Published online Jun 28, 2025. doi: 10.4329/wjr.v17.i6.108247
Revised: April 19, 2025
Accepted: May 26, 2025
Published online: June 28, 2025
Processing time: 78 Days and 23.6 Hours
Dual-phenotype hepatocellular carcinoma (HCC) is a relatively new subtype of HCC. Studies have shown that in the context of chronic hepatitis, liver cirrhosis, and other liver conditions, some intrahepatic cholangiocarcinomas (ICCs) exhibit an enhancement pattern similar to that of HCC. Both dual-phenotype HCC (DPHCC) and ICC can express biliary markers, making imaging and pathology differentiation difficult. Currently, radiomics is widely used in the differentiation, clinical staging, and prognosis assessment of various diseases. Radiomics can effectively differentiate DPHCC and ICC preoperatively.
To evaluate the value of radiomics in the differential diagnosis of DPHCC and ICC and to validate its clinical applicability
In this retrospective study, the data of 53 DPHCC patients and 124 ICC patients were collected retrospectively and randomly divided into training and testing sets at a ratio of 7: 3. After delineation of regions of interest and feature extraction and selection, radiomics models were constructed. Receiver operating characteristic curve analysis was conducted to calculate the area under the curve (AUC) for each model. The AUC values of radiologists with and without assistance from the model were also assessed.
In the training set, the AUC value of the radiomic model was the highest, and the combined model and the radiomic model had similar AUC (P > 0.05); the differences in the AUC values between the combined model and the clinical-sign model was statistically significant (P < 0.05). In the testing set, the AUC value of the combined model was the highest, and the differences in the AUC values between the combined model and the clinical-sign model was statistically significant (P < 0.05). With model assistance, the AUC values of Doctor D (10 years of experience in abdominal imaging diagnosis) and Doctor E (5 years of experience in abdominal imaging diagnosis) both increased.
Radiomics can differentiate DPHCC and ICC, and with assistance from the developed model, the accuracy of less experienced doctors in the differential diagnosis of these two diseases can be improved.
Core Tip: In the present study, the clinical and imaging data of 53 dual-phenotype hepatocellular carcinoma (DPHCC) patients and 124 intrahepatic cholangiocarcinoma (ICC) patients were collected, the regions of interest were delineated slice by slice, and relevant information was extracted to construct a clinical-sign model, a radiomic model, and a combined model. Subsequently, the performance of the predictive models was evaluated to explore their clinical applicability. The study found that radiomics can effectively differentiate DPHCC and ICC preoperatively, and with assistance from the developed model, the accuracy of less experienced doctors in the differential diagnosis of these two diseases can be improved.
- Citation: Zhang CC, Lu D, Yang J, Zhang L, Zeng XF, Fang XM, Fan CG. Clinical value and applicability of radiomics in differential diagnosis of dual-phenotype hepatocellular carcinoma and intrahepatic cholangiocarcinoma. World J Radiol 2025; 17(6): 108247
- URL: https://www.wjgnet.com/1949-8470/full/v17/i6/108247.htm
- DOI: https://dx.doi.org/10.4329/wjr.v17.i6.108247
Dual-phenotype hepatocellular carcinoma (DPHCC) is a relatively new subtype of hepatocellular carcinoma (HCC), first reported in 2011. Although it only involves the presence of a single histological component of HCC in a tumour nodule, the cells show bidirectional differentiation and gene activation, and can simultaneously express HCC and intrahepatic cholangiocarcinoma (ICC) protein markers[1,2]. Compared with ordinary HCC, cancer cells in DPHCC not only have stronger proliferation and migration ability, but also have stronger drug resistance and hypoxia resistance. The incidence of DPHCC in the United States has increased by more than 140%[3-5]. For example, Rhee et al[6] found that DPHCC exhibited more frequent microvascular invasion than ordinary HCC (62% vs 18%), and that DPHCC had more residual HCC cells (57% vs 15%) than ordinary HCC after hepatic arterial chemoembolization, with three cases having extrahepatic recurrence within 2 years. ICC is a malignant tumour that occurs in the epithelial lining and peribiliary glands of the secondary intrahepatic bile duct to the smallest intrahepatic bile duct branch. Its morbidity and mortality have increased in recent years[7].
Studies have shown that in the context of chronic hepatitis, liver cirrhosis, and other liver conditions, some ICCs exhibit an enhancement pattern similar to that of HCC[8,9]. Small duct cholangiocarcinoma and small duct ICC, which are composed of polygonal cancer cells, may be morphologically and radiologically similar to HCC[10]. Both DPHCC and ICC can express biliary markers such as cytokeratin 19 (CK19), making imaging and pathology differentiation difficult. However, the two differ considerably in terms of pathogenesis, treatment methods, and prognosis[11]. For patients with DPHCC, due to the characteristics of cancer cells metastasizing mainly along blood vessels, anatomic liver resection is usually recommended, and prophylactic lymph node dissection is not recommended unless the imaging suggests. In contrast, there is no special requirement for the resection method for ICC patients, but intraoperative lymph node dissection should be routinely performed[12-14]. DPHCC is highly resistant to radiotherapy and chemotherapy, so combined targeted therapy is often employed postoperatively[15], while chemotherapy is the preferred adjuvant treatment for ICC postoperatively[16]. Misdiagnosis between the two can delay treatment and adversely affect patient outcomes.
