Published online Mar 28, 2023. doi: 10.4329/wjr.v15.i3.56
Peer-review started: December 18, 2022
First decision: January 31, 2023
Revised: February 12, 2023
Accepted: March 22, 2023
Article in press: March 22, 2023
Published online: March 28, 2023
Processing time: 99 Days and 5.1 Hours
Primary liver cancer is the fourth most common malignancy worldwide, with hepatocellular carcinoma (HCC) comprising up to 90% of cases. Imaging is a staple for surveillance and diagnostic criteria for HCC in current guidelines. Because early diagnosis can impact treatment approaches, utilizing new imaging methods and protocols to aid in differentiation and tumor grading provides a unique opportunity to drastically impact patient prognosis. Within this review manuscript, we provide an overview of imaging modalities used to screen and evaluate HCC. We also briefly discuss emerging uses of new imaging techniques that offer the potential for improving current paradigms for HCC characterization, management, and treatment monitoring.
Core Tip: Successful tumor assessment can be a critical component to patient management and prognosis. The expansion of imaging techniques beyond conventional modalities (e.g. ultrasound, computed tomography, magnetic resonance imaging) provides an opportunity to improve the identification of small or well-differentiated hepatocellular carcinoma tumors, along with the capability to monitor treatment responses to surgery or locoregional therapy.
- Citation: Criss C, Nagar AM, Makary MS. Hepatocellular carcinoma: State of the art diagnostic imaging. World J Radiol 2023; 15(3): 56-68
- URL: https://www.wjgnet.com/1949-8470/full/v15/i3/56.htm
- DOI: https://dx.doi.org/10.4329/wjr.v15.i3.56
Hepatocellular carcinoma (HCC) is the most common liver malignancy, accounting for 90% of liver tumors, and a leading cause of mortality worldwide[1,2]. Global risk factors for HCC include cirrhosis, existing in up to 90% of new cases[3], or patients with long-standing liver infections such as viral hepatitis B and C[4]. East Asia and sub-Saharan Africa account for greater than 80% of cases, and incidence within the United States continues to rise[3,4]. Unfortunately, many patients are diagnosed in the advanced stages of the disease, which emphasizes the importance of early detection and surveillance[5]. Surveillance and early tumor detection using ultrasonography is recommended by the American Association for the study of Liver Diseases (AASLD) for high-risk populations driven by ultrasound-guided imaging. Unlike other malignancies, HCC expresses distinctive characteristics that can be diagnosed based on imaging features alone, without the need for confirmation from tissue sampling[6]. In this review, we provide a summary of diagnostic criteria and imaging modalities used to detect and stage HCC, as well as emerging methods to further assist in the surveillance and characterization of the disease.
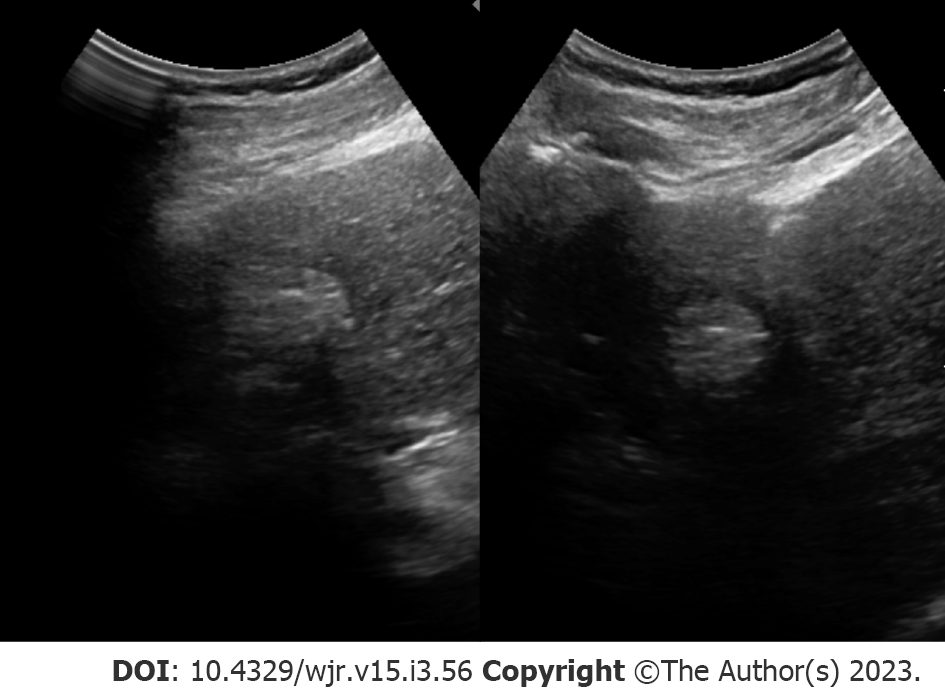
Tumor burden can significantly impact management, where patients with small, localized tumors can receive curative methods such as liver transplantation, resection, or locoregional therapies. On the other hand, treatment options are limited in patients with HCC displaying more aggressive features (e.g., extrahepatic metastases, multifocal tumors, and vascular invasion). As a result, early detection may offer a significant benefit in select patients. Patient populations recommended for HCC surveillance differs between AASLD[7], the European Association for the Study of the Liver (EASL)[8] and the Asian Pacific Association for the Study of the Liver (APASL)[9], but largely consist of adults with cirrhosis or patients with hepatitis B virus (HBV)[10]. Across surveillance recommendations, biannual abdominal ultrasonography (Figure 1) is the standard modality for HCC detection with major advantages including accessibility, cost-effectiveness, and safety[10].
Surveillance programs typically consist of ultrasound examination performed at either 6- or 12-mo intervals. Across all stages of HCC, ultrasound detection carries 84% sensitivity. A number of investigations have found a mortality and cost-benefit of biannual ultrasonography for imaging surveillance of HCC[10-12]. For example, a study in HBV-infected patients in China found a 37% reduction in mortality in those receiving biannual ultrasonography examinations compared to the control group[12]. Suboptimal visualization poses a major limitation for ultrasound screening. Poor visualization and sonographic sensitivity to HCC lesions can be caused by a number of different extrinsic factors such as morbid obesity, patient inability to suspend respiration, obscured portions of the liver by bowel gas or rib shadowing, and intrinsic factors such as hepatic steatosis or fibrosis causing parenchymal heterogenicity[13]. A recent investigation reported approximately 20% of scans were inadequate to exclude liver lesions[14]. Detecting smaller nodules (< 2 cm) appears to be a major limitation of ultrasound, with studies reporting detection rates as low as approximately 28%[15]. In response, the American College of Radiology (ACR) screening guidelines have recommended the use of systematic documentation and scoring for visualization (A: No or minimal limitations; B: Moderate limitations; C: Severe limitations). The use of tumor biomarkers, such as alpha-fetoprotein (AFP), in concert with ultrasound examination appears to have an additive effect on detection rate[16]. For example, a recent meta-analysis of prospective studies found the sensitivity for ultrasound alone to detect any stage of HCC was 78% compared to 97% when adding AFP[17]. Interestingly, the synergistic impact of combining ultrasound with AFP also exists for detection of earlier, smaller nodules (45% vs 63% sensitivity, respectively)[17] which is particularly salient given the limitations of ultrasound and earlier stages of HCC. This is not without controversy, however. The 2018 AASLD guidelines do not designate any preferences between adjunctive use of AFP while the 2017 APASL guidelines recommend the combination of ultrasound and AFP[18]. In contrast, the 2018 EASL guidelines discourage the use of ultrasound with AFP for 6-mo HCC surveillance, citing concerns of false-positives in the setting of active liver inflammation with infection[18].
While the objective of this review is to elucidate the latest advancements in technological imaging for the screening and diagnosis of HCC, it is important to note the efficacy of detection can be limited due to multifactorial screening challenges. In fact, less than 1 in 5 patients with cirrhosis receive surveillance screening for HCC[19]. Previous reviews have extensively examined the numerous challenges encountered during the screening process, including the inability to properly stratify high-risk patients, the presence of socio-economic and logistical impediments to accessing healthcare, as well as training and detection limitations using conventional imaging techniques, as previously discussed. One of the most common attributable factors to surveillance underuse includes lack of surveillance orders or unrecognized cirrhosis[5]. Therefore, strategies to improve education and integrating primary care providers in surveillance efforts can have a drastic and meaningful effect on rates of patients undergoing HCC screening[20]. The implementation of patient-centered outreach programs such as reminder protocols or embedding best-practice advisories within the electronic health record may be solutions to improve barriers of patients undergoing surveillance[5,20]. The decision to select which patients to screen also been discussed and studies have developed scoring systems across different risk factors (e.g. hepatitis or cirrhosis) to refine and improve risk stratification have been proposed[21]. Other methods have also focused on improving surveillance outcomes and detection rates, such as utilizing serological biomarkers (e.g. AFP) either as a single screening modality or in concert with imaging to improve sensitivity, at the potential cost of increased rates of false positivity. The use of biomarkers may also be especially helpful for smaller HCCs, not easily visible with ultrasound[21].
