulting in a mortality rate of 1.6%. Four major procedure-related complications were seen; one case with pneumothorax that required insertion of an intercostal tube, another case had a duodenal perforation which was managed by insertion of a jejunal feeding tube, one patient had a hepatic hematoma that subsequently resolved and one patient had hepatic infarction. Minor complications in the form of immediate post-procedure sepsis were seen in seven patients.
Surgery is currently the accepted method of treatment for most cases of acute biliary tract disorders. However, sometimes a surgical approach may be technically difficult or associated with an unacceptable level of morbidity and mortality. Such situations include emergency surgery in the elderly as well as elective surgery among high-risk patients. Percutaneous biliary intervention plays an important role in these conditions. It is considered a particularly valuable complement in patients who are not candidates for ERCP or surgery. Current percutaneous biliary interventions include PTC and biliary drainage to manage benign and malignant obstruction, and percutaneous cholecystostomy. Other applications include cholangioplasty for biliary strictures, biopsy of biliary duct masses, and management of complications from laparoscopic cholecystectomy and liver transplantation. In this study, we demonstrated the value of percutaneous imaging-guided biliary interventions in the management of three acute biliary disorders; acute biliary obstruction, acute biliary infection and abnormal intra-abdominal collections related to acute biliary tract disorders.
In this work we evaluated the efficacy of 139 percutaneous imaging-guided biliary interventions in the management of acute biliary disorders in 122 high surgical risk patients. In 17 patients, more than one procedure was performed as in 2 cases of cholangitis where CT-guided drainage was performed after failure of US-guided drainage, or combined procedures such as combined US/fluoroscopy-guidance in 5 cases of percutaneous cholecystostomy.
In cases of biliary obstruction, ERCP usually allows visualization of the biliary tract but may not always fully depict the extent of a stricture, may neglect to show anomalous intrahepatic bile duct anatomy, and may not fully show the proximal biliary tree. In addition, induction of anesthesia is needed, which may not be suitable for some critically ill patients[19]. PTC is rarely needed to demonstrate the presence of an obstruction, but it is needed to accurately define the length of a stricture or of anomalous anatomy[20].
Among 59 cases of acute biliary obstruction in this study, ERCP was the first imaging modality to opacify the biliary tree. The preceding ERCP was able to demonstrate the cause and level of obstruction in 21 patients (35.5%) and failed to opacify the biliary tree in the remaining 38 patients (64.5%). This relatively high failure rate of ERCP was explained later by reviewing the results of PTC, which showed a high level of obstruction (bifurcational and supra-bifurcational) in 30 patients (51% of cases). Our results confirm the advantage of PTC over ERCP in the diagnosis of high-level biliary obstruction where the obstructing lesion prevents contrast material from opacifying the cephalic portions of the biliary system[21]. In another eight patients, the cause of failure of ERCP was the presence of a previous history of anatomy-altering surgeries (Billroth II procedure) which make using an endoscope to cannulate the ampulla difficult. This observation is consistent with that in the study by Faylona et al[22] in 1999, who noted that successful selective cannulation during ERCP, was achieved in only 66% of attempts in patients with anatomy-altering surgeries. The role of biliary decompression in patients with acute biliary obstruction is well known. In addition to relief of pain and pruritus, it also prevents the development of further biliary complications. In addition, preoperative placement of a biliary drainage catheter provides an important intraoperative landmark to define the anatomy preoperatively and provide postoperative stent management[23].