Currently, radiomics is widely used in the differentiation, clinical staging, and prognosis assessment of various diseases[17-19]. This study aimed to investigate the value of radiomics in the differential diagnostic of DPHCC and ICC.
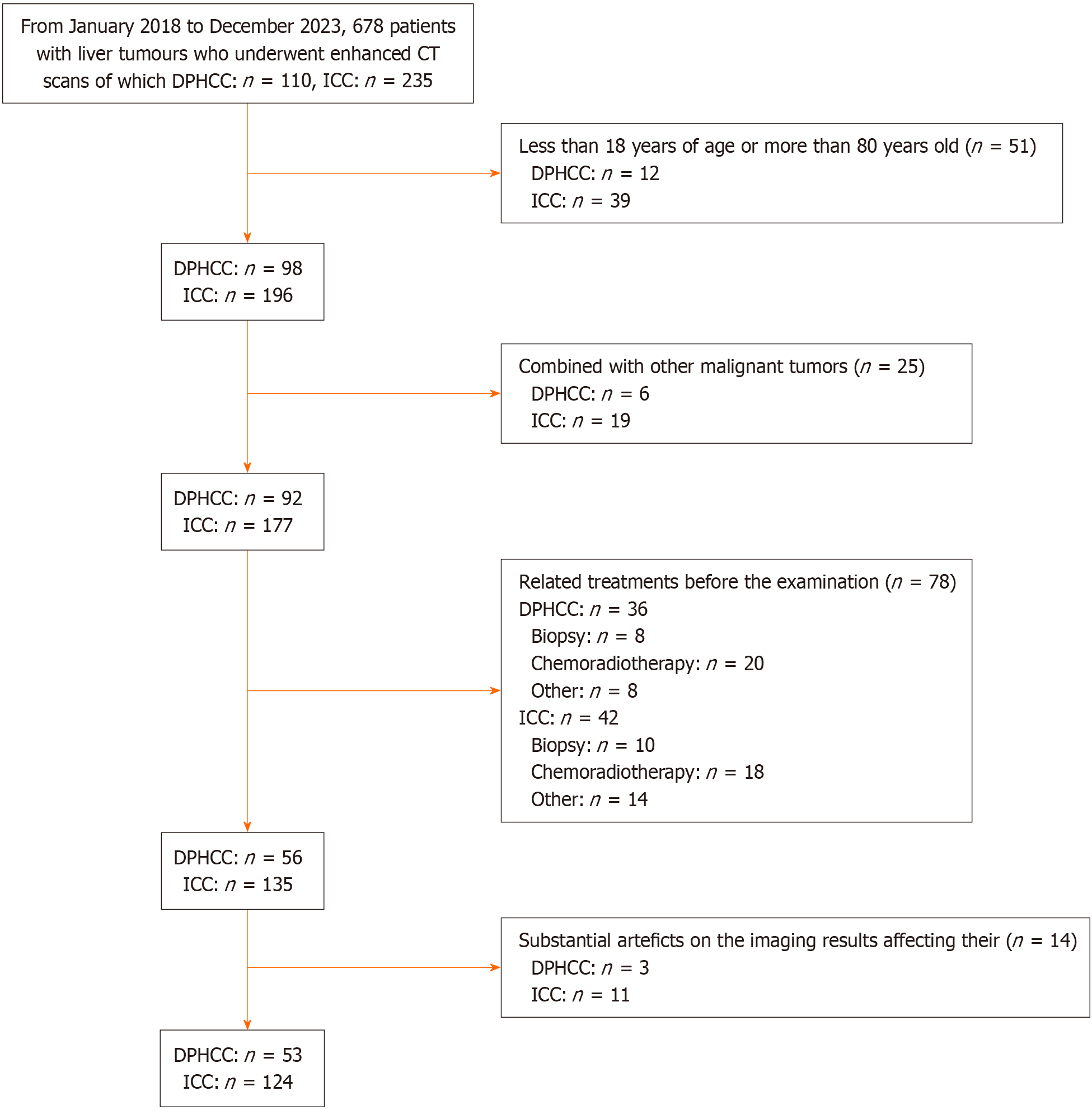
The clinical and imaging data of 678 patients with liver tumors who underwent computed tomography (CT) scans with contrast from January 2018 to December 2023 in Nanfang Hospital of Southern Medical University were retrospectively collected, of which 110 DPHCC and 235 ICC patients were obtained, and 53 DPHCC and 124 ICC patients were finally included in the study.
The inclusion criteria were as follows: (1) Age 18 to 80 years; (2) Liver tumour diameter ≥ 1 cm; (3) Previously conducted enhanced CT examination and complete clinical and imaging records; and (4) Postoperative pathologic confirmation of DPHCC or ICC following surgical resection of a liver tumour (Figure 1).
The exclusion criteria were as follows: (1) Other malignant tumours; (2) Related treatments such as biopsy or chemoradiotherapy before the examination; and (3) Substantial artefacts on the imaging results affecting their analysis.
This was a retrospective study approved by the Ethics Committee of Nanfang Hospital of Southern Medical University. Because the data were analyzed retrospectively and anonymously, the need to obtain informed consent from the patients was waived.