Established by the ACR, standardized methods for imaging interpretation and reporting are defined using the liver imaging reporting and data systems (LI-RADS). The application of the LI-RADS diagnostic algorithm was initially developed for computed tomography (CT)/magnetic resonance imaging (MRI). LI-RADS is subdivided into 8 categories and ranges in greater probability of malignancy from LR-1 to LR-5 with additional categories including LI-RADS M (LR-M) (probably or definitely malignant but not HCC specific), LR-definite tumor in vein, and LR-cannot be categorized (NC)[13,22]. Observation of lesions categorized in LR-5 is designated as almost certainly HCC, with a systematic review of 454 studies reporting 94% of LR-5 lesions confirmed to be HCC and 97% malignant[23]. The Organ Procurement and Transplantation Network (OPTN) is another diagnostic criterion established by National Organ Transplant Act, with subcategories ranging from class 0-5. Specificities are similar between LI-RADSv2018 LR-5 and OPTN class 5[24]. However, inter-reader agreement and sensitivity of LI-RADS (sensitivity: 63.9%) is higher than that of OPTN (sensitivity: 53.6%)[24].
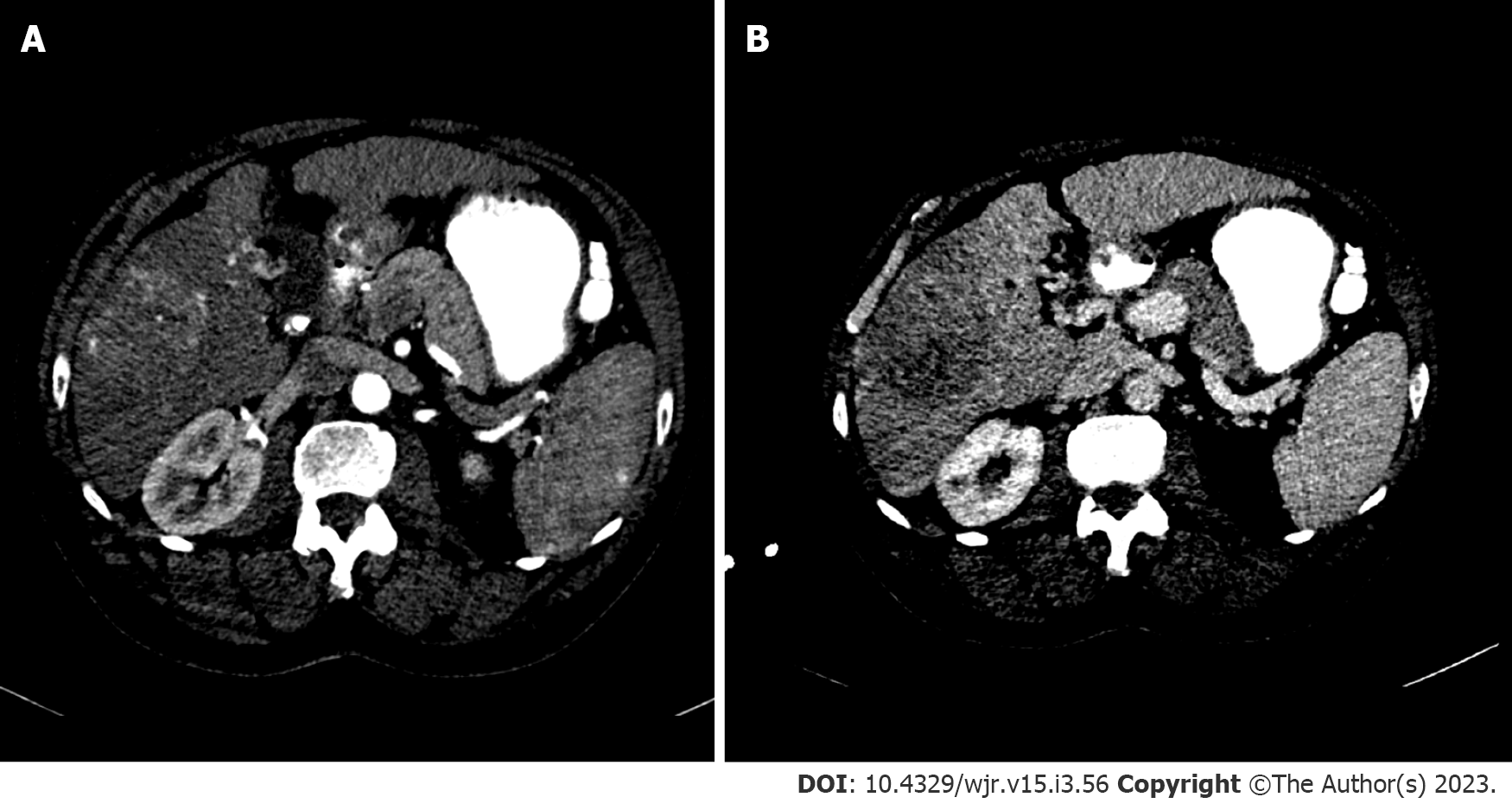
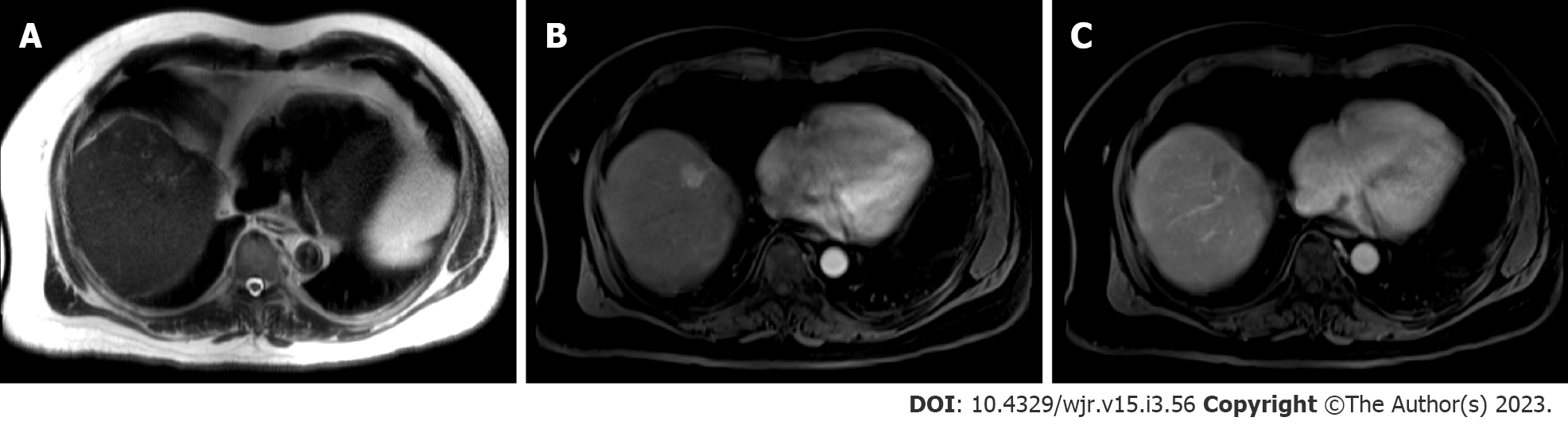
Standard recommendations for HCC diagnosis include multiphase CT or MRI which are beneficial modalities for highlighting unique features of HCC (Figures 2 and 3). Physiological differences in blood perfusion between hepatocarcinogenic lesions and non-neoplastic tissue display distinguishing differences in imaging characteristics using multiphasic contrast examinations[25]. Phases consist of late hepatic arterial (20-40 s), portal venous (60-90 s), and delayed (3-5 min). Late arterial phase is useful for detecting hypervascular lesions with HCC lesions characteristically enhancing relative to surrounding liver parenchyma. Arterial lesion enhancement can be appreciated within lesions as small as 1 cm. Within the portal venous and delayed phases, washout or hypointensity is commonly observed for HCC lesions[26]. During the delayed or equilibrium phase, other characteristics of HCC such as capsule features (e.g. lesion washout with pseudocapsule enhancement) and mosaic architecture can be visualized[26]. The introduction of gadolinium-based contrast agents (Gadobenate dimeglumine and gadoxetate acid) may aid LI-RADS categorization. These agents are taken up by hepatocytes of normal liver parenchyma and there is little uptake in non-functioning or dysfunctional hepatocytes, such as the case for HCC. Gadolinium-based agents function similarly to extracellular agents, but can aid in the diagnosis of lesions with atypical features (e.g. without washout, arterial hyperenhancement) or distinguish HCC from pseudolesions[27,28]. For example, these agents permit an additional post-contrast hepatobiliary phase, which will display a majority of HCC lesions (90%-95%) as hypointense relative to surrounding hyperintense liver parenchyma[27,29].
MRI is recommended for staging of HCC disease given that some reports have estimated CT to underestimate 52% of cases[1,2]. MRI also has superior diagnostic efficiency to CT in the detection of small (≤ 3cm) lesions[30]. However, CT is more readily available than MRI, and limitations to using MRI including greater costs and technical complexity make CT a complementary diagnostic alternative[31,32]. A report showed that the combined use of CT/MRI provides better diagnostic accuracy in characterizing liver lesions using LI-RADS (91.29%) than MRI (85.37%) or CT (67.6%) alone, but combined protocols should be limited to difficult or uncertain cases in order to warrant use[32].