In this study, after demonstrating the cause, level, and extent of biliary obstruction, PTD procedures were carried out under fluoroscopy- , US- and CT-guidance. We noticed that the success rates in patients drained by CT-guidance were higher (93.3%) compared to those drained under fluoroscopy or ultrasound-guidance (62.5% and 46.1%, respectively) with significant P values (P = 0.026 and 0.002, respectively). CT-guidance was used for ductal puncture followed by immediate external biliary drainage. We prefer the use of percutaneous biliary interventions under CT-guidance to ensure immediate relief of obstruction and sepsis, and this may explain why we had a high number of patients in our study who were managed under CT-guidance in contrast to the literature where US-guidance is the predominant modality used for guidance. This can be explained by the fact that under CT-guidance, the bile ducts can be punctured easily and more selectively with precise placement of drainage catheters within the pre-selected sub-segmental bile ducts. This conclusion is supported by the study of Froelich et al[24] who stated that CT facilitates percutaneous biliary drainage procedures and is superior to conventional fluoroscopically-guided biliary interventions because the number of hepatic punctures, procedure times, and radiation exposure times are significantly reduced. They also concluded that because the number of hepatic punctures can be reduced and bile ducts can be punctured more selectively, procedure-related safety may be improved with decreased complication rates. Safety during the procedure and decreased post-procedure complications are important targets in managing critically ill patients.
Acute biliary infection is a serious clinical problem, especially in elderly and critically ill patients and immediate drainage of the biliary tree is essential. Management of acute biliary infection can be performed by means of surgical, endoscopic, or percutaneous interventional procedures. Emergency surgery in these patients is associated with unacceptable morbidity and mortality. Endoscopic papillotomy and duct clearance or placement of a stent can be life-saving in these conditions. However, endoscopic interventions are unsuccessful in 10%-15% of cases due to duodenal diverticula, edema of the papilla, biliary strictures, large impacted stones or previous gastric surgery. Percutaneous transhepatic external biliary drainage has few limitations and can be performed easily if the biliary tree is dilated[4].
In our study, percutaneous biliary drainage served as a diagnostic and therapeutic maneuver in 26 patients with acute biliary sepsis (group 2). These patients were managed either by US- or CT-guided percutaneous drainage in conjunction with systemic antibiotics. Biliary drainage in cases of biliary sepsis was successful in 22 patients with a total success rate of 84.6%. This success rate in the drainage of biliary sepsis is slightly lower than the results obtained in the study by Chopra et al[1], in which successful treatment of acute biliary inflammation was obtained in 52 of 53 (98.1%) of patients. On the other hand, our results are similar to another study comparing percutaneous drainage with surgery in the management of 66 critically ill patients with acute gallbladder sepsis, which concluded that percutaneous drainage has a relatively low complication rate and is rapidly effective[25]. These findings indicate that percutaneous imaging-guided biliary drainage is a useful therapeutic intervention for acute biliary sepsis in critically ill patients or patients unsuitable for immediate surgery.
We observed that the success rate in our patients drained under US-guidance (46.15%) was similar to another retrospective study performed by Andrén-Sandberg et al[26] in 2001 on the safety and efficacy of US-guided percutaneous drainage of the gallbladder in 86 patients with acute calcular cholecystitis. They observed that two thirds of their patients recovered within 36 h after the procedure and 27 (45%) of these were asymptomatic during the follow-up period for 6 mo. The success rate of drainage under US-guidance in our study was slightly lower than the results obtained by Cozzi et al[6] in 1999, who observed a dramatic improvement in the clinical condition of 48 out of 82 patients (59%) with gallbladder sepsis within 48 h after percutaneous cholecystostomy. In addition, our success rate was lower than that of Sosna et al[27] in 2004, who showed improvement in 30 (86%) of 35 patients who underwent US-guided percutaneous cholecystostomy. We think that our lower success rate was due to the specific nature of our patients who were critically-ill elderly patients. Also, we noted that our success rate of drainage under CT-guidance was higher than that under US-guidance and this can be explained by the same reasons mentioned previously regarding the advantages of CT-guidance of biliary drainage in cases of obstruction.
In recent years, the drainage of abnormal abdominal collections has been changed from surgical to nonsurgical management using imaging-guided catheter aspiration and drainage with antibiotic therapy, and are associated with high cure rates. The third group of patients in our study consisted of 37 cases with intra-abdominal pathological fluid collections (bilomas, hepatic abscess and pancreatic pseudocysts) as a complication of underlying acute biliary disorders such as biliary trauma, post-cholecystectomy complications, post-biliary interventions or following acute biliary pancreatitis.