A Siemens Somatom Definition 64-slice CT scanner was used to scan the upper abdomen (from the top of the liver to the bottom of the kidneys). Images in the arterial and venous phases were obtained 35-40 seconds and 65-70 seconds after contrast agent injection, respectively, with a reconstruction slice thickness of 5 mm, slice spacing of 5 mm, matrix of 512 × 512, tube voltage of 120 kV, tube current of 80 mAs, and rotation time of 0.5 s/0.8 s. A 1.0-1.5 mL/kg dose of Ultravist 370 contrast agent was injected into a peripheral vein at a flow rate of 2.0-3.0 mL/s.
Two radiologists specializing in abdominal imaging diagnosis (Doctor A, with 5 years of diagnostic experience, and Doctor B, with 10 years of diagnostic experience) analysed all CT images on the Picture Archiving and Communication System (PACS) of the institution without knowledge of the histopathological results of the patients. In cases of disagreement, they discussed their findings and reached a consensus with another radiologist (Doctor C, with more than 15 years of experience).
The whole tumour was segmented by Doctor A using Insight Toolkit - Segmentation and Registration Application (ITK-SNAP) software (http://www.itksnap.org) by delineating regions of interest (ROI) slice by slice along the lesion edge on the arterial and venous phase images, and the results were verified by Doctor C; any disagreements were resolved through consensus. To examine reproducibility, after a 4-week washout period, Doctor A randomly selected 25 patients from each of the two groups to repeat segmentation of their tumours. An intraclass correlation coefficient of ≥ 0.8 was considered to be consistent. The data of all the patients were divided into training and testing sets in a 7:3 ratio. A prediction model was constructed using the random forest algorithm, whose parameters were selected using 10-fold cross-validation with a grid search. The testing set data were used to evaluate the predictive performance of the model. In the training set, 2818 features were extracted from the ROIs using open-source software including Python 3.7, PyRadiomics 3.0.1, and Sklearn 0.21.4. Features with an intraclass correlation coefficient of ≥ 0.8 and a feature variance of ≥ 0.1 were retained. Finally, feature dimensionality reduction was conducted by screening the features using the least absolute shrinkage and selection operator (LASSO). The screened features were included into the random forest model to construct a clinical-sign model, a radiomic model, and a model combining the two.
Doctor D (with 10 years of experience in abdominal imaging diagnosis) and Doctor E (with 5 years of experience in abdominal imaging diagnosis) performed image analysis independently and with model assistance: (1) Independent analysis method: Without knowledge of the patient’s clinical history, imaging reports, or pathological results, the radiologist acquired the patient’s abdominal CT images from the PACS system for diagnosis and recorded the diagnostic results; and (2) Model-aided analysis method: After a two-week washout period, given the probabilities of DPHCC and ICC provided by the best model, the radiologist made the final diagnosis based on their experience combined with the model output and recorded the diagnostic results.
Two experienced pathologists (both of whom have been engaged in pathological diagnosis of liver cancer for 20 years) collected the postoperative tumor tissues, performed HE staining and immunohistochemistry, and classified and diagnosed DPHCC and ICC according to the 2019 version of the World Health Organization classification of digestive tumors[20]. Any disagreement was addressed through discussion to achieve consensus.
SPSS 26.0 and R 4.0.3 were used for statistical analyses. Measurement data were tested for normality using the Kolmogorov-Smirnov test. Data following a normal distribution are described as the mean ± SD, and between-group comparisons were conducted by the t-test. Data not conforming to a normal distribution are expressed as the median (P25-P75), and between-group comparisons were conducted using the nonparametric Mann–Whitey U test. Categorical data are presented as percentages, and between-group comparisons were conducted with using the c2 test. The receiver operating characteristic curve of each model was plotted, and the area under the curve (AUC) was calculated to evaluate the predictive performance of the models. The DeLong test was used to compare the AUC values of the prediction models. For all statistical tests above, P < 0.05 indicated statistical significance. The calibration performance of the best model was evaluated by generating a calibration curve, and the clinical utility of the best model was assessed by decision curve analysis.
In the training set, age, total bilirubin, hepatitis B virus infection, tumour margin, the presence of cholangiectasis, nonrim arterial phase hyperenhancement, nonperipheral washout, the halo sign, and the degree of peritumoral enhancement were significantly different between the two groups of patients (P < 0.05). In the testing set, there were statistically significant differences in α-fetoprotein (AFP) level, tumour margin, and the degree of peritumoral enhancement between the two groups (P < 0.05). There were no significant differences in any of the patient characteristics between the training set and the testing set (P > 0.05; Table 1). The AFP level, the presence of cirrhosis, and the degree of peritumoral enhancement were independent risk factors for differentiating DPHCC from ICC patients (Table 2).