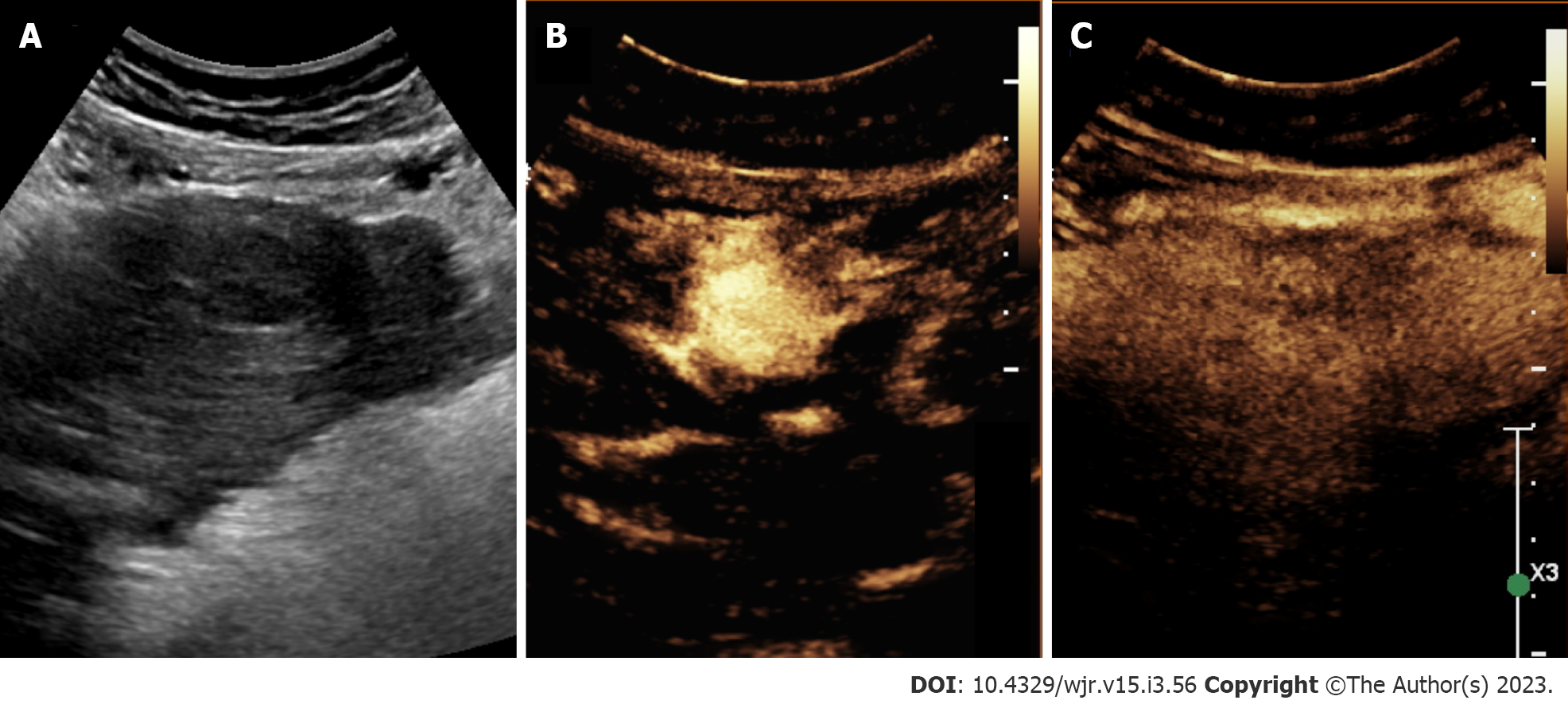
In recent years, there has been an emerging use of contrast-enhanced ultrasound (CEUS) for the evaluation of focal liver lesions (Figure 4). CEUS combines the benefit of accessible, non-invasive assessment without ionizing radiation as well as improvements in temporal resolution. Given some of the limitations in ultrasound sensitivity, CEUS may offer a useful solution. CEUS utilizes highly echogenic microbubble contrast agents (such as SonoVue®, Definity®) which are rapidly injected via the antecubital vein. These agents circulate freely among capillary beds, and the use of dynamic phases of contrast enhancement [e.g. arterial (start 10-20 s), portal (start 30-45 s), late (start > 120 s) phases] can help differentiate liver lesions[33].
The reporting system was initially developed for CT and MRI; however, in 2016, CEUS-LIRADS was released to improve standardization in reporting and interpretation specific to CEUS for nodule evaluation[33]. CEUS LI-RADS consists of 8 categories and ranges in greater levels of severity from LR-1 to LR-5, with additional categories such as LR-M (probably or definitely malignant but not HCC specific), LR-TIV, and LR-NC[33]. Diagnostic features of CEUS LI-RADS are based on arterial phase hyperenhancement and washout, or reductions in enhancement relative to the liver[33]. Features specific to HCC include arterial phase hypervascularity and late or low washout[34,35]. HCC displays earlier levels of enhancement compared to native liver tissue, and detection rates are greater for larger lesions (2-3 cm) compared to smaller ones (≤ 1 cm)[36]. Within nodules approximately 2 cm or greater, detection rates for ultrasound approach that of CT or MRI. For example, Gaiani et al[37] reported a detection rate of 91%-97.3% for evaluating hypervascularity in 103 cirrhotic nodules > 2 cm using CEUS[37]. Limitations of CEUS are like that of conventional ultrasound. Disadvantages of CEUS include user-dependent accuracy, the requirement for multiple contrast injections to survey or investigate separate liver lesions, and restricted ability to distinguish HCC from cholangiocarcinoma and stage disease[34,38].
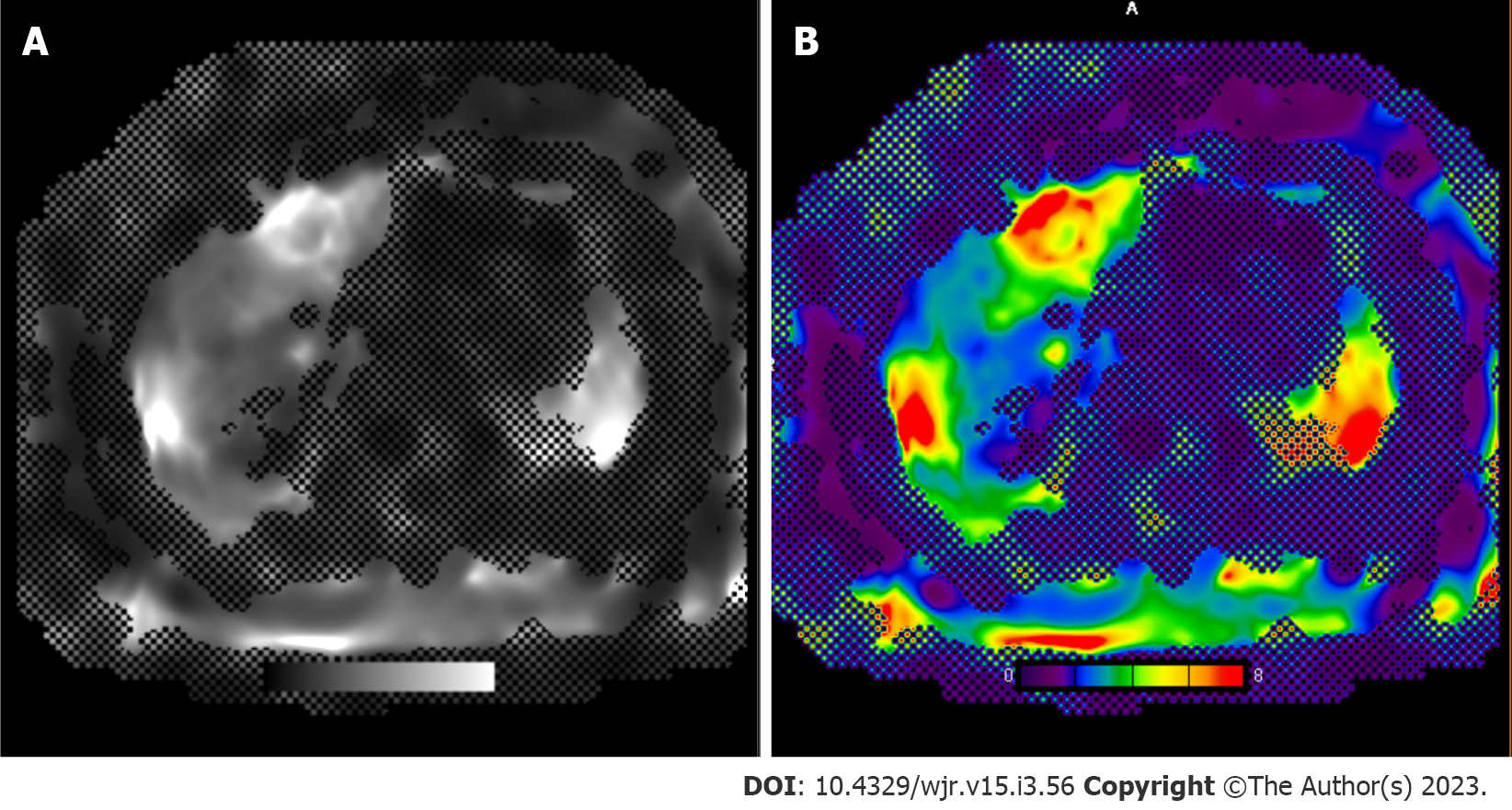
The acquisition of sequential imaging combined with IV contrast administration by multidetector can permit the assessment of tissue perfusion. The distribution of contrast media between the intravascular and interstitial compartments depends on the extent of blood flow and capillary permeability. Using kinetic modules, perfusion parameters across liver tissue can be calculated (blood flow, volume, permeability, hepatic arterial perfusion, portal venous perfusion, perfusion index, slope of increase/decrease). Quantitative color-graded perfusion maps can be used to localize lesions with abnormal tissue perfusion with a high degree of spatial resolution[39-42]. Tumor angiogenesis mediates differences in blood supply between normal liver parenchyma and HCC[42]. Quantitative parameters from CT perfusion, such as hepatic perfusion index, carries high sensitivity and specificity (≥ 99%) for HCC detection in patients with cirrhosis[43]. Early HCC lesions or hepatocellular nodules will demonstrate increased arterial supply as hepatocarcinogenesis progresses, reflected in increased hepatic arterial perfusion and perfusion indices[40]. Other common focal liver lesions can exhibit unique CT perfusion behavior and therefore be used to identify HCC from hemangiomas, liver metastases, and arterioportal shunts[44]. Bai et al[45] reported histopathological features such as microvascular density, a poor prognostic factor for HCC, is correlated with multiple perfusion parameters[45]. A major prognostic factor for HCC, microvascular density has been shown to be correlated with multiple CT perfusion parameters.