For many years, interventional radiologists as well as endoscopists have also reported success with nonoperative therapy for bile leaks. Kaufman et al[28] treated 12 patients who had biliary leaks with percutaneous transhepatic biliary drainage. The biliary leaks healed in six patients, while surgery was required in five patients. Liguory et al[29] used percutaneous transhepatic biliary drainage in seven patients after failure of endoscopic biliary drainage and attained closure of the biliary leak in six patients. In our study, bilomas were detected in 13 high surgical risk patients as complications of biliary leak secondary to trauma, surgery, or previous intervention. Our success rate in the percutaneous management of bile duct injuries with bilomas was 84.6%. This result confirms the efficacy of percutaneous transhepatic biliary drainage in the treatment of bilomas. It is consistent with the results of Ernst et al[30] who reported complete cure of biliary leaks in 13 of 16 (81.2%) patients. Our success rate was higher than that in the study by Misra et al[31], who reported a 58.8% success rate in 51 patients with major bile duct stricture or injury without the need for subsequent intervention. The higher success rate in our study may be due to the limited number of patients.
Management of hepatic abscess has evolved rapidly during the past decade. For many years, the traditional treatment was surgical drainage. Imaging-guided percutaneous drainage, along with appropriate antibiotics, is an effective approach to treat hepatic abscesses. In our study, hepatic abscesses were drained percutaneously under CT and US-guidance in 12 patients with a total success rate of 83.3%. The percutaneous procedures were not curative in two of the 12 patients. Our success rate was higher than that of Mehendiratta et al[32] who reported cure of 67 of 92 (73%) abscesses managed by percutaneous aspiration without the need for open surgical drainage. The success rate of abscess drainage under CT-guidance in our study was 87.5% and was similar to that of Cinat et al[33] who showed resolution of hepatic abscess in 17 out of 20 patients with a success rate of 85% and that of Thomas et al[34] who showed resolution in 17/19 patients (89%).
The third subgroup of intra-abdominal collections in this study included 12 patients with pancreatic pseudocysts. Percutaneous or endoscopic drainage of peripancreatic fluid collections, pseudocysts, and abscesses have been well established as diagnostic and therapeutic standards in the management of acute pancreatitis[35]. In an older study, Heider et al[36] compared the success rates of surgical and percutaneous management of pancreatic pseudocysts. They observed that percutaneous drainage was successful only in 42% and surgical treatment was successful in 88%. They also noted that percutaneous drainage was also associated with a high death rate (16% vs 0%) and a high rate of complications (64% vs 27%). The low success rate of percutaneous drainage in their study may be related to the nature of unselected patients in their study and the lack of recently developed catheters and tubes. In most of the recent studies, the success rates of percutaneous drainage were higher. In the study by Nealon et al[37], 50 patients with a diagnosis of pancreatic pseudocysts had percutaneous drainage and 37 (74%) of them responded to this treatment. In another study, percutaneous external drainage was successful in two of three patients with pancreatic pseudocysts. They concluded that percutaneous external drainage is a good first choice for patients with unilocular pancreatic pseudocysts[38]. This conclusion is clearer in our current study, where the total clinical success rate of percutaneous catheter drainage was 83.3% (10/12) in these patients. The success rate under CT-guidance was 100%, this is consistent with the conclusion of Ferrucci et al[39] who reported that imaging guidance is best performed under CT control which allows precise definition of access route, catheter placement, and response. With regard to access, routes are chosen to avoid traversing vital intervening structures, especially the pleural space, colon, and small bowel.
Although imaging-guided percutaneous interventions appear safe and relatively simple, they are not completely free of risk. Two deaths occurred within 30 d among the patients in this study resulting in a mortality rate of 1.6%. This mortality rate was similar to that in the study by vanSonnenberg et al[19], who reported a 2.2% mortality rate following 104 interventional procedures in 45 patients who underwent cholecystostomy. In the present study, we also reported acceptable procedure-related complications (four major and seven minor) in 11 patients (9%).