| Variable | Training set | Testing set | P value | ||
| t/z or χ2 | P value | t/z or χ2 | P value | ||
| Sex | 0.724 | 0.395 | 0.047 | 0.828 | 0.592 |
| Age | -2.628 | 0.010 | -0.197 | 0.845 | 0.676 |
| TBIL | 0.458 | 0.647 | 0.066 | 0.947 | 0.500 |
| ALT | 0.409 | 0.683 | -0.104 | 0.917 | 0.322 |
| AST | -2.637 | 0.008 | -0.717 | 0.474 | 0.254 |
| ALB | -1.013 | 0.313 | -0.913 | 0.366 | 0.763 |
| AFP | 26.78 | 0.000 | 16.544 | 0.000 | 0.758 |
| PLT | -0.677 | 0.499 | -0.405 | 0.685 | 0.083 |
| HBV | 12.510 | 0.000 | 2.422 | 0.120 | 0.480 |
| Cirrhosis | 19.279 | 0.000 | 3.878 | 0.049 | 0.270 |
| MTD | 0.652 | 0.515 | 0.038 | 0.970 | 0.750 |
| Margin | 7.978 | 0.005 | 0.007 | 0.931 | 0.190 |
| Shape | 0.862 | 0.353 | 4.561 | 0.033 | 0.128 |
| Dense | 0.547 | 0.459 | 0.476 | 0.490 | 0.089 |
| Cholangiectasis | 5.872 | 0.015 | 0.158 | 0.691 | 0.592 |
| Artery within tumor | 0.250 | 0.617 | 0.011 | 0.918 | 0.376 |
| Nonrim APHE | 8.801 | 0.003 | 2.422 | 0.119 | 0.311 |
| Nonperipheral washout | 21.524 | 0.000 | 2.354 | 0.125 | 0.705 |
| Halo sign | 1.787 | 0.181 | 2.191 | 0.139 | 0.612 |
| Capsule | 2.433 | 0.296 | 1.886 | 0.389 | 0.879 |
| Cystoid variation and necrosis | 0.358 | 0.549 | 3.362 | 0.067 | 0.793 |
| Peritumoral enhancement | 8.947 | 0.003 | 9.477 | 0.002 | 0.730 |
| Variable | b value | OR value | 95%CI | P value |
| Sex | -0.018 | 0.917 | 0.954-1.003 | 0.587 |
| Age | 0.008 | 1.008 | 0.977-1.041 | 0.601 |
| TBIL | -0.002 | 0.998 | 0.992-1.000 | 1.351 |
| ALT | 1.358 | 3.889 | 0.629-24.049 | 0.144 |
| AST | 0.936 | 2.55 | 1.949-3.335 | 2.934 |
| ALB | -0.671 | 0.511 | 0.108-2.413 | 0.397 |
| AFP | 1.076 | 2.933 | 1.225-7.021 | 0.016 |
| PLT | 0.388 | 1.474 | 0.423-5.142 | 0.542 |
| HBV | -0.988 | 0.372 | 0.089-1.561 | 0.177 |
| Cirrhosis | 0.103 | 1.109 | 1.062-1.157 | 0.000 |
| MTD | 0.001 | 1.001 | 0.977-1.024 | 0.06 |