Similar to CT perfusion, dynamic contrast-enhanced MRI (DCE-MRI) permits the quantification of perfusion characteristics of liver lesions[46]. This approach is accomplished using gadolinium IV contrast administration followed by image acquisition with high temporal resolution and kinetic modules to quantify contrast distribution perfusion to reflect focal perfusion differences[47]. DCE-MRI perfusion parameters have highlighted unique physiological characteristics in HCC lesions, including increased arterial hepatic blood flow, arterial fraction, and lower portal hepatic blood flow, compared to normal liver parenchyma[46]. Pahwa et al[48] reported, arterial fraction and distribution volume are high in HCC and metastatic lesions compared to normal liver parenchyma[48]. Metastatic lesions may also be distinguished from HCC and normal liver parenchyma by the perfusion parameter mean transit time[48]. By evaluating tumor vascularity pre- and post-treatment, DCE-MRI has shown efficacy for treatment monitoring (e.g. following administration of anti-angiogenic agents, transarterial chemoembolization or radiotherapy) and predicting survival outcomes for HCC patients[49].
Elastography is an imaging method to quantify mechanical properties, notably stiffness, to evaluate focal fibrotic cirrhotic changes (Figure 5). Either MRI or ultrasound, coupled with a device that generates low frequency vibrations (i.e. shear waves) and wave propagation, can be quantified in order to calculate levels of stiffness in a focal area of interest[50]. First introduced with ultrasound, there are multiple elastography methods which include transient elastography, point shear wave, two-dimensional sheer wave, and quasi-static elastography. Ultrasound elastography has shown to provide satisfactory sensitivity and specificity for identifying histological stages of severe fibrosis (sensitivity: 81.9%, specificity: 84.7%) and cirrhosis (sensitivity: 84.8%, specificity 87.5%)[51]. MRI elastography may also aid to differentiate focal liver lesions. For example, malignant tumors have been reported to have greater levels of stiffness relative to benign lesions, focal fibrotic regions, and normal liver parenchyma[52].
Evaluation of liver stiffness may also offer a prognostic biomarker for determining risk of HCC development and survival. A meta-analysis of 9 studies by Singh et al[53] reported increased liver stiffness is associated with an elevated risk of HCC[53]. A more recent metanalysis of 1735 patients reported a varied sensitivity (31%-100%) and high specificity (81%-94%) for predicting HCC development. The use of MR elastography can also be used as a biomarker to predict treatment response and tumor recurrence[54-56]. A prospective investigation assessed 192 patients undergoing HCC treatment (e.g. transarterial chemboembolization, ablation, or resection) found liver parenchymal stiffness to be an independent predictor of early recurrence[54]. A recent investigation also reported efficacy for predicting both early and late recurrence in 180 patients with HBV-related HCC prior to undergoing hepatectomy[56]. Qayyum et al[57] reported the use of MR elastography to evaluate stiffness changes in patients treated with immunotherapy (i.e. Pembrolizumab). HCC tumor stiffness significantly correlated with survival outcomes, including overall survival and time to disease progression, as well as intratumoral T-lymphocyte abundance[57].
Similarly, T1 mapping is an MR method by which T1 relaxation time is measured and can be useful for identifying liver fibrosis. In the setting of inflammation and fibrosis, T1 relaxation time will be increased and has been used extensively to evaluate myocardial edema and scarring[58]. In fact, there is a moderate correlation between T1 relaxation time and elastography-measured stiffness[59]. T1 relaxation time can also be measured before and after administering hepatobiliary contrast agents such as GD-EOB-DTPA (Eovist®) to provide a more reliable, quantitative evaluation of contrast media uptake within the liver parenchyma[60]. Given that T1 relaxation is influenced by intrinsic properties of tissue, T1 mapping can overcome some of the traditional limitations of conventional MR signal intensity, which can be influenced by technical factors and imaging parameters. Combining T1 mapping and GD-EOB-DTPA has shown to be useful for identifying and classifying differentiated lesions (e.g. reduced uptake associated with increased HCC grade), and can be used to distinguish HCC from other focal liver lesions, including hepatic cysts, focal nodular hyperplasia, and hemangiomas[61-63]. HCCs with microvascular invasion have also shown reductions in T1 relaxation relative to lesions without evidence of microvascular invasion[64], showing promise of another imaging method for predicting prognosis.
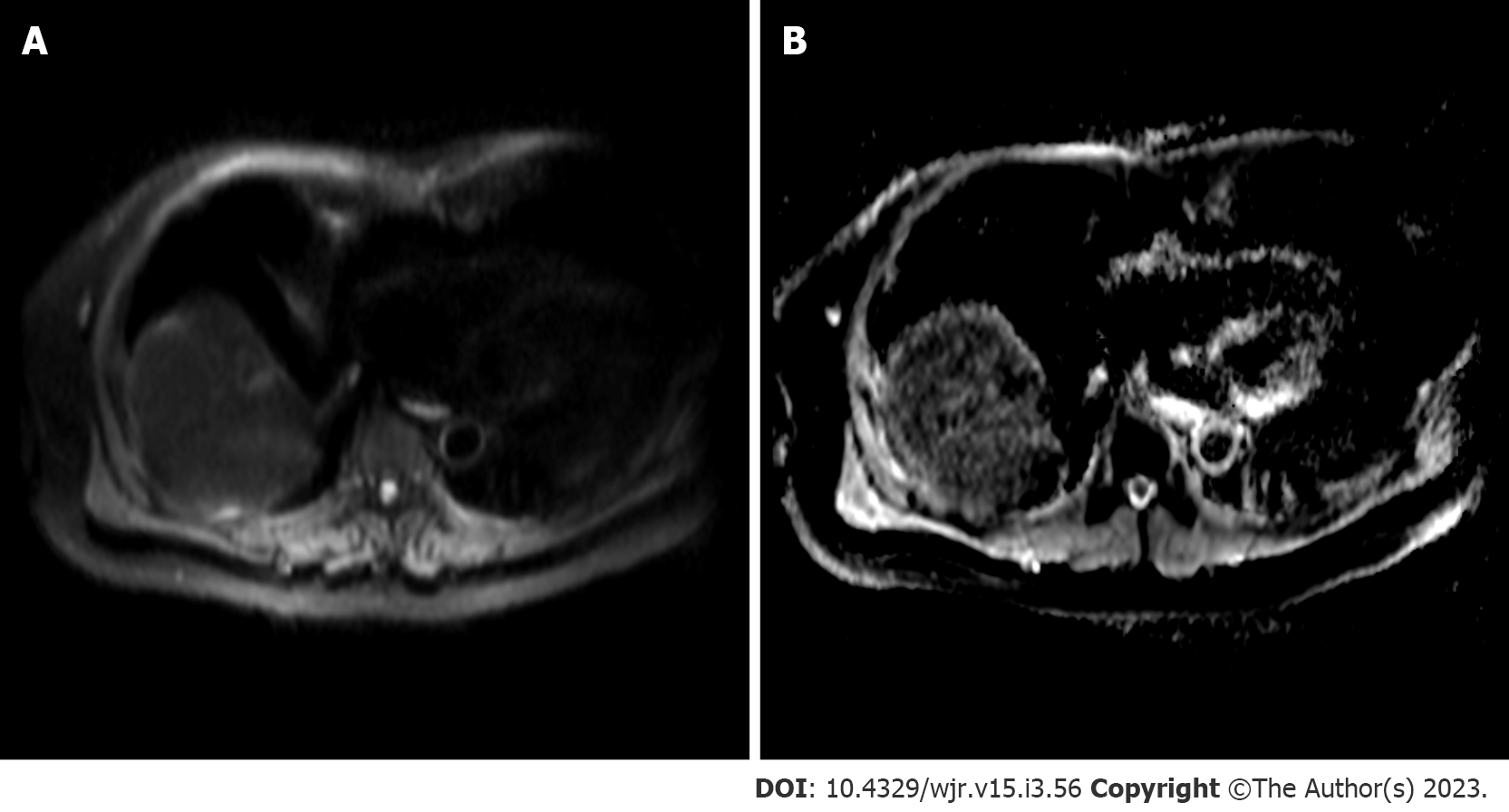
Diffusion weighted imaging (DWI) is a non-invasive MR sequence which can characterize focal liver lesions without the need for contrast media by measuring diffusion properties of water molecules within tissues (Figure 6). Gradations of diffusion are measured using b-values, with greater values denoting more sensitivity to diffusion and higher signal intensity. These values are used to calculate and generate apparent diffusion coefficient (ADC) maps, used clinically to assess local changes in liver tissue diffusion[65]. Changes in cellularity, cell architecture and extracellular space, combined with necrosis and vascularization, can restrict diffusion and thus, HCC will appear hyperintense on DWI[65,66]. DWI has exhibited exceptionally high sensitivity and specificity for a single HCC lesion (100%) and moderate sensitivity and high specificity for multiple lesions (75% and 100%, respectively)[67]. The detection of early, smaller HCC (≤ 2 cm) is a clinically useful feature of DWI, especially when combined with contrast use. Generally, poorly differentiated lesions will exhibit lower ADC