In conclusion, percutaneous imaging-guided interventional biliary procedures help to promptly diagnose and effectively treat major acute biliary disorders such as acute biliary obstruction, acute biliary infection, and abnormal intra-abdominal collections related to acute biliary tract disorders. These interventional procedures can either cure the disorder with a high success rate and obviate surgery or aid the surgeon by relieving sepsis and jaundice before operation when the clinical condition of these patients improve. In these conditions, the success rate is sufficiently high to justify the use of percutaneous intervention procedures as the technique of choice in the management of high surgical risk patients with acute biliary disorders. To increase the effectiveness of the procedure, we need to improve our criteria in patient selection, choice, and performing the procedure. Close follow-up, for monitoring and management of intervention-related problems is appropriate to avoid complications.
Acute disorders of the biliary tract affect a significant portion of the population. These conditions include biliary obstruction, biliary sepsis, hepato-biliary trauma, and their complications. In this study, we illustrate the spectrum of acute biliary disorders in patients with high surgical risk and evaluate the success rate and complications of percutaneous imaging-guided interventions in their diagnosis and management.
Percutaneous imaging interventional procedures can either cure the disorder with a high success rate and obviate operation or aid the surgeon by relieving sepsis and jaundice before operation when the clinical condition of these patients improve
In our study, the success rate was sufficiently high to justify the use of percutaneous intervention procedures as the technique of choice in the management of high surgical risk patients with acute biliary disorders.
To increase the effectiveness of the procedure, we need to improve our criteria in patient selection, choice, and performing the procedure. Close follow-up, for monitoring and management of intervention-related problems is appropriate to avoid complications.
Percutaneous imaging-guided interventional biliary procedures are imaging modalities like CT, US and fluoroscopy which are used to guide puncture of biliary ducts, collections or gallbladder and to guide manipulation of guide-wires, catheters and stents to ensure their correct position.
Congratulations for discussing the role of interventions especially in surgically high risk individuals.
Peer reviewers: Shivanand Gamanagatti, MD, Assistant Professor of Radiology, Department of Radiodiagnosis, All India Institute of Medical Sciences, New Delhi, 110029, India; John L Nosher, MD, Clinical Professor and Chairman, Department of Radiology, UMDNJ-Robert Wood Johnson Medical School, 1 Robert Wood Johnson Place, PO Box 19, Medical Education Bldg., Room 404, New Brunswick, NJ 08903-0019, United States
S- Editor Cheng JX L- Editor Webster JR E- Editor Zheng XM
| 1. | Chopra S, Dodd GD 3rd, Mumbower AL, Chintapalli KN, Schwesinger WH, Sirinek KR, Dorman JP, Rhim H. Treatment of acute cholecystitis in non-critically ill patients at high surgical risk: comparison of clinical outcomes after gallbladder aspiration and after percutaneous cholecystostomy. AJR Am J Roentgenol. 2001;176:1025-1031. |
| 2. | Magnuson TH, Bender JS, Duncan MD, Ahrendt SA, Harmon JW, Regan F. Utility of magnetic resonance cholangiography in the evaluation of biliary obstruction. J Am Coll Surg. 1999;189:63-71; discussion 71-72. |