| Margin | -0.988 | 0.372 | 0.089-1.561 | 0.177 |
| Shape | 0.996 | 2.707 | 0.830-8.828 | 2.707 |
| Dense | -1.406 | 0.245 | 0.042-1.428 | 0.245 |
| Cholangiectasis | 0.985 | 2.678 | 0.997-7.191 | 0.051 |
| Artery within tumor | 1.421 | 4.137 | 0.924-18.503 | 0.064 |
| Nonrim APHE | 0.081 | 1.084 | 0.314-3.741 | 0.898 |
| Nonperipheral washout | 0.598 | 1.765 | 0.785-3.965 | 0.068 |
| Halo sign | -0.172 | 0.73 | 0.39-1.49 | 0.267 |
| Capsule | 0.228 | 1.07 | 0.29-1.84 | 0.098 |
| Cystoid variation and necrosis | 0.073 | 1.076 | 0.238-4.864 | 0.924 |
| Peritumoral enhancement | 0.097 | 1.102 | 1.033-1.176 | 0.003 |
Among the 2818 features extracted in the training set, 2154 radiomic features had good consistency (intraclass correlation coefficient ≥ 0.80). After features with a variance ≥ 0.1 were retained, the remaining 984 features were screened by LASSO regression with 10-fold cross-validation. Eventually, 28 radiomic features were selected to develop models.
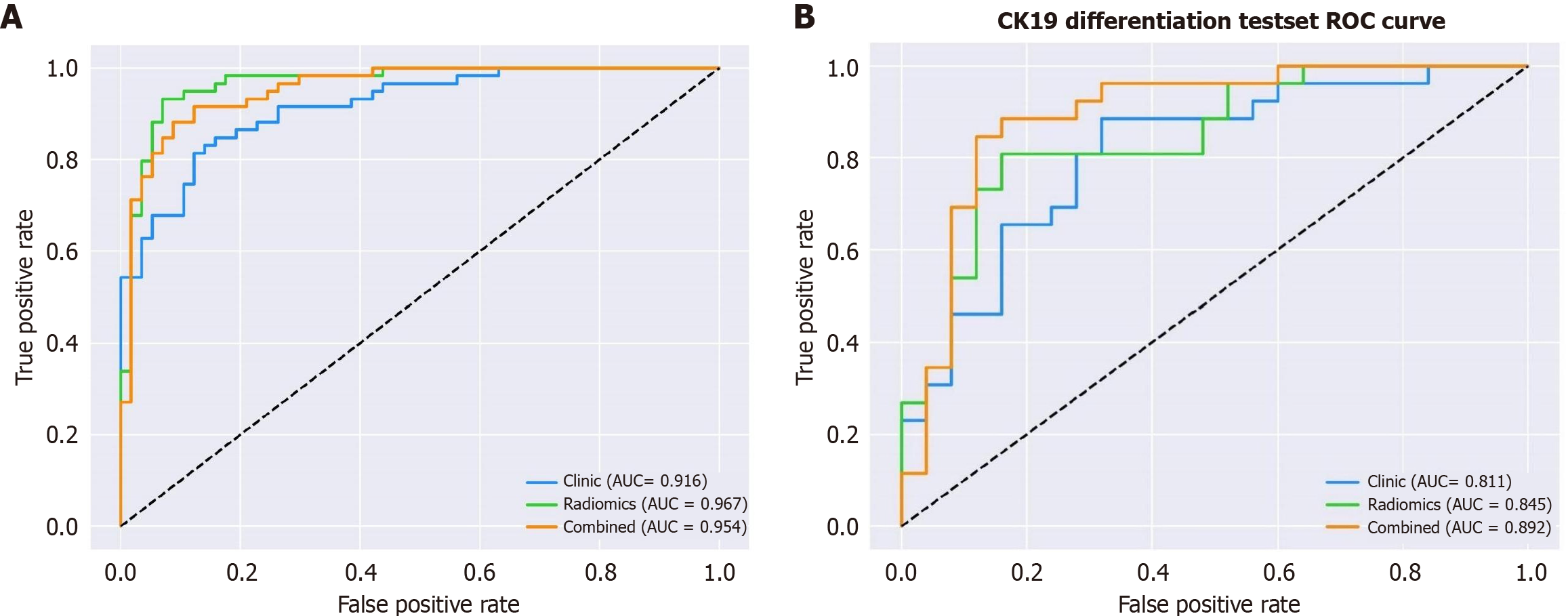
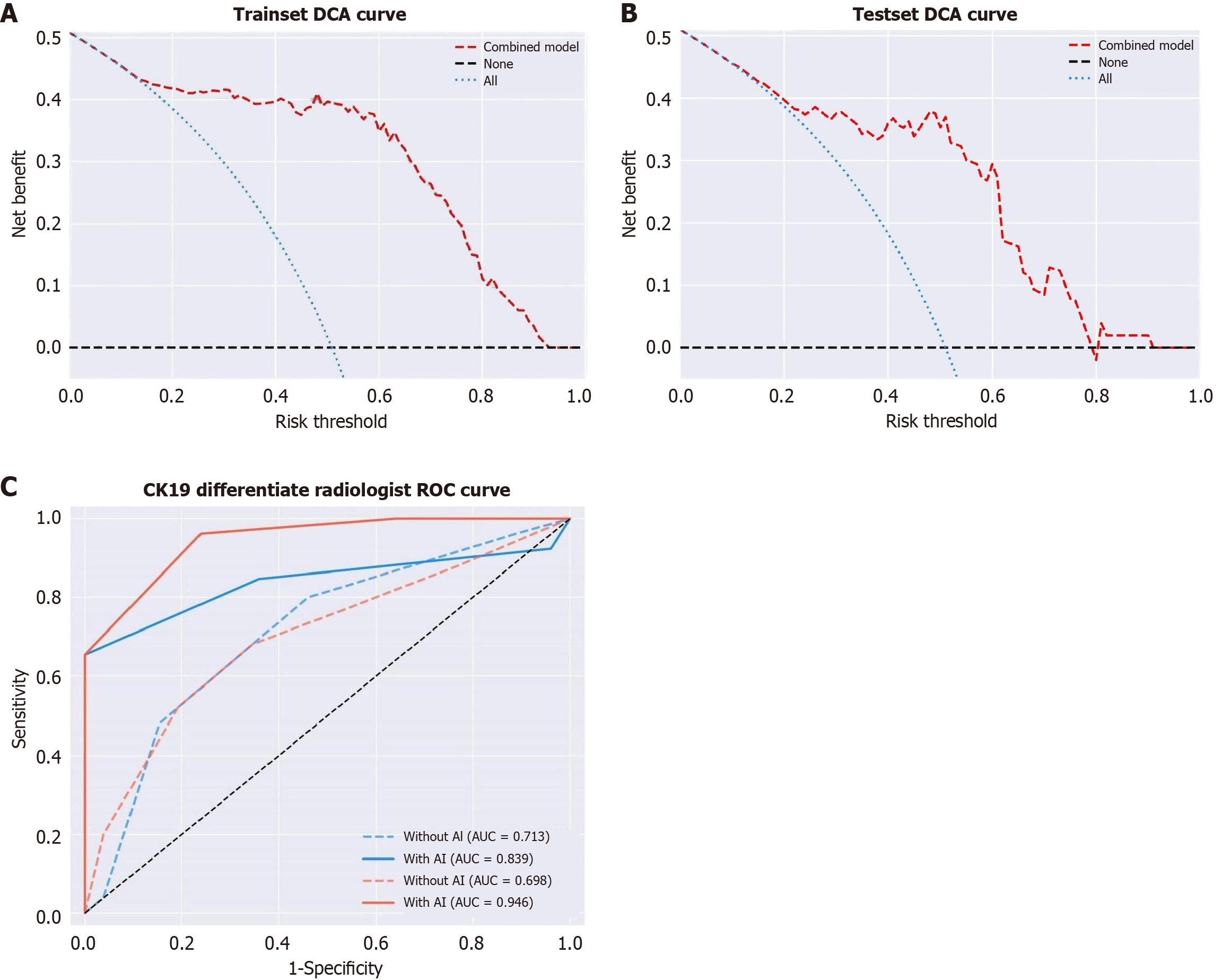
The AUC, sensitivity, specificity, and accuracy of the three models are shown in Table 3 and Figure 2. In the test set, the AUC values of the clinical-sign model, the radiomics model, and the combined model were 0.811 (95% confidence interval [CI]: 0.677-0.907), 0.845 (95%CI: 0.716-0.931), and 0.892 (95%CI: 0.774-0.962), respectively, and the differences between the three models were statistically significant (P < 0.05). In the testing set, the differences between the combined model and both the clinical-sign model and the radiomic model were statistically significant (P < 0.05). The combined model demonstrated net clinical benefits in differentiating DPHCC and ICC at threshold probabilities of > 15% in the training set and > 20% in the testing set, and the model predictions showed a good fit with the actual observed values (Figure 3A and B).