compared to well-differentiated lesions[66]. A meta-analysis of 21 studies (1799 HCC lesions) by Surov et al[68] reported that DWI can provide grading and prognostic utility by demonstrating use of ADC values can predict tumor grade and microvascular invasiveness (specifically, minimum ADC values)[68]. A recent study in 81 patients with HCC also reported microvascular invasiveness to be associated with ADC with a receiver operating characteristic curve AUC values ranging from 0.860-0.909[69]. DWI has been used as a biomarker for monitoring and predicting tumor response following locoregional therapy[70,71]. ADC correlates with tumor response, according to mRECIST, 6 mo after TACE[70]. ADC values of 1.84 × 10−3 mm2/s10 have been reported to have high sensitivity (92.3%) and specificity values (100%) for identifying evidence of necrosis following TACE[71]. For Y90-radioembolization, increases in ADC value (> 30%) can predict objective mRECIST response with 90% sensitivity and 100% specificity. Further, a > 30% change in ADC following TACE is associated with prolonged overall survival[72]. A prospective investigation of 40 patients treated with radiofrequency ablation found an ADC value of 1.01 × 10−3 mm2/s yields the highest sensitivity (80%) and specificity (100%) for detecting residual HCCs 3 mo following treatment[73]. However, differentiation between benign and malignant lesions are difficult in the setting of cirrhosis, as the ADC between both lesion types exhibit considerable overlap[65]. Further, standardization for DWI sequences may be needed since different study protocols can alter ADC calculation[74].
MR spectroscopy is an analytical technique that permits the characterization and quantification of tissue metabolite composition in vivo. For each given voxel, a plot of signal intensity and metabolites/chemicals are expressed by their frequencies[75]. Malignant hepatic lesions have been found to have elevated choline levels relative to normal liver parenchyma. Changes in metabolite frequencies exist between healthy and cirrhotic livers, namely choline and lipid levels[76]. Choline is a component of phospholipid membranes, which increases in states of cell proliferation and carcinogenesis. Zhang et al[77] reported the diagnostic efficacy of measuring choline-containing compounds using MR spectroscopy is high for discriminating malignant and benign tumors (sensitivity: 94.3% and specificity: 93.3%)[77]. Determining ratios of choline and lipids within a given lesion has also been used to monitor treatment responses after locoregional therapy[78]. For example, prospective investigations have found choline levels to decline following TACE therapy[79,80].
The integration of artificial intelligence and diagnostic imaging modalities has led to an exponential rise in radiogenomics or radiomics, which collectively refers to processes that aim to bridge quantitative radiologic data with immunobiological or clinical characteristics to inform prognosis or predict treatment outcomes[81]. The computing process consists of extracting quantitative features from medical images (CT, MRI or positron emission tomography) into large analyzable databases[82]. This process is carried out in multiple steps, including: (1) Defining volumes or regions of interest; (2) image segmentation (performed manually, semi-automatic or automatic tools); (3) image processing used to normalize grey-level intensities, denoise and improve data quality (e.g. signal intensity normalization, motion correction, filtering, image interpolation, and bias field correction); (4) feature extraction; and (5) statistical model building[83,84]. The final process, specific to oncology, includes generating association maps to correlate radiomic-based models with clinical outcome data, microvascular invasion, histological grade, or genomic/molecular data (e.g. immune marker expression).
Multiple studies have utilized either radiogenomic or radiomic models to diagnose and differentiate liver tumors or predict treatment efficacy for HCC[85-87]. For example, Lewis et al[88] reported greater prediction accuracy when combining LI-RADS and DWI-derived radiomics models using the ADC values than LI-RADS alone for distinguishing HCC from other primary liver cancers[88]. Banerjee et al[89] evaluated radiomic-models using contrast-enhanced CT to predict prognostic factors, such as microvascular invasion[89]. Radiogenomic venous invasion, an imaging biomarker, can predict microvascular invasion and correlates with lower overall and recurrence-free survival[89]. A recent meta-analysis/systematic review including 4947 patients showed promising predictive potential of radiomic models for microvascular invasion, reporting a pooled area under the curve (AUC) of 0.85, 0.87, and 0.74 across studies using CT, MR, and ultrasound-based models, respectively[90].
Given the link between gene expression and immunotherapy response, the ability to predict HCC immunoprofiles can be critical for delineating appropriate treatment. Hectors et al[91] retrospectively evaluated the relationship between MRI radiomic features (e.g. models using tumor size, enhancement ratios, fat content, ADC, texture features) and HCC immunhistochemical and genomic makers[91]. In particular, multiple relationships were found to exist between radiomic features and immunotherapy targets, cytotoxic T-lymphocyte-associated antigen 4 and programmed death 1[91]. Radiomic models have also shown promise for predicting treatment response and potential adverse outcomes after locoregional therapy use[92-96]. For example, MRI-based radiomics models have a reported AUC of 0.861 and 0.884 for predicting tumor response at 3-mo post TACE, evaluated using the mRECIST criterion. While larger, multi-center cohort models should be investigated in the future, the use of radiogenomics or radiomic models provide a novel method that can improve tumor grading, predicting prognosis and clinical decision-making strategies.
