Published online Dec 28, 2010. doi: 10.4329/wjr.v2.i12.468
Revised: October 20, 2010
Accepted: October 27, 2010
Published online: December 28, 2010
Miriplatin, a cisplatin derivative with a high affinity for iodized oil, is a novel chemotherapeutic agent designed for use in the transarterial treatment of hepatocellular carcinoma. This case report describes our experience with transarterial chemoembolization (TACE) using miriplatin in 2 patients with neuroendocrine liver metastases. A 38-year-old man with multiple neuroendocrine liver metastases was treated by whole liver chemoembolization, and a 35-year-old woman with a single hepatic lesion was treated by superselective chemoembolization. No serious adverse events were noted during the interventional procedures, or during the observation period of 3 mo in either patient. Sufficient iodized oil uptake was observed in the hypervascular lesions on the unenhanced computed tomography (CT) at 7 d after the procedure. Contrast-enhanced CT obtained at 3 mo after chemoembolization revealed that all hepatic lesions were substantially reduced in size irrespective of tumor vascularity or degree of cystic degeneration, although iodized oil accumulation was only marginal for lesions with cystic degeneration. Thus, TACE with miriplatin can be a safe and effective therapeutic option for the treatment of neuroendocrine metastases of the liver.
- Citation: Iwazawa J, Ohue S, Yasumasa K, Mitani T. Transarterial chemoembolization with miriplatin-lipiodol emulsion for neuroendocrine metastases of the liver. World J Radiol 2010; 2(12): 468-471
- URL: https://www.wjgnet.com/1949-8470/full/v2/i12/468.htm
- DOI: https://dx.doi.org/10.4329/wjr.v2.i12.468
Neuroendocrine tumors (NET) are a group of carcinomas that secrete various polypeptides with hormonal activity[1]. NETs are sometimes indolent but most cases eventually present with systemic metastases. A significant percentage of patients already have hepatic metastases at the time of initial diagnosis[2]. Hepatic metastases from NET are intrinsically hypervascular, thus necessitating transarterial chemoembolization (TACE) as an alternative option for managing these unresectable liver lesions.
Miriplatin, a novel lipophilic platinum complex with a high affinity for iodized oil, has been developed to treat hepatocellular carcinoma[3]. Miriplatin suspended in iodized oil has demonstrated antitumor effects in hepatic tumors after intrahepatic arterial administration in several animal models[4-6] as well as in humans[7]. However, transarterial administration of miriplatin in patients with hepatic metastases from NET has not been previously documented. Here, we describe the treatment of two patients with hepatic metastases from NET using TACE with miriplatin suspended in iodized oil, along with the safety and efficacy of miriplatin during the course of this therapy.
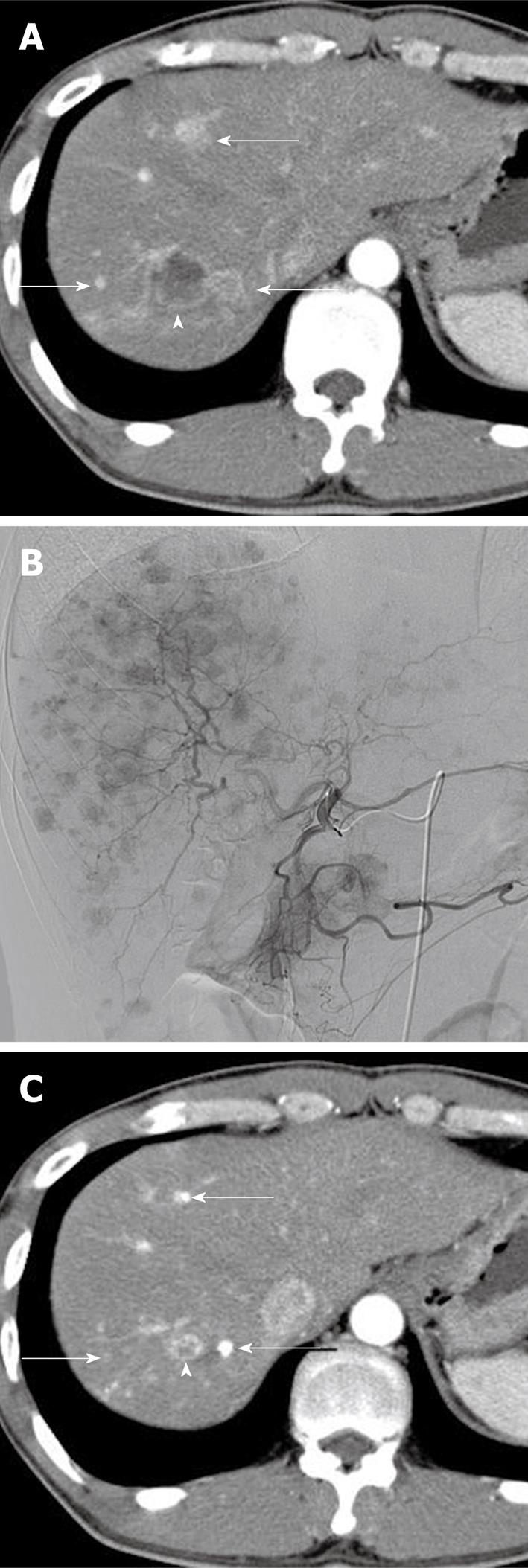