| Variables | Clinical-sign model | Radiomic model | Combined model | |
| Training set | AUC | 0.916 | 0.967 | 0.954 |
| 95%CI | 0.850-0.960 | 0.916-0.991 | 0.898-0.984 | |
| Sensitivity | 0.831 | 0.949 | 0.864 | |
| 95%CI | 0.735-0.926 | 0.893-1.000 | 0.777-0.952 | |
| Specificity | 0.842 | 0.842 | 0.912 | |
| 95%CI | 0.747-0.937 | 0.747-0.937 | 0.839-0.986 | |
| Accuracy | 0.836 | 0.897 | 0.888 | |
| 95%CI | 0.834-0.839 | 0.895-0.898 | 0.886-0.890 | |
| Testing set | AUC | 0.811 | 0.845 | 0.892 |
| 95%CI | 0.677-0.907 | 0.716-0.931 | 0.774-0.962 | |
| Sensitivity | 0.731 | 0.808 | 0.846 | |
| 95%CI | 0.560-0.901 | 0.656-0.959 | 0.707-0.985 | |
| Specificity | 0.720 | 0.840 | 0.840 | |
| 95%CI | 0.544-0.896 | 0.696-0.984 | 0.696-0.984 | |
| Accuracy | 0.726 | 0.824 | 0.843 | |
| 95%CI | 0.718-0.733 | 0.818-0.829 | 0.838-0.846 |
With model assistance, the AUC value of Doctor D increased from 0.713 (95%CI: 0.569-0.831) to 0.839 (95%CI: 0.710-0.927), while that of Doctor E increased from 0.698 (95%CI: 0.554-0.819) to 0.946 (95%CI: 0.844-0.990); the increase in the AUC value following model assistance for Doctor E was significant (P < 0.05; Figure 3C).
In the present study, the clinical and imaging data of 53 DPHCC patients and 124 ICC patients were collected, the ROIs were delineated slice by slice, and relevant information was extracted to construct a clinical-sign model, a radiomic model, and a combined model. Subsequently, the performance of the predictive models was evaluated to explore their clinical applicability.
Previous studies have shown that radiomic models can effectively predict the CK19 status in HCC preoperatively[21], but currently, there is a lack of research on the differentiation of DPHCC from other liver tumours. Most studies have focused on magnetic resonance imaging (MRI) radiomics[22], and when lesions are delineated, only two-dimensional barcode ROIs are delineated at the largest tumour slice, and so the overall heterogeneity of the tumour cannot be adequately assessed[23].
The results of the present study revealed that AFP, liver cirrhosis, and peritumoral enhancement are independent factors for distinguishing ICC from DPHCC. AFP is synthesized mainly in the foetal liver and is important for the diagnosis of HCC[24]. DPHCC cells can turn on HCC housekeeping genes, thus leading to an increase in AFP[25], while ICC predominantly expresses CK19 and is almost AFP-negative[26], making AFP an independent factor for distinguishing the two diseases. Liver cirrhosis is a critical step in the development of HCC[27]. DPHCC is composed of liver cancer cells, so it is prone to developing into liver cirrhosis. Finally, it is generally believed that peritumoral enhancement in the arterial phase is caused by tumour thrombi generated by microvascular invasion blocking the portal vein branches around the tumour, resulting in compensatory arterial collaterals[28]. DPHCC has a stronger microvascular invasion ability and, therefore, is more likely to demonstrate arterial peritumoral enhancement.
The radiomic model yielded high AUC values in both the training and testing sets. The possible reason is that DPHCC originates from liver cells, whereas ICC originates from intrahepatic bile duct cells, resulting in different manifestations of the two lesions. The radiomic model extracts features hidden in the image and invisible to the naked eye to achieve a differential diagnosis. A comparison of the three models revealed that the radiomic model had the highest AUC value in the training set, while the combined model had the highest AUC value in the testing set. However, the difference between the combined model and the radiomic model in the training set was not significant (P > 0.05). We therefore believe that the combined model has better generalizability and diagnostic value.
With the use of model-aided analysis, the AUC values of Doctors D and E both increased, and the difference in the AUC values of Doctor E was significant (P < 0.05). These results indicate that the use of model assistance can improve the diagnostic accuracy of clinicians, especially those with limited diagnostic experience. Regarding the little improvement in the diagnostic accuracy of Doctor D with model assistance, we believe that because Doctor D has a rich knowledge base and clinical diagnostic experience, with an already high diagnostic accuracy, the use of the model resulted in little improvement.