In summary, the use of imaging is an essential component to the diagnosis and management of HCC. Ultrasound is cost-effective modality for the screening. The utility of diagnostic modalities such as MR or CT for differentiation and grading of HCC continues to expand, especially with the advancement of new techniques and image analyses. The implementation of techniques, including elastography, T1 mapping, perfusion imaging and CEUS provide multiple unique benefits to further aid in the characterization of HCC. Other methods, such as radiomics/radiogenomics, which seek to integrate imaging data to predict prognostic risk factors and determine treatment response probability, will be a new frontier for informing clinical decision-making with the ultimate goal to generate more precise and personalized treatment management strategies for patients with HCC.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Radiology, nuclear medicine and medical imaging
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Kumar SKY, India; Papadopoulos N, Greece S-Editor: Fan JR L-Editor: A P-Editor: Fan JR
| 1. | Villanueva A. Hepatocellular Carcinoma. N Engl J Med. 2019;380:1450-1462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2066] [Cited by in RCA: 3173] [Article Influence: 528.8] [Reference Citation Analysis (37)] |
| 2. | Chakraborty E, Sarkar D. Emerging Therapies for Hepatocellular Carcinoma (HCC). Cancers (Basel). 2022;14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 134] [Cited by in RCA: 201] [Article Influence: 67.0] [Reference Citation Analysis (0)] |
| 3. | Harris PS, Hansen RM, Gray ME, Massoud OI, McGuire BM, Shoreibah MG. Hepatocellular carcinoma surveillance: An evidence-based approach. World J Gastroenterol. 2019;25:1550-1559. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 156] [Cited by in RCA: 153] [Article Influence: 25.5] [Reference Citation Analysis (4)] |
| 4. | McGlynn KA, Petrick JL, El-Serag HB. Epidemiology of Hepatocellular Carcinoma. Hepatology. 2021;73 Suppl 1:4-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 820] [Cited by in RCA: 1343] [Article Influence: 335.8] [Reference Citation Analysis (1)] |
| 5. | Parikh ND, Tayob N, Al-Jarrah T, Kramer J, Melcher J, Smith D, Marquardt P, Liu PH, Tang R, Kanwal F, Singal AG. Barriers to Surveillance for Hepatocellular Carcinoma in a Multicenter Cohort. JAMA Netw Open. 2022;5:e2223504. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 61] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 6. | Willatt J, Ruma JA, Azar SF, Dasika NL, Syed F. Imaging of hepatocellular carcinoma and image guided therapies - how we do it. Cancer Imaging. 2017;17:9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 24] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 7. | Marrero JA, Kulik LM, Sirlin CB, Zhu AX, Finn RS, Abecassis MM, Roberts LR, Heimbach JK. Diagnosis, Staging, and Management of Hepatocellular Carcinoma: 2018 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology. 2018;68:723-750. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2121] [Cited by in RCA: 3241] [Article Influence: 463.0] [Reference Citation Analysis (1)] |
| 8. | European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J Hepatol. 2018;69:182-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5593] [Cited by in RCA: 6059] [Article Influence: 865.6] [Reference Citation Analysis (3)] |
| 9. | Omata M, Cheng AL, Kokudo N, Kudo M, Lee JM, Jia J, Tateishi R, Han KH, Chawla YK, Shiina S, Jafri W, Payawal DA, Ohki T, Ogasawara S, Chen PJ, Lesmana CRA, Lesmana LA, Gani RA, Obi S, Dokmeci AK, Sarin SK. Asia-Pacific clinical practice guidelines on the management of hepatocellular carcinoma: a 2017 update. Hepatol Int. 2017;11:317-370. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1628] [Cited by in RCA: 1644] [Article Influence: 205.5] [Reference Citation Analysis (0)] |
| 10. | Ahn JC, Lee YT, Agopian VG, Zhu Y, You S, Tseng HR, Yang JD. Hepatocellular carcinoma surveillance: current practice and future directions. Hepatoma Res. 2022;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (1)] |
| 11. | Pocha C, Dieperink E, McMaken KA, Knott A, Thuras P, Ho SB. Surveillance for hepatocellular cancer with ultrasonography vs. computed tomography -- a randomised study. Aliment Pharmacol Ther. 2013;38:303-312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 130] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 12. | Zhang BH, Yang BH, Tang ZY. Randomized controlled trial of screening for hepatocellular carcinoma. J Cancer Res Clin Oncol. 2004;130:417-422. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 960] [Cited by in RCA: 944] [Article Influence: 45.0] [Reference Citation Analysis (1)] |
| 13. | Morgan TA, Maturen KE, Dahiya N, Sun MRM, Kamaya A; American College of Radiology Ultrasound Liver Imaging and Reporting Data System (US LI-RADS) Working Group. US LI-RADS: ultrasound liver imaging reporting and data system for screening and surveillance of hepatocellular carcinoma. Abdom Radiol (NY). 2018;43:41-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 83] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 14. | Schoenberger H, Chong N, Fetzer DT, Rich NE, Yokoo T, Khatri G, Olivares J, Parikh ND, Yopp AC, Marrero JA, Singal AG. Dynamic Changes in Ultrasound Quality for Hepatocellular Carcinoma Screening in Patients With Cirrhosis. Clin Gastroenterol Hepatol. 2022;20:1561-1569.e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 80] [Article Influence: 26.7] [Reference Citation Analysis (0)] |
| 15. | Kim SY, An J, Lim YS, Han S, Lee JY, Byun JH, Won HJ, Lee SJ, Lee HC, Lee YS. MRI With Liver-Specific Contrast for Surveillance of Patients With Cirrhosis at High Risk of Hepatocellular Carcinoma. JAMA Oncol. 2017;3:456-463. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 263] [Article Influence: 32.9] [Reference Citation Analysis (0)] |
| 16. | Tzartzeva K, Singal AG. Testing for AFP in combination with ultrasound improves early liver cancer detection. Expert Rev Gastroenterol Hepatol. 2018;12:947-949. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 29] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 17. | Tzartzeva K, Obi J, Rich NE, Parikh ND, Marrero JA, Yopp A, Waljee AK, Singal AG. Surveillance Imaging and Alpha Fetoprotein for Early Detection of Hepatocellular Carcinoma in Patients With Cirrhosis: A Meta-analysis. Gastroenterology. 2018;154:1706-1718.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 638] [Cited by in RCA: 811] [Article Influence: 115.9] [Reference Citation Analysis (0)] |
| 18. | Yilmaz N, Yilmaz UE, Suer K, Goral V, Cakir N. Screening for hepatocellular carcinoma: summary of current guidelines up to 2018. Hepatoma Research. 2018;4:46. DOI:10.20517/2394-5079.2018.49. [RCA] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 19. | Choi DT, Kum HC, Park S, Ohsfeldt RL, Shen Y, Parikh ND, Singal AG. Hepatocellular Carcinoma Screening Is Associated With Increased Survival of Patients With Cirrhosis. Clin Gastroenterol Hepatol. 2019;17:976-987.e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 95] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 20. | Beste LA, Ioannou GN, Yang Y, Chang MF, Ross D, Dominitz JA. Improved surveillance for hepatocellular carcinoma with a primary care-oriented clinical reminder. Clin Gastroenterol Hepatol. 2015;13:172-179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 101] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 21. | Sherman M. How to improve HCC surveillance outcomes. JHEP Rep. 2019;1:460-467. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 22. | Kanmaniraja D, Dellacerra G, Holder J, Erlichman D, Chernyak V. Liver Imaging Reporting and Data System (LI-RADS) v2018: Review of the CT/MRI Diagnostic Categories. Can Assoc Radiol J. 2021;72:142-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 23. | van der Pol CB, Lim CS, Sirlin CB, McGrath TA, Salameh JP, Bashir MR, Tang A, Singal AG, Costa AF, Fowler K, McInnes MDF. Accuracy of the Liver Imaging Reporting and Data System in Computed Tomography and Magnetic Resonance Image Analysis of Hepatocellular Carcinoma or Overall Malignancy-A Systematic Review. Gastroenterology. 2019;156:976-986. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 257] [Article Influence: 42.8] [Reference Citation Analysis (0)] |
| 24. | Kierans AS, Song C, Gavlin A, Roudenko A, Lu L, Askin G, Hecht EM. Diagnostic Performance of LI-RADS Version 2018, LI-RADS Version 2017, and OPTN Criteria for Hepatocellular Carcinoma. AJR Am J Roentgenol. 2020;215:1085-1092. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 25. | Choi JY, Lee JM, Sirlin CB. CT and MR imaging diagnosis and staging of hepatocellular carcinoma: part I. Development, growth, and spread: key pathologic and imaging aspects. Radiology. 2014;272:635-654. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 371] [Cited by in RCA: 351] [Article Influence: 31.9] [Reference Citation Analysis (1)] |
| 26. | Kulkarni NM, Fung A, Kambadakone AR, Yeh BM. Computed Tomography Techniques, Protocols, Advancements, and Future Directions in Liver Diseases. Magn Reson Imaging Clin N Am. 2021;29:305-320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 33] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 27. | Hope TA, Fowler KJ, Sirlin CB, Costa EA, Yee J, Yeh BM, Heiken JP. Hepatobiliary agents and their role in LI-RADS. Abdom Imaging. 2015;40:613-625. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 105] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 28. | Niendorf E, Spilseth B, Wang X, Taylor A. Contrast Enhanced MRI in the Diagnosis of HCC. Diagnostics (Basel). 2015;5:383-398. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 27] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 29. | Schima W, Heiken J. LI-RADS v2017 for liver nodules: how we read and report. Cancer Imaging. 2018;18:14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 30. | Wang G, Zhu S, Li X. Comparison of values of CT and MRI imaging in the diagnosis of hepatocellular carcinoma and analysis of prognostic factors. Oncol Lett. 2019;17:1184-1188. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 19] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 31. | Roberts LR, Sirlin CB, Zaiem F, Almasri J, Prokop LJ, Heimbach JK, Murad MH, Mohammed K. Imaging for the diagnosis of hepatocellular carcinoma: A systematic review and meta-analysis. Hepatology. 2018;67:401-421. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 244] [Cited by in RCA: 342] [Article Influence: 48.9] [Reference Citation Analysis (0)] |