A 38-year-old man, who had undergone surgical resection of NET of the pancreas 15 mo before, was admitted to our hospital for the treatment of multiple liver metastases from NET. The liver lesions were first observed at 8 mo after surgery and gradually increased in size and number despite systemic chemotherapy with octreotide (Sandostatin LAR; Novartis Pharma) and tegafur, gimeracil, and oteracil (TS-1; Taiho Pharmaceutical, Tokyo, Japan). Contrast-enhanced computed tomography (CT) at the time of admission showed numerous early-enhanced tumors throughout the liver, accompanied by several hypovascular tumors with cystic degeneration (Figure 1A). Histopathological examination of needle biopsy specimens of one of the hypovascular tumors revealed a glucagon-producing islet cell carcinoma, a finding compatible with the histopathological results for the surgical specimens of the primary pancreatic tumor. Since systemic chemotherapy had been ineffective, TACE was scheduled 16 mo after the initial surgical resection of the pancreatic NET: the patient opted to receive whole liver treatment instead of lobar treatment in two sessions, despite the increased risk of adverse events. TACE was performed using a coaxially placed 2.4-F microcatheter (Sniper 2; Terumo Clinical Supply, Gifu, Japan) through a 4-F catheter via the femoral artery. Selective proper hepatic angiography revealed innumerable hypervascular tumors located throughout the liver (Figure 1B). In order to treat lesions in the whole liver, the right and left hepatic arteries were separately embolized with gelatin particles (Gelpart; Astellas Pharma, Tokyo, Japan) after infusion with 120 mg of miriplatin (Miripla; Dainippon Sumitomo Pharma, Osaka, Japan) suspended in 10 mL of iodized oil (Lipiodol Ultrafluid; Guerbet, Aulnay-sous-Bois, France) at a ratio of 5:2, respectively. The maximum permissible dose in a single session of 120 mg of miriplatin was used, as per the recommendation of the Ministry of Health, Labour and Welfare of Japan, for transarterial administration to the liver. During intraarterial infusion of miriplatin, the patient complained of slight transient numbness of both limbs; however, no other adverse effects were observed during the course of the therapy. Serum creatinine levels (1.0 mg/dL) were unchanged after treatment. Serum aspartate transaminase, alanine transaminase, and bilirubin levels were transiently elevated from 31 to 214 IU/L, from 26 to 116 IU/L, and from 1.2 to 2.7 mg/dL, respectively, at one day after the treatment, but returned to normal within 6 d. Serum neuron-specific enolase, a tumor marker, remained unchanged (5.7 ng/mL) before and after treatment with miriplatin. Other tumor markers such as 5-hydroxyindole acetic acid were not investigated. Follow-up CT at 3 mo after TACE revealed a significant reduction in the size of all metastatic lesions, irrespective of tumor vascularity or the degree of cystic degeneration (Figure 1C): the diameter of the largest tumor decreased from 26 mm to 12 mm (size reduction rate, 54%). Prior to TACE, the patient complained of diarrhea, nausea, and epigastric pain. After chemoembolization, however, the patient was almost symptom-free.