| 3. | Killeen RP, Harte S, Maguire D, Malone DE. Achievable outcomes in the management of proximal cholangiocarcinoma: an update prepared using "evidence-based practice" techniques. Abdom Imaging. 2008;33:54-57. |
| 4. | Bae JI, Park AW, Choi SJ, Kim HP, Lee SJ, Park YM, Yoon JH. Crisscross-configured dual stent placement for trisectoral drainage in patients with advanced biliary hilar malignancies. J Vasc Interv Radiol. 2008;19:1614-1619. |
| 5. | van Delden OM, Laméris JS. Percutaneous drainage and stenting for palliation of malignant bile duct obstruction. Eur Radiol. 2008;18:448-456. |
| 6. | Cozzi G, Alasio L, Civelli E, Colnago MF, Salvetti M, Pilotti S, Rilke F, Severini A. Percutaneous intraductal sampling for cyto-histologic diagnosis of biliary duct strictures. Tumori. 1999;85:153-156. |
| 7. | Menu Y, Vuillerme MP. Non-traumatic abdominal emergencies: imaging and intervention in acute biliary conditions. Eur Radiol. 2002;12:2397-2406. |
| 8. | Burke DR, Lewis CA, Cardella JF, Citron SJ, Drooz AT, Haskal ZJ, Husted JW, McCowan TC, Van Moore A, Oglevie SB. Quality improvement guidelines for percutaneous transhepatic cholangiography and biliary drainage. J Vasc Interv Radiol. 2003;14:S243-S246. |
| 9. | Tse F, Barkun JS, Romagnuolo J, Friedman G, Bornstein JD, Barkun AN. Nonoperative imaging techniques in suspected biliary tract obstruction. HPB (Oxford). 2006;8:409-425. |
| 10. | George C, Byass OR, Cast JEI. Interventional radiology in the management of malignant biliary obstruction. World J Gastrointest Oncol. 2010;2:146-150. |
| 11. | Born P, Rosch T, Bruhl K, Ulm K, Sandschin W, Frimberger E, Allescher H, Classen M. Long-term results of endoscopic treatment of biliary duct obstruction due to pancreatic disease. Hepatogastroenterology. 1998;45:833-839. |
| 12. | Radder RW. Ultrasonically guided percutaneous catheter drainage for gallbladder empyema. Diagn Imaging. 1980;49:330-333. |
| 13. | McGahan JP, Lindfors KK. Acute cholecystitis: diagnostic accuracy of percutaneous aspiration of the gallbladder. Radiology. 1988;167:669-671. |
| 14. | Patel M, Miedema BW, James MA, Marshall JB. Percutaneous cholecystostomy is an effective treatment for high-risk patients with acute cholecystitis. Am Surg. 2000;66:33-37. |
| 15. | Lohela P, Soiva M, Suramo I, Taavitsainen M, Holopainen O. Ultrasonic guidance for percutaneous puncture and drainage in acute cholecystitis. Acta Radiol Diagn (Stockh). 1986;27:543-546. |
| 16. | Borzellino G, de Manzoni G, Ricci F, Castaldini G, Guglielmi A, Cordiano C. Emergency cholecystostomy and subsequent cholecystectomy for acute gallstone cholecystitis in the elderly. Br J Surg. 1999;86:1521-1525. |
| 17. | Bortoff GA, Chen MY, Ott DJ, Wolfman NT, Routh WD. Gallbladder stones: imaging and intervention. Radiographics. 2000;20:751-766. |
| 18. | Laganà D, Carrafiello G, Mangini M, Ianniello A, Giorgianni A, Nicotera P, Fontana F, Dionigi G, Fugazzola C. Image-guided percutaneous treatment of abdominal-pelvic abscesses: a 5-year experience. Radiol Med. 2008;113:999-1007. |
| 19. | vanSonnenberg E, Casola G, Wittich GR, Christensen R, Varney RR, Neff CC, D'Agostino HB, Moossa AR. The role of interventional radiology for complications of cholecystectomy. Surgery. 1990;107:632-638. |
| 20. | Lillemoe KD, Pitt HA, Cameron JL. Current management of benign bile duct strictures. Adv Surg. 1992;25:119-174. |
| 21. | Mallery S, Van Dam J. Advances in diagnostic and therapeutic endoscopy. Med Clin North Am. 2000;84:1059-1083. |