| 32. | Basha MAA, AlAzzazy MZ, Ahmed AF, Yousef HY, Shehata SM, El Sammak DAEA, Fathy T, Obaya AA, Abdelbary EH. Does a combined CT and MRI protocol enhance the diagnostic efficacy of LI-RADS in the categorization of hepatic observations? Eur Radiol. 2018;28:2592-2603. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 47] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 33. | Bartolotta TV, Terranova MC, Gagliardo C, Taibbi A. CEUS LI-RADS: a pictorial review. Insights Imaging. 2020;11:9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 43] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 34. | Bartolotta TV, Taibbi A, Midiri M, Lagalla R. Contrast-enhanced ultrasound of hepatocellular carcinoma: where do we stand? Ultrasonography. 2019;38:200-214. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 27] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 35. | D'Onofrio M, Crosara S, De Robertis R, Canestrini S, Mucelli RP. Contrast-Enhanced Ultrasound of Focal Liver Lesions. AJR Am J Roentgenol. 2015;205:W56-W66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 142] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 36. | Zheng SG, Xu HX, Liu LN. Management of hepatocellular carcinoma: The role of contrast-enhanced ultrasound. World J Radiol. 2014;6:7-14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 45] [Cited by in RCA: 52] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 37. | Gaiani S, Celli N, Piscaglia F, Cecilioni L, Losinno F, Giangregorio F, Mancini M, Pini P, Fornari F, Bolondi L. Usefulness of contrast-enhanced perfusional sonography in the assessment of hepatocellular carcinoma hypervascular at spiral computed tomography. J Hepatol. 2004;41:421-426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 86] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 38. | Schwarze V, Marschner C, Völckers W, Grosu S, Negrão de Figueiredo G, Rübenthaler J, Clevert DA. Diagnostic value of contrast-enhanced ultrasound versus computed tomography for hepatocellular carcinoma: a retrospective, single-center evaluation of 234 patients. J Int Med Res. 2020;48:300060520930151. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 39. | Navin PJ, Venkatesh SK. Hepatocellular Carcinoma: State of the Art Imaging and Recent Advances. J Clin Transl Hepatol. 2019;7:72-85. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 28] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 40. | Kim SH, Kamaya A, Willmann JK. CT perfusion of the liver: principles and applications in oncology. Radiology. 2014;272:322-344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 141] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 41. | Oğul H, Kantarcı M, Genç B, Pirimoğlu B, Cullu N, Kızrak Y, Yılmaz O, Karabulut N. Perfusion CT imaging of the liver: review of clinical applications. Diagn Interv Radiol. 2014;20:379-389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 30] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 42. | Nakamura Y, Higaki T, Honda Y, Tatsugami F, Tani C, Fukumoto W, Narita K, Kondo S, Akagi M, Awai K. Advanced CT techniques for assessing hepatocellular carcinoma. Radiol Med. 2021;126:925-935. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 50] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 43. | Fischer MA, Kartalis N, Grigoriadis A, Loizou L, Stål P, Leidner B, Aspelin P, Brismar TB. Perfusion computed tomography for detection of hepatocellular carcinoma in patients with liver cirrhosis. Eur Radiol. 2015;25:3123-3132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 45] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 44. | Chartampilas E, Rafailidis V, Georgopoulou V, Kalarakis G, Hatzidakis A, Prassopoulos P. Current Imaging Diagnosis of Hepatocellular Carcinoma. Cancers (Basel). 2022;14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 39] [Reference Citation Analysis (0)] |
| 45. | Bai RJ, Li JP, Ren SH, Jiang HJ, Liu XD, Ling ZS, Huang Q, Feng GL. A correlation of computed tomography perfusion and histopathology in tumor edges of hepatocellular carcinoma. Hepatobiliary Pancreat Dis Int. 2014;13:612-617. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 46. | Taouli B, Johnson RS, Hajdu CH, Oei MT, Merad M, Yee H, Rusinek H. Hepatocellular carcinoma: perfusion quantification with dynamic contrast-enhanced MRI. AJR Am J Roentgenol. 2013;201:795-800. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 72] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 47. | Aronhime S, Calcagno C, Jajamovich GH, Dyvorne HA, Robson P, Dieterich D, Fiel MI, Martel-Laferriere V, Chatterji M, Rusinek H, Taouli B. DCE-MRI of the liver: effect of linear and nonlinear conversions on hepatic perfusion quantification and reproducibility. J Magn Reson Imaging. 2014;40:90-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 64] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 48. | Pahwa S, Liu H, Chen Y, Dastmalchian S, O'Connor G, Lu Z, Badve C, Yu A, Wright K, Chalian H, Rao S, Fu C, Vallines I, Griswold M, Seiberlich N, Zeng M, Gulani V. Quantitative perfusion imaging of neoplastic liver lesions: A multi-institution study. Sci Rep. 2018;8:4990. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 49. | Chen BB, Shih TT. DCE-MRI in hepatocellular carcinoma-clinical and therapeutic image biomarker. World J Gastroenterol. 2014;20:3125-3134. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 43] [Cited by in RCA: 51] [Article Influence: 4.6] [Reference Citation Analysis (1)] |
| 50. | Guglielmo FF, Venkatesh SK, Mitchell DG. Liver MR Elastography Technique and Image Interpretation: Pearls and Pitfalls. Radiographics. 2019;39:1983-2002. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 42] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 51. | Malik P, Pillai S, Agarwal K, Abdelwahed S, Bhandari R, Singh A, Chidharla A, Patel K, Singh P, Manaktala P, Rabbani R, Koritala T, Gupta S. Diagnostic Accuracy of Elastography and Liver Disease: A Meta-Analysis. Gastroenterology Res. 2022;15:232-239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 52. | Venkatesh SK, Yin M, Glockner JF, Takahashi N, Araoz PA, Talwalkar JA, Ehman RL. MR elastography of liver tumors: preliminary results. AJR Am J Roentgenol. 2008;190:1534-1540. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 259] [Cited by in RCA: 214] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 53. | Singh S, Fujii LL, Murad MH, Wang Z, Asrani SK, Ehman RL, Kamath PS, Talwalkar JA. Liver stiffness is associated with risk of decompensation, liver cancer, and death in patients with chronic liver diseases: a systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2013;11:1573-84.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 210] [Cited by in RCA: 238] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 54. | Cho HJ, Kim B, Kim HJ, Huh J, Kim JK, Lee JH, Seo CW, Ahn HR, Eun JW, Kim SS, Cho SW, Cheong JY. Liver stiffness measured by MR elastography is a predictor of early HCC recurrence after treatment. Eur Radiol. 2020;30:4182-4192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 36] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 55. | Gordic S, Ayache JB, Kennedy P, Besa C, Wagner M, Bane O, Ehman RL, Kim E, Taouli B. Value of tumor stiffness measured with MR elastography for assessment of response of hepatocellular carcinoma to locoregional therapy. Abdom Radiol (NY). 2017;42:1685-1694. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 34] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 56. | Zhang L, Chen J, Jiang H, Rong D, Guo N, Yang H, Zhu J, Hu B, He B, Yin M, Venkatesh SK, Ehman RL, Wang J. MR elastography as a biomarker for prediction of early and late recurrence in HBV-related hepatocellular carcinoma patients before hepatectomy. Eur J Radiol. 2022;152:110340. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 57. | Qayyum A, Hwang KP, Stafford J, Verma A, Maru DM, Sandesh S, Sun J, Pestana RC, Avritscher R, Hassan MM, Amin H, Rashid A, Wistuba II, Ehman RL, Ma J, Kaseb AO. Immunotherapy response evaluation with magnetic resonance elastography (MRE) in advanced HCC. J Immunother Cancer. 2019;7:329. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 36] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 58. | Taylor AJ, Salerno M, Dharmakumar R, Jerosch-Herold M. T1 Mapping: Basic Techniques and Clinical Applications. JACC Cardiovasc Imaging. 2016;9:67-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 271] [Cited by in RCA: 406] [Article Influence: 45.1] [Reference Citation Analysis (0)] |
| 59. | Hoffman DH, Ayoola A, Nickel D, Han F, Chandarana H, Shanbhogue KP. T1 mapping, T2 mapping and MR elastography of the liver for detection and staging of liver fibrosis. Abdom Radiol (NY). 2020;45:692-700. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 74] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 60. | Katsube T, Okada M, Kumano S, Hori M, Imaoka I, Ishii K, Kudo M, Kitagaki H, Murakami T. Estimation of liver function using T1 mapping on Gd-EOB-DTPA-enhanced magnetic resonance imaging. Invest Radiol. 2011;46:277-283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 174] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 61. | Peng Z, Jiang M, Cai H, Chan T, Dong Z, Luo Y, Li ZP, Feng ST. Gd-EOB-DTPA-enhanced magnetic resonance imaging combined with T1 mapping predicts the degree of differentiation in hepatocellular carcinoma. BMC Cancer. 2016;16:625. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 31] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 62. | Peng Z, Li C, Chan T, Cai H, Luo Y, Dong Z, Li ZP, Feng ST. Quantitative evaluation of Gd-EOB-DTPA uptake in focal liver lesions by using T1 mapping: differences between hepatocellular carcinoma, hepatic focal nodular hyperplasia and cavernous hemangioma. Oncotarget. 2017;8:65435-65444. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 63. | Mio M, Fujiwara Y, Tani K, Toyofuku T, Maeda T, Inoue T. Quantitative evaluation of focal liver lesions with T1 mapping using a phase-sensitive inversion recovery sequence on gadoxetic acid-enhanced MRI. Eur J Radiol Open. 2021;8:100312. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 64. | Rao C, Wang X, Li M, Zhou G, Gu H. Value of T1 mapping on gadoxetic acid-enhanced MRI for microvascular invasion of hepatocellular carcinoma: a retrospective study. BMC Med Imaging. 2020;20:43. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (1)] |