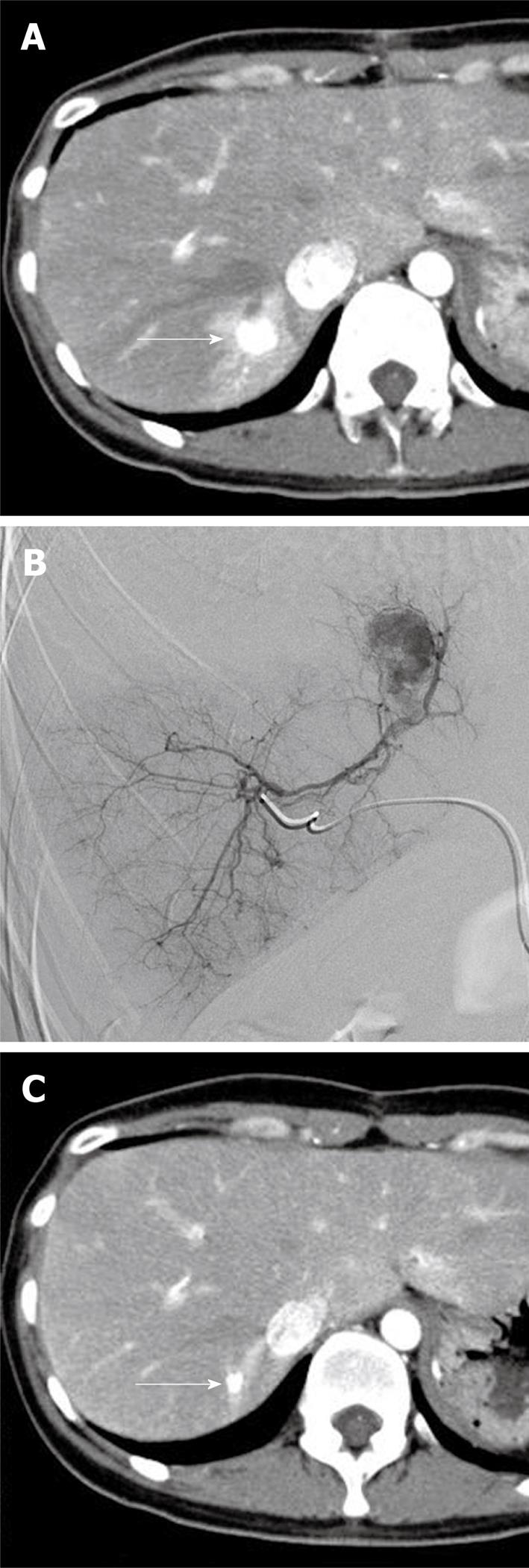
A 35-year-old woman with no prior medical history presented at our hospital for the treatment of a pancreatic tumor detected by abdominal ultrasound in a general screening examination. Contrast-enhanced CT at admission showed a hypervascular tumor at the pancreatic tail and a single hypervascular tumor at hepatic segment VII accompanied by distal arterioportal shunting (Figure 2A). The pancreatic tumor was surgically removed 1 mo after initial diagnosis. Concurrent needle biopsy and histopathological examination of the liver tumor during surgery revealed the tumor to be a well-differentiated neuroendocrine carcinoma compatible with metastasis from the pancreatic NET. One month after surgery, we performed TACE for the hepatic metastasis, using a coaxially placed 2.4-F microcatheter (Sniper 2) through a 4-F catheter via the femoral artery. Selective angiography from the right posterior arterial branch revealed a single hypervascular tumor at hepatic segment VII accompanied by arterioportal shunting (Figure 2B). We embolized the right posterior arterial segment with gelatin particles (Gelpart) following infusion of 70 mg of miriplatin (Miripla) suspended in 3.5 mL of iodized oil. No adverse events were noted during the interventional therapy. Serum creatinine levels (0.5 mg/dL) remained unchanged after the treatment. Serum aspartate transaminase, alanine transaminase, and bilirubin levels were elevated from 15 to 295 IU/L at day 1, from 13 to 389 IU/L at day 4, and from 0.4 to 0.9 mg/dL at day 1 after the treatment, respectively, and returned to normal within 20 d. The eosinophil count gradually increased from 3.3% (before treatment) to 39.8% (at 20 d after treatment) and decreased to 7.8% at 61 d after treatment. Eosinophilia observed in this patient may be attributed to an allergic reaction to miriplatin; however, the precise reason remains unknown. Levels of serum neuron-specific enolase, a tumor marker, remained normal (4.8 ng/mL) after the treatment. Other tumor markers were not investigated. Follow-up CT at 3 mo after TACE confirmed dense iodized oil accumulation in the metastatic lesion with a significant reduction in tumor size (Figure 2C): the diameter of the tumor decreased from 14 mm to 6 mm (size reduction rate, 57%). No local recurrence was evident over a follow-up period of 3 mo.