| 22. | Faylona JM, Qadir A, Chan AC, Lau JY, Chung SC. Small-bowel perforations related to endoscopic retrograde cholangiopancreatography (ERCP) in patients with Billroth II gastrectomy. Endoscopy. 1999;31:546-549. |
| 23. | Ahmed A, Cheung RC, Keeffe EB. Management of gallstones and their complications. Am Fam Physician. 2000;61:1673-1680, 1687-1688. |
| 24. | Froelich JJ, Wagner HJ, Ishaque N, Alfke H, Scherf C, Klose KJ. Comparison of C-arm CT fluoroscopy and conventional fluoroscopy for percutaneous biliary drainage procedures. J Vasc Interv Radiol. 2000;11:477-482. |
| 25. | Wise JN, Gervais DA, Akman A, Harisinghani M, Hahn PF, Mueller PR. Percutaneous cholecystostomy catheter removal and incidence of clinically significant bile leaks: a clinical approach to catheter management. AJR Am J Roentgenol. 2005;184:1647-1651. |
| 26. | Andrén-Sandberg A, Haugsvedt T, Larssen TB, Søndenaa K. Complications and late outcome following percutaneous drainage of the gallbladder in acute calculous cholecystitis. Dig Surg. 2001;18:393-398. |
| 27. | Sosna J, Kruskal JB, Copel L, Goldberg SN, Kane RA. US-guided percutaneous cholecystostomy: features predicting culture-positive bile and clinical outcome. Radiology. 2004;230:785-791. |
| 28. | Kaufman SL, Kadir S, Mitchell SE, Chang R, Kinnison ML, Cameron JL, White RI Jr. Percutaneous transhepatic biliary drainage for bile leaks and fistulas. AJR Am J Roentgenol. 1985;144:1055-1058. |
| 29. | Liguory C, Vitale GC, Lefebre JF, Bonnel D, Cornud F. Endoscopic treatment of postoperative biliary fistulae. Surgery. 1991;110:779-783; discussion 783-784. |
| 30. | Ernst O, Sergent G, Mizrahi D, Delemazure O, L'Herminé C. Biliary leaks: treatment by means of percutaneous transhepatic biliary drainage. Radiology. 1999;211:345-348. |
| 31. | Misra S, Melton GB, Geschwind JF, Venbrux AC, Cameron JL, Lillemoe KD. Percutaneous management of bile duct strictures and injuries associated with laparoscopic cholecystectomy: a decade of experience. J Am Coll Surg. 2004;198:218-226. |
| 32. | Mehendiratta V, McCarty BC, Gomez L, Graviss EA, Musher DM. Computerized tomography (CT)-guided aspiration of abscesses: outcome of therapy at a tertiary care hospital. J Infect. 2007;54:122-128. |
| 33. | Cinat ME, Wilson SE, Din AM. Determinants for successful percutaneous image-guided drainage of intra-abdominal abscess. Arch Surg. 2002;137:845-849. |
| 34. | Thomas J, Turner SR, Nelson RC, Paulson EK. Postprocedure sepsis in imaging-guided percutaneous hepatic abscess drainage: how often does it occur? AJR Am J Roentgenol. 2006;186:1419-1422. |
| 35. | Werner J, Feuerbach S, Uhl W, Büchler MW. Management of acute pancreatitis: from surgery to interventional intensive care. Gut. 2005;54:426-436. |
| 36. | Heider R, Meyer AA, Galanko JA, Behrns KE. Percutaneous drainage of pancreatic pseudocysts is associated with a higher failure rate than surgical treatment in unselected patients. Ann Surg. 1999;229:781-787; discussion 787-789. |
| 37. | Nealon WH, Walser E. Main pancreatic ductal anatomy can direct choice of modality for treating pancreatic pseudocysts (surgery versus percutaneous drainage). Ann Surg. 2002;235:751-758. |
| 38. | Naoum E, Zavos A, Goudis K, Sarros C, Pitsargiotis E, Karamouti M, Tsikrikis P, Karantanas A. Pancreatic pseudocysts: 10 years of experience. J Hepatobiliary Pancreat Surg. 2003;10:373-376. |
| 39. | Ferrucci JT 3rd, Mueller PR. Interventional approach to pancreatic fluid collections. Radiol Clin North Am. 2003;41:1217-1226, vii. |