The main limitations of this study are as follows: (1) This study was a retrospective analysis, and there may be potential selection bias; (2) Features were extracted only from the tumour lesions and not from the surrounding environment; (3) The sample was relatively small; and (4) The research data only came from a single medical institution, and the model was not validated with multi-centre data.
Radiomics can effectively differentiate DPHCC and ICC preoperatively. With assistance from the developed model, the accuracy of less experienced doctors in the differential diagnosis of these two diseases can be improved.
| 1. | Zheng QL, Feng CY, Lian YE, Wu L, Yang YH, Xiao H, Chen YX. [Clinical and pathological analysis of 6 cases of diphenotypic hepatocellular carcinoma]. Zhonghua Bing Li Xue Za Zhi. 2020;49:1320-1322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 2. | Chen Y, Chen J, Zhang Y, Lin Z, Wang M, Huang L, Huang M, Tang M, Zhou X, Peng Z, Huang B, Feng ST. Preoperative Prediction of Cytokeratin 19 Expression for Hepatocellular Carcinoma with Deep Learning Radiomics Based on Gadoxetic Acid-Enhanced Magnetic Resonance Imaging. J Hepatocell Carcinoma. 2021;8:795-808. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 22] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 3. | Wu Q, Yu YX, Zhang T, Zhu WJ, Fan YF, Wang XM, Hu CH. Preoperative Diagnosis of Dual-Phenotype Hepatocellular Carcinoma Using Enhanced MRI Radiomics Models. J Magn Reson Imaging. 2023;57:1185-1196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 11] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 4. | Zhang J, Qi YP, Ma N, Lu F, Gong WF, Chen B, Ma L, Zhong JH, Xiang BD, Li LQ. Overexpression of Epcam and CD133 Correlates with Poor Prognosis in Dual-phenotype Hepatocellular Carcinoma. J Cancer. 2020;11:3400-3406. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 5. | Moris D, Palta M, Kim C, Allen PJ, Morse MA, Lidsky ME. Advances in the treatment of intrahepatic cholangiocarcinoma: An overview of the current and future therapeutic landscape for clinicians. CA Cancer J Clin. 2023;73:198-222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 262] [Cited by in RCA: 258] [Article Influence: 129.0] [Reference Citation Analysis (0)] |
| 6. | Rhee H, Nahm JH, Kim H, Choi GH, Yoo JE, Lee HS, Koh MJ, Park YN. Poor outcome of hepatocellular carcinoma with stemness marker under hypoxia: resistance to transarterial chemoembolization. Mod Pathol. 2016;29:1038-1049. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 54] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 7. | Vij M, Puri Y, Rammohan A, G G, Rajalingam R, Kaliamoorthy I, Rela M. Pathological, molecular, and clinical characteristics of cholangiocarcinoma: A comprehensive review. World J Gastrointest Oncol. 2022;14:607-627. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 21] [Cited by in RCA: 22] [Article Influence: 7.3] [Reference Citation Analysis (4)] |
| 8. | Viganò L, Lleo A, Muglia R, Gennaro N, Samà L, Colapietro F, Roncalli M, Aghemo A, Chiti A, Di Tommaso L, Solbiati L, Colombo M, Torzilli G. Intrahepatic cholangiocellular carcinoma with radiological enhancement patterns mimicking hepatocellular carcinoma. Updates Surg. 2020;72:413-421. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 9. | Çelebi F, Yaghouti K, Cindil E, Dogusoy GB, Tokat Y, Balcı C. The Role of 18F-FDG PET/MRI in the Assessment of Primary Intrahepatic Neoplasms. Acad Radiol. 2021;28:189-198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 10. | Komuta M, Govaere O, Vandecaveye V, Akiba J, Van Steenbergen W, Verslype C, Laleman W, Pirenne J, Aerts R, Yano H, Nevens F, Topal B, Roskams T. Histological diversity in cholangiocellular carcinoma reflects the different cholangiocyte phenotypes. Hepatology. 2012;55:1876-1888. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 228] [Cited by in RCA: 250] [Article Influence: 19.2] [Reference Citation Analysis (0)] |
| 11. | Ni T, Shang XS, Wang WT, Hu XX, Zeng MS, Rao SX. Different MR features for differentiation of intrahepatic mass-forming cholangiocarcinoma from hepatocellular carcinoma according to tumor size. Br J Radiol. 2018;91:20180017. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 28] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 12. | Gilligan T, Lin DW, Aggarwal R, Chism D, Cost N, Derweesh IH, Emamekhoo H, Feldman DR, Geynisman DM, Hancock SL, LaGrange C, Levine EG, Longo T, Lowrance W, McGregor B, Monk P, Picus J, Pierorazio P, Rais-Bahrami S, Saylor P, Sircar K, Smith DC, Tzou K, Vaena D, Vaughn D, Yamoah K, Yamzon J, Johnson-Chilla A, Keller J, Pluchino LA. Testicular Cancer, Version 2.2020, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2019;17:1529-1554. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 179] [Article Influence: 35.8] [Reference Citation Analysis (0)] |
| 13. | Benson AB, D'Angelica MI, Abrams T, Abbott DE, Ahmed A, Anaya DA, Anders R, Are C, Bachini M, Binder D, Borad M, Bowlus C, Brown D, Burgoyne A, Castellanos J, Chahal P, Cloyd J, Covey AM, Glazer ES, Hawkins WG, Iyer R, Jacob R, Jennings L, Kelley RK, Kim R, Levine M, Palta M, Park JO, Raman S, Reddy S, Ronnekleiv-Kelly S, Sahai V, Singh G, Stein S, Turk A, Vauthey JN, Venook AP, Yopp A, McMillian N, Schonfeld R, Hochstetler C. NCCN Guidelines® Insights: Biliary Tract Cancers, Version 2.2023. J Natl Compr Canc Netw. 2023;21:694-704. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 95] [Reference Citation Analysis (0)] |