Although several studies have established the beneficial therapeutic effects of TACE for hepatic metastases from NET, there is no consensus on the most effective chemotherapeutic agent for use in this procedure. Various chemotherapeutic agents including doxorubicin, streptozocin, 5-FU, mitomycin C, cisplatin, and a combination of these agents have been used to perform TACE for hepatic metastases of NETs[8]. However, there were no significant differences in the response rate to these agents[8].
Miriplatin is a lipophilic platinum complex designed for the transarterial treatment of hepatocellular carcinoma. This agent can be easily suspended in iodized oil and is gradually released after the oil accumulates in the target tumor[6]. This novel feature of miriplatin can be potentially beneficial for long-acting antitumor effects, thus making it a superior chemotherapeutic agent as compared to other hydrophilic agents. In addition, since the oil-suspended miriplatin remains in the tumor for a long period, its rapid release into the systemic circulation is inhibited, resulting in reduced systemic side effects such as nausea/vomiting, renal damage, and other acute toxic events[7].
In this report, we have described the treatment of hepatic metastases from NET using TACE with miriplatin in 2 patients, who achieved a significant reduction in the size of each lesion as well as sufficient uptake of the iodized oil in hypervascular lesions, without major complications. TACE with miriplatin was effective not only for treating hypervascular metastases but also for hypovascular lesions with cystic degeneration. This might be predominantly attributable to the ischemic effect of arterial embolization, but also to the long-acting antitumor effect of miriplatin accumulated in the wall of the cystic lesions. Furthermore, it is possible that miriplatin accumulated in the hypervascular tumors might be released gradually into the surrounding liver parenchyma or into the bloodstream, resulting in a persistent steady platinum concentration adequate for inhibiting tumor growth within the hypovascular lesions.
In contrast to hepatocellular carcinoma, TACE for NET poses a potential risk of crisis or other hormonal symptoms attributable to acute hormone release due to tumor necrosis. We treated a patient with progressive tumor burden throughout the liver in a single session. This treatment procedure carried the potential risk of hormonal crisis or tumor lysis syndrome. We suggest that NET patients with massive tumor involvement should be treated by selective TACE for separate lesions in multiple treatment sessions: selective TACE has the added benefit of increasing the total amount of chemotherapeutic agent delivered to each lesion as well as reducing the risk of post-embolization syndrome.