| 65. | Kele PG, van der Jagt EJ. Diffusion weighted imaging in the liver. World J Gastroenterol. 2010;16:1567-1576. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 106] [Cited by in RCA: 110] [Article Influence: 7.3] [Reference Citation Analysis (2)] |
| 66. | Saito K, Tajima Y, Harada TL. Diffusion-weighted imaging of the liver: Current applications. World J Radiol. 2016;8:857-867. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 19] [Cited by in RCA: 22] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 67. | Shankar S, Kalra N, Bhatia A, Srinivasan R, Singh P, Dhiman RK, Khandelwal N, Chawla Y. Role of Diffusion Weighted Imaging (DWI) for Hepatocellular Carcinoma (HCC) Detection and its Grading on 3T MRI: A Prospective Study. J Clin Exp Hepatol. 2016;6:303-310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 32] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 68. | Surov A, Pech M, Omari J, Fischbach F, Damm R, Fischbach K, Powerski M, Relja B, Wienke A. Diffusion-Weighted Imaging Reflects Tumor Grading and Microvascular Invasion in Hepatocellular Carcinoma. Liver Cancer. 2021;10:10-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 58] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 69. | Jing M, Cao Y, Zhang P, Zhang B, Lin X, Deng L, Han T, Zhou J. The Benefit of Apparent Diffusion Coefficient in Evaluating the Invasiveness of Hepatocellular Carcinoma. Front Oncol. 2021;11:719480. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 70. | Ludwig JM, Camacho JC, Kokabi N, Xing M, Kim HS. The Role of Diffusion-Weighted Imaging (DWI) in Locoregional Therapy Outcome Prediction and Response Assessment for Hepatocellular Carcinoma (HCC): The New Era of Functional Imaging Biomarkers. Diagnostics (Basel). 2015;5:546-563. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 24] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 71. | Yuan Z, Zhang J, Yang H, Ye XD, Xu LC, Li WT. Diffusion-Weighted MR Imaging of Hepatocellular Carcinoma: Current Value in Clinical Evaluation of Tumor Response to Locoregional Treatment. J Vasc Interv Radiol. 2016;27:20-30; quiz 31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 72. | Kokabi N, Camacho JC, Xing M, Qiu D, Kitajima H, Mittal PK, Kim HS. Apparent diffusion coefficient quantification as an early imaging biomarker of response and predictor of survival following yttrium-90 radioembolization for unresectable infiltrative hepatocellular carcinoma with portal vein thrombosis. Abdom Imaging. 2014;39:969-978. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 42] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 73. | Elrefaey Hasan BM, Abd ElHamid HAE, Khater NH, ElGendy W, Abdelrahman AS. Role of DWI in evaluation of HCC after radiofrequency ablation compared to dynamic MRI using MRI (3 T). Egyptian J Radiol Nucl Med. 2021;52:267. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 74. | Granata V, Fusco R, Filice S, Incollingo P, Belli A, Izzo F, Petrillo A. Comment on "State of the art in magnetic resonance imaging of hepatocellular carcinoma": the role of DWI. Radiol Oncol. 2019;53:369-370. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 75. | Qayyum A. MR spectroscopy of the liver: principles and clinical applications. Radiographics. 2009;29:1653-1664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 87] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 76. | Wang D, Li Y. 1H Magnetic Resonance Spectroscopy Predicts Hepatocellular Carcinoma in a Subset of Patients With Liver Cirrhosis: A Randomized Trial. Medicine (Baltimore). 2015;94:e1066. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 77. | Zhang L, Zhao X, Ouyang H, Wang S, Zhou C. Diagnostic value of 3.0T (1)H MRS with choline-containing compounds ratio (∆CCC) in primary malignant hepatic tumors. Cancer Imaging. 2016;16:25. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 78. | Yang K, Zhang XM, Yang L, Xu H, Peng J. Advanced imaging techniques in the therapeutic response of transarterial chemoembolization for hepatocellular carcinoma. World J Gastroenterol. 2016;22:4835-4847. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 22] [Cited by in RCA: 23] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 79. | Kuo YT, Li CW, Chen CY, Jao J, Wu DK, Liu GC. In vivo proton magnetic resonance spectroscopy of large focal hepatic lesions and metabolite change of hepatocellular carcinoma before and after transcatheter arterial chemoembolization using 3.0-T MR scanner. J Magn Reson Imaging. 2004;19:598-604. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 62] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 80. | Chen CY, Li CW, Kuo YT, Jaw TS, Wu DK, Jao JC, Hsu JS, Liu GC. Early response of hepatocellular carcinoma to transcatheter arterial chemoembolization: choline levels and MR diffusion constants--initial experience. Radiology. 2006;239:448-456. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 134] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 81. | Jeong WK, Jamshidi N, Felker ER, Raman SS, Lu DS. Radiomics and radiogenomics of primary liver cancers. Clin Mol Hepatol. 2019;25:21-29. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 52] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 82. | Gillies RJ, Kinahan PE, Hricak H. Radiomics: Images Are More than Pictures, They Are Data. Radiology. 2016;278:563-577. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4541] [Cited by in RCA: 5550] [Article Influence: 616.7] [Reference Citation Analysis (3)] |
| 83. | Shur JD, Doran SJ, Kumar S, Ap Dafydd D, Downey K, O'Connor JPB, Papanikolaou N, Messiou C, Koh DM, Orton MR. Radiomics in Oncology: A Practical Guide. Radiographics. 2021;41:1717-1732. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 212] [Article Influence: 53.0] [Reference Citation Analysis (0)] |
| 84. | van Timmeren JE, Cester D, Tanadini-Lang S, Alkadhi H, Baessler B. Radiomics in medical imaging-"how-to" guide and critical reflection. Insights Imaging. 2020;11:91. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 588] [Cited by in RCA: 767] [Article Influence: 153.4] [Reference Citation Analysis (0)] |
| 85. | Gong XQ, Tao YY, Wu YK, Liu N, Yu X, Wang R, Zheng J, Huang XH, Li JD, Yang G, Wei XQ, Yang L, Zhang XM. Progress of MRI Radiomics in Hepatocellular Carcinoma. Front Oncol. 2021;11:698373. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 31] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 86. | Fu J, Cao SJ, Song L, Tong XQ, Wang J, Yang M, Zou YH. Radiomics/Radiogenomics in hepatocellular carcinoma: Applications and challenges in interventional management. iLIVER. 2022;1:96-100. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 6] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 87. | Yao S, Ye Z, Wei Y, Jiang HY, Song B. Radiomics in hepatocellular carcinoma: A state-of-the-art review. World J Gastrointest Oncol. 2021;13:1599-1615. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 21] [Cited by in RCA: 18] [Article Influence: 4.5] [Reference Citation Analysis (3)] |
| 88. | Lewis S, Peti S, Hectors SJ, King M, Rosen A, Kamath A, Putra J, Thung S, Taouli B. Volumetric quantitative histogram analysis using diffusion-weighted magnetic resonance imaging to differentiate HCC from other primary liver cancers. Abdom Radiol (NY). 2019;44:912-922. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 42] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 89. | Banerjee S, Wang DS, Kim HJ, Sirlin CB, Chan MG, Korn RL, Rutman AM, Siripongsakun S, Lu D, Imanbayev G, Kuo MD. A computed tomography radiogenomic biomarker predicts microvascular invasion and clinical outcomes in hepatocellular carcinoma. Hepatology. 2015;62:792-800. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 203] [Cited by in RCA: 277] [Article Influence: 27.7] [Reference Citation Analysis (0)] |
| 90. | Zhong X, Long H, Su L, Zheng R, Wang W, Duan Y, Hu H, Lin M, Xie X. Radiomics models for preoperative prediction of microvascular invasion in hepatocellular carcinoma: a systematic review and meta-analysis. Abdom Radiol (NY). 2022;47:2071-2088. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 23] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 91. | Hectors SJ, Lewis S, Besa C, King MJ, Said D, Putra J, Ward S, Higashi T, Thung S, Yao S, Laface I, Schwartz M, Gnjatic S, Merad M, Hoshida Y, Taouli B. MRI radiomics features predict immuno-oncological characteristics of hepatocellular carcinoma. Eur Radiol. 2020;30:3759-3769. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 113] [Article Influence: 22.6] [Reference Citation Analysis (0)] |
| 92. | Niu XK, He XF. Development of a computed tomography-based radiomics nomogram for prediction of transarterial chemoembolization refractoriness in hepatocellular carcinoma. World J Gastroenterol. 2021;27:189-207. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 27] [Cited by in RCA: 31] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 93. | Kong C, Zhao Z, Chen W, Lv X, Shu G, Ye M, Song J, Ying X, Weng Q, Weng W, Fang S, Chen M, Tu J, Ji J. Prediction of tumor response via a pretreatment MRI radiomics-based nomogram in HCC treated with TACE. Eur Radiol. 2021;31:7500-7511. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 86] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 94. | Sheen H, Kim JS, Lee JK, Choi SY, Baek SY, Kim JY. A radiomics nomogram for predicting transcatheter arterial chemoembolization refractoriness of hepatocellular carcinoma without extrahepatic metastasis or macrovascular invasion. Abdom Radiol (NY). 2021;46:2839-2849. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 20] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 95. | Bai H, Meng S, Xiong C, Liu Z, Shi W, Ren Q, Xia W, Zhao X, Jian J, Song Y, Ni C, Gao X, Li Z. Preoperative CECT-based Radiomic Signature for Predicting the Response of Transarterial Chemoembolization (TACE) Therapy in Hepatocellular Carcinoma. Cardiovasc Intervent Radiol. 2022;45:1524-1533. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 96. | Zhao Y, Wang N, Wu J, Zhang Q, Lin T, Yao Y, Chen Z, Wang M, Sheng L, Liu J, Song Q, Wang F, An X, Guo Y, Li X, Wu T, Liu AL. Radiomics Analysis Based on Contrast-Enhanced MRI for Prediction of Therapeutic Response to Transarterial Chemoembolization in Hepatocellular Carcinoma. Front Oncol. 2021;11:582788. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 28] [Article Influence: 7.0] [Reference Citation Analysis (0)] |