| 14. | European Association for the Study of the Liver. EASL-ILCA Clinical Practice Guidelines on the management of intrahepatic cholangiocarcinoma. J Hepatol. 2023;79:181-208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 126] [Article Influence: 63.0] [Reference Citation Analysis (0)] |
| 15. | Kawai T, Yasuchika K, Ishii T, Katayama H, Yoshitoshi EY, Ogiso S, Kita S, Yasuda K, Fukumitsu K, Mizumoto M, Hatano E, Uemoto S. Keratin 19, a Cancer Stem Cell Marker in Human Hepatocellular Carcinoma. Clin Cancer Res. 2015;21:3081-3091. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 135] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 16. | Shridhar R, Blinn P, Huston J, Meredith KL. Outcomes of adjuvant radiation therapy after chemotherapy in resected extrahepatic cholangiocarcinoma: A National Cancer Database analysis. Cancer. 2023;129:890-900. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 17. | Bathla G, Dhruba DD, Soni N, Liu Y, Larson NB, Kassmeyer BA, Mohan S, Roberts-Wolfe D, Rathore S, Le NH, Zhang H, Sonka M, Priya S. AI-based classification of three common malignant tumors in neuro-oncology: A multi-institutional comparison of machine learning and deep learning methods. J Neuroradiol. 2024;51:258-264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 8] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 18. | Wu Y, Ma Q, Fan L, Wu S, Wang J. An Automated Breast Volume Scanner-Based Intra- and Peritumoral Radiomics Nomogram for the Preoperative Prediction of Expression of Ki-67 in Breast Malignancy. Acad Radiol. 2024;31:93-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 10] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 19. | Qu J, Zhang T, Zhang X, Zhang W, Li Y, Gong Q, Yao L, Lui S. MRI radiomics for predicting intracranial progression in non-small-cell lung cancer patients with brain metastases treated with epidermal growth factor receptor tyrosine kinase inhibitors. Clin Radiol. 2024;79:e582-e591. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 20. | Nagtegaal ID, Odze RD, Klimstra D, Paradis V, Rugge M, Schirmacher P, Washington KM, Carneiro F, Cree IA; WHO Classification of Tumours Editorial Board. The 2019 WHO classification of tumours of the digestive system. Histopathology. 2020;76:182-188. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2554] [Cited by in RCA: 2438] [Article Influence: 487.6] [Reference Citation Analysis (3)] |
| 21. | Hu X, Wang Q, Huang G, He X, Sparrelid E, Brismar TB, Fan Y. Gadoxetic Acid-Enhanced MRI-Based Radiomics Signature: A Potential Imaging Biomarker for Identifying Cytokeratin 19-Positive Hepatocellular Carcinoma. Comput Math Methods Med. 2023;2023:5424204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 22. | Yoon JK, Choi JY, Rhee H, Park YN. MRI features of histologic subtypes of hepatocellular carcinoma: correlation with histologic, genetic, and molecular biologic classification. Eur Radiol. 2022;32:5119-5133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 13] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 23. | Ng F, Kozarski R, Ganeshan B, Goh V. Assessment of tumor heterogeneity by CT texture analysis: can the largest cross-sectional area be used as an alternative to whole tumor analysis? Eur J Radiol. 2013;82:342-348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 245] [Cited by in RCA: 305] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 24. | Tian S, Chen Y, Zhang Y, Xu X. Clinical value of serum AFP and PIVKA-II for diagnosis, treatment and prognosis of hepatocellular carcinoma. J Clin Lab Anal. 2023;37:e24823. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 24] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 25. | Lu XY, Xi T, Lau WY, Dong H, Zhu Z, Shen F, Wu MC, Cong WM. Hepatocellular carcinoma expressing cholangiocyte phenotype is a novel subtype with highly aggressive behavior. Ann Surg Oncol. 2011;18:2210-2217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 49] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 26. | Eschrich J, Kobus Z, Geisel D, Halskov S, Roßner F, Roderburg C, Mohr R, Tacke F. The Diagnostic Approach towards Combined Hepatocellular-Cholangiocarcinoma-State of the Art and Future Perspectives. Cancers (Basel). 2023;15. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 19] [Reference Citation Analysis (0)] |
| 27. | Zhong X, Guan T, Tang D, Li J, Lu B, Cui S, Tang H. Differentiation of small (≤ 3 cm) hepatocellular carcinomas from benign nodules in cirrhotic liver: the added additive value of MRI-based radiomics analysis to LI-RADS version 2018 algorithm. BMC Gastroenterol. 2021;21:155. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 21] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 28. | Lee S, Kim SH, Lee JE, Sinn DH, Park CK. Preoperative gadoxetic acid-enhanced MRI for predicting microvascular invasion in patients with single hepatocellular carcinoma. J Hepatol. 2017;67:526-534. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 205] [Cited by in RCA: 348] [Article Influence: 43.5] [Reference Citation Analysis (0)] |