At present, there are no data on the effects of TACE on survival benefits in patients with hepatic metastases from NET. The reported median survival times in patients with metastatic NETs after TACE or bland embolization vary considerably, ranging from 13 to 80 mo in various studies[8]. Moreover, the prognostic factors for survival in patients with NET undergoing TACE have not been studied. Since we have treated only 2 cases with a follow-up of 3 mo, the long-term outcomes of TACE with miriplatin for liver metastases from NET, including chronic toxicities, local control, and survival benefits are not yet known. Long-term observations are necessary in order to assess whether miriplatin is superior to other chemotherapeutic agents in the intraarterial treatment of hepatic metastases from NET.
In conclusion, we performed TACE using miriplatin-iodized oil emulsion in 2 patients with neuroendocrine liver metastases and achieved an acceptable response in short-term observation periods, with no serious adverse events. TACE with miriplatin can be a safe and effective therapeutic option for the management of NET hepatic metastases. However, further investigations are required to assess the long-term outcomes of chemoembolization with miriplatin for liver metastases from NET.
Peer reviewers: Barbaros E Çil, MD, Associated Professor of Radiology, Department of Radiology, Hacettepe University School of Medicine, Sihhiye 06100, Ankara, Turkey; Wei Lu, MD, PhD, Associate Professor, Department of Interventional Radiology, Nanfang Hospital, Southern Medical University, Guangzhou, Guangdong 510515, Province, China
S- Editor Cheng JX L- Editor Webster JR E- Editor Zheng XM
| 1. | Rindi G, Capella C, Solcia E. Cell biology, clinicopathological profile, and classification of gastro-enteropancreatic endocrine tumors. J Mol Med. 1998;76:413-420. |
| 2. | Chamberlain RS, Canes D, Brown KT, Saltz L, Jarnagin W, Fong Y, Blumgart LH. Hepatic neuroendocrine metastases: does intervention alter outcomes? J Am Coll Surg. 2000;190:432-445. |
| 3. | Maeda M, Uchida NA, Sasaki T. Liposoluble platinum(II) complexes with antitumor activity. Jpn J Cancer Res. 1986;77:523-525. |
| 4. | Kishimoto S, Noguchi T, Yamaoka T, Fukushima S, Takeuchi Y. Antitumor effects of a novel lipophilic platinum complex (SM-11355) against a slowly-growing rat hepatic tumor after intra-hepatic arterial administration. Biol Pharm Bull. 2000;23:344-348. |
| 5. | Hanada M, Baba A, Tsutsumishita Y, Noguchi T, Yamaoka T, Chiba N, Nishikaku F. Intra-hepatic arterial administration with miriplatin suspended in an oily lymphographic agent inhibits the growth of tumors implanted in rat livers by inducing platinum-DNA adducts to form and massive apoptosis. Cancer Chemother Pharmacol. 2009;64:473-483. |
| 6. | Hanada M, Baba A, Tsutsumishita Y, Noguchi T, Yamaoka T. Intra-hepatic arterial administration with miriplatin suspended in an oily lymphographic agent inhibits the growth of human hepatoma cells orthotopically implanted in nude rats. Cancer Sci. 2009;100:189-194. |
| 7. | Okusaka T, Okada S, Nakanishi T, Fujiyama S, Kubo Y. Phase II trial of intra-arterial chemotherapy using a novel lipophilic platinum derivative (SM-11355) in patients with hepatocellular carcinoma. Invest New Drugs. 2004;22:169-176. |
| 8. | Madoff DC, Gupta S, Ahrar K, Murthy R, Yao JC. Update on the management of neuroendocrine hepatic metastases. J Vasc Interv Radiol. 2006;17:1235-1249; quiz 